Abstract
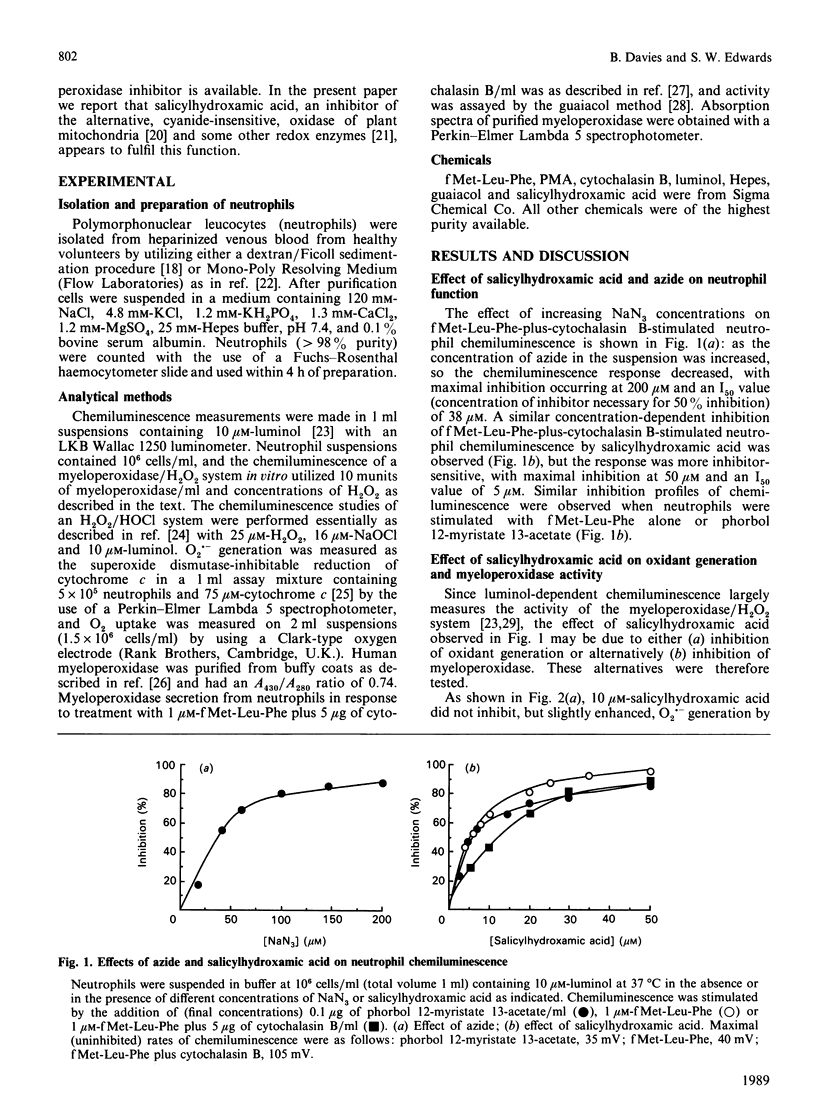
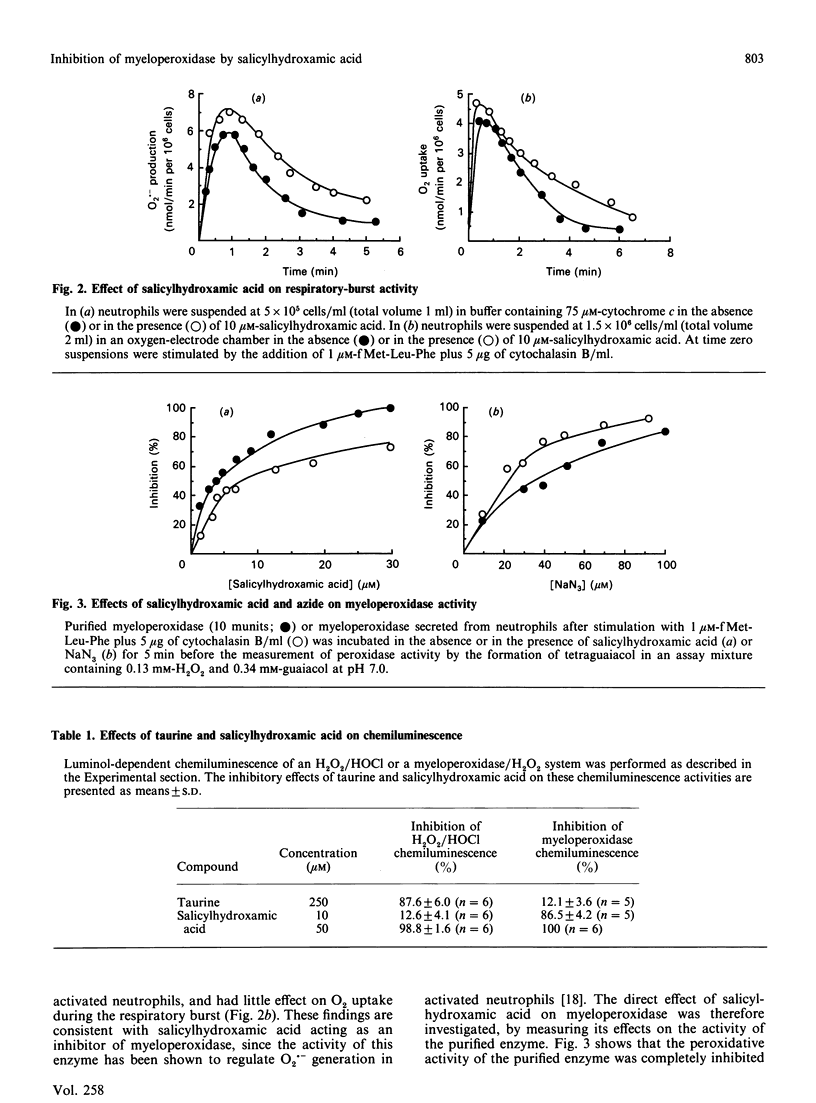
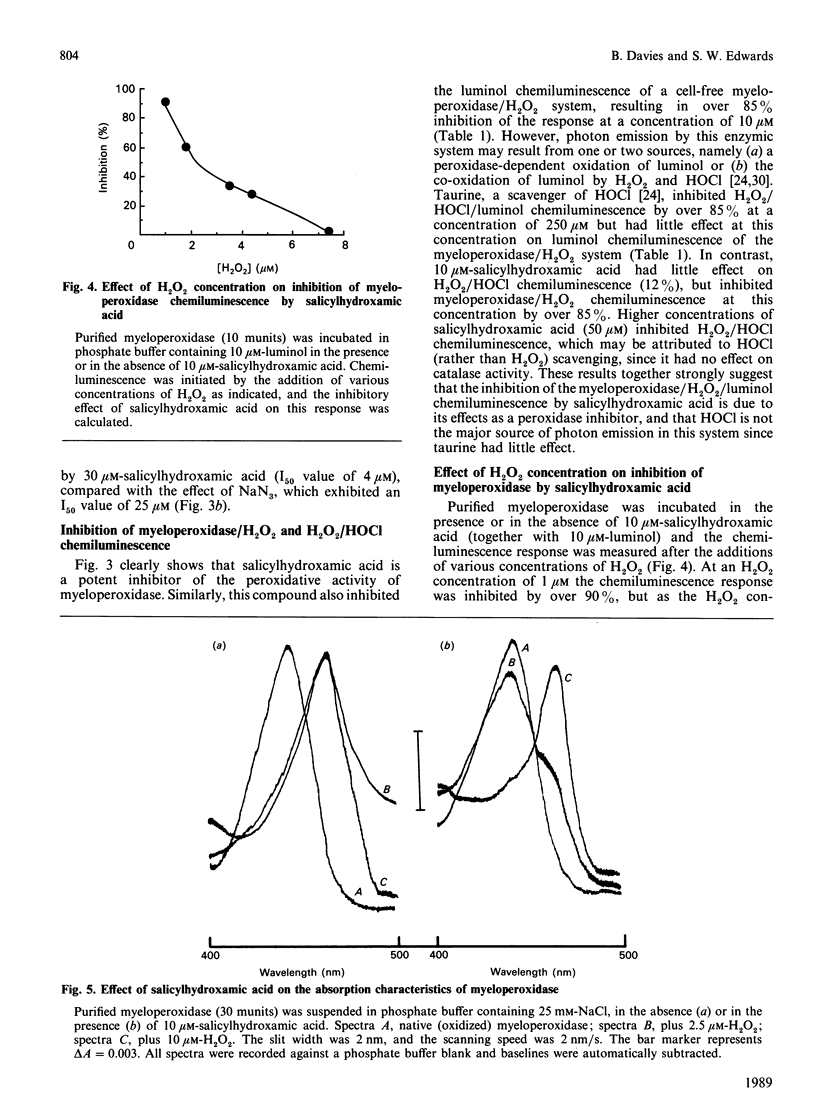
Salicylhydroxamic acid inhibited the luminol-dependent chemiluminescence of human neutrophils stimulated by phorbol 12-myristate 13-acetate or the chemotactic peptide N-formylmethionyl-leucyl-phenylalanine (fMet-Leu-Phe). This compound had no inhibitory effect on the kinetics of O2.- generation or O2 uptake during the respiratory burst, but inhibited both the peroxidative activity of purified myeloperoxidase and the chemiluminescence generated by a cell-free myeloperoxidase/H2O2 system. The concentration of salicylhydroxamic acid necessary for complete inhibition of myeloperoxidase activity was 30-50 microM (I50 values of 3-5 microM) compared with the non-specific inhibitor NaN3, which exhibited maximal inhibition at 100-200 microM (I50 values of 30-50 microM). Whereas taurine inhibited the luminol chemiluminescence of an H2O2/HOC1 system by HOC1 scavenging, this compound had little effect on myeloperoxidase/H2O2-dependent luminol chemiluminescence; in contrast, 10 microM-salicylhydroxamic acid did not quench HOC1 significantly but greatly diminished myeloperoxidase/H2O2-dependent luminol chemiluminescence, indicating that its effects on myeloperoxidase chemiluminescence were largely due to peroxidase inhibition rather than non-specific HOC1 scavenging. Salicylhydroxamic acid prevented the formation of myeloperoxidase Compound II, but only at low H2O2 concentrations, suggesting that it may compete for the H2O2-binding site on the enzyme. These data suggest that salicylhydroxamic acid may be used as a potent inhibitor to delineate the function of myeloperoxidase in neutrophil-mediated inflammatory events.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestel E. P. Co-oxidation of luminol by hypochlorite and hydrogen peroxide implications for neutrophil chemiluminescence. Biochem Biophys Res Commun. 1985 Jan 16;126(1):482–488. doi: 10.1016/0006-291x(85)90631-x. [DOI] [PubMed] [Google Scholar]
- Breton-Gorius J., Houssay D., Dreyfus B. Partial myeloperoxidase deficiency in a case of preleukaemia. I. Studies of fine structure and peroxidase synthesis of promyelocytes. Br J Haematol. 1975 Jul;30(3):273–278. doi: 10.1111/j.1365-2141.1975.tb00542.x. [DOI] [PubMed] [Google Scholar]
- Briheim G., Stendahl O., Dahlgren C. Intra- and extracellular events in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect Immun. 1984 Jul;45(1):1–5. doi: 10.1128/iai.45.1.1-5.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuperus R. A., Muijsers A. O., Wever R. The superoxide dismutase activity of myeloperoxidase; formation of compound III. Biochim Biophys Acta. 1986 May 12;871(1):78–84. doi: 10.1016/0167-4838(86)90135-4. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Lloyd D. CO-reacting haemoproteins of neutrophils: evidence for cytochrome b-245 and myeloperoxidase as potential oxidases during the respiratory burst. Biosci Rep. 1987 Mar;7(3):193–199. doi: 10.1007/BF01124789. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Lloyd D. Formation of myeloperoxidase compound II during aerobic stimulation of rat neutrophils. Biosci Rep. 1986 Mar;6(3):275–282. doi: 10.1007/BF01115156. [DOI] [PubMed] [Google Scholar]
- Edwards S. W. Luminol- and lucigenin-dependent chemiluminescence of neutrophils: role of degranulation. J Clin Lab Immunol. 1987 Jan;22(1):35–39. [PubMed] [Google Scholar]
- Edwards S. W., Nurcombe H. L., Hart C. A. Oxidative inactivation of myeloperoxidase released from human neutrophils. Biochem J. 1987 Aug 1;245(3):925–928. doi: 10.1042/bj2450925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Say J. E., Hart C. A. Oxygen-dependent killing of Staphylococcus aureus by human neutrophils. J Gen Microbiol. 1987 Dec;133(12):3591–3597. doi: 10.1099/00221287-133-12-3591. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Swan T. F. Regulation of superoxide generation by myeloperoxidase during the respiratory burst of human neutrophils. Biochem J. 1986 Jul 15;237(2):601–604. doi: 10.1042/bj2370601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Kitahara M., Eyre H. J., Simonian Y., Atkin C. L., Hasstedt S. J. Hereditary myeloperoxidase deficiency. Blood. 1981 May;57(5):888–893. [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Pincus S. H. Hydrogen peroxide utilization in myeloperoxidase-deficient leukocytes: a possible microbicidal control mechanism. J Clin Invest. 1971 Oct;50(10):2226–2229. doi: 10.1172/JCI106718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza F., Fietta A., Spisani S., Castoldi G. L., Traniello S. Does a relationship exist between neutrophil myeloperoxidase deficiency and the occurrence of neoplasms? J Clin Lab Immunol. 1987 Apr;22(4):175–180. [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao S., Stevens F. J., Ito A., Huberman E. Myeloperoxidase: a myeloid cell nuclear antigen with DNA-binding properties. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1232–1236. doi: 10.1073/pnas.85.4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef W. M. Myeloperoxidase deficiency. Hematol Oncol Clin North Am. 1988 Mar;2(1):135–158. [PubMed] [Google Scholar]
- Odajima T., Yamazaki I. Myeloperoxidase of the leukocyte of normal blood. I. Reaction of myeloperoxidase with hydrogen peroxide. Biochim Biophys Acta. 1970 Apr 22;206(1):71–77. doi: 10.1016/0005-2744(70)90083-5. [DOI] [PubMed] [Google Scholar]
- Parry M. F., Root R. K., Metcalf J. A., Delaney K. K., Kaplow L. S., Richar W. J. Myeloperoxidase deficiency: prevalence and clinical significance. Ann Intern Med. 1981 Sep;95(3):293–301. doi: 10.7326/0003-4819-95-3-293. [DOI] [PubMed] [Google Scholar]
- Pember S. O., Shapira R., Kinkade J. M., Jr Multiple forms of myeloperoxidase from human neutrophilic granulocytes: evidence for differences in compartmentalization, enzymatic activity, and subunit structure. Arch Biochem Biophys. 1983 Mar;221(2):391–403. doi: 10.1016/0003-9861(83)90158-3. [DOI] [PubMed] [Google Scholar]
- Pui C. H., Behm F. G., Kalwinsky D. K., Murphy S. B., Butler D. L., Dahl G. V., Mirro J. Clinical significance of low levels of myeloperoxidase positivity in childhood acute nonlymphoblastic leukemia. Blood. 1987 Jul;70(1):51–54. [PubMed] [Google Scholar]
- Rich P. R., Wiegand N. K., Blum H., Moore A. L., Bonner W. D., Jr Studies on the mechanism of inhibition of redox enzymes by substituted hydroxamic acids. Biochim Biophys Acta. 1978 Aug 7;525(2):325–337. doi: 10.1016/0005-2744(78)90227-9. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Cline M. J., Schultz J., Lehrer R. I. Myeloperoxidase deficiency. Immunologic study of a genetic leukocyte defect. N Engl J Med. 1970 Jan 29;282(5):250–253. doi: 10.1056/NEJM197001292820505. [DOI] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., Garcia R. C., Segal A. W. Production of the superoxide adduct of myeloperoxidase (compound III) by stimulated human neutrophils and its reactivity with hydrogen peroxide and chloride. Biochem J. 1985 Jun 15;228(3):583–592. doi: 10.1042/bj2280583. [DOI] [PMC free article] [PubMed] [Google Scholar]


