Abstract
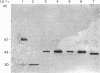
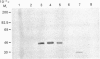
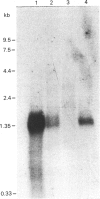
The secreted carbonic anhydrases, CA VI, are high molecular mass, oligomeric enzymes originally found in the sheep parotid gland and saliva. The enzymes have been purified from the saliva or parotid glands of several different species. All the CA VI enzymes studied have an apparent subunit Mr of about 45,000 as previously reported for the sheep enzyme. By Western analysis, CA VI from human, cow and dog cross-reacted with antibody raised against the purified sheep enzyme whereas that of the mouse did not. The N-terminal sequences of the sheep, human, cow and mouse enzymes are reported. The sheep, cow and human N-terminal sequences are similar to one another while the mouse sequence is substantially different. Nevertheless, the amino acids in the aromatic cluster I (Trp-5, Tyr-7, Trp-16 and Tyr/Phe-20) have all been conserved, as is the case with the cytoplasmic carbonic anhydrases. Eighteen tissues from the sheep have been examined for the presence of CA VI by Western analysis but it has been found only in the salivary glands. Northern analysis and hybridization histochemistry show that the mRNA for CA VI in sheep is expressed specifically in the acinar cells of the parotid and submandibular glands.
Full text
PDF

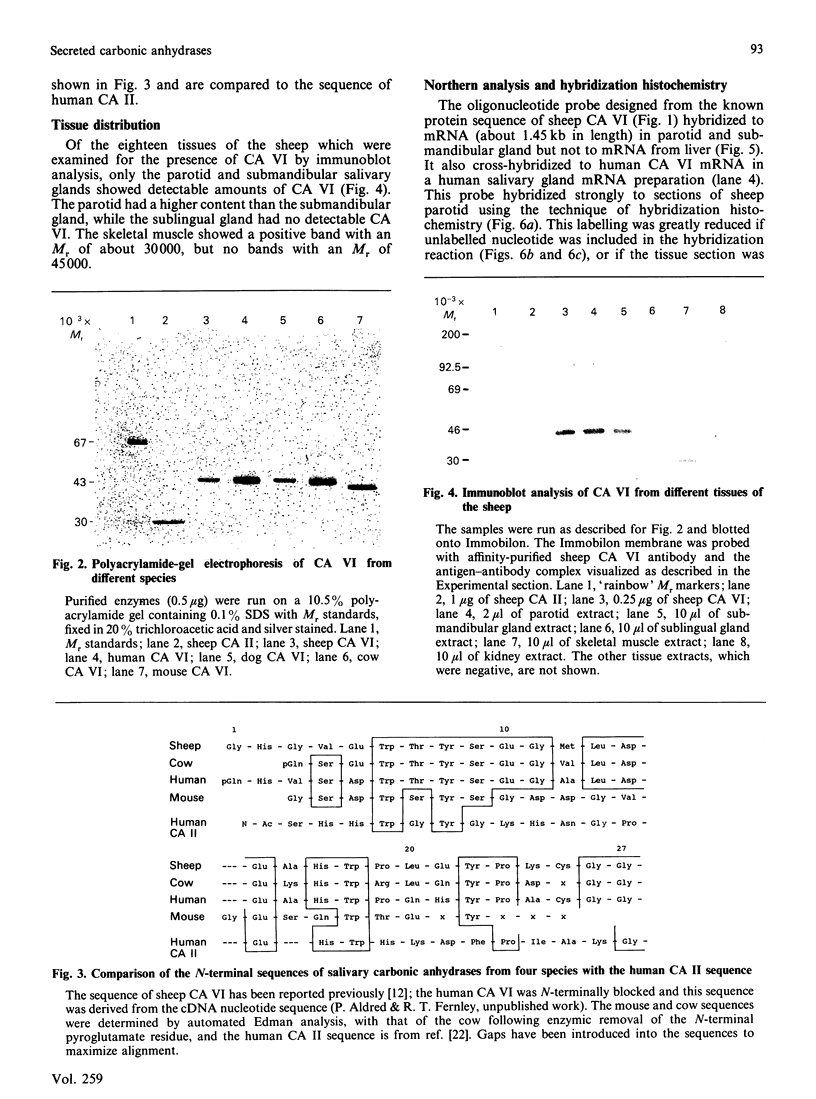
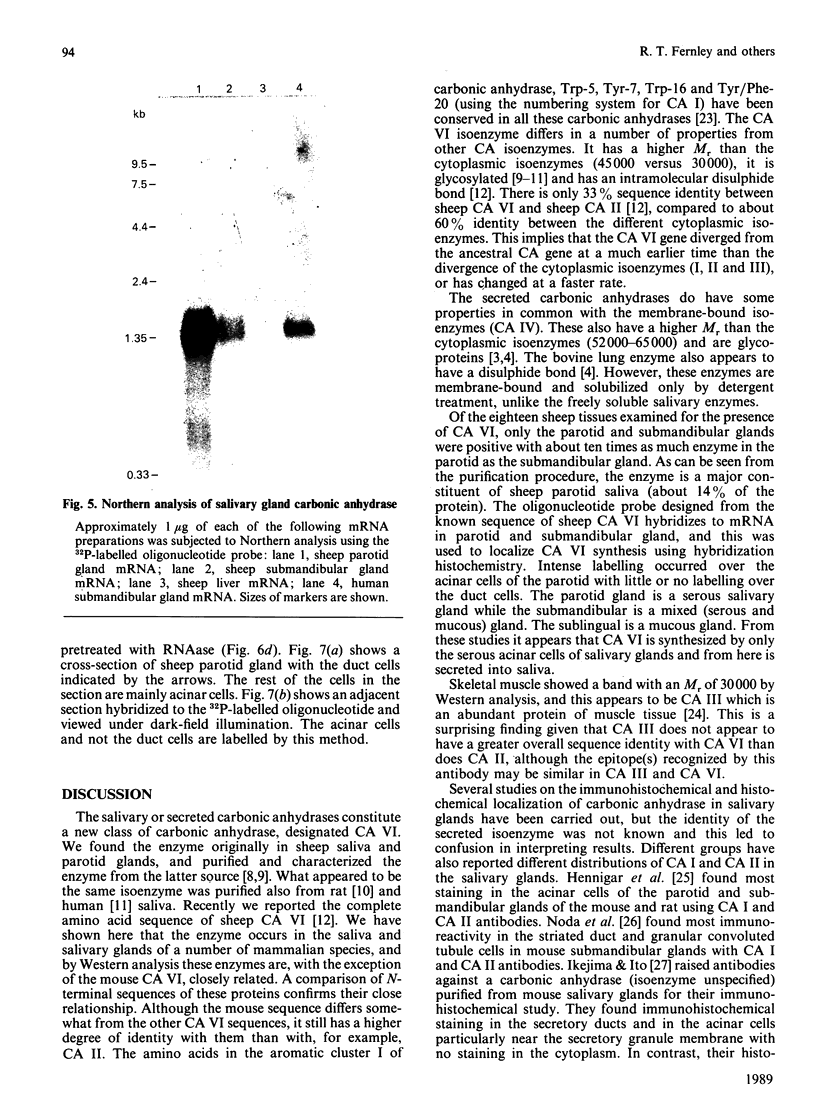
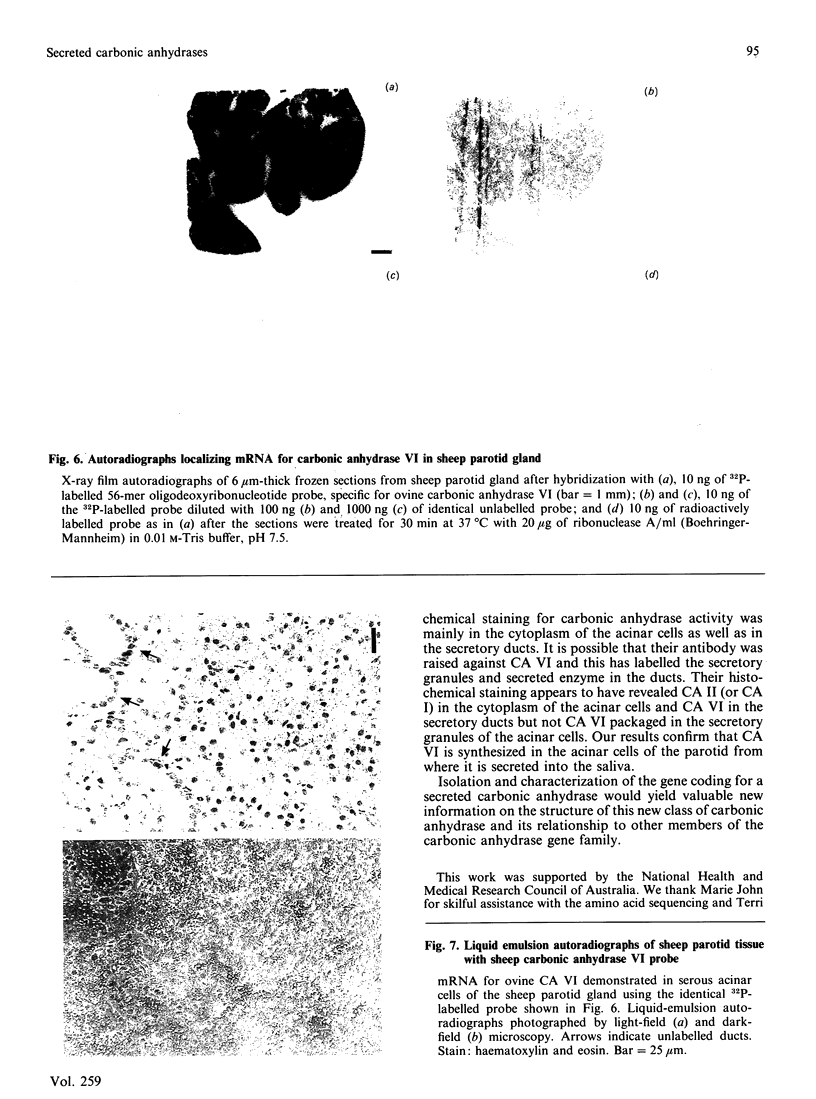

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butkus A., Roche P. J., Fernley R. T., Haralambidis J., Penschow J. D., Ryan G. B., Trahair J. F., Tregear G. W., Coghlan J. P. Purification and cloning of a corpuscles of Stannius protein from Anguilla australis. Mol Cell Endocrinol. 1987 Dec;54(2-3):123–133. doi: 10.1016/0303-7207(87)90149-3. [DOI] [PubMed] [Google Scholar]
- Carter N. D., Hewett-Emmett D., Jeffrey S., Tashian R. E. Testosterone-induced, sulfonamide-resistant carbonic anhydrase isozyme of rat liver is indistinguishable from skeletal muscle carbonic anhydrase III. FEBS Lett. 1981 Jun 1;128(1):114–118. doi: 10.1016/0014-5793(81)81094-0. [DOI] [PubMed] [Google Scholar]
- Caruthers M. H., Beaucage S. L., Efcavitch J. W., Fisher E. F., Goldman R. A., deHaseth P. L., Mandecki W., Matteucci M. D., Rosendahl M. S., Stabinsky Y. Chemical synthesis and biological studies on mutated gene-control regions. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):411–418. doi: 10.1101/sqb.1983.047.01.048. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dodgson S. J., Forster R. E., 2nd, Storey B. T., Mela L. Mitochondrial carbonic anhydrase. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5562–5566. doi: 10.1073/pnas.77.9.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein J. B., Silverman D. N. Purification and characterization of carbonic anhydrase from the saliva of the rat. J Biol Chem. 1984 May 10;259(9):5447–5453. [PubMed] [Google Scholar]
- Fernley R. T., Coghlan J. P., Wright R. D. Purification and characterization of a high-Mr carbonic anhydrase from sheep parotid gland. Biochem J. 1988 Jan 1;249(1):201–207. doi: 10.1042/bj2490201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernley R. T., Wright R. D., Coghlan J. P. A novel carbonic anhydrase from the ovine parotid gland. FEBS Lett. 1979 Sep 15;105(2):299–302. doi: 10.1016/0014-5793(79)80634-1. [DOI] [PubMed] [Google Scholar]
- Fernley R. T., Wright R. D., Coghlan J. P. Complete amino acid sequence of ovine salivary carbonic anhydrase. Biochemistry. 1988 Apr 19;27(8):2815–2820. doi: 10.1021/bi00408a023. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Henriksson D., Nyman P. O. Primary structure of human carbonic anhydrase C. J Biol Chem. 1976 Sep 25;251(18):5457–5463. [PubMed] [Google Scholar]
- Hennigar R. A., Schulte B. A., Spicer S. S. Immunolocalization of carbonic anhydrase isozymes in rat and mouse salivary and exorbital lacrimal glands. Anat Rec. 1983 Dec;207(4):605–614. doi: 10.1002/ar.1092070408. [DOI] [PubMed] [Google Scholar]
- Ikejima T., Ito S. Carbonic anhydrase in mouse salivary glands and saliva: a histochemical, immunohistochemical, and enzyme activity study. J Histochem Cytochem. 1984 Jun;32(6):625–635. doi: 10.1177/32.6.6427328. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Murakami H., Sly W. S. Purification and characterization of human salivary carbonic anhydrase. J Biol Chem. 1987 Jan 25;262(3):1382–1388. [PubMed] [Google Scholar]
- Noda Y., Takai Y., Hikosaka N., Meenaghan M. A., Mori M. Immunohistochemical localization of carbonic anhydrase in submandibular salivary glands of mice and hamsters treated with phenylephrine, testosterone or duct-ligation. Arch Oral Biol. 1986;31(7):441–447. doi: 10.1016/0003-9969(86)90017-8. [DOI] [PubMed] [Google Scholar]
- Penschow J. D., Haralambidis J., Aldred P., Tregear G. W., Coghlan J. P. Location of gene expression in CNS using hybridization histochemistry. Methods Enzymol. 1986;124:534–548. doi: 10.1016/0076-6879(86)24038-0. [DOI] [PubMed] [Google Scholar]
- Tashian R. E., Hewett-Emmett D., Goodman M. On the evolution and genetics of carbonic anhydrases I, II, and III. Isozymes Curr Top Biol Med Res. 1983;7:79–100. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Wood W. I., Hayflick J., Seeburg P. H. Isolation of a cDNA clone coding for the gamma-subunit of mouse nerve growth factor using a high-stringency selection procedure. DNA. 1984 Oct;3(5):387–392. doi: 10.1089/dna.1984.3.387. [DOI] [PubMed] [Google Scholar]
- Venta P. J., Montgomery J. C., Tashian R. E. Molecular genetics of carbonic anhydrase isozymes. Isozymes Curr Top Biol Med Res. 1987;14:59–72. [PubMed] [Google Scholar]
- Whitney P. L., Briggle T. V. Membrane-associated carbonic anhydrase purified from bovine lung. J Biol Chem. 1982 Oct 25;257(20):12056–12059. [PubMed] [Google Scholar]
- Wistrand P. J. Properties of membrane-bound carbonic anhydrase. Ann N Y Acad Sci. 1984;429:195–206. doi: 10.1111/j.1749-6632.1984.tb12333.x. [DOI] [PubMed] [Google Scholar]