Abstract
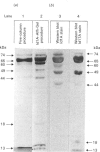
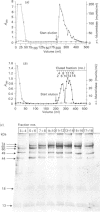
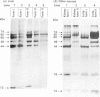
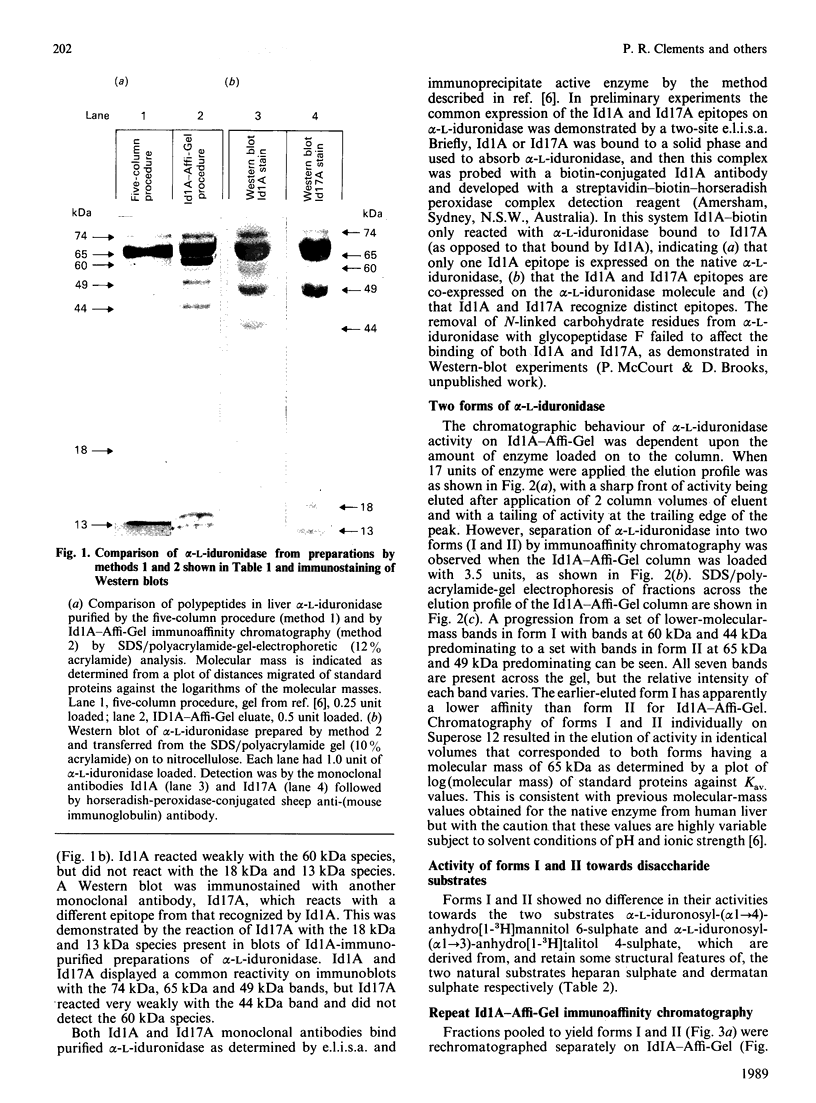
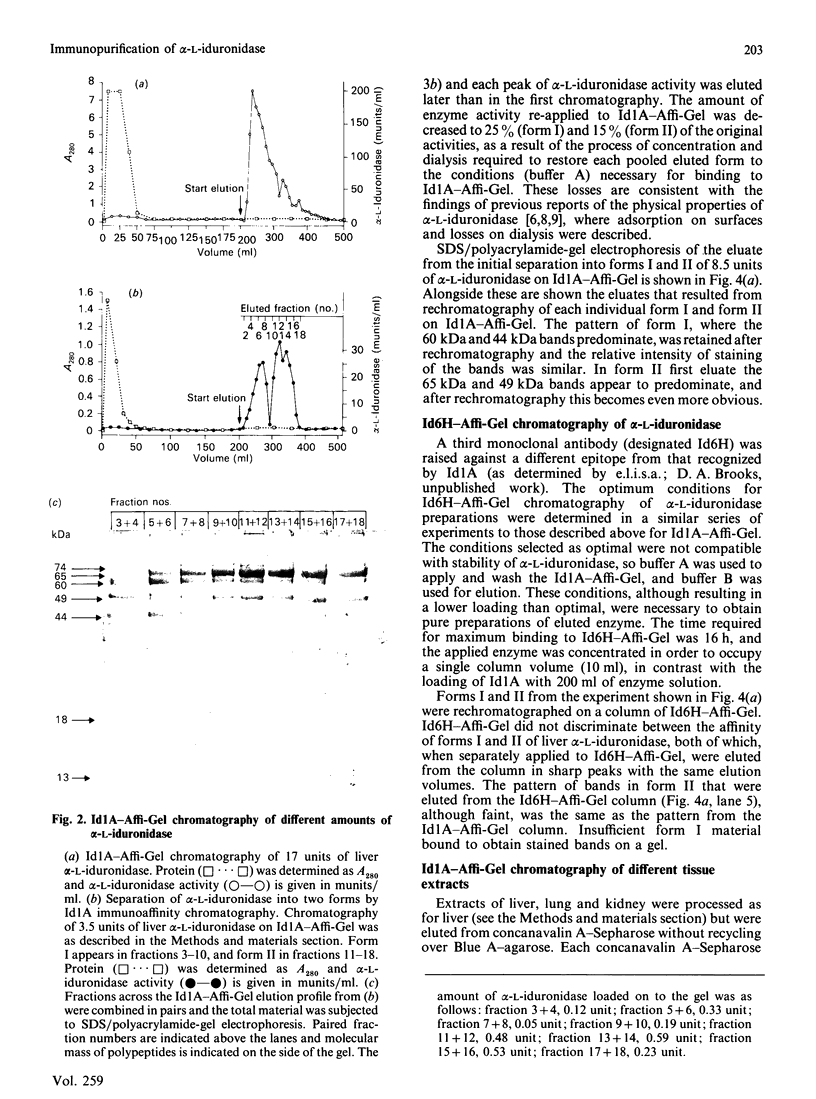
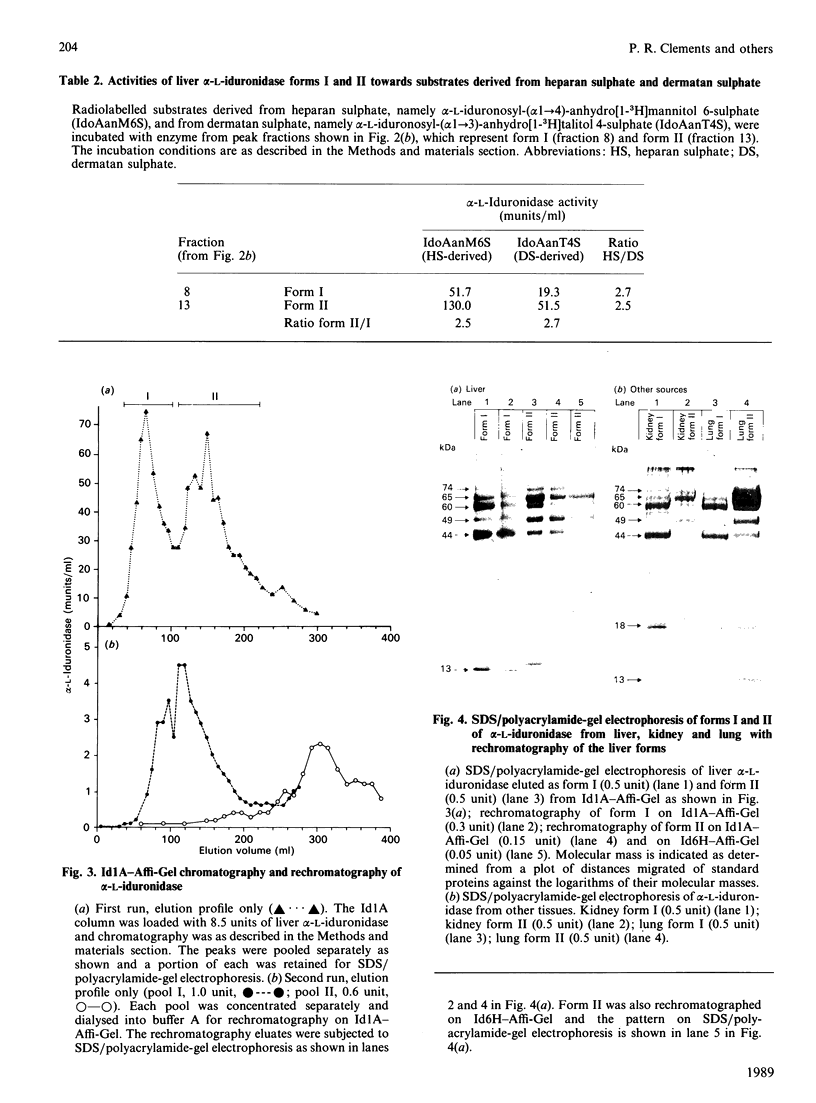
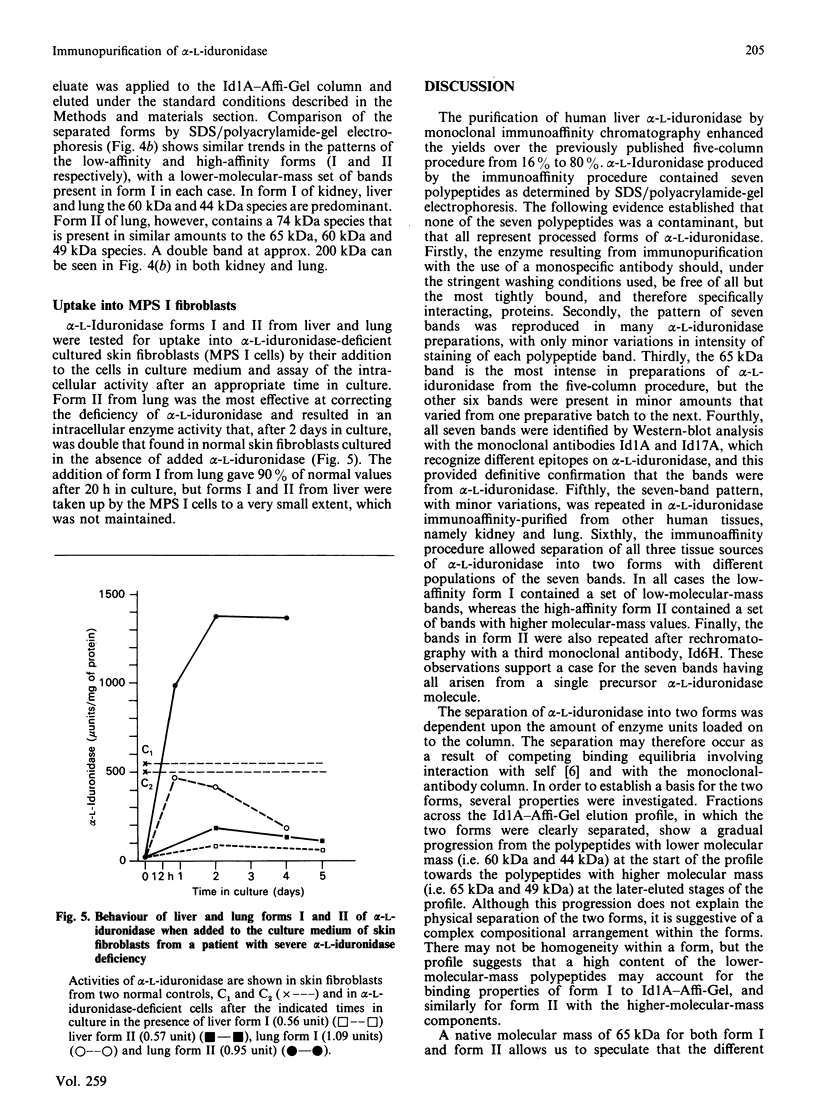
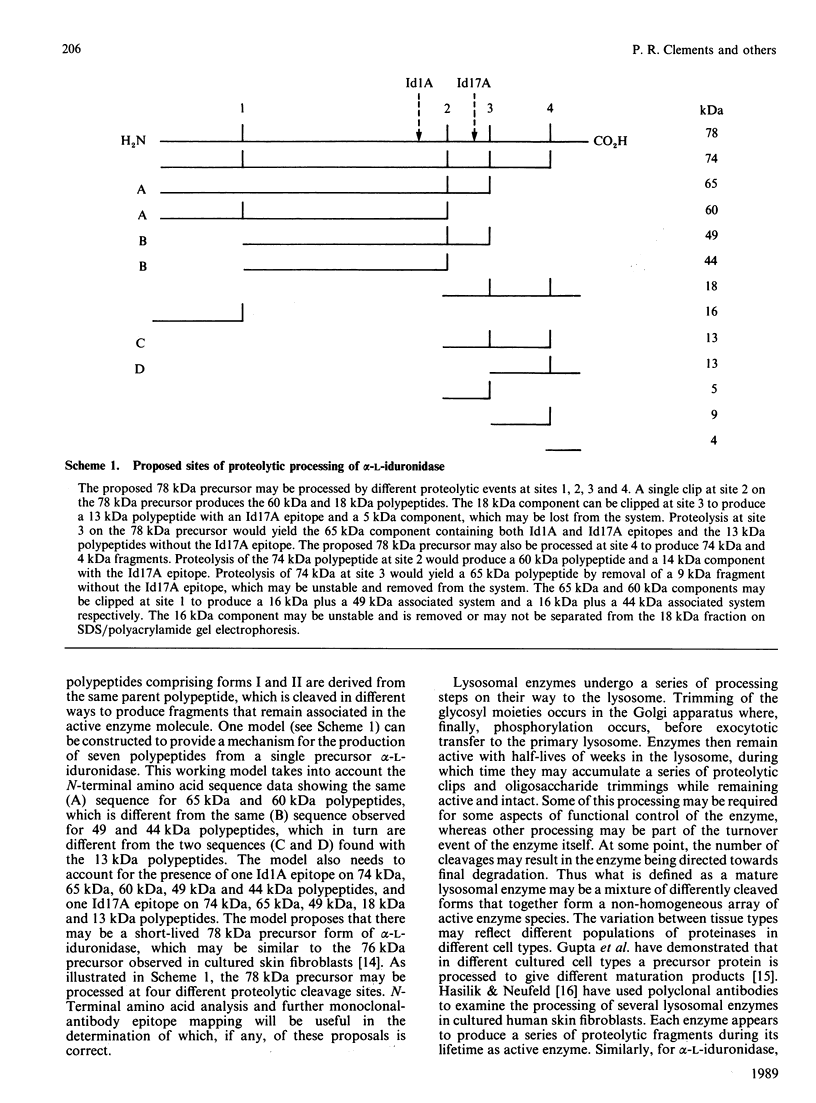
alpha-L-Iduronidase from human liver was purified by a three-step five-column procedure and by immunoaffinity chromatography with a monoclonal antibody raised against purified enzyme. Seven bands identified by staining with Coomassie Blue had molecular masses of 74, 65, 60, 49, 44, 18 and 13 kDa and were present in both preparations of the liver enzyme. However, relative to the immunopurification procedure, alpha-L-iduronidase purified by the five-column procedure was considerably enriched in the 65 kDa polypeptide band. The seven bands were identified by Western-blot analysis with two different monoclonal antibodies raised against alpha-L-iduronidase. The chromatographic behaviour of alpha-L-iduronidase on the antibody column was dependent upon the quantity of enzyme loaded. Above a particular load concentration a single peak of enzyme activity was eluted, whereas at load concentrations below the critical value alpha-L-iduronidase was eluted in two peaks of activity, designated form I (eluted first) and form II (eluted second). The following properties of the two forms of alpha-L-iduronidase were determined. (1) The two forms from liver were composed of different proportions of the same seven polypeptides. (2) When individually rechromatographed on the antibody column, each form from liver shifted to a more retarded elution position but essentially retained its chromatographic behaviour relative to the other form. (3) Forms I and II of liver alpha-L-iduronidase showed no difference in their activities towards disaccharide substrates derived from two glycosaminoglycan sources, heparan sulphate and dermatan sulphate. (4) The native molecular size of forms I and II of liver alpha-L-iduronidase was 65 kDa as determined by gel-permeation chromatography. (5) Immunoaffinity chromatography of extracts of human lung and kidney resulted in the separation of alpha-L-iduronidase into two forms, each with different proportions of the seven common polypeptide species. (6) Lung forms I and II were taken up readily into cultured skin fibroblasts taken from a patient with alpha-L-iduronidase deficiency. Liver forms I and II were not taken up to any significant extent. Lung form II gave intracellular contents of alpha-L-iduronidase that were more than double those of normal control fibroblasts, whereas lung form I gave contents approximately equal to normal control values. We propose that all seven polypeptides are derived from a single alpha-L-iduronidase gene product, and that different proportions of these polypeptides can function as a single alpha-L-iduronidase entity.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown W. E., Seamon K. B. Quantitation and characterization of the trifluoroacetonyl derivative of cysteine: a useful NMR probe. Anal Biochem. 1978 Jun 15;87(1):211–222. doi: 10.1016/0003-2697(78)90587-0. [DOI] [PubMed] [Google Scholar]
- Carey W. F., Pollard A. C. Variability of fibroblast lysosomal acid hydrolases with reference to the detection of enzyme deficiencies. Aust J Exp Biol Med Sci. 1977 Jun;55(3):245–252. doi: 10.1038/icb.1977.19. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Brooks D. A., Saccone G. T., Hopwood J. J. Human alpha-L-iduronidase. 1. Purification, monoclonal antibody production, native and subunit molecular mass. Eur J Biochem. 1985 Oct 1;152(1):21–28. doi: 10.1111/j.1432-1033.1985.tb09158.x. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Muller V., Hopwood J. J. Human alpha-L-iduronidase. 2. Catalytic properties. Eur J Biochem. 1985 Oct 1;152(1):29–34. doi: 10.1111/j.1432-1033.1985.tb09159.x. [DOI] [PubMed] [Google Scholar]
- Freeman C., Clements P. R., Hopwood J. J. Human liver N-acetylglucosamine-6-sulphate sulphatase. Purification and characterization. Biochem J. 1987 Sep 1;246(2):347–354. doi: 10.1042/bj2460347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman C., Hopwood J. J. Human liver N-acetylglucosamine-6-sulphate sulphatase. Catalytic properties. Biochem J. 1987 Sep 1;246(2):355–365. doi: 10.1042/bj2460355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman C., Hopwood J. J. Human liver sulphamate sulphohydrolase. Determinations of native protein and subunit Mr values and influence of substrate agylcone structure on catalytic properties. Biochem J. 1986 Feb 15;234(1):83–92. doi: 10.1042/bj2340083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujibayashi S., Minami R., Ishikawa Y., Wagatsuma K., Nakao T., Tsugawa S. Properties of alpha-L-iduronidase in cultured skin fibroblasts from alpha-L-iduronidase-deficient patients. Hum Genet. 1984;65(3):268–272. doi: 10.1007/BF00286515. [DOI] [PubMed] [Google Scholar]
- Gibson G. J., Saccone G. T., Brooks D. A., Clements P. R., Hopwood J. J. Human N-acetylgalactosamine-4-sulphate sulphatase. Purification, monoclonal antibody production and native and subunit Mr values. Biochem J. 1987 Dec 15;248(3):755–764. doi: 10.1042/bj2480755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D. K., Schmidt A., von Figura K., Hasilik A. Processing and transport of lysosomal enzymes in human monocyte line U937. Hoppe Seylers Z Physiol Chem. 1984 Aug;365(8):867–876. doi: 10.1515/bchm2.1984.365.2.867. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hopwood J. J., Muller V. Biochemical discrimination of Hurler and Scheie syndromes. Clin Sci (Lond) 1979 Sep;57(3):265–272. doi: 10.1042/cs0570265. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes in normal and disease states. J Clin Invest. 1986 Jan;77(1):1–6. doi: 10.1172/JCI112262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahuran D., Clements P., Carrella M., Strasberg P. M. A high recovery method for concentrating microgram quantities of protein from large volumes of solution. Anal Biochem. 1983 Mar;129(2):513–516. doi: 10.1016/0003-2697(83)90585-7. [DOI] [PubMed] [Google Scholar]
- Muller V. J., Hopwood J. J. alpha-L-Iduronidase deficiency in mucopolysaccharidosis type I against a radiolabelled sulfated disaccharide substrate derived from dermatan sulfate. Clin Genet. 1984 Nov;26(5):414–421. doi: 10.1111/j.1399-0004.1984.tb01081.x. [DOI] [PubMed] [Google Scholar]
- Myerowitz R., Neufeld E. F. Maturation of alpha-L-iduronidase in cultured human fibroblasts. J Biol Chem. 1981 Mar 25;256(6):3044–3048. [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Neufeld E. F. Human kidney alpha-L-iduronidase: purification and characterization. Arch Biochem Biophys. 1978 Aug;189(2):344–353. doi: 10.1016/0003-9861(78)90221-7. [DOI] [PubMed] [Google Scholar]
- Sando G. N., Neufeld E. F. Recognition and receptor-mediated uptake of a lysosomal enzyme, alpha-l-iduronidase, by cultured human fibroblasts. Cell. 1977 Nov;12(3):619–627. doi: 10.1016/0092-8674(77)90262-8. [DOI] [PubMed] [Google Scholar]
- Schuchman E. H., Guzman N. A., Desnick R. J. Human alpha-L-iduronidase. I. Purification and properties of the high uptake (higher molecular weight) and the low uptake (processed) forms. J Biol Chem. 1984 Mar 10;259(5):3132–3140. [PubMed] [Google Scholar]