Abstract
This study was undertaken to demonstrate the feasibility of whole-body 62Cu-ethylglyoxal bis(thiosemicarbazonato)copper(II) (62Cu-ETS) PET/CT tumor perfusion imaging in patients with metastatic renal carcinoma and to validate 62Cu-ETS as a quantitative marker of tumor perfusion by direct comparison with 15O-water perfusion imaging.
Methods:
PET/CT imaging of 10 subjects with stage IV renal cell cancer was performed after intravenous administration of 15O-water (10-min dynamic list-mode study) with the heart and at least 1 tumor in the PET field of view, followed 10 min later by intravenous 62Cu-ETS (6-min list-mode study). Whole-body 62Cu imaging was then performed from 6 to 20 min at 2–3 min/bed position. Blood flow (K1) was quantified with both agents for normal and malignant tissues in the 21.7-cm dynamic field of view. The required arterial input functions were derived from the left atrium and, in the case of 62Cu-ETS, corrected for partial decomposition of the agent by blood with data from an in vitro analysis using a sample of each patient’s blood. This imaging protocol was repeated at an interval of 3–4 wk after initiation of a standard clinical treatment course of the antiangiogenic agent sunitinib.
Results:
All subjects received the scheduled 62Cu-ETS doses for the dynamic and subsequent whole-body PET/CT scans, but technical issues resulted in no baseline 15O-water data for 2 subjects. Direct comparisons of the perfusion estimates for normal tissues and tumor metastases were made in 18 paired baseline and treatment studies (10 subjects; 8 baseline studies, 10 repeated studies during treatment). There was an excellent correlation between the blood flow estimates made with 62Cu-ETS and 15O-water for normal tissues (muscle, thyroid, myocardium) and malignant lesions (pulmonary nodules, bone lesions); the regression line was y = 0.85x + 0.15, R2 = 0.83, for the 88 regions analyzed.
Conclusion:
62Cu-ETS provided high-quality whole-body PET/CT images, and 62Cu-ETS measures of blood flow were highly and linearly correlated with 15O-water–derived K1 values (mL−1·min−1·g). This tracer is suitable for use as a PET tracer of tumor perfusion in patients with metastatic renal cell carcinoma.
Keywords: tumor perfusion, 62Cu PET, 62Cu-ETS [ethylglyoxal bis(thiosemicarbazonato)copper(II)], 15O-water, renal cell carcinoma
Because of the varied and distributed nature of metastatic disease, the clinical care of cancer patients often demands diagnostic methods that are capable for whole-body imaging. Thus, whole-body scanning is a clinical standard in metabolic imaging of tumors with 18F-FDG as well as in imaging to detect skeletal metastases based on rates of bone remodeling and in the imaging of neuroendocrine tumors using somatostatin receptor–targeted agents. A technique for whole-body assessment of tumor perfusion would naturally complement metabolic imaging in the definition of the tumor microenvironment and could also assist in evaluation of patient response to antiangiogenic therapies.
The growth rates of solid tumors are often limited by their capacity to recruit vasculature for nutritive perfusion (1–3), leading to widespread interest in tumor vascular endothelium as a target for the action of chemotherapeutic drugs (4–6). Tumor vasculature is morphologically abnormal (2,7), and unlike normal tissues the rates of tumor perfusion and metabolism are often uncoupled and unpredictably heterogeneous. The heterogeneity of tumor perfusion affects the efficiency of drug delivery while also leading to zones of hypoxia that may be quite resistant to treatment by radiation therapy (8–10). A whole-body scanning technique for assessment of tumor perfusion would potentially complement standard whole-body 18F-FDG imaging of tumor metabolism, offering further insights into the physiology of a patient’s tumors and their response to treatment (10).
15O-labeled water is the gold standard for PET quantification of regional tissue perfusion, having been validated to behave as a freely diffusible tracer in myocardium (11–14) and to also robustly allow quantification of cerebral blood flow (15,16). As a freely diffusible tracer, 15O-water is also the standard for noninvasive imaging to quantify tumor perfusion (17–20). The 2-min physical half-life of 15O is ideally suited to imaging at high activity levels over the intrinsically brief time interval in which the distribution of a freely diffusible tracer is dominated by its perfusion rate-limited delivery to tissue. However, for routine clinical imaging of tumor perfusion, 15O-water faces significant technical and practical barriers, including the need for an on-site cyclotron for 15O production; intrinsic need to image 15O-water at high counting rates to obtain good counting statistics; imaging being constrained to a single position of the scanner bed, precluding flow assessment using whole-body imaging protocols of the type routinely used in clinical oncology studies with 18F-FDG; and the need for invasive arterial blood sampling after each 15O-water injection, if flow is to be quantified and the heart, or another large arterial blood pool region, falls outside the camera’s field of view.
The 62Zn/62Cu generator system has the potential to provide widespread routine clinical access to short-lived 62Cu (9.7-min half-life) for PET/CT imaging without the limitations of 15O (21–23). Copper(II) bis(thiosemicarbazone) complexes, most notably Cu-PTSM (pyruvaldehyde bis[N4-methylthiosemicarbazonato] copper[II]) and Cu-ETS (ethylglyoxal bis[thiosemicarbazonato]copper[II]), have shown promise for use as 62Cu radiopharmaceuticals for perfusion imaging with PET (21,22,24–33). In addition to exhibiting the desired high first-pass tissue extraction of tracer, these agents also desirably afford prolonged microsphere-like tissue retention of the radiolabel (24,33–35) as they undergo rapid intracellular reductive decomposition, liberating ionic 62Cu into the endogenous intracellular copper pool (36,37). For human studies, 62Cu-ETS should be superior to 62Cu-PTSM for perfusion assessment in high-flow tissues because it avoids Cu-PTSM’s strong species-specific association with human serum albumin (38,39).
The purpose of the present study was to demonstrate the feasibility of whole-body 62Cu-ETS PET/CT tumor perfusion imaging in patients with metastatic renal carcinoma and to begin validation of 62Cu-ETS as a quantitative marker of tumor perfusion by direct comparison to 15O-water perfusion imaging.
MATERIALS AND METHODS
The study protocol was approved by the Indiana University institutional review board. All patients had a diagnosis of advanced-stage IV renal cell cancer and agreed to participate in the study after informed consent. The primary renal cell cancer had been surgically removed in all of our subjects.
Radiopharmaceuticals
Proportional Technologies, Inc., produced the required 62Zn/62Cu generators and the lyophilized cold kits and reagents for on-demand preparation of the 62Cu-ETS radiopharmaceutical. Details of generator and radiopharmaceutical kit performance are reported elsewhere (23). Patient imaging with 62Cu-ETS was performed under investigational new drug #75,018 held by Proportional Technologies, Inc. 15O-water was prepared to the specifications of its U.S. Pharmacopeia monograph by the PET Radiochemistry Core of the Indiana Institute for Biomedical Imaging Sciences.
Imaging Protocols and Procedures
All imaging studies were performed on a 64-slice Siemens True-D Biograph PET/CT scanner. After a CT examination for attenuation correction in a single bed position centered over the thorax (with the field of view including at least 1 known tumor site and the heart for image-based reconstruction of the 15O-water arterial blood time–activity curve), a 10-min dynamic list-mode acquisition was performed on intravenous administration of 15O-water (0.766 ± 0.118 GBq [20.7 ± 3.2 mCi]; n = 18). This acquisition was followed by a 10-min delay to allow for decay of the 15O. A dynamic list-mode acquisition was then performed for 6 min in the same bed position after intravenous administration of 62Cu-ETS (0.64 ± 0.13 GBq [17.2 ± 3.5 mCi]; n = 20). An additional CT examination of the whole body was then performed to provide attenuation correction for the whole-body PET/CT 62Cu-ETS examination. Whole-body 62Cu images were then acquired from 6 to 20 min after 62Cu-ETS injection (2–3 min per 21.6-cm bed position), allowing assessment of the perfusion distribution in normal tissue and tumor sites outside the initial, single-bed field of view. This imaging protocol was repeated after initiation of a standard 3- to 4-wk clinical treatment course of sunitinib.
In Vitro Assay to Define Kinetics of 62Cu-ETS Decomposition by Patient Blood
Because a portion of the injected 62Cu-ETS radiopharmaceutical will be reductively decomposed by the patient’s blood cells, in vitro assays were performed to directly examine the rate of 62Cu-ETS decomposition by patient blood, allowing correction of the PET-derived arterial blood 62Cu time–activity curve to represent the fraction of blood radioactivity expected to remain present as intact 62Cu-ETS (27,33,40). Details of this method are presented in the supplemental materials (available at http://jnm.snmjournals.org).
PET/CT Data Analysis
Three-dimensional volumes of interest were generated using the MIM software PET Edge tool (MIM Software, Inc.) for normal tissues (muscle, thyroid, myocardium, ventricular blood pools) and any malignant lesions that were identified in the 21.7-cm field of view used for dynamic list-mode acquisition. The standard 2-compartment model routinely used for the measurement of blood flow in the brain and heart was applied to the measurement of tumor perfusion with 15O-water (11–17). Details are presented in the accompanying supplemental materials. Briefly, in this model the transport of the tracer from the vasculature to the interstitial space is assumed to be sufficiently rapid that the single-pass extraction fraction of water is approximately equal to 1.0 across the range of perfusion values. This model consists of 2 constants, K1 and k2, representing the tissue perfusion and tracer washout rates, respectively. Both model rate constants are related to tissue perfusion with:
In these equations, RBF is regional blood flow, EF is the tracer single-pass extraction fraction, and Vd is the tracer volume of distribution in tissue. For the lung metastases, if the dynamic data showed radiotracer arriving at the tumor before arrival in the left ventricular blood pool, the radionuclide in the right ventricular blood volume was used to define that lesion’s arterial input function. For all other tissues, modeling for flow quantification was based on an input function derived from a region of interest for the blood pool of the left ventricle or atrium.
For 62Cu-ETS PET, flow was quantified with a 2-compartment model that was previously used with success in myocardial flow quantification with 62Cu-PTSM (27,29). The model was fit using an image-derived input function, analogous to the 15O-water study, corrected to account for partial 62Cu-ETS decomposition in the blood (27,33,40).
In addition to the modeling to obtain quantitative tumor perfusion measurements from the dynamic 62Cu-ETS data, we also quantified normal-tissue and tumor uptake of 62Cu-ETS as standardized uptake values (SUVs) using the attenuation-corrected whole-body examination and 3-dimensional volumes of interest. SUVs were obtained for normal tissues (muscle, thyroid, myocardium, right ventricular blood pool, lung, left atrial blood pool, liver, pancreas, and kidney) and for any areas of increased tracer localization representing metastatic renal cell cancer in the lungs or bone. Mean and maximum SUV measurements were obtained and normalized to body weight and lean body mass.
RESULTS
The study enrolled 10 subjects, 2 women and 8 men. Average age was 62.8 ± 8.5 y (range, 49–77 y) and body mass 86.4 ± 21 kg (range, 60.7–113.5 kg). All subjects had a diagnosis of advanced stage renal cell cancer with metastatic skeletal or metastatic pulmonary nodular disease. The average time interval between the baseline and repeated (during sunitinib treatment) PET/CT studies was 32 ± 9 d (range, 21–49 d).
Eighteen dynamic 15O-water studies were successfully performed. Technical difficulties prevented baseline 15O-water imaging in 2 subjects. All 10 subjects received the scheduled pretreatment and during-treatment doses of 62Cu-ETS for dynamic list-mode imaging and the subsequent companion whole-body PET/CT acquisition. Vital signs were monitored before, during, and after administration of the 62Cu-ETS, and no changes were observed. No adverse events were noted.
Examples of images from dynamic list-mode acquisitions of the 2 tracers and a whole-body 62Cu-ETS acquisition are shown in Figures 1 and 2. The 62Cu-ETS radiopharmaceutical is rapidly cleared from the blood (Fig. 3), but the image-derived arterial input function does need to be corrected for the partial decomposition of tracer by blood cells. This correction was made on a study-by-study basis, using the rate of 62Cu-ETS decomposition measured with a sample of that patient’s blood at 37°C in vitro. The decomposition of 62Cu-ETS by blood always exhibited first-order kinetics, but the reaction rate was quite variable (half-time of decomposition averaging 115 ± 87 s, n = 19, or 96 ± 27 s with the omission of a blood sample for which the decomposition rate appeared anomalously slow).
FIGURE 1.
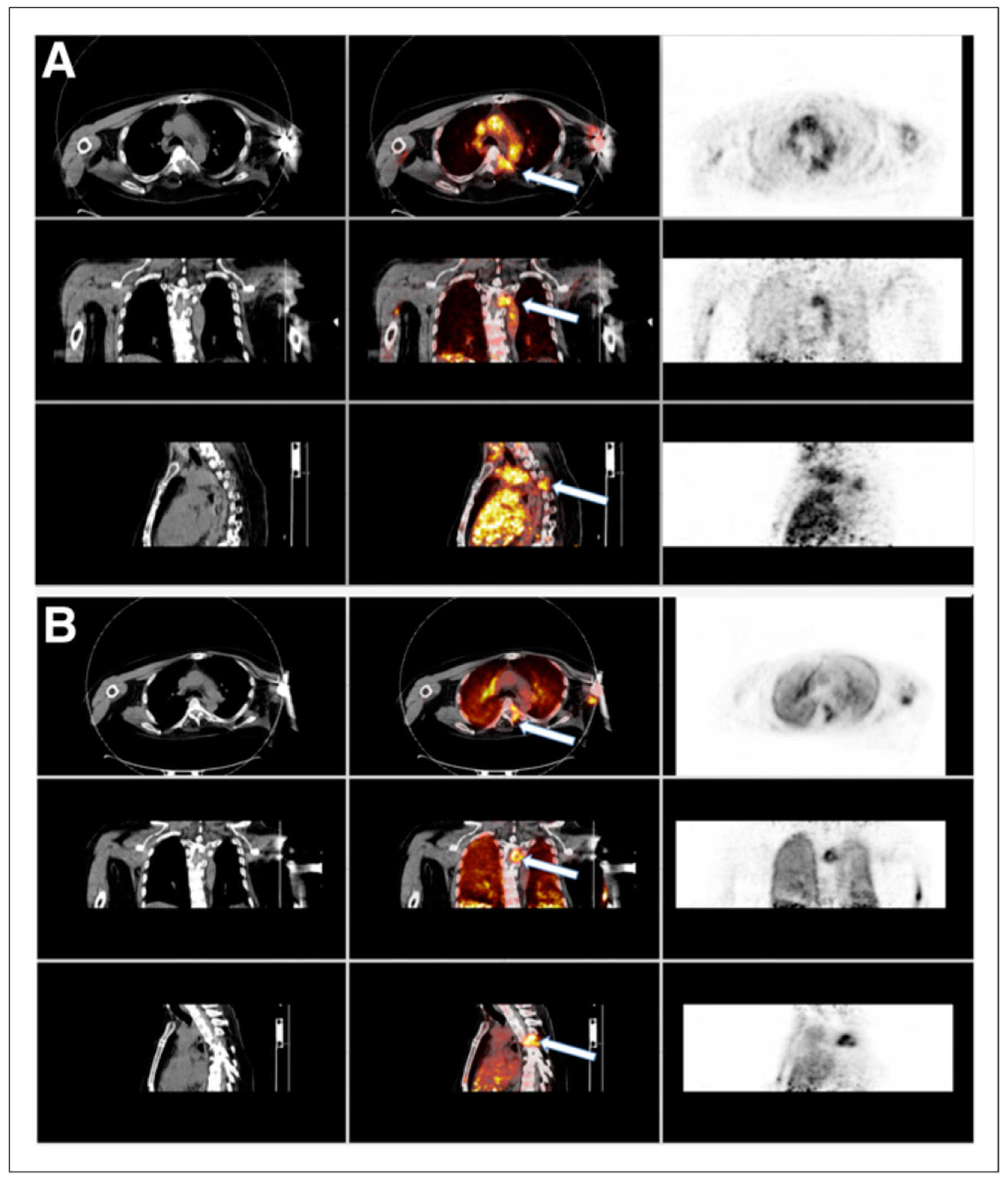
Summed images formed after rebinning dynamic list-mode data from 0 to 3 min after injection of 15O-water and 62Cu-ETS. In this example, and in all other patients, dynamic list-mode PET data were obtained in 21.7-cm z-axis field of view to ensure inclusion of cardiac blood pool for determination of arterial input function used to find K1 for both tracers. (A) Images from 15O-water dynamic study, in which CT on left shows hypodense lesion at T4 that is also seen on fused images (center, white arrow) and PET images (right). (B) Images from 62Cu-ETS dynamic study, in which again CT on left shows hypodense lesion at T4 that is seen on fused images (center, white arrow) and PET images (left). Both summed 15O-water and 62Cu-ETS demonstrate similar image findings in normal tissues and metastatic T4 vertebral lesion. Elevated perfusion seen in metastatic bone lesion was typical of all other skeletal lesions.
FIGURE 2.
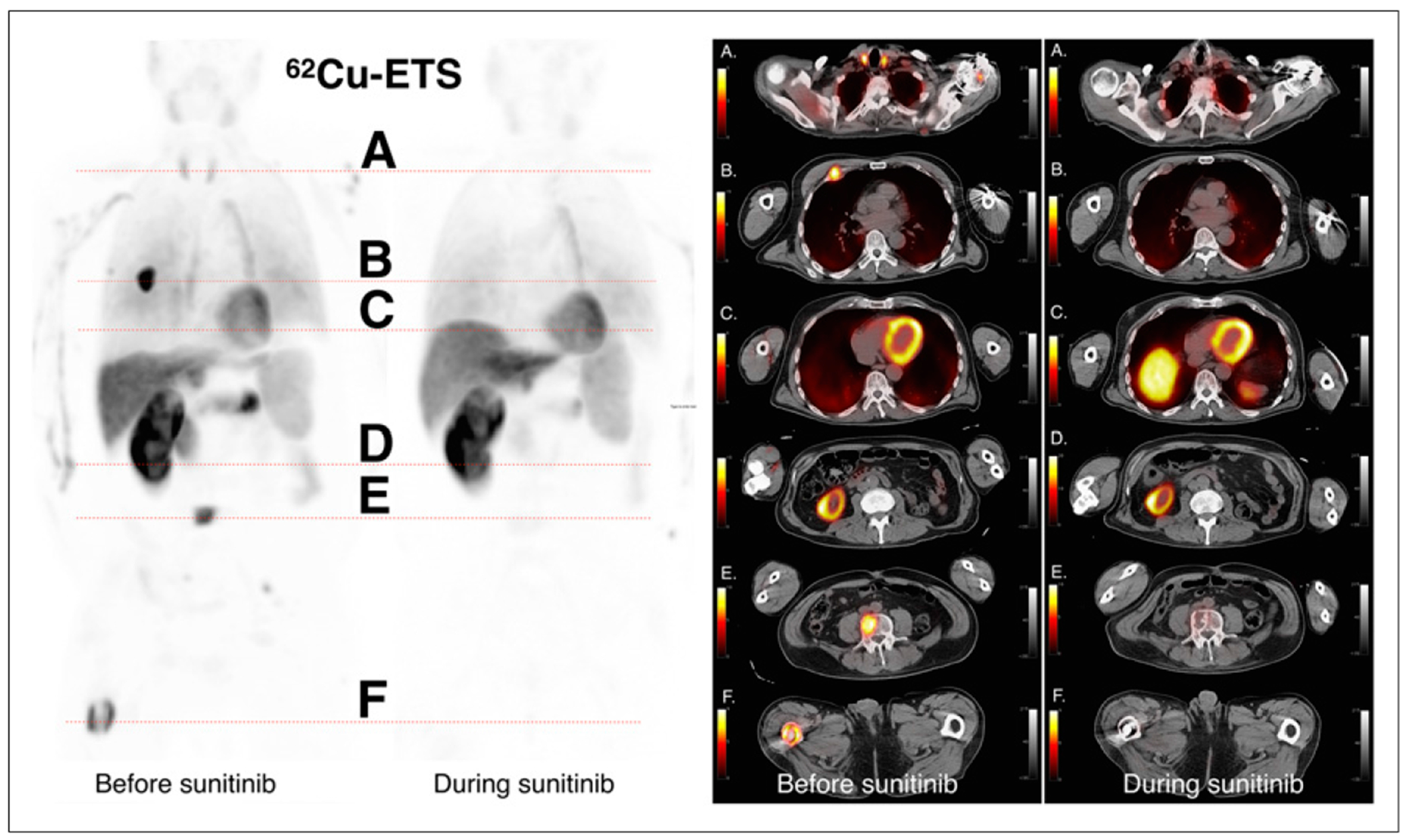
Anterior whole-body 62Cu-ETS images are shown on left, at baseline and during sunitinib therapy, with 6 corresponding transverse slices of fused PET and CT images on right. The 6 levels correspond to the following structures: level A, normal thyroid tissue; level B, hyperemic anterior rib lesion on right; level C, normal myocardium and dome of liver; level D, inferior pole of remaining right kidney; level E, hyperemic metastases in L4 lumbar vertebrae; and level F, hyperemic metastases in right distal femur. Hyperemic signals in metastatic lesions at baseline are no longer identified on images obtained during therapy with antiangiogenesis agent, whereas normal tissue signals are similar to baseline. Thyroid tissue signal decreased during therapy and may relate to previously observed decreases in thyroid function in patients receiving sunitinib. Similar changes were seen in other subjects.
FIGURE 3.
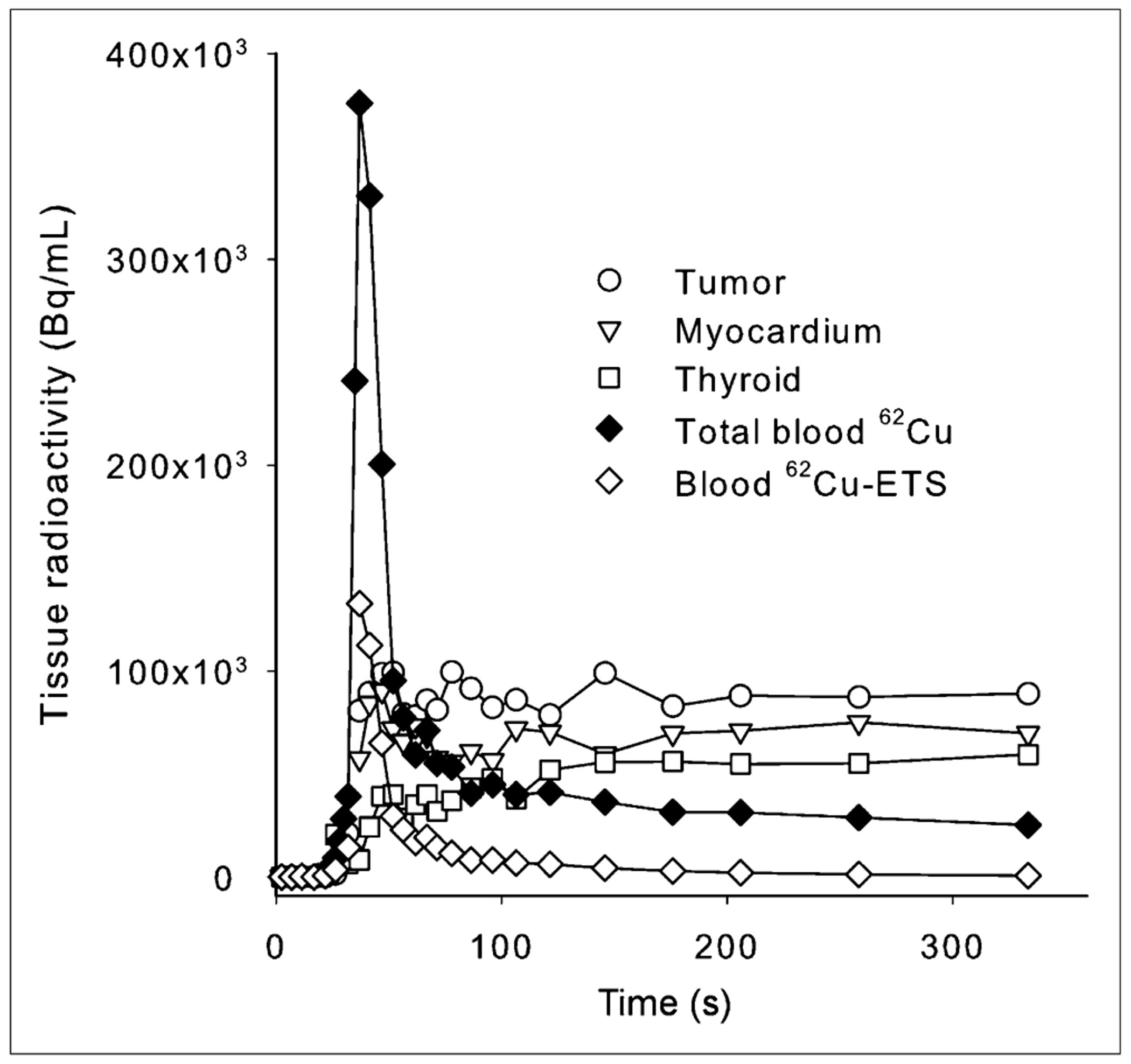
Example of observed kinetics of blood clearance and tissue uptake after intravenous administration of 62Cu-ETS, derived from dynamic PET data. Observed left atrial blood 62Cu concentration was corrected to blood concentration of intact 62Cu-ETS based on independently measured rate of 62Cu-ETS decomposition by patient’s blood at 37°C in vitro.
Direct comparison of the 15O-water and 62Cu-ETS perfusion estimates were made for 88 regions from the 18 paired dynamic 15O–62Cu scans, representing normal tissues (myocardium, thyroid, and muscle, n = 18, 17, and 18, respectively) plus 35 metastatic lesions (Fig. 4). An excellent linear correlation was observed between the blood flow measurements obtained with 62Cu-ETS and the 15O-water reference standard (y = 0.85x + 0.15, R2 = 0.83). The data for the tumors increase the observed variance in this analysis. When restricted to only the data for myocardial, thyroid, and muscle regions of interest, the regression line becomes y = 0.97x + 0.09, R2 = 0.90, whereas the tumor data alone are fit by the line y = 0.70x + 0.27, R2 = 0.72 (Fig. 5).
FIGURE 4.
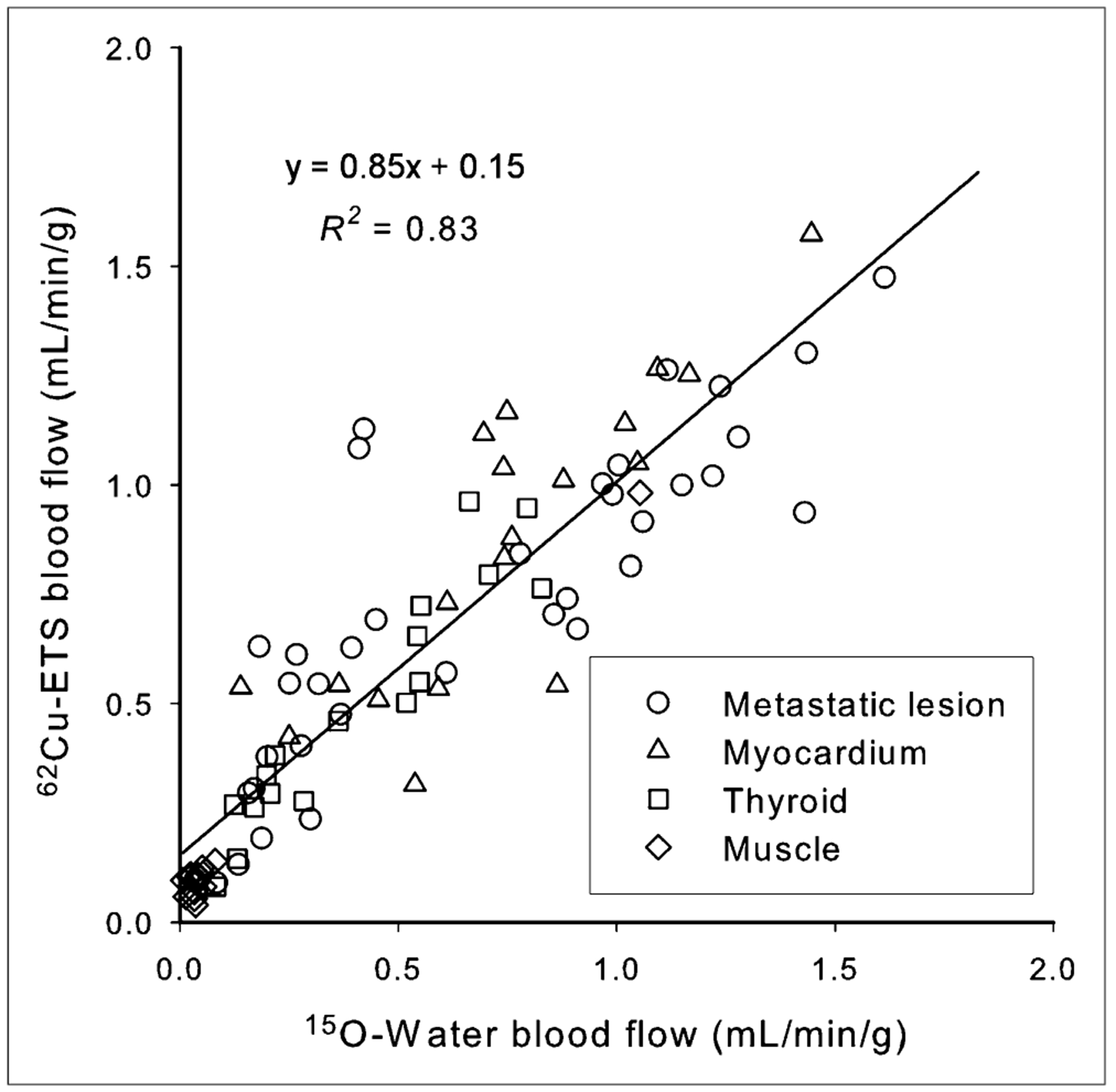
Correlation of perfusion rate measurements (K1 values, mL·min−1·g−1) obtained with 62Cu-ETS and 15O-water for normal tissues and metastases (n = 88 tissue regions from 18 paired dynamic PET images).
FIGURE 5.
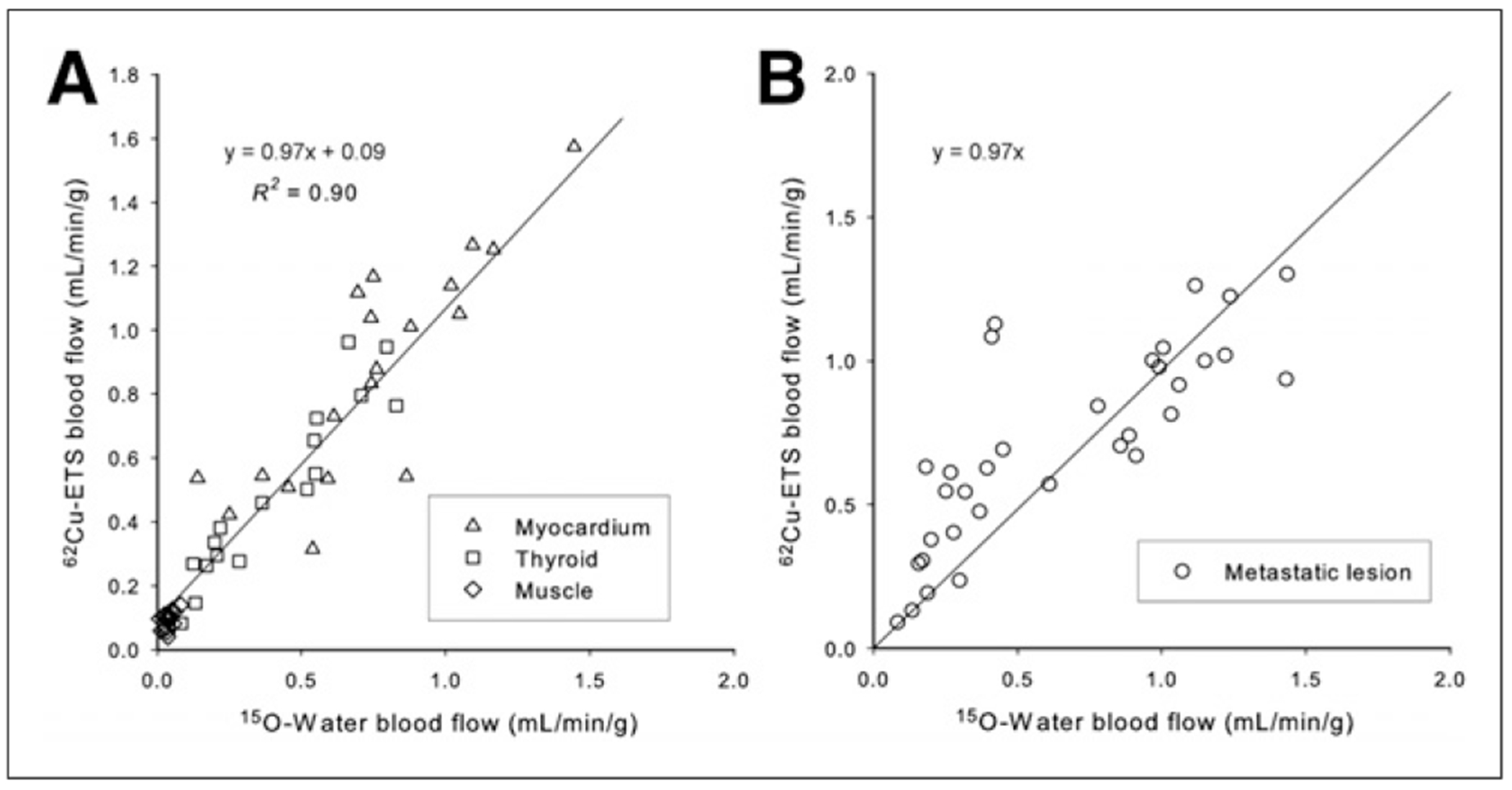
Correlation of perfusion rate measurements (K1 values, mL·min−1·g−1) obtained with 62Cu-ETS and 15O-water when analyses are restricted to tissue regions from normal tissues alone (A, n = 53) and metastases alone (B, n = 35) in the 18 paired dynamic PET studies. For metastatic lesion data, regression line shown was forced through origin, illustrating that several lower flow lesions (all in lung) are largest source of variance. Unforced regression line for 5B is y = 0.70x + 0.27; R2 = 0.72.
Table 1 compares the K1 values of both tracers in normal tissue and for the metastatic lesions. Paired 2-tailed t test values for all tissues and lesions, with the exception of skeletal muscle (for which the flow values are low), also indicate no significant difference between the 15O-water and 62Cu-ETS measurements of tissue perfusion. The 62Cu-ETS measurements of blood flow (mL−1·min−1·g) from dynamic list-mode data are also well correlated with whole-body 62Cu-ETS SUVs (Fig. 6; y = 0.20x + 0.06, R2 = 0.73).
TABLE 1.
t Test Statistics for K1 Values from 15O-Water and 62Cu-ETS for Normal Tissues and Metastatic Lesions
| 15O K1 vs. 62Cu K1 | 15O K1 | 62Cu K1 | n | P |
|---|---|---|---|---|
| All samples | ||||
| Baseline and therapy | 0.55 ± 0.45 | 0.63 ± 0.42 | 88 | 0.28 |
| Baseline | 0.66 ± 0.49 | 0.71 ± 0.43 | 42 | 0.65 |
| Treatment | 0.45 ± 0.36 | 0.55 ± 0.38 | 46 | 0.25 |
| Normal tissues | ||||
| Baseline and therapy | 0.46 ± 0.42 | 0.54 ± 0.43 | 53 | 0.33 |
| Baseline | 0.53 ± 0.42 | 0.58 ± 0.42 | 24 | 0.66 |
| Treatment | 0.41 ± 0.42 | 0.51 ± 0.45 | 29 | 0.37 |
| All lesions | ||||
| Baseline and therapy | 0.69 ± 0.46 | 0.75 ± 0.38 | 35 | 0.56 |
| Baseline | 0.84 ± 0.53 | 0.87 ± 0.40 | 18 | 0.81 |
| Treatment | 0.53 ± 0.33 | 0.61 ± 0.32 | 17 | 0.47 |
| Muscle | ||||
| Baseline and therapy | 0.05 ± 0.04 | 0.10 ± 0.05 | 18 | 0.00011 |
| Baseline | 0.05 ± 0.04 | 0.11 ± 0.02 | 8 | 0.0034 |
| Treatment | 0.05 ± 0.04 | 0.10 ± 0.06 | 10 | 0.072 |
| Thyroid | ||||
| Baseline and therapy | 0.46 ± 0.25 | 0.54 ± 0.22 | 17 | 0.36 |
| Baseline | 0.61 ± 0.19 | 0.69 ± 0.24 | 8 | 0.79 |
| Treatment | 0.30 ± 0.17 | 0.41 ± 0.12 | 9 | 0.12 |
| Myocardium | ||||
| Baseline and therapy | 0.88 ± 0.35 | 0.99 ± 0.32 | 18 | 0.31 |
| Baseline | 0.90 ± 0.32 | 0.97 ± 0.28 | 8 | 0.64 |
| Treatment | 0.86 ± 0.39 | 1.00 ± 0.37 | 10 | 0.39 |
FIGURE 6.
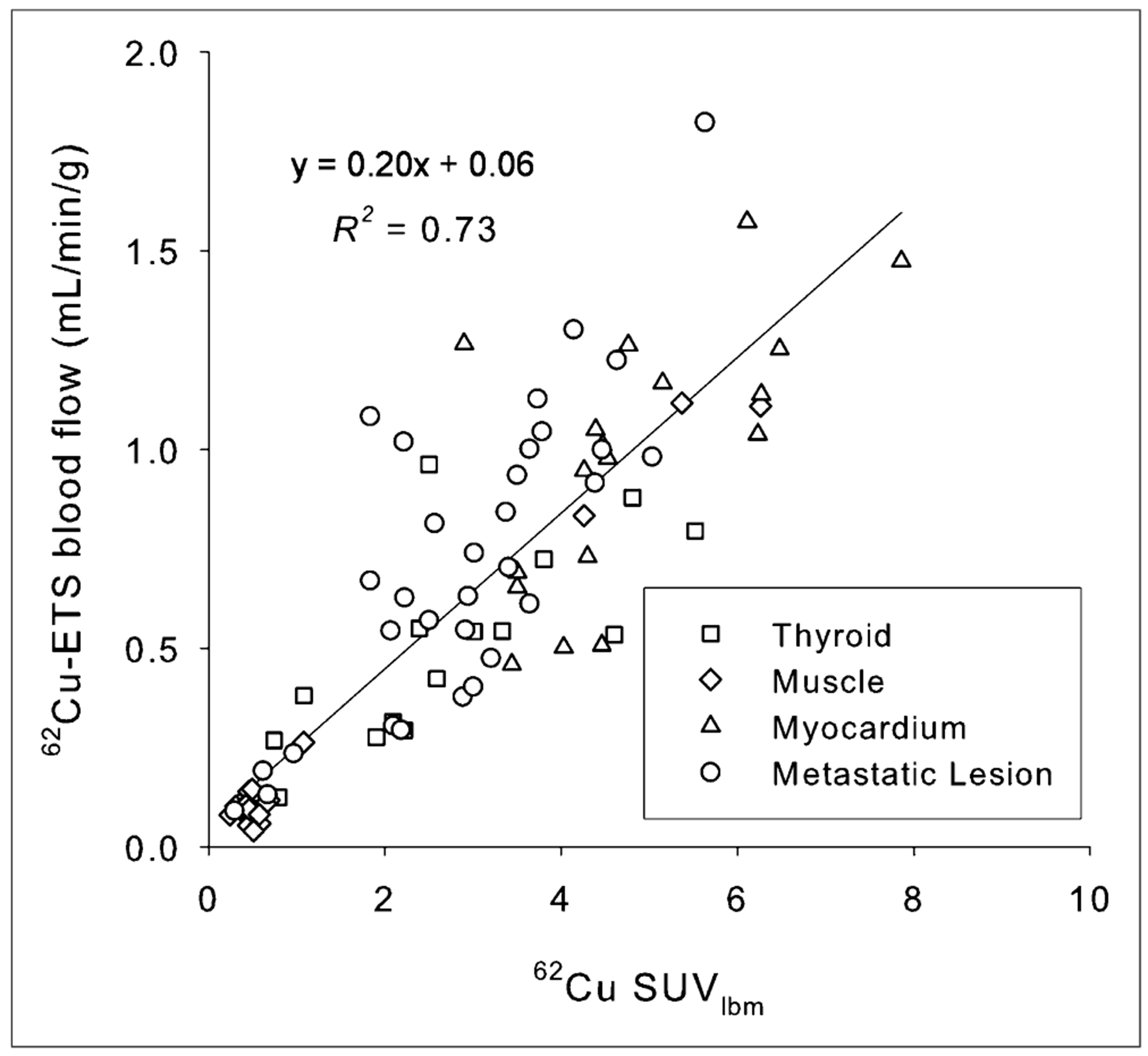
Correlation of mean SUV measurement normalized to lean body mass (SUVmeanlbm) in attenuation-corrected whole-body 62Cu-ETS scans with blood flow values obtained from preceding dynamic 62Cu-ETS data acquisition.
Nine of the patients had pulmonary nodules. Five of these had solitary nodules. The average size (Response Evaluation Criteria in Solid Tumors long diameter) of the nodules at baseline and during the conclusion of therapy is shown in Table 2. An average decrease in size of 29.4% was noted.
TABLE 2.
Characteristics of Pulmonary Nodules
| Statistic | Initial tumor diameter (cm) | Tumor diameter at repeated imaging (cm) | Percentage change |
|---|---|---|---|
| Mean | 2.3 ± 1.4 | 1.8 ± 1.5 | −29% |
| Range | 1.14–6.29 | 0–5.04 | |
| Median | 1.8 | 1.6 |
DISCUSSION
The current study provides general validation of 62Cu-ETS as a PET tracer of tumor perfusion in patients with metastatic renal cell carcinoma, with the 62Cu-ETS radiopharmaceutical delivering flow values equivalent to those from the best available reference standard—15O-water. The model-derived K1 values from the sequential 15O-water and 62Cu-ETS studies were highly correlated, both by linear regression and by paired 2-tailed t test, for the tumors as well as the concurrently imaged myocardium and thyroid (Figs. 4 and 5; Table 1). Deviation of the correlation of 62Cu-ETS and 15O-water blood flow measurements from the ideal slope of 1.0 (Figs. 4 and 5) is largely driven by the data from the lower flow metastatic lesions in the lungs, perhaps reflecting the need for a more refined approach to 62Cu-ETS modeling in pulmonary lesions (e.g., better correcting for count spill-over into the tumor from the less dense surrounding lung).
Despite the short 9.7-min half-life of 62Cu, high-quality whole-body images were easily obtained with 62Cu-ETS (Fig. 2) after initial imaging with the heart in the field of view to define the arterial blood time–activity curve. Blood clearance of 62Cu-ETS was rapid, with virtually no intact radiopharmaceutical remaining in blood at the conclusion of our initial 6-min dynamic acquisition (Fig. 3). We believe further work will allow validation of a modeling approach for conveniently extracting quantitative perfusion values from such whole-body scans. The ability to perform whole-body scans is particularly important in the evaluation of patients with known or possible metastatic disease, because of the inherently varied and distributed nature of potential lesion presentation.
Laking and Price (10) have presented an excellent review of the current status of physiologic PET and SPECT imaging of perfusion and hypoxia as related to angiogenesis. In brief, they concluded that measurement of perfusion with radionuclides is the best biomarker for angiogenesis and that PET functional imaging has the potential to reveal much about tumor circulation and the effect of treatment. The most successful results have been seen using 15O-water. But there will always be substantial technical limitations to widespread implementation of the 15O-water method, including the requirements for an on-site cyclotron; restriction to a single bed position by the required dynamic imaging; and the need for invasive arterial blood sampling to obtain arterial input function, if the heart and tumors are not in a common field of view. Despite these limitations, 15O-water remains the reference standard for perfusion measurements in humans.
The reported results indicate that, as a short-lived microsphere-like agent, 62Cu-ETS potentially overcomes the practical limitations of 15O-water for quantitative clinical imaging of perfusion. The centrally manufactured and distributed 62Zn/62Cu generator, and companion kits for rapid on-demand radiopharmaceutical synthesis, can make 62Cu-ETS perfusion imaging available to virtually any clinical PET site in the United States. Additionally, the kinetics of 62Cu-ETS enable whole-body scanning, with high-quality images being obtained despite the short radionuclide half-life (Fig. 2). Furthermore, the 9.7-min half-life of 62Cu ideally complements companion imaging with 18F-FDG for the assessment of tissue metabolism, because the 62Cu will be essentially completely decayed during a standard 18F-FDG uptake period, if the 18F-FDG is simply administered at the conclusion of 62Cu image acquisition.
Whole-body 62Cu-ETS SUVs (Fig. 6) may be useful for evaluating relative perfusion within a given subject; however, the assessment of perfusion with 62Cu-ETS will be most robustly modeled using the arterial blood input function as the basis for normalizing tissue levels of 62Cu in the whole-body images. The use of the SUV calculation to normalize tissue concentrations of radiotracer is expected to be somewhat unsatisfactory when using a tracer that exhibits essentially perfusion-rate–limited delivery to tissue, because the fractional distribution of cardiac output to major organs should largely be independent of total body mass. Although more technically demanding than a simple SUV calculation, the microsphere-like tissue trapping of the 62Cu radiolabel should allow robust, and reasonably convenient, 62Cu-ETS perfusion measurements in the whole-body scans by applying an image-derived arterial input function of the type acquired in the present dynamic studies.
The lung lesions were somewhat demanding in the modeling for flow quantification, due to the need to define whether individual tumors derived their arterial blood flow from the pulmonary, or brachial, artery. This is a problem that will be unique to lung metastases, and differentiation of the perfusion source proved readily feasible by kinetic analysis of the dynamic data acquired for image-based definition of the blood input function based on cardiac regions of interest.
Limitations of the present study include the limited total number of subjects and the focus solely on metastatic renal cell carcinoma. Because of the study design, repeatability was not defined. The 62Cu half-life certainly allows a study design involving replicate imaging to directly assess test–retest reproducibility. But in consideration of the medical condition of these volunteers, that additional data collection was judged to be best deferred to a subsequent study. Expansion of this validation protocol is now under way for the assessment of 62Cu-ETS performance in a broader array of tumors.
CONCLUSION
This study demonstrates that 62Cu-ETS can be used as an alternative to 15O-water for tumor perfusion imaging in patients with metastatic renal cell carcinoma and illustrates the feasibility of whole-body PET/CT imaging with 62Cu, despite its short half-life, using a radiopharmaceutical that produces tissue trapping of the radionuclide.
Supplementary Material
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This work was supported by grants from the Melvin and Bren Simon Cancer Center and the National Cancer Institute of the National Institutes of Health (R01-CA140299). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Hirst DG. Blood flow and its modulation in malignant tumors. In: Willmott N, Daly JM, eds. Microspheres and Regional Cancer Chemotherapy. Boca Raton, FL: CRC Press; 1994:31–56. [Google Scholar]
- 2.Ruoslahti E. Specialization of tumor vasculature. Nat Rev Cancer. 2002;2:83–90. [DOI] [PubMed] [Google Scholar]
- 3.Plank MJ, Sleeman BD. Tumor-induced angiogenesis: a review. J Theor Med. 2003;5:137–153. [Google Scholar]
- 4.Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–424. [DOI] [PubMed] [Google Scholar]
- 5.Molema G. Design of vascular endothelium-specific drug-targeting strategies for the treatment of cancer. Acta Biochim Pol. 2005;52:301–310. [PubMed] [Google Scholar]
- 6.Featherstone J, Griffiths S. Drugs that target angiogenesis. Nat Rev Drug Discov. 2002;1:413–414. [DOI] [PubMed] [Google Scholar]
- 7.Gillies RJ, Schornack PA, Secomb YW, Raghunand N. Causes and effects of heterogeneous perfusion in tumors. Neoplasia. 1999;1:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moeller BJ, Cao Y, Vujaskovic Z, Li C, Haroon Z, Dewhirst M. The relationship between hypoxia and angiogenesis. Semin Radiat Oncol. 2004;14:215–221. [DOI] [PubMed] [Google Scholar]
- 9.Eriksen JG, Horsman MR. Tumour hypoxia: a characteristic feature with a complex molecular background. Radiother Oncol. 2006;81:119–121. [DOI] [PubMed] [Google Scholar]
- 10.Laking G, Price P. Radionuclide imaging of perfusion and hypoxia. Eur J Nucl Med Mol Imaging. 2010;37(suppl 1):S20–S29. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann SR, Fox KA, Rand AL, et al. Quantification of regional myocardial blood flow in vivo with H215O. Circulation. 1984;70:724–733. [DOI] [PubMed] [Google Scholar]
- 12.Huang S-C, Schwaiger M, Carson RE, et al. Quantitative measurement of myocardial blood flow with oxygen-15 water and positron computed tomography: an assessment of potential and problems. J Nucl Med. 1985;26:616–625. [PubMed] [Google Scholar]
- 13.Herrero P, Markham J, Bergmann SR. Quantitation of myocardial blood-flow with (H2O)-O-15 and positron emission tomography: assessment and error analysis of a mathematical approach. J Comput Assist Tomogr. 1989;13:862–873. [DOI] [PubMed] [Google Scholar]
- 14.Hutchins GD, Caraher J, Raylman RR. A region of interest strategy for minimizing resolution distortions in quantitative myocardial PET studies. J Nucl Med. 1992;33:1243–1250. [PubMed] [Google Scholar]
- 15.Frackowiak RSJ, Lenzi G-L, Jones T, Heather JD. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using O-15 and positron emission tomography: theory, procedure, and normal values. J Comput Assist Tomogr. 1980;4:727–736. [DOI] [PubMed] [Google Scholar]
- 16.Herscovitch P, Markham J, Raichle ME. Brain blood flow measured with intravenous H215O. I. Theory and error analysis. J Nucl Med. 1983;24:782–789. [PubMed] [Google Scholar]
- 17.Bacharach SL, Libutti SK, Carrasquillo JA. Measuring tumor blood flow with H215O: practical considerations. Nucl Med Biol. 2000;27:671–676. [DOI] [PubMed] [Google Scholar]
- 18.Lodge MA, Carson RE, Carrasquillo JA, Whatley M, Libutti SK, Bacharach SL. Parametric images of blood flow in oncology PET studies using 15O-water. J Nucl Med. 2000;41:1784–1792. [PubMed] [Google Scholar]
- 19.Lehtiö K, Eskola O, Viljanen T, et al. Imaging perfusion and hypoxia with PET to predict radiotherapy response in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;59:971–982. [DOI] [PubMed] [Google Scholar]
- 20.Logan TF, Jadali F, Egorin MJ, et al. Decreased tumor blood flow as measured by positron emission tomography in cancer patients treated with interleukin-1 and carboplatin on a phase I trial. Cancer Chemother Pharmacol. 2002;50:433–444. [DOI] [PubMed] [Google Scholar]
- 21.Green MA, Klippenstein DL, Tennison JR. Copper(II) bis(thiosemicarbazone) complexes as potential tracers for evaluation of cerebral and myocardial blood flow with PET. J Nucl Med. 1988;29:1549–1557. [PubMed] [Google Scholar]
- 22.Green MA, Mathias CJ, Welch MJ, et al. [62Cu]-labeled pyruvaldehyde bis(N4-methylthiosemicarbazonato)copper(II): synthesis and evaluation as a positron emission tomography tracer for cerebral and myocardial perfusion. J Nucl Med. 1990;31:1989–1996. [PubMed] [Google Scholar]
- 23.Ng Y, Lacy JL, Fletcher JW, Green MA. Performance of a 62Zn/62Cu micro-generator in kit-based synthesis and delivery of 62Cu–ETS for PET perfusion imaging. Appl Radiat Isot. 2014;91:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.John EK, Green MA. Structure-activity relationships for metal-labeled blood flow tracers: comparison of ketoaldehyde bis(thiosemicarbazonato)copper(II) derivatives. J Med Chem. 1990;33:1764–1770. [DOI] [PubMed] [Google Scholar]
- 25.Mathias CJ, Welch MJ, Perry DJ, et al. Investigation of copper-PTSM as a PET tracer for tumor blood flow. Int J Rad Appl Instrum B. 1991;18:807–811. [DOI] [PubMed] [Google Scholar]
- 26.Mathias CJ, Green MA, Morrison WB, Knapp DW. Evaluation of Cu-PTSM as a tracer of tumor perfusion: comparison with labeled microspheres in spontaneous canine neoplasms. Nucl Med Biol. 1994;21:83–87. [DOI] [PubMed] [Google Scholar]
- 27.Herrero P, Markham J, Weinheimer CJ, et al. Quantification of regional myocardial perfusion with generator-produced 62Cu-PTSM and positron emission tomography. Circulation. 1993;87:173–183. [DOI] [PubMed] [Google Scholar]
- 28.Beanlands RSB, Muzik O, Hutchins GD, Woulfe ER, Schwaiger M. Heterogeneity of regional nitrogen-13-labeled ammonia tracer distribution in the normal human heart: comparison with rubidium-82 and copper-62-labeled PTSM. J Nucl Cardiol. 1994;1:225–235. [DOI] [PubMed] [Google Scholar]
- 29.Herrero P, Hartman JJ, Green MA, et al. Assessment of regional myocardial perfusion with generator-produced 62Cu-PTSM and PET in human subjects. J Nucl Med. 1996;37:1294–1300. [PubMed] [Google Scholar]
- 30.Melon PG, Brihaye C, Degueldre C, et al. Myocardial kinetics of K-38 in humans and comparison with Cu-62-PTSM. J Nucl Med. 1994;35:1116–1122. [PubMed] [Google Scholar]
- 31.Okazawa H, Yonekura Y, Fujibayashi Y, et al. Clinical application and quantitative evaluation of generator-produced copper-62-PTSM as a brain perfusion tracer for PET. J Nucl Med. 1994;35:1910–1915. [PubMed] [Google Scholar]
- 32.Wallhaus TR, Lacy J, Stewart R, Bianco J, Green MA, Stone CK. Copper-62-pyruvaldehyde bis(N4-methylthiosemicarbazone) PET imaging in the detection of coronary artery disease in humans. J Nucl Cardiol. 2001;8:67–74. [DOI] [PubMed] [Google Scholar]
- 33.Green MA, Mathias CJ, Willis LR, et al. Assessment of Cu-ETS as a PET radiopharmaceutical for evaluation of regional renal perfusion. Nucl Med Biol. 2007;34:247–255. [DOI] [PubMed] [Google Scholar]
- 34.Shelton ME, Green MA, Mathias CJ, Welch MJ, Bergmann SR. Kinetics of copper-PTSM in isolated hearts: a novel tracer for measuring blood flow with positron emission tomography. J Nucl Med. 1989;30:1843–1847. [PubMed] [Google Scholar]
- 35.Mathias CJ, Welch MJ, Raichle ME, et al. Evaluation of a potential generator-produced PET tracer for cerebral perfusion imaging: single-pass cerebral extraction measurements and imaging with radiolabeled Cu-PTSM. J Nucl Med. 1990;31:351–359. [PubMed] [Google Scholar]
- 36.Minkel DT, Saryan LA, Petering DH. Structure-function correlations in the reaction of bis(thiosemicarbazone) copper(II) complexes with Ehrlich ascites tumor cells. Cancer Res. 1978;38:124–129. [PubMed] [Google Scholar]
- 37.Baerga ID, Maickel RP, Green MA. Subcellular distribution of tissue radiocopper following intravenous administration of 67Cu-labeled Cu-PTSM. Int J Rad Appl Instrum B. 1992;19:697–701. [DOI] [PubMed] [Google Scholar]
- 38.Basken NE, Mathias CJ, Green MA. Elucidation of the human serum albumin (HSA) binding site for the Cu-PTSM and Cu-ATSM radiopharmaceuticals. J Pharm Sci. 2009;98:2170–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basken NE, Green MA. Cu(II) bis(thiosemicarbazone) radiopharmaceutical binding to serum albumin: further definition of species-dependence and associated substituent effects. Nucl Med Biol. 2009;36:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathias CJ, Bergmann SR, Green MA. Development and validation of a solvent extraction technique for determination of Cu-PTSM in blood. Nucl Med Biol. 1993;20:343–349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


