Abstract
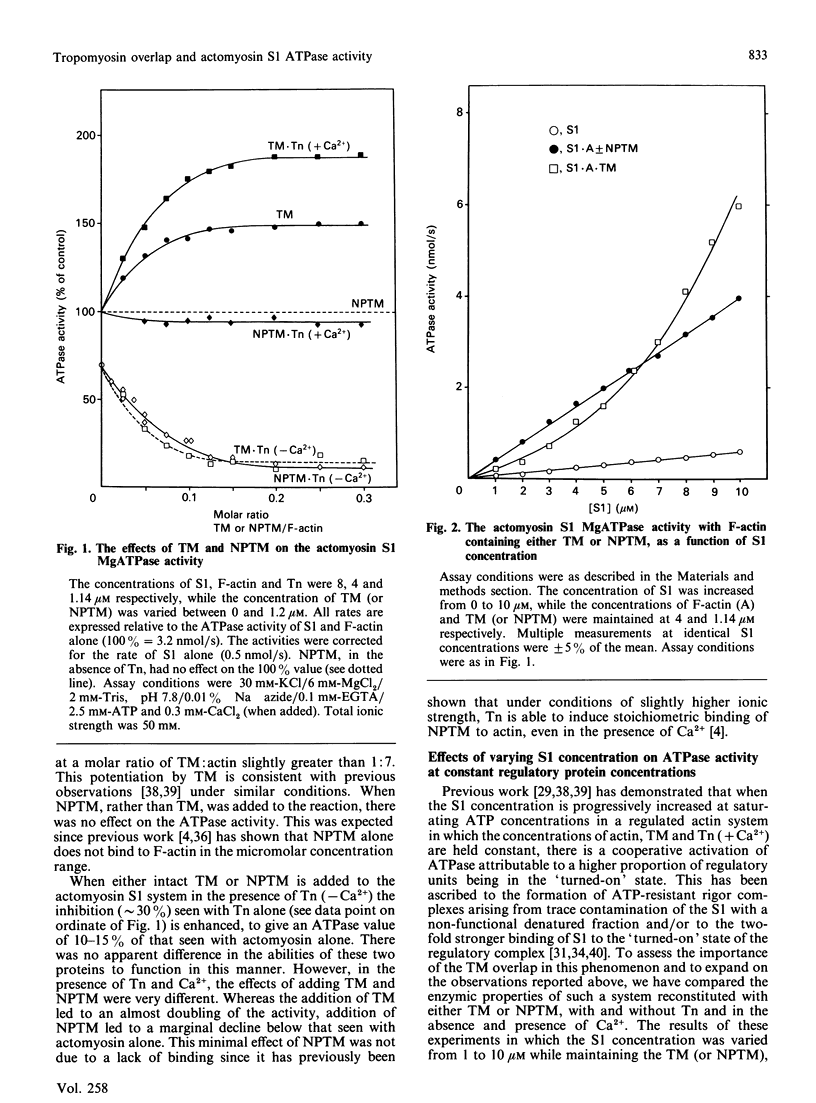
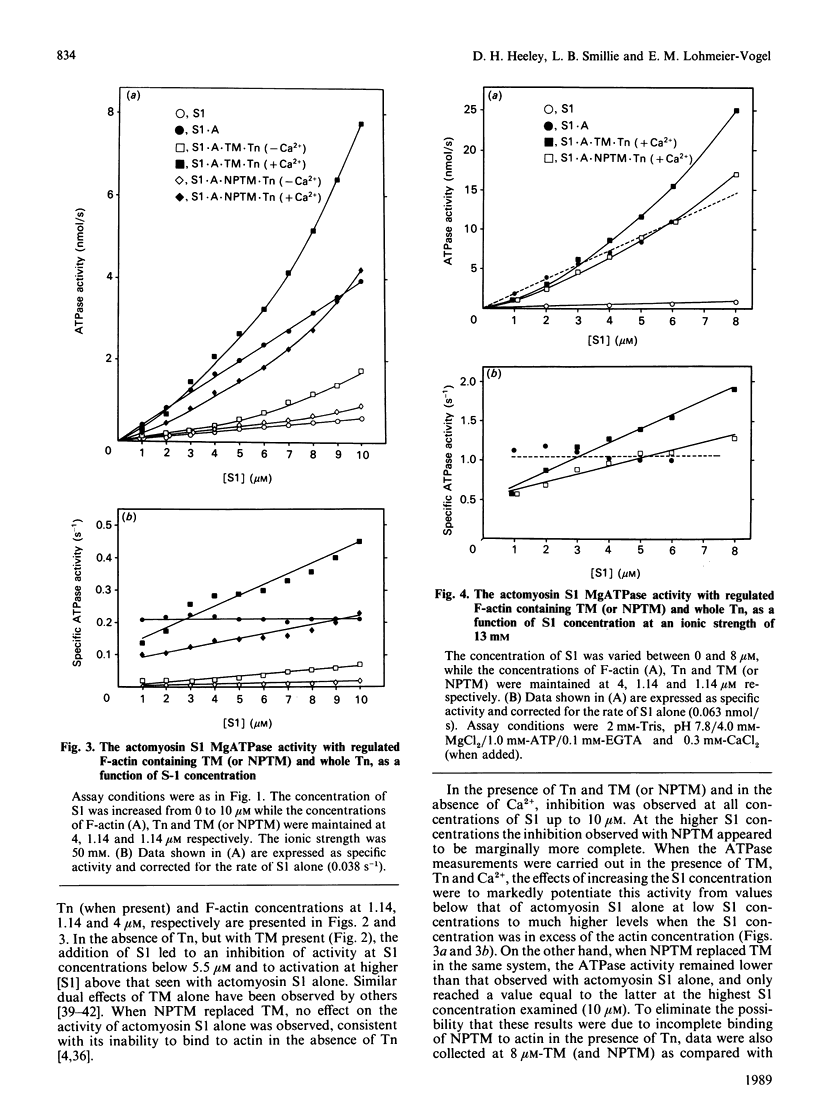
The role of the overlap region at the ends of tropomyosin molecules in the properties of regulated thin filaments has been investigated by substituting nonpolymerizable tropomyosin for tropomyosin in a reconstituted troponin-tropomyosin-actomyosin subfragment 1 ATPase assay system. A previous study [Heeley, Golosinka & Smillie (1987) J. Biol. Chem. 262, 9971-9978] has shown that at an ionic strength of 70 mM, troponin will induce full binding of nonpolymerizable tropomyosin to F-actin both in the presence and absence of calcium. At a myosin subfragment 1-to-actin ratio of 2:1 ([actin] = 4 microM) and an ionic strength of 50 mM, comparable levels of ATPase inhibition were observed with increasing levels of tropomyosin or the truncated derivative in the presence of troponin (-Ca2+). Large differences were noted, however, in the activation by Ca2+. Significantly lower ATPase activities were observed with nonpolymerizable tropomyosin and troponin (+Ca2+) over a range of subfragment 1-to-actin ratios from 0.25 to 2.5. The concentration of subfragment 1 required to generate ATPase activities exceeding those seen with actomyosin subfragment 1 alone under these conditions was 3-4-fold greater when nonpolymerizable tropomyosin was used. Similar effects were seen at the much lower ionic strength of 13 mM and are consistent with the reduced ATPase activity with nonpolymerizable tropomyosin observed previously [Walsh, Trueblood, Evans & Weber (1985) J. Mol. Biol. 182, 265-269] at low ionic strength and a subfragment 1-to-actin ratio of 1:100. Little cooperativity in activity as a function of subfragment 1 concentration with either intact tropomyosin or its truncated derivative was observed under the present conditions. Further studies are directed towards an understanding of these effects in terms of the two-state binding model for the attachment of myosin heads to regulated thin filaments.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt P. W., Diamond M. S., Schachat F. H. The thin filament of vertebrate skeletal muscle co-operatively activates as a unit. J Mol Biol. 1984 Dec 5;180(2):379–384. doi: 10.1016/s0022-2836(84)80010-8. [DOI] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Brisson J. R., Golosinska K., Smillie L. B., Sykes B. D. Interaction of tropomyosin and troponin T: a proton nuclear magnetic resonance study. Biochemistry. 1986 Aug 12;25(16):4548–4555. doi: 10.1021/bi00364a014. [DOI] [PubMed] [Google Scholar]
- Chong P. C., Hodges R. S. Photochemical cross-linking between rabbit skeletal troponin and alpha-tropomyosin. Attachment of the photoaffinity probe N-(4-azidobenzoyl-[2-3H]glycyl)-S-(2-thiopyridyl)-cysteine to cysteine 190 of alpha-tropomyosin. J Biol Chem. 1982 Aug 10;257(15):9152–9160. [PubMed] [Google Scholar]
- Eaton B. L., Kominz D. R., Eisenberg E. Correlation between the inhibition of the acto-heavy meromyosin ATPase and the binding of tropomyosin to F-actin: effects of Mg2+, KCl, troponin I, and troponin C. Biochemistry. 1975 Jun 17;14(12):2718–2725. doi: 10.1021/bi00683a025. [DOI] [PubMed] [Google Scholar]
- Eaton B. L. Tropomyosin binding to F-actin induced by myosin heads. Science. 1976 Jun 25;192(4246):1337–1339. doi: 10.1126/science.131972. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Wakabayashi T., Ebashi F. Troponin and its components. J Biochem. 1971 Feb;69(2):441–445. doi: 10.1093/oxfordjournals.jbchem.a129486. [DOI] [PubMed] [Google Scholar]
- Edwards B. F., Sykes B. D. Analysis of cooperativity observed in pH titrations of proton nuclear magnetic resonances of histidine residues of rabbit cardiac tropomyosin. Biochemistry. 1981 Jul 7;20(14):4193–4198. doi: 10.1021/bi00517a037. [DOI] [PubMed] [Google Scholar]
- Edwards B. F., Sykes B. D. Assignment and characterization of the histidine resonances in the 1H nuclear magnetic resonance spectra of rabbit tropomyosins. Biochemistry. 1978 Feb 21;17(4):684–689. doi: 10.1021/bi00597a020. [DOI] [PubMed] [Google Scholar]
- Edwards B. F., Sykes B. D. Nuclear magnetic resonance evidence for the coexistence of several conformational states of rabbit cardiac and skeletal tropomyosins. Biochemistry. 1980 Jun 10;19(12):2577–2583. doi: 10.1021/bi00553a007. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. Muscle contraction and free energy transduction in biological systems. Science. 1985 Mar 1;227(4690):999–1006. doi: 10.1126/science.3156404. [DOI] [PubMed] [Google Scholar]
- Grabarek Z., Grabarek J., Leavis P. C., Gergely J. Cooperative binding to the Ca2+-specific sites of troponin C in regulated actin and actomyosin. J Biol Chem. 1983 Dec 10;258(23):14098–14102. [PubMed] [Google Scholar]
- Graceffa P., Lehrer S. S. The excimer fluorescence of pyrene-labeled tropomyosin. A probe of conformational dynamics. J Biol Chem. 1980 Dec 10;255(23):11296–11300. [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 May;77(5):2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Relationship between regulated actomyosin ATPase activity and cooperative binding of myosin to regulated actin. Cell Biophys. 1988 Jan-Jun;12:59–71. doi: 10.1007/BF02918350. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Williams D. L., Jr, Eisenberg E. Regulation of actomyosin ATPase activity by troponin-tropomyosin: effect of the binding of the myosin subfragment 1 (S-1).ATP complex. Proc Natl Acad Sci U S A. 1987 May;84(10):3102–3106. doi: 10.1073/pnas.84.10.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeley D. H., Golosinska K., Smillie L. B. The effects of troponin T fragments T1 and T2 on the binding of nonpolymerizable tropomyosin to F-actin in the presence and absence of troponin I and troponin C. J Biol Chem. 1987 Jul 25;262(21):9971–9978. [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Greene L. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3186–3190. doi: 10.1073/pnas.77.6.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama E. Interaction of troponin-I with troponin-T and its fragment. J Biochem. 1979 May;85(5):1379–1381. [PubMed] [Google Scholar]
- Kay C. M., McCubbin W. D., Sykes B. D. Biophysical studies on the calcium trigger of muscle contraction. Biopolymers. 1987;26 (Suppl):S123–S144. doi: 10.1002/bip.360260013. [DOI] [PubMed] [Google Scholar]
- Kress M., Huxley H. E., Faruqi A. R., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol. 1986 Apr 5;188(3):325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- Leavis P. C., Gergely J. Thin filament proteins and thin filament-linked regulation of vertebrate muscle contraction. CRC Crit Rev Biochem. 1984;16(3):235–305. doi: 10.3109/10409238409108717. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S., Morris E. P. Dual effects of tropomyosin and troponin-tropomyosin on actomyosin subfragment 1 ATPase. J Biol Chem. 1982 Jul 25;257(14):8073–8080. [PubMed] [Google Scholar]
- Mak A. S., Golosinska K., Smillie L. B. Induction of nonpolymerizable tropomyosin binding to F-actin by troponin and its components. J Biol Chem. 1983 Dec 10;258(23):14330–14334. [PubMed] [Google Scholar]
- Mak A. S., Smillie L. B. Non-polymerizable tropomyosin: preparation, some properties and F-actin binding. Biochem Biophys Res Commun. 1981 Jul 16;101(1):208–214. doi: 10.1016/s0006-291x(81)80032-0. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Smillie L. B. Structural interpretation of the two-site binding of troponin on the muscle thin filament. J Mol Biol. 1981 Jul 5;149(3):541–550. doi: 10.1016/0022-2836(81)90486-1. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Morris E. P., Lehrer S. S. Troponin-tropomyosin interactions. Fluorescence studies of the binding of troponin, troponin T, and chymotryptic troponin T fragments to specifically labeled tropomyosin. Biochemistry. 1984 May 8;23(10):2214–2220. doi: 10.1021/bi00305a018. [DOI] [PubMed] [Google Scholar]
- Murray J. M., Knox M. K., Trueblood C. E., Weber A. Potentiated state of the tropomyosin actin filament and nucleotide-containing myosin subfragment 1. Biochemistry. 1982 Mar 2;21(5):906–915. doi: 10.1021/bi00534a015. [DOI] [PubMed] [Google Scholar]
- Murray J. M., Weber A. Cooperativity of the calcium switch of regulated rabbit actomyosin system. Mol Cell Biochem. 1981 Feb 26;35(1):11–15. doi: 10.1007/BF02358183. [DOI] [PubMed] [Google Scholar]
- Murray J. M., Weber A., Knox M. K. Myosin subfragment 1 binding to relaxed actin filaments and steric model of relaxation. Biochemistry. 1981 Feb 3;20(3):641–649. doi: 10.1021/bi00506a030. [DOI] [PubMed] [Google Scholar]
- Ohtsuki I. Molecular arrangement of troponin-T in the thin filament. J Biochem. 1979 Aug;86(2):491–497. doi: 10.1093/oxfordjournals.jbchem.a132549. [DOI] [PubMed] [Google Scholar]
- Otsuk I. Distribution of troponin components in the thin filament studied by immunoelectron microscopy. J Biochem. 1975 Mar;77(3):633–639. doi: 10.1093/oxfordjournals.jbchem.a130765. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Squire J. M. Structural role of tropomyosin in muscle regulation: analysis of the x-ray diffraction patterns from relaxed and contracting muscles. J Mol Biol. 1973 Mar 25;75(1):33–55. doi: 10.1016/0022-2836(73)90527-5. [DOI] [PubMed] [Google Scholar]
- Pearlstone J. R., Smillie L. B. The binding sites of rabbit skeletal troponin-I on troponin-T. Can J Biochem. 1980 Aug;58(8):649–654. doi: 10.1139/o80-090. [DOI] [PubMed] [Google Scholar]
- Pearlstone J. R., Smillie L. B. Troponin T fragments: physical properties and binding to troponin C. Can J Biochem. 1978 Jun;56(6):521–527. doi: 10.1139/o78-080. [DOI] [PubMed] [Google Scholar]
- Rosenfeld S. S., Taylor E. W. Kinetic studies of calcium and magnesium binding to troponin C. J Biol Chem. 1985 Jan 10;260(1):242–251. [PubMed] [Google Scholar]
- Rosenfeld S. S., Taylor E. W. Kinetic studies of calcium binding to regulatory complexes from skeletal muscle. J Biol Chem. 1985 Jan 10;260(1):252–261. [PubMed] [Google Scholar]
- Solaro R. J., Shiner J. S. Modulation of Ca2+ control of dog and rabbit cardiac myofibrils by Mg2+. Comparison with rabbit skeletal myofibrils. Circ Res. 1976 Jul;39(1):8–14. doi: 10.1161/01.res.39.1.8. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stone D., Smillie L. B. The amino acid sequence of rabbit skeletal alpha-tropomyosin. The NH2-terminal half and complete sequence. J Biol Chem. 1978 Feb 25;253(4):1137–1148. [PubMed] [Google Scholar]
- Tobacman L. S. Activation of actin-cardiac myosin subfragment 1 MgATPase rate by Ca2+ shows cooperativity intrinsic to the thin filament. Biochemistry. 1987 Jan 27;26(2):492–497. doi: 10.1021/bi00376a022. [DOI] [PubMed] [Google Scholar]
- Walsh T. P., Trueblood C. E., Evans R., Weber A. Removal of tropomyosin overlap and the co-operative response to increasing calcium concentrations of the acto-subfragment-1 ATPase. J Mol Biol. 1985 Mar 20;182(2):265–269. doi: 10.1016/0022-2836(85)90344-4. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Wegner A. Equilibrium of the actin-tropomyosin interaction. J Mol Biol. 1979 Jul 15;131(4):839–853. doi: 10.1016/0022-2836(79)90204-3. [DOI] [PubMed] [Google Scholar]
- White S. P., Cohen C., Phillips G. N., Jr Structure of co-crystals of tropomyosin and troponin. 1987 Feb 26-Mar 4Nature. 325(6107):826–828. doi: 10.1038/325826a0. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Jr, Greene L. E., Eisenberg E. Comparison of effects of smooth and skeletal muscle tropomyosins on interactions of actin and myosin subfragment 1. Biochemistry. 1984 Aug 28;23(18):4150–4155. doi: 10.1021/bi00313a022. [DOI] [PubMed] [Google Scholar]
- Zot A. S., Potter J. D. Structural aspects of troponin-tropomyosin regulation of skeletal muscle contraction. Annu Rev Biophys Biophys Chem. 1987;16:535–559. doi: 10.1146/annurev.bb.16.060187.002535. [DOI] [PubMed] [Google Scholar]


