Abstract
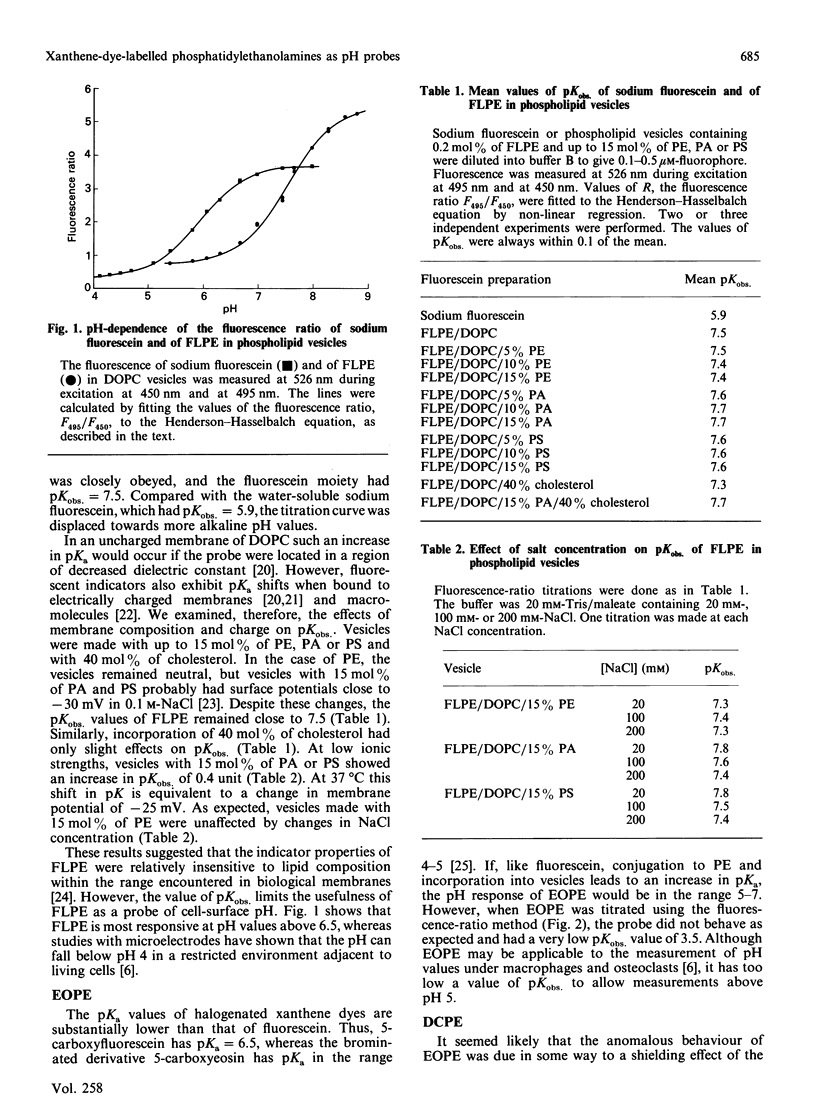
We have been developing the use of plasma-membrane-bound fluorescent probes to measure the pH values at the surfaces of living chondrocytes. For this purpose, three lipophilic pH indicators were made by covalently binding the xanthene dyes fluorescein, eosin or dichlorofluorescein to the amino group of phosphatidylethanolamine. The probes were incorporated into phospholipid vesicles and the effect of pH on the fluorescence was characterized. Fluorescence was measured at a single emission wavelength during excitation at two wavelengths, and the ratio of the intensities was calculated. The experimentally observed pKobs. values were determined by fitting the fluorescence ratios to the Henderson-Hasselbalch equation. All three probes acted as pH indicators, and the eosinyl-, dichlorofluoresceinyl- and fluoresceinylphosphatidylethanolamines had pKobs. values of 3.5, 6.3 and 7.5 respectively. At physiological salt concentrations, changes in the composition of the vesicle membrane had little effect on these values. We concluded that these probes were promising candidates for the measurement of pH values at cell surfaces.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright G. R., Fisher G. W., Rogowska J., Taylor D. L. Fluorescence ratio imaging microscopy: temporal and spatial measurements of cytoplasmic pH. J Cell Biol. 1987 Apr;104(4):1019–1033. doi: 10.1083/jcb.104.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner J., Skrabal P., Hauser H. Single bilayer vesicles prepared without sonication. Physico-chemical properties. Biochim Biophys Acta. 1976 Dec 2;455(2):322–331. doi: 10.1016/0005-2736(76)90308-4. [DOI] [PubMed] [Google Scholar]
- Dingle J. T., Dingle T. T. The site of cartilage matrix degradation. Biochem J. 1980 Aug 15;190(2):431–438. doi: 10.1042/bj1900431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T. The secretion of enzymes into the pericellular environment. Philos Trans R Soc Lond B Biol Sci. 1975 Jul 17;271(912):315–324. doi: 10.1098/rstb.1975.0055. [DOI] [PubMed] [Google Scholar]
- Duggleby R. G. Regression analysis of nonlinear Arrhenius plots: an empirical model and a computer program. Comput Biol Med. 1984;14(4):447–455. doi: 10.1016/0010-4825(84)90045-3. [DOI] [PubMed] [Google Scholar]
- Etherington D. J., Pugh D., Silver I. A. Collagen degradation in an experimental inflammatory lesion: studies on the role of the macrophage. Acta Biol Med Ger. 1981;40(10-11):1625–1636. [PubMed] [Google Scholar]
- Frelin C., Vigne P., Ladoux A., Lazdunski M. The regulation of the intracellular pH in cells from vertebrates. Eur J Biochem. 1988 May 16;174(1):3–14. doi: 10.1111/j.1432-1033.1988.tb14055.x. [DOI] [PubMed] [Google Scholar]
- Friedrich K., Woolley P. Electrostatic potential of macromolecules measured by pKa shift of a fluorophore. 1. The 3' terminus of 16S RNA. Eur J Biochem. 1988 Apr 5;173(1):227–231. doi: 10.1111/j.1432-1033.1988.tb13988.x. [DOI] [PubMed] [Google Scholar]
- Fromherz P. A new method for investigation of lipid assemblies with a lipoid pH indicator in monomolecular films. Biochim Biophys Acta. 1973 Oct 11;323(2):326–334. doi: 10.1016/0005-2736(73)90155-7. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Stryer L. Surface density determination in membranes by fluorescence energy transfer. Biochemistry. 1978 Nov 28;17(24):5241–5248. doi: 10.1021/bi00617a025. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hembry R. M., Knight C. G., Dingle J. T., Barrett A. J. Evidence that extracellular cathepsin D is not responsible for the resorption of cartilage matrix in culture. Biochim Biophys Acta. 1982 Feb 2;714(2):307–312. doi: 10.1016/0304-4165(82)90338-5. [DOI] [PubMed] [Google Scholar]
- Jubb R. W., Fell H. B. The breakdown of collagen by chondrocytes. J Pathol. 1980 Mar;130(3):159–167. doi: 10.1002/path.1711300304. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The pH in the neighborhood of membranes generating a protonmotive force. J Theor Biol. 1986 May 7;120(1):73–84. doi: 10.1016/s0022-5193(86)80018-2. [DOI] [PubMed] [Google Scholar]
- Madshus I. H. Regulation of intracellular pH in eukaryotic cells. Biochem J. 1988 Feb 15;250(1):1–8. doi: 10.1042/bj2500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Green G. D., Barrett A. J. Human liver cathepsin L. Biochem J. 1985 Feb 15;226(1):233–241. doi: 10.1042/bj2260233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales T. I., Wahl L. M., Hascall V. C. The effect of bacterial lipopolysaccharides on the biosynthesis and release of proteoglycans from calf articular cartilage cultures. J Biol Chem. 1984 Jun 10;259(11):6720–6729. [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe A., Tyler J. A., Hardingham T. E. Articular cartilage cultured with interleukin 1. Increased release of link protein, hyaluronate-binding region and other proteoglycan fragments. Biochem J. 1986 Sep 1;238(2):571–580. doi: 10.1042/bj2380571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver I. A., Murrills R. J., Etherington D. J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988 Apr;175(2):266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- Soucaille P., Prats M., Tocanne J. F., Teissié J. Use of a fluorescein derivative of phosphatidylethanolamine as a pH probe at water/lipid interfaces. Biochim Biophys Acta. 1988 Apr 7;939(2):289–294. doi: 10.1016/0005-2736(88)90073-9. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Pagano R. E. Insertion of fluorescent phospholipids into the plasma membrane of a mammalian cell. J Biol Chem. 1980 Jun 10;255(11):5404–5410. [PubMed] [Google Scholar]
- Thelen M., Petrone G., O'Shea P. S., Azzi A. The use of fluorescein-dipalmitoylphosphatidylethanolamine for measuring pH-changes in the internal compartment of phospholipid vesicles. Biochim Biophys Acta. 1984 Jul 27;766(1):161–168. doi: 10.1016/0005-2728(84)90228-7. [DOI] [PubMed] [Google Scholar]
- Tsui F. C., Ojcius D. M., Hubbell W. L. The intrinsic pKa values for phosphatidylserine and phosphatidylethanolamine in phosphatidylcholine host bilayers. Biophys J. 1986 Feb;49(2):459–468. doi: 10.1016/S0006-3495(86)83655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. A. Chondrocyte-mediated depletion of articular cartilage proteoglycans in vitro. Biochem J. 1985 Jan 15;225(2):493–507. doi: 10.1042/bj2250493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Nisksch A., Jähnig F. Electrostatic interactions at charged lipid membranes. Measurement of surface pH with fluorescent lipoid pH indicators. Eur J Biochem. 1978 Feb 1;83(1):299–305. doi: 10.1111/j.1432-1033.1978.tb12094.x. [DOI] [PubMed] [Google Scholar]


