Summary
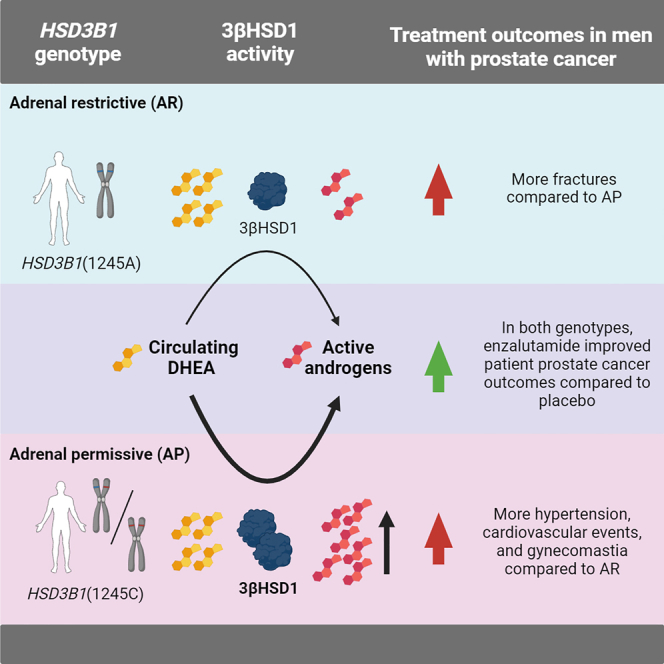
HSD3B1 encodes 3β-hydroxysteroid dehydrogenase-1, which converts adrenal dehydroepiandrosterone to 5α-dihydrotestosterone and is inherited in adrenal-permissive (AP) or adrenal-restrictive forms. The AP allele is linked to castration resistance, mainly in low-volume tumors. Here, we investigate the association of HSD3B1 alleles with outcomes in ARCHES, a multinational, double-blind, randomized, placebo-controlled phase 3 trial that demonstrated clinical benefit with enzalutamide plus androgen deprivation therapy (ADT) in men with metastatic hormone-sensitive prostate cancer (mHSPC) compared to those treated with placebo plus ADT. There are no significant differences between genotypes for clinical efficacy endpoints. Enzalutamide significantly improves radiographic progression-free survival and overall survival vs. placebo irrespective of HSD3B1 status. Men with the AP genotype have higher post-progression mortality and treatment-emergent adverse events, including hypertension, cardiovascular events, and gynecomastia, but a lower fracture rate. Overall, enzalutamide is beneficial in men with mHSPC independent of the HSD3B1 genotype. Inherited polymorphisms of HSD3B1 may account for differential toxicities.
Keywords: metabolism, androgens, enzalutamide, HSD3B1, metastatic hormone-sensitive prostate cancer
Graphical abstract
Highlights
-
•
Enzalutamide improves OS and rPFS in patients with mHSPC irrespective of HSD3B1 genotype
-
•
Clinical events are driven by high-volume tumors
-
•
Clinical outcomes do not significantly differ by HSD3B1 genotype
-
•
TEAEs and post-progression mortality are higher with the HSD3B1 AP genotype
Sharifi et al. demonstrate that, in metastatic hormone-sensitive prostate cancer (mHSPC), there are no differences in clinical outcomes, largely evaluable in high-volume disease, between men with the adrenal-permissive genotype and those with the adrenal-restrictive genotype of HSD3B1. Enzalutamide therapy provides clinical benefit independent of the HSD3B1 genotype.
Introduction
Prostate cancer is a highly androgen-dependent disease.1 Testosterone is an androgen receptor agonist that is converted by steroid-5α-reductase (SRD5A) within prostate cancer cells to 5α-dihydrotestosterone (DHT), a more potent androgen receptor activator.2 Androgen deprivation therapy (ADT) with medical or surgical castration depletes circulating testosterone and intratumoral DHT.3 However, tumors that are initially responsive to ADT usually evolve into castration-resistant prostate cancer (CRPC) through adoption of intratumoral androgen biosynthesis from non-gonadal (largely adrenal) precursors and other mechanisms that enable androgen receptor stimulation to drive tumor progression.4,5
Enzalutamide is a highly potent direct antagonist that impedes androgen receptor action in CRPC to improve the overall survival (OS) of patients with CRPC.6,7 Enzalutamide has been shown to confer an even greater survival benefit when used in combination with ADT in the setting of metastatic (m) hormone-sensitive prostate cancer (HSPC) (mHSPC).8,9 The ARCHES trial (NCT02677896) demonstrated the efficacy of enzalutamide plus ADT in patients with mHSPC.10 Enzalutamide plus ADT significantly improved OS by 34% (p < 0.001)8 and reduced the risk of radiographic progression or death by 61% (p < 0.001) compared to placebo plus ADT.10 However, other treatments combined with ADT also confer a survival benefit for HSPC, and biomarkers are needed that match genetic and metabolic drivers of disease to a therapy that will maximize individual treatment benefit and/or lower the risk of adverse effects.11
The first and rate-limiting step in the conversion from adrenal dehydroepiandrosterone (DHEA) to DHT is catalyzed by the enzyme 3β-hydroxysteroid dehydrogenase-1 (3βHSD1), which is encoded by the HSD3B1 gene.12,13 A common missense-encoding germline single-nucleotide polymorphism regulates 3βHSD1 protein stability and the conversion of adrenal precursor steroids to DHT.14 The wild-type adrenal-restrictive (AR) HSD3B1(1245A) allele encodes an enzyme that undergoes rapid proteasome-mediated degradation and therefore impedes conversion from DHEA to DHT, whereas the adrenal-permissive (AP) HSD3B1(1245C) allele encodes an enzyme that maintains higher steady-state protein levels, thereby enabling greater DHT synthesis.15,16 Inheritance of a single allele of the AP HSD3B1(1245C) form is therefore mechanistically associated with more rapid progression on ADT and shorter survival, particularly in men with non-metastatic HSPC or low-volume mHSPC.17,18 With more advanced mCRPC, homozygous AP HSD3B1(1245C) inheritance is associated with worse outcomes following enzalutamide treatment and inconsistent results with abiraterone treatment.19,20,21
The impact of inherited HSD3B1 alleles on clinical outcomes following treatment with enzalutamide plus ADT to treat mHSPC has not been established. The objective of this post hoc analysis was to evaluate the potential association of germline HSD3B1 alleles and clinical outcomes in the phase 3 ARCHES trial. We hypothesized that the AP version of HSD3B1 is associated with worse outcomes in men treated with ADT alone in ARCHES but that enzalutamide would overcome this poor prognostic germline genotype through blockade of downstream androgen receptor signaling.
Results
Patient characteristics
A total of 1,150 patients were randomized in ARCHES; 1,146 patients received at least one dose of enzalutamide plus ADT (n = 572) or placebo plus ADT (n = 574) (Figure S1; Table S1). Germline DNA was collected from 660 patients (57%) who consented to pharmacogenetic testing: 243 had low disease volume (enzalutamide, 125; placebo, 118) and 417 had high disease volume (enzalutamide, 206; placebo, 211); 331 received enzalutamide plus ADT (HSD3B1 genotype AR, 168; AP, 163) and 329 received placebo plus ADT (HSD3B1 genotype AR, 172; AP, 157). Most consented patients were from Europe (64.5%), followed by North America (16.8%), Asia-Pacific (12.7%), and South America (5.9%). Overall, baseline demographics and disease characteristics were similar between treatment arms and between genotype groups (Table 1). De novo disease status and disease volume were also similar between genotype groups (Table S2). Of the 660 patients with HSD3B1 genotyping, 340 (51.5%) inherited wild-type AR alleles and 320 (48.5%) inherited AP alleles (245 [37.1%] heterozygous [A/C] and 75 [11.4%] homozygous [C/C] genotypes). The proportion of patients with AR vs. AP genotypes varied per region: 66.7% vs. 33.3% in Asia-Pacific, 47.9% vs. 52.1% in Europe, 51.4% vs. 48.6% in North America, and 59.0% vs. 41.0% in South America (Figure S2).
Table 1.
Baseline demographics and disease characteristics in patients by HSD3B1 AR and AP genotype
| Characteristics | Enzalutamide + ADT (n = 331) |
Placebo + ADT (n = 329) |
||
|---|---|---|---|---|
| AR (A/A) (n = 168) |
AP (A/C or C/C) (n = 163) |
AR (A/A) (n = 172) |
AP (A/C or C/C) (n = 157) |
|
| Median age, years (Q1–Q3) | 68.5 (63–75) | 70 (65–75.0) | 69.5 (64–75) | 70 (65.0–76) |
| Median PSA, μg/L (Q1–Q3) | 3.17 (0.6–35.3) | 5.27 (1.0–21.0) | 5.64 (1.0–34.3) | 5.67 (0.9–5.7) |
| Racea | ||||
| White | 143 (85.1) | 152 (93.3) | 139 (80.8) | 142 (90.4) |
| Black or African American | 3 (1.8) | 1 (0.6) | 4 (2.3) | 0 |
| Asian | 17 (10.1) | 3 (1.8) | 26 (15.1) | 4 (2.5) |
| Missing | 5 (3.0) | 7 (4.3) | 3 (1.7) | 11 (7.0) |
| Ethnicity | ||||
| Hispanic or Latino | 16 (9.5) | 11 (6.7) | 16 (9.3) | 6 (3.8) |
| Not Hispanic or Latino | 147 (87.5) | 145 (89.0) | 153 (89.0) | 140 (89.2) |
| Missing | 5 (3.0) | 7 (4.3) | 3 (1.7) | 11 (7.0) |
| Volume of disease | ||||
| Low | 64 (38.1) | 61 (37.4) | 56 (32.6) | 62 (39.5) |
| High | 104 (61.9) | 102 (62.6) | 116 (67.4) | 95 (60.5) |
| ECOG PS at study entry | ||||
| 0 | 133 (79.2) | 120 (73.6) | 133 (77.3) | 112 (71.3) |
| 1 | 35 (20.8) | 42 (25.8) | 39 (22.7) | 45 (28.7) |
| Unknown | 0 (0) | 1 (0.6) | 0 (0) | 0 (0) |
| Gleason score at initial diagnosis | ||||
| <8 | 52 (31.0) | 52 (31.9) | 56 (32.6) | 60 (38.2) |
| ≥8 | 111 (66.1) | 108 (66.3) | 111 (64.5) | 93 (59.2) |
| Unknown | 5 (3.0) | 3 (1.8) | 5 (2.9) | 4 (2.5) |
| Distant metastasis at initial diagnosis | ||||
| M1 | 123 (73.2) | 104 (63.8) | 109 (63.4) | 92 (58.6) |
| M0 | 28 (16.7) | 26 (16.0) | 22 (12.8) | 30 (19.1) |
| MX/unknown | 17 (10.1) | 33 (20.2) | 41 (23.8) | 35 (22.3) |
| Localization of metastasisb | ||||
| Bone only | 85 (50.6) | 78 (47.9) | 77 (44.8) | 62 (39.5) |
| Soft tissue only | 13 (17.7) | 16 (9.8) | 15 (8.7) | 15 (9.6) |
| Bone and soft tissue | 60 (35.7) | 65 (39.9) | 71 (41.3) | 63 (40.1) |
| No metastases | 10 (6.0) | 4 (2.5) | 9 (5.2) | 17 (10.8) |
| Prior docetaxel therapy | ||||
| None | 138 (82.1) | 138 (84.7) | 147 (85.5) | 125 (79.6) |
| 1–5 cycles | 3 (1.8) | 1 (0.6) | 4 (2.3) | 1 (0.6) |
| 6 cycles | 27 (16.1) | 24 (14.7) | 21 (12.2) | 31 (19.7) |
| Previous use of ADT | ||||
| None | 11 (6.5) | 9 (5.5) | 21 (12.2) | 18 (11.5) |
| ≤3 months | 125 (74.4) | 124 (76.1) | 122 (70.9) | 103 (65.6) |
| >3 months | 32 (19.0) | 30 (18.4) | 29 (16.9) | 36 (22.9) |
| Median duration of prior ADT, months (Q1–Q3) | 1.6 (0.7–2.8) | 1.6 (0.7–2.8) | 1.4 (0.7–2.7) | 1.8 (0.9–3.2) |
A, HSD3B1(1245A); ADT, androgen deprivation therapy; AP, adrenal-permissive; AR, adrenal-restrictive; C, HSD3B1(1245C); ECOG PS, Eastern Cooperative Oncology Group performance status; M0, no distant metastases; M1, distant metastases; MX, distant metastases cannot be assessed; PSA, prostate-specific antigen; Q, quartile. n (%) unless otherwise stated.
Race is not collected in France according to country regulations.
Based on investigator-reported results.
Efficacy
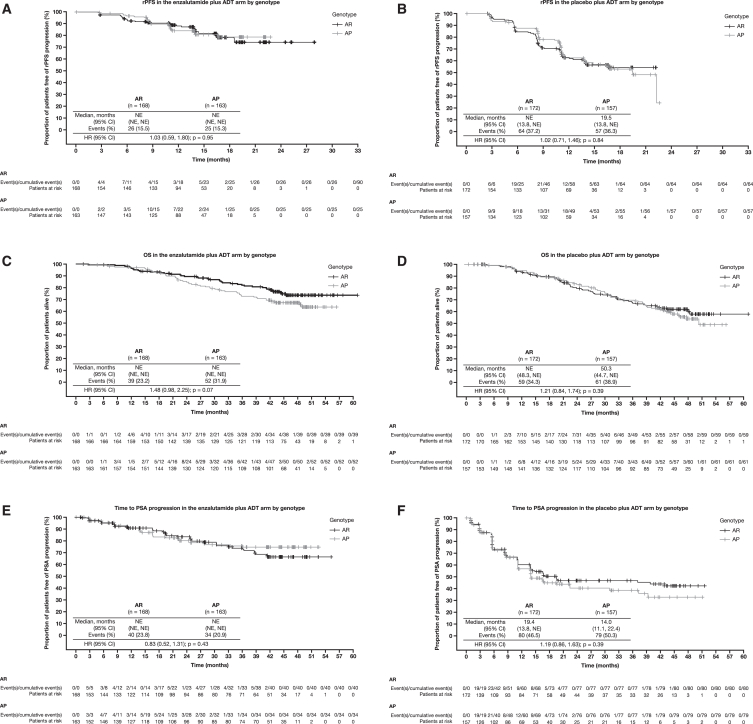
We did not observe a significant association in outcomes between patients based on HSD3B1 genotypes either overall or in the placebo plus ADT arm. In all patients, there were no significant differences in outcomes between patients with the AP genotype vs. those with the AR genotype for the clinical efficacy endpoints of radiographic progression-free survival (rPFS) (hazard ratio [HR] for all patients: 1.02; 95% confidence interval [CI]: 0.75, 1.37; p = 0.88; Figures 1A, enzalutamide, and 1B, placebo plus ADT), OS (HR for all patients: 1.29; 95% CI: 0.99, 1.70; p = 0.07; Figures 1C, enzalutamide, and 1D, placebo plus ADT), and time to prostate-specific antigen (PSA) progression (HR for all patients: 1.00; 95% CI: 0.77, 1.30; p = 0.96; Figures 1E, enzalutamide, and 1F, placebo plus ADT; Table S3). In the enzalutamide arm, median length of follow-up for OS was 46.0 months in patients with the AR genotype and 46.8 months in patients with the AP genotype. In the placebo arm, median length of follow-up for OS was 45.9 and 46.1 months for the AR and AP cohorts, respectively.
Figure 1.
Kaplan-Meier curves displaying clinical outcomes by HSD3B1 genotype associations
(A) rPFS in the enzalutamide plus ADT arm between HSD3B1 genotypes. The data cutoff date for the rPFS curve was October 14, 2018, before crossover. HR comparisons were made for patients with AP vs. AR genotypes.
(B) rPFS in the placebo plus ADT arm between HSD3B1 genotypes. The data cutoff date for the rPFS curve was October 14, 2018, before crossover. HR comparisons were made for patients with AP vs. AR genotypes.
(C) OS in the enzalutamide plus ADT arm between HSD3B1 genotypes. The data cutoff date for OS was May 28, 2021, after crossover. HR comparisons were made for patients with AP vs. AR genotypes.
(D) OS in the placebo plus ADT arm between HSD3B1 genotypes. The data cutoff date for OS was May 28, 2021, after crossover. HR comparisons were made for patients with AP vs. AR genotypes.
(E) Time to PSA progression in the enzalutamide plus ADT arm between HSD3B1 genotypes. The data cutoff date for time to PSA progression was May 28, 2021, after crossover. HR comparisons were made for patients with AP vs. AR genotypes.
(F) Time to PSA progression in the placebo plus ADT arm between HSD3B1 genotypes. The data cutoff date for time to PSA progression was May 28, 2021, after crossover. HR comparisons were made for patients with AP vs. AR genotypes.
Analyses were conducted using Cox proportional hazards models that were stratified for prior docetaxel use and disease volume for HRs, 95% CIs, and nominal (unadjusted) p values.
ADT, androgen deprivation therapy; AP, adrenal-permissive; AR, adrenal-restrictive; CI, confidence interval; HR, hazard ratio; NE, not estimable; OS, overall survival; PSA, prostate-specific antigen; rPFS, radiographic progression-free survival.
We next examined whether there was differential efficacy with the addition of enzalutamide to ADT according to the HSD3B1 genotype. No significant interactions were observed between genotype and treatment arm for rPFS (interaction p = 0.76), OS (p = 0.44), and time to PSA progression (p = 0.15). In a subgroup analysis of the AR cohort, patients treated with enzalutamide plus ADT showed a benefit in rPFS (HR: 0.66; 95% CI: 0.48, 0.92; p = 0.01) and OS (HR: 0.55; 95% CI: 0.37, 0.83; p < 0.01) compared to those with placebo plus ADT. This treatment benefit was similarly observed in the AP cohort for rPFS (HR: 0.62; 95% CI: 0.44, 0.88; p = 0.01) and OS (HR: 0.67; 95% CI: 0.46, 0.98; p = 0.04). After adjusting for genotype, HRs for enzalutamide plus ADT vs. placebo plus ADT were as follows: rPFS: 0.64 (95% CI: 0.51, 0.81; p < 0.01), OS: 0.62 (95% CI: 0.47, 0.81; p < 0.01), and time to PSA progression: 0.27 (95% CI: 0.21, 0.36; p < 0.01), which were similar to results reported from the overall ARCHES trial.10
Among patients with high-volume disease, there was no difference in rPFS between AR and AP in the enzalutamide plus ADT group or placebo plus ADT group (enzalutamide plus ADT: HR: 1.01; 95% CI: 0.69, 1.48; p = 0.96; placebo plus ADT: HR: 1.01; 95% CI: 0.69, 1.47; p = 0.97; Figures 2A and 2E). Patients receiving enzalutamide plus ADT produced a higher median rPFS (AR: not estimable [NE]; 95% CI: 18.56, NE; AP: NE; 95% CI: NE, NE) compared to placebo plus ADT (AR: 12.45; 95% CI: 11.04, NE; AP: 13.77; 95% CI: 11.04, 22.24), irrespective of HSD3B1 genotype (Figures 2A and 2E). In patients with low-volume disease, rPFS was similar between the AR and AP groups in the enzalutamide plus ADT treatment arm (HR: 1.15; 95% CI: 0.60, 2.20; p = 0.67; Figures 2B and 2E). For patients with low-volume disease treated with placebo plus ADT, risk of rPFS events was comparable between the AP group and AR group (HR: 0.85; 95% CI: 0.45, 1.62; p = 0.63).
Figure 2.
rPFS and OS based on HSD3B1 genotype by volume of disease and metastasis status
(A) Kaplan-Meier curve of rPFS in patients with high-volume disease between the AP and AR HSD3B1 genotypes. The data cutoff date for the rPFS curve was October 14, 2018, before crossover.
(B) Kaplan-Meier curve of rPFS in patients with low-volume disease between HSD3B1 genotypes. The data cutoff date for the rPFS curve was October 14, 2018, before crossover.
(C) Kaplan-Meier curve of OS in patients with high-volume disease between HSD3B1 genotypes. The data cutoff date for the OS curve was May 28, 2021, after crossover was allowed.
(D) Kaplan-Meier curve of OS in patients with low-volume disease between HSD3B1 genotypes. The data cutoff date for the OS curve was May 28, 2021, after crossover was allowed.
(E) Forest plot of rPFS by volume of disease and metastasis status.
(F) Forest plot of OS by volume of disease and metastasis status.
Analyses were conducted using Cox proportional hazards models that were stratified for prior docetaxel use and disease volume for HRs, 95% CIs, and nominal (unadjusted) p values.
AP, adrenal-permissive; AR, adrenal-restrictive; CI, confidence interval; E, number of events; ENZA, enzalutamide; HR, hazard ratio; M0, non-metastatic; M1, metastatic; N, number of patients; NE, not estimable; OS, overall survival; PBO, placebo; rPFS, radiographic progression-free survival.
Survival outcomes were similar to the rPFS results between genotypes in men with high-volume disease treated with placebo plus ADT, as curves were superimposable between the AR and AP groups and event rates were 41.4% and 46.3%, respectively (HR: 0.89; 95% CI: 0.59, 1.35; p = 0.59; Figures 2C and 2F; Table S4). However, in the enzalutamide plus ADT treatment arm, there was a non-significant separation between the genotype groups in AR vs. AP men with high-volume disease (HR: 0.67; 95% CI: 0.42, 1.07; p = 0.096), with 29.8% and 39.2% event rates, respectively, and a smaller non-significant separation in men with low-volume disease (HR: 0.69; 95% CI: 0.28, 1.69; p = 0.42), with 12.5% and 19.7% event rates, respectively (Figures 2C, 2D, and 2F). A similar non-significant separation was observed between the AR and AP groups in patients with low-volume disease who received placebo plus ADT (HR: 0.70; 95% CI: 0.33, 1.50; p = 0.36), with 19.6% and 27.4% event rates, respectively (Figures 2D and 2F). The Kaplan-Meier event-free rate estimate of OS of men with high-volume disease in the enzalutamide plus ADT arm for the AR vs. AP genotype was 0.78 vs. 0.64 at 3 years and 0.67 vs. 0.58 at 4 years (Table S5). Given that rPFS on enzalutamide plus ADT did not differ according to HSD3B1 genotype, these data suggest worse survival following enzalutamide plus ADT progression for men with high-volume disease and an AP genotype when compared to the AR genotype.
The median maximal reduction in PSA from baseline, defined as the largest decrease in PSA from baseline after the start of treatment, was similar between patients with AR vs. AP genotypes within the enzalutamide plus ADT arm (median [range]: AR, −96.94% [−100.0 to 88.2]; AP, −98.00% [−100.0 to 858.0]) and the placebo plus ADT arm (AR, −62.5% [−100.0 to 207.7]; AP, −55.17 [−100.0 to 282.4]). Higher proportions of patients reached a PSA reduction of ≥50% from baseline (PSA 50) in the enzalutamide plus ADT arm (AR, 91.7%; AP, 93.3%) vs. the placebo plus ADT arm (AR, 58.1%; AP, 54.8%), irrespective of HSD3B1 genotype (Figure 3). The proportion of patients who reached a PSA reduction of ≥90% from baseline (PSA 90) and undetectable PSA responses was similarly higher in the enzalutamide plus ADT arm, regardless of HSD3B1 genotype.
Figure 3.
Proportion of patients reaching a PSA reduction of ≥50% from baseline, a PSA reduction of ≥90% from baseline, and undetectable PSA responses
Undetectable PSA rate was defined as the percentage of patients with detectable PSA (≥0.2 ng/mL) at baseline, which becomes undetectable (<0.2 ng/mL) during the study treatment. AP, adrenal-permissive; AR, adrenal-restrictive; ENZA, enzalutamide; PBO, placebo; PSA, prostate-specific antigen.
Safety
Median treatment duration was similar between patients with AR and AP HSD3B1 genotypes within the enzalutamide plus ADT arm (AR, 39.2 months; AP, 39.1 months) and the placebo plus ADT arm (AR, 15.0 months; AP, 14.6 months).
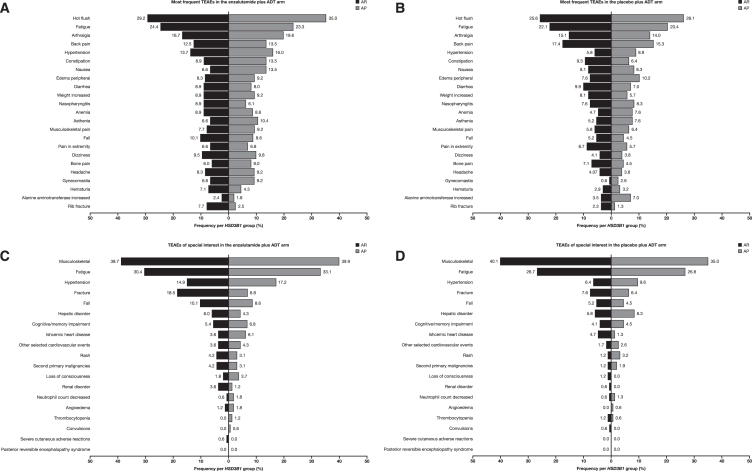
Treatment-emergent adverse events (TEAEs) and serious TEAEs were also similar between patients with AR and AP genotypes within both treatment arms (Table 2). However, with enzalutamide plus ADT treatment, the number of TEAEs leading to treatment withdrawal was higher with the AP genotype (25 [15.3%]) than that with the AR genotype (17 [10.1%]). More patients with the AP genotype experienced more hot flush (AP, 57 [35.0%] vs. AR, 49 [29.2%]), nausea (AP, 22 [13.5%] vs. AR, 11 [6.5%]), gynecomastia (AP, 15 [9.2%] vs. AR, 11 [6.5%]), arthralgia (AP, 32 [19.6%] vs. AR, 28 [16.7%]), and constipation (AP, 22 [13.5%] vs. AR, 15 [8.9%]) but fewer rib fractures (AP, 4 [2.5%] vs. AR, 13 [7.7%]) than patients with the AR genotype (Table 2: Figure 4A). Irrespective of treatment arm, hypertension, asthenia, gynecomastia, and nausea occurred more frequently with the AP genotype than with the AR genotype (Figures 4A and 4B).
Table 2.
TEAEs based on genotypic effects
| Characteristics, n (%) | Enzalutamide + ADT (n = 331) |
Placebo + ADT (n = 329) |
||
|---|---|---|---|---|
| AR (A/A) (n = 168) |
AP (A/C or C/C) (n = 163) |
AR (A/A) (n = 172) |
AP (A/C or C/C) (n = 157) |
|
| TEAEs | 150 (89.3) | 151 (92.6) | 155 (90.1) | 136 (86.6) |
| Drug-related TEAEs | 92 (54.8) | 100 (61.3) | 85 (49.4) | 76 (48.4) |
| Serious TEAEs | 55 (32.7) | 51 (31.3) | 40 (23.3) | 31 (19.7) |
| Drug-related serious TEAEs | 10 (6.0) | 9 (5.5) | 5 (2.9) | 2 (1.3) |
| TEAEs leading to withdrawal of treatment | 17 (10.1) | 25 (15.3) | 10 (5.8) | 9 (5.7) |
| TEAEs leading to death | 5 (3.0) | 9 (5.5) | 2 (1.2) | 4 (2.5) |
| Selected TEAEs | ||||
| Hot flush | 49 (29.2) | 57 (35.0) | 40 (23.3) | 38 (24.2) |
| Fatigue | 41 (24.4) | 38 (23.3) | 26 (15.1) | 28 (17.8) |
| Arthralgia | 28 (16.7) | 32 (19.6) | 22 (12.8) | 21 (13.4) |
| Hypertension | 23 (13.7) | 26 (16.0) | 9 (5.2) | 11 (7.0) |
| Fall | 17 (10.1) | 14 (8.6) | 7 (4.1) | 6 (3.8) |
| Dizziness | 16 (9.5) | 16 (9.8) | 7 (4.1) | 6 (3.8) |
| Anemia | 15 (8.9) | 14 (8.6) | 7 (4.1) | 11 (7.0) |
| Constipation | 15 (8.9) | 22 (13.5) | 13 (7.6) | 8 (5.1) |
| Diarrhea | 15 (8.9) | 13 (8.0) | 14 (8.1) | 10 (6.4) |
| Weight increase | 15 (8.9) | 15 (9.2) | 14 (8.1) | 8 (5.1) |
| Headache | 14 (8.3) | 15 (9.2) | 7 (4.1) | 5 (3.2) |
| Edema peripheral | 14 (8.3) | 15 (9.2) | 10 (5.8) | 14 (8.9) |
| Rib fracture | 13 (7.7) | 4 (2.5) | 2 (1.2) | 2 (1.3) |
| Hematuria | 12 (7.1) | 7 (4.3) | 3 (1.7) | 5 (3.2) |
| Asthenia | 11 (6.5) | 17 (10.4) | 8 (4.7) | 12 (7.6) |
| Gynecomastia | 11 (6.5) | 15 (9.2) | 0 (0) | 3 (1.9) |
| Nausea | 11 (6.5) | 22 (13.5) | 9 (5.2) | 13 (8.3) |
| Ischemic heart disease | 6 (3.6) | 10 (6.1) | 6 (3.5) | 0 |
| Myalgia | 8 (4.8) | 6 (3.7) | 11 (6.4) | 3 (1.9) |
| Other selected cardiovascular events | 6 (3.6) | 7 (4.3) | 2 (1.2) | 2 (1.3) |
A, HSD3B1(1245A); ADT, androgen deprivation therapy; AP, adrenal-permissive; AR, adrenal-restrictive; C, HSD3B1(1245C); TEAE, treatment-emergent adverse event.
Figure 4.
Tornado plots by genotype and treatment group
(A) Most frequent TEAEs in the enzalutamide plus ADT arm.
(B) Most frequent TEAEs in the placebo plus ADT arm.
(C) TEAEs of special interest in the enzalutamide plus ADT arm.
(D) TEAEs of special interest in the placebo plus ADT arm.
ADT, androgen deprivation therapy; AP, adrenal-permissive; AR, adrenal-restrictive; TEAE, treatment-emergent adverse event.
Cardiovascular toxicity within the enzalutamide plus ADT arm, indicated by ischemic heart disease and other selected cardiovascular events, was more frequent in patients with the AP genotype (10 [6.1%] ischemic heart disease events and seven [4.3%] other selected cardiovascular events) compared to the AR genotype (six [3.6%] ischemic heart disease events and six [3.6%] other selected cardiovascular events) (Figures 4C and 4D). In the enzalutamide plus ADT arm, patients with the AP genotype had seven grade 3/4 cardiovascular events (four [2.5%] ischemic heart disease events and three [1.8%] other selected cardiovascular events) compared to five events in patients with the AR genotype (two [1.2%] ischemic heart disease events and three [1.8%] other selected cardiovascular events). In the placebo plus ADT arm, there were five grade 3/4 cardiovascular events in patients with the AR genotype (five [2.9%] ischemic heart disease events) compared to one patient with the AP genotype (one [0.6%] other selected cardiovascular event).
In patients who received enzalutamide plus ADT treatment, a greater incidence of fractures was reported by those with the AR genotype (31 [18.5%]) compared to those with the AP genotype (11 [6.8%]), and a difference in the incidence of falls was observed in patients with the AR genotype (17 [10.1%]) vs. patients with the AP genotype (14 [8.6%]) (Figures 4C and 4D).
TEAEs leading to death in the enzalutamide plus ADT arm occurred in 5.5% (nine patients) and 3.0% (five patients) of patients with the AP and AR genotypes, respectively (Table 2). The causes of death for those in the enzalutamide arm with the AP genotype were two by malignant neoplasm progression and one each by pulmonary embolism, myocardial infarction, cardiopulmonary failure, cerebral infarction, thermal burn, suicide, and drowning. The causes of death for those in the enzalutamide arm with the AR genotype were two each by malignant neoplasm progression and pulmonary embolism and one by sepsis. There was one death reported (0.6%) in the AP group in the placebo plus ADT arm that was deemed to be related to the study drug by the investigator.
Discussion
In this prospectively defined and retrospectively conducted analysis of the ARCHES trial, we assessed whether germline HSD3B1 genotypes impacted clinical outcomes or response to enzalutamide plus ADT in patients with mHSPC. We found no significant differences in rPFS, OS, PSA decline, or time to PSA progression between patients with HSD3B1 AP and AR alleles in either treatment arm. In addition, enzalutamide significantly improved rPFS and OS as well as PSA outcomes equally in both HSD3B1 genotype groups. These data suggest that HSD3B1 genotype is not a predictive biomarker for potent androgen receptor inhibitor therapy with ADT over ADT alone in men with mHSPC, irrespective of disease volume.
Several interesting observations arose from our analysis of HSD3B1 genotype with long-term clinical outcomes in the ARCHES trial. First, OS events were more frequent among patients with the AP genotype than those with the AR genotype, and there were especially more mortality events in men with high-volume mHSPC treated with enzalutamide plus ADT with the AP genotype vs. those with the AR genotype. Enzalutamide plus ADT significantly improved all evaluated endpoints compared to placebo vs. ADT, irrespective of genotype, and given that rPFS did not differ by genotype, we conclude that post-progression survival (from mCRPC to death) may be shorter in men with an AP HSD3B1 genotype. This probably coincides with the cessation of the potent androgen receptor inhibitor (in this case, enzalutamide), and this release in the setting of an abundance of adrenally derived androgens in men with AP mCRPC leads to more rapid progression.
Combination approaches that further reduce androgen levels beyond what is achieved with ADT alone in patients with high-risk genotypes may improve clinical outcomes, suggesting a benefit from prospective genomic testing, similar to the ACIS or A031201 trials, which evaluated the combination of apalutamide or enzalutamide with abiraterone acetate compared to androgen receptor inhibition monotherapy.22,23 We speculate that patients with HSD3B1 AP genotypes benefit from adrenal androgen inhibition or even a combination of adrenal androgen inhibition with androgen receptor inhibition. As these studies have demonstrated improved rPFS with combination therapy over monotherapy in the mCRPC setting,22,23 biomarker-defined precision oncology trials will define subgroups of patients who are most likely to benefit from combination androgen receptor blockade.
One interesting finding in our study was that patients with an HSD3B1 AP genotype also reported higher frequencies of hypertension, asthenia, cardiovascular events, gynecomastia, and nausea and reduced fracture risks compared to those with the AR genotype. In both treatment arms, certain TEAEs, including hypertension, gynecomastia, asthenia, and nausea, were reported more frequently in patients with the AP genotype compared to those with the AR genotype. Within the enzalutamide arm, the incidence of hot flush, arthralgia, and constipation was increased in patients with the AP genotype compared to those with the AR genotype, but there were fewer incidences of rib fractures. As the enzyme that is encoded by HSD3B1 is not only upstream of SRD5A but also one step upstream of aromatase, this enzyme probably regulates the synthesis of estrogen from adrenal precursor steroids.24 The increase in TEAEs in patients with an AP genotype treated with enzalutamide plus ADT appears to indicate a link with higher estrogen exposure, which is consistent with the function of the AP allele and is associated with the higher rate of TEAEs leading to withdrawal.
Among patients with low-volume prostate cancer, the rPFS outcomes for the AR and AP groups were superimposable in the enzalutamide plus ADT treatment arm, reflecting the setting in which non-gonadal androgens are blocked by enzalutamide. However, in the placebo plus ADT treatment arm, there was divergence of the rPFS Kaplan-Meier curves, with a greater proportion of events in the AP arm compared with the AR arm. Although not statistically significant, this finding is consistent with the previously observed associations between AP inheritance and clinical outcomes in low-volume prostate cancer with ADT (±docetaxel) treatment in E3805, in which freedom from CRPC at 2 years was 51% for the AP genotype and 70.5% for the AR genotype.18 The HSD3B1 genotype comparison in men with low-volume prostate cancer in the placebo plus ADT arm of ARCHES is not as well powered as E3805, which explains why the same comparison is not statistically significant. The similarity in rPFS outcomes with ADT treatment (without enzalutamide) in men with high-volume metastatic disease and AP or AR allele inheritance is consistent between ARCHES and E3805. Collectively, these data suggest that persistent adrenal androgen production in men with AP inheritance and low-volume metastatic disease leads to worsening outcomes with ADT in the absence of adrenal androgen blockage or other potentially effective therapies such as docetaxel.
Previous studies have also suggested that HSD3B1 AP genotypes are associated with worse clinical outcomes in men with CRPC treated with enzalutamide or abiraterone.19,20,21 An important question addressed in the current analyses was whether such adverse outcomes were reversible with upfront use of enzalutamide plus ADT for HSPC. There was a qualitative difference in survival outcomes, with a non-significant separation in the OS curves observed between the high-volume-disease AP and AR groups in the enzalutamide plus ADT treatment arm. While patients with HSD3B1 AP and AR genotypes all benefited from treatment intensification with the addition of enzalutamide to ADT, results from the high-volume disease cohort suggest that OS outcomes are worse in men with an HSD3B1 AP genotype and that treatment intensification with triple therapy (docetaxel plus ADT and an androgen receptor signaling inhibitor) or subsequent adrenal androgen suppression is beneficial when tumors harbor more active androgen biosynthesis machinery.25,26
Men with high-volume mHSPC and an HSD3B1 AR genotype had similar rPFS times as those with an AP genotype, but their post-progression survival was longer after enzalutamide plus ADT. This finding suggests that prostate cancer progresses more rapidly in men with an AP genotype after cessation of enzalutamide plus ADT. Tumor-specific mechanisms very likely drive these outcomes, including the possibilities that AP inheritance primes tumors for progression because of (1) HSD3B1 transcriptional upregulation that occurs with enzalutamide exposure,27,28 (2) BMX-driven phosphorylation and activation of 3βHSD1 that occurs preferentially under conditions of androgen receptor blockade,29,30 (3) loss of heterozygosity of the HSD3B1 AR allele so that tumors only express the AP HSD3B1 allele,14 or (4) other tumor-driven mechanisms that augment baseline 3βHSD1 activity.12 Furthermore, differences in post-progression survival based on HSD3B1 AP or AR genotype are associated with the different aggregate adverse event profiles, including the more frequent TEAEs reported for patients with the AP genotype. The former possibilities are attributable to the effects of HSD3B1 genotype and its expression in the tumors, whereas the latter occurs because of genotype-specific effects that impact non-cancer cells. Another alternative explanation is that patients with an AP genotype have worse post-progression outcomes during subsequent therapy with abiraterone acetate or docetaxel, as reported.19,21 This possibility warrants further mechanistic and clinical validation studies.
In conclusion, the results of this retrospective analysis of the phase 3, double-blind, ARCHES trial demonstrate no significant differences between HSD3B1 AP and AR genotype groups in rPFS or OS. Patients with mHSPC in the ARCHES trial who received enzalutamide plus ADT had improved OS and rPFS compared to those who received placebo plus ADT, irrespective of the HSD3B1 genotype. However, although not statistically significant, men with low-volume disease and an inherited AP HSD3B1 genotype did have worse rPFS when treated with placebo plus ADT. Larger prospective studies are therefore required to better characterize the impact of HSD3B1 genotypes on prostate cancer progression and potential combination or post-progression salvage treatments for these men to further optimize outcomes.
Limitations of the study
The small sample size available for the current biomarker analyses is a limitation of this study, as the cohort of patients with low-volume disease may have insufficient events or patient follow-up to draw conclusions regarding differences in outcomes for patients with an HSD3B1 AP vs. AR genotype following upfront enzalutamide use. Furthermore, while the rPFS results were not affected by crossover because PSA progression precedes radiographic progression, the OS data were impacted by patients crossing over from the placebo arm to the enzalutamide arm. Another limitation of this study is the lack of inclusivity in the data, as the majority of the study cohort was White and non-Hispanic. A final limitation is the lack of sequential tumor analysis for patients in ARCHES given that somatic HSD3B1 alterations may develop over time and create an AP genotype within the tumor, resulting in misclassification of the HSD3B1 genotype in some men.14
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| LightScanner Master Mix | BioFire Defense, LLC | HRLS-ASY-003 |
| Magnesium Chloride (10 mM) | BioFire Defense, LLC | Component from HRLS-ASY-003 |
| Nuclease-Free Water | BioFire Defense, LLC | Component from HRLS-ASY-003 |
| Dimethyl Sulfoxide | Sigma-Aldrich | D2650 |
| Oligonucleotides | ||
| IDT HSD3B1 Forward Primer (1 μM) (5′- GTCAA ATAGCGTATTCACCTTCTCTTAT-3′) |
Hearn et al.31 | N/A |
| IDT HSD3B1 Reverse Primer (10 μM) (5′- GAGGG TGGAGCTTGATGACATCT-3′) |
Hearn et al.31 | N/A |
| Qiagen Custom LNA Oligonucleotide Probe (10 μM)(5′3′):GGAGA+ACCTGAAGTCCAAGA CTCAGTGATTTAAGG/Phos |
Hearn et al.31 | 339413 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to, and will be fulfilled by, the lead contact, Dr. Nima Sharifi (nimasharifi@miami.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Trial design and participants
ARCHES was a multinational, double-blind, randomized, placebo-controlled phase 3 trial and its results have been previously published.10 In ARCHES, patients with metastatic hormone-sensitive prostate cancer (mHSPC) were centrally randomized 1:1 to enzalutamide (160 mg/day) plus androgen deprivation therapy (ADT) or placebo plus ADT through an interactive response technology system. Patients were stratified at randomization by disease volume (high or low) and prior docetaxel therapy. The present analysis is a prospectively designed but retrospective biomarker analysis of a randomized controlled trial in which the biomarker statistical analysis plan for HSD3B1 germline genotyping was developed prior to overall survival results being available, genotyping analyses were blinded to clinical outcomes, and clinicians were blinded to HSD3B1 genotype. The statistical analysis plan for the ARCHES trial has been previously published.10
Method details
Study protocol
For patients who consented to pharmacogenomic testing, germline DNA was collected prior to therapy and used for blinded HSD3B1 genotyping at a central laboratory. To appropriately detect the variant sequence, samples were tested with a melting assay using an unlabeled locked nucleic acid oligonucleotide probe in an asymmetric polymerase chain reaction.32 Results were included for all patients in the intent-to-treat population with an HSD3B1 determination. The analysis was separated into two groups based on HSD3B1 genotype: the adrenal-restrictive (AR) group, with two HSD3B1(1245A) alleles, and the adrenal-permissive (AP) group, with either one or two HSD3B1(1245C) alleles. Although inheritance of two HSD3B1(1245C) alleles has previously been found to be associated with worse clinical outcomes compared with one allele, the lower number (<10%) of homozygous HSD3B1(1245C) men is a limitation. We therefore elected to use the two group AP and AR definitions above to avoid this limitation and be consistent with prior HSD3B1 analysis in another phase 3 trial.18
Assessments
The ARCHES primary and secondary endpoint results were previously reported.10 The current analyses were performed in the final ARCHES dataset and compared radiographic progression-free survival (rPFS), overall survival (OS), time to prostate-specific antigen (PSA) progression, PSA response rates [PSA reduction of ≥50% from baseline (PSA 50), PSA reduction of ≥90% from baseline (PSA 90), and undetectable PSA], and safety and toxicity outcomes between patients in ARCHES with mHSPC who inherited AR vs. AP HSD3B1 genotypes (endpoints defined previously10). In brief, rPFS was calculated in patients with an rPFS event as the time from randomization to the first objective evidence of radiographic disease progression (based on blinded independent central review) or death, whichever occurred first. In patients with no rPFS event, rPFS was censored on the date of last evaluable radiographic assessment prior to the data analysis cut-off. OS was defined as the time from randomization to death from any cause. For patients who were alive at the time of the data cut-off date, OS time was censored on the last date the patient was known to be alive or the cut-off date, whichever occurred first. Time to PSA progression was calculated as the time from randomization to the first observation of PSA progression, defined as a ≥25% increase and an absolute increase of ≥2 ng/mL above the nadir, which was confirmed by a second consecutive value at least 3 weeks later. In patients with no PSA progression, time to PSA progression was censored on the date of the last PSA sample. Patients without PSA progression after two or more consecutive missed PSA assessments were censored on the date of the last PSA assessment prior to the assessments missed. Comparison of efficacy between genotype groups was analyzed by disease volume as well as metastasis status, both defined previously.10 Treatment-emergent adverse events (TEAEs) of all grades were assessed within this analysis.
Quantification and statistical analysis
The primary endpoint of this study was rPFS; secondary endpoints were OS, time to PSA progression, PSA response rates (PSA 50, PSA 90, and undetectable PSA), and safety. Kaplan–Meier estimates were used to summarize time-to-event endpoints of rPFS, OS, and PSA progression. Statistical analyses were conducted using Cox proportional hazards models that were stratified for prior docetaxel use and disease volume for HRs, 95% (CIs), and nominal (unadjusted) p values. The main hypothesis tested was whether the AR HSD3B1(1245A) and the AP HSD3B1(1245C) genotypes have the same or different survival distributions over time in the entire biomarker-defined population of ARCHES. Cox proportional hazards models included terms for the HSD3B1 genotype, treatment group, volume of disease, and prior docetaxel use. Additionally, the presence of an interaction between HSD3B1 genotype and treatment was assessed. If a significant interaction was identified indicating a differential benefit of enzalutamide over placebo based on HSD3B1 genotype, then the emphasis would change to testing within each treatment arm. If no significant interactions were noted, the results would be reported by treatment arm as descriptive data summaries. Significance of interaction tests were assessed at a 0.10 level and other tests at a 0.05 level. The PSA response rates were assessed using logistic regression models in place of Cox proportional hazards models. Subgroups were assessed for rPFS and OS using separate Cox proportional hazards models for each category and the results were presented in forest plots. Stratification factors were not included in the subgroup models due to the reduced number of events. Metastasis status was added as a post hoc subgroup. Safety was assessed in patients who received at least one dose of study drug.
Acknowledgments
This study was funded by Astellas Pharma Inc. and Pfizer Inc., the co-developers of enzalutamide. Data analyses were performed by the study sponsors and provided to all authors. Medical writing and editorial assistance were provided by Terrance Ku, MSc, and Jane Beck, MA, from Complete HealthVizion, IPG Health Medical Communications, funded by the study sponsors. N.S. is supported by grants from the National Institutes of Health (R01CA236780, R01CA261995, R01CA172382, and R01CA249279) and Congressionally Directed Medical Research Programs (W81XWH2010137 and W81XWH-22-1-0082).
Author contributions
N.S.: conceptualization, resources, supervision, funding acquisition, investigation, methodology, writing – original draft, and writing – review & editing. A.A.A.: conceptualization, resources, writing – original draft, and writing – review & editing. M.P.: formal analysis, validation, investigation, and writing – review & editing. J.W.D.H.: formal analysis and writing – review & editing. M.W.: conceptualization, data curation, formal analysis, methodology, and writing – review & editing. F.Z.: formal analysis, supervision, funding acquisition, validation, investigation, visualization, methodology, writing – original draft, and writing – review & editing. J.S.: resources, data curation, formal analysis, investigation, visualization, methodology, and writing – review & editing. G.P.H.: conceptualization, data curation, investigation, visualization, project administration, and writing – review & editing. A.S.: conceptualization, visualization, and writing – review & editing. A.J.A.: conceptualization, resources, formal analysis, supervision, funding acquisition, investigation, writing – original draft, and writing – review & editing.
Declaration of interests
N.S. reports consulting for Celgene and Roivant, research funding from Astellas, and a filed patent application by Cleveland Clinic for a method of steroid-dependent disease treatment based on HSD3B1. A.A.A. reports honoraria from Aculeus Therapeutics, Amgen, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck, Merck Serono, Novartis, Noxopharm, Pfizer, Sanofi, Telix, and Tolmar; consulting for Aculeus Therapeutics, Amgen, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck, Merck Serono, Novartis, Noxopharm, Pfizer, Sanofi, Telix, and Tolmar; speakers’ bureau for Amgen, Astellas, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck Serono, and Novartis; grants from Aptevo (institutional), Astellas (institutional), Astellas (investigator), AstraZeneca (institutional), AstraZeneca (investigator), Bionomics (institutional), Bristol Myers Squibb (institutional), Exelixis (institutional), Gilead Sciences (institutional), GlaxoSmithKline (institutional), Hinova (institutional), Ipsen (institutional), Janssen (institutional), Lilly (institutional), MedImmune (institutional), Merck Serono (institutional), Merck Serono (investigator), Merck Sharpe & Dohme (institutional), Novartis (institutional), Pfizer (institutional), Sanofi Aventis (institutional), and Synthorx (institutional); travel/accommodation reimbursement from Amgen, Astellas, Bayer, Janssen, Merck Serono, Pfizer, Sanofi, and Tolmar; participation on an advisory board for Amgen, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck, Merck Serono, Novartis, Noxopharm, Pfizer, Sanofi, Telix, and Tolmar; and participation as the chair of the Urologic Oncology Group for the Clinical Oncology Society of Australia and as a chair of the Translational Research Subcommittee and member of the Scientific Advisory Committee for the ANZUP Cancer Trials Group. J.W.D.H. reports consulting for Astellas. M.W. is an employee of Astellas and reports ownership of stock of ADMA Biologics, CASI, Merck, and Seelos. F.Z. is an employee of Pfizer and reports ownership of stock of Pfizer and AlloVir and patents with AlloVir. J.S. is an employee of Astellas and reports ownership of stock of AstraZeneca. G.P.H. is an employee of Astellas. A.S. reports consulting for Alere, Astellas, Bayer, Bristol Myers Squibb, Ferring, Ipsen, Janssen, Roche, Steba Biotech, and Synergo; research funding from Amgen, Astellas, AstraZeneca, Bayer, Cepheid, GenomeDx, Immatics, Janssen, Johnson & Johnson, Karl Storz, Medivation, Novartis, and Roche; patents for A290/99 implantable incontinence device, AT00/0001:C-Trap implantable device to treat urinary incontinence, and 2018/6579 gene-expression signature for subtype and prognostic prediction of renal cell carcinoma; expert testimony for GBA Pharma; and travel support from Amgen, Astellas, AstraZeneca, CureVac, Ferring, Ipsen, Janssen, and Sanofi/Aventis. A.J.A. reports research support (institutional) to Duke from the NIH/NCI, PCF/Movember, DOD, Astellas, Pfizer, Bayer, Janssen, Dendreon, BMS, AstraZeneca, Merck, Forma, Celgene, Amgen, and Novartis and personal compensation from consulting or advising relationships with Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Celgene, Clovis, Dendreon, Epic Sciences, Exact Sciences, Exelixis, Forma, GoodRx, Janssen, Merck, Myovant, Novartis, Pfizer, and Point.
Published: August 20, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2024.101644.
Supplemental information
References
- 1.Auchus R.J., Sharifi N. Sex hormones and prostate cancer. Annu. Rev. Med. 2020;71:33–45. doi: 10.1146/annurev-med-051418-060357. [DOI] [PubMed] [Google Scholar]
- 2.Bruchovsky N., Wilson J.D. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J. Biol. Chem. 1968;243:2012–2021. [PubMed] [Google Scholar]
- 3.Desai K., McManus J.M., Sharifi N. Hormonal therapy for prostate cancer. Endocr. Rev. 2021;42:354–373. doi: 10.1210/endrev/bnab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard M., Mostaghel E.A., Auchus R.J., Storbeck K.H. The role of adrenal derived androgens in castration resistant prostate cancer. J. Steroid Biochem. Mol. Biol. 2020;197 doi: 10.1016/j.jsbmb.2019.105506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai C., Heemers H., Sharifi N. Androgen signaling in prostate cancer. Cold Spring Harb. Perspect. Med. 2017;7 doi: 10.1101/cshperspect.a030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scher H.I., Fizazi K., Saad F., Taplin M.E., Sternberg C.N., Miller K., de Wit R., Mulders P., Chi K.N., Shore N.D., et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 7.Beer T.M., Tombal B. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014;371:1755–1756. doi: 10.1056/NEJMc1410239. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong A.J., Azad A.A., Iguchi T., Szmulewitz R.Z., Petrylak D.P., Holzbeierlein J., Villers A., Alcaraz A., Alekseev B., Shore N.D., et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J. Clin. Oncol. 2022;40:1616–1622. doi: 10.1200/jco.22.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis I.D., Martin A.J., Stockler M.R., Begbie S., Chi K.N., Chowdhury S., Coskinas X., Frydenberg M., Hague W.E., Horvath L.G., et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl. J. Med. 2019;381:121–131. doi: 10.1056/NEJMoa1903835. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong A.J., Szmulewitz R.Z., Petrylak D.P., Holzbeierlein J., Villers A., Azad A., Alcaraz A., Alekseev B., Iguchi T., Shore N.D., et al. ARCHES: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J. Clin. Oncol. 2019;37:2974–2986. doi: 10.1200/jco.19.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmann M.R., Hussain M., Dehm S.M., Beltran H., Wyatt A.W., Halabi S., Sweeney C., Scher H.I., Ryan C.J., Feng F.Y., et al. Prostate Cancer Foundation Hormone-Sensitive Prostate Cancer Biomarker Working Group meeting summary. Urology. 2021;155:165–171. doi: 10.1016/j.urology.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Qin L., Chung Y.M., Berk M., Naelitz B., Zhu Z., Klein E., Chakraborty A.A., Sharifi N. Hypoxia-reoxygenation couples 3βHSD1 enzyme and cofactor upregulation to facilitate androgen biosynthesis and hormone therapy resistance in prostate cancer. Cancer Res. 2022;82:2417–2430. doi: 10.1158/0008-5472.Can-21-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui D., Li J., Zhu Z., Berk M., Hardaway A., McManus J., Chung Y.M., Alyamani M., Valle S., Tiwari R., et al. Cancer-associated fibroblast-secreted glucosamine alters the androgen biosynthesis program in prostate cancer via HSD3B1 upregulation. J. Clin. Invest. 2023;133 doi: 10.1172/jci161913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang K.-H., Li R., Kuri B., Lotan Y., Roehrborn C.G., Liu J., Vessella R., Nelson P.S., Kapur P., Guo X., et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–1084. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naelitz B.D., Sharifi N. Through the looking-glass: reevaluating DHEA metabolism through HSD3B1 genetics. Trends Endocrinol. Metabol. 2020;31:680–690. doi: 10.1016/j.tem.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabharwal N., Sharifi N. HSD3B1 genotypes conferring adrenal-restrictive and adrenal-permissive phenotypes in prostate cancer and beyond. Endocrinology. 2019;160:2180–2188. doi: 10.1210/en.2019-00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas L., Sharifi N. Germline HSD3B1 genetics and prostate cancer outcomes. Urology. 2020;145:13–21. doi: 10.1016/j.urology.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hearn J.W.D., Sweeney C.J., Almassi N., Reichard C.A., Reddy C.A., Li H., Hobbs B., Jarrard D.F., Chen Y.H., Dreicer R., et al. HSD3B1 genotype and clinical outcomes in metastatic castration-sensitive prostate cancer. JAMA Oncol. 2020;6 doi: 10.1001/jamaoncol.2019.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalaf D.J., Aragón I.M., Annala M., Lozano R., Taavitsainen S., Lorente D., Finch D.L., Romero-Laorden N., Vergidis J., Cendón Y., et al. HSD3B1 (1245A>C) germline variant and clinical outcomes in metastatic castration-resistant prostate cancer patients treated with abiraterone and enzalutamide: results from two prospective studies. Ann. Oncol. 2020;31:1186–1197. doi: 10.1016/j.annonc.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Sharifi N. Homozygous HSD3B1(1245C) inheritance and poor outcomes in metastatic castration-resistant prostate cancer with abiraterone or enzalutamide: what does it mean? Ann. Oncol. 2020;31:1103–1105. doi: 10.1016/j.annonc.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Lu C., Terbuch A., Dolling D., Yu J., Wang H., Chen Y., Fountain J., Bertan C., Sharp A., Carreira S., et al. Treatment with abiraterone and enzalutamide does not overcome poor outcome from metastatic castration-resistant prostate cancer in men with the germline homozygous HSD3B1 c.1245C genotype. Ann. Oncol. 2020;31:1178–1185. doi: 10.1016/j.annonc.2020.04.473. [DOI] [PubMed] [Google Scholar]
- 22.Saad F., Efstathiou E., Attard G., Flaig T.W., Franke F., Goodman O.B., Jr., Oudard S., Steuber T., Suzuki H., Wu D., et al. Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2021;22:1541–1559. doi: 10.1016/s1470-2045(21)00402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris M.J., Heller G., Bryce A.H., Armstrong A.J., Beltran H., Hahn O.M., McGary E.C., Mehan P.T., Goldkorn A., Roth B.J., et al. Alliance A031201: a phase III trial of enzalutamide (ENZ) versus enzalutamide, abiraterone, and prednisone (ENZ/AAP) for metastatic castration resistant prostate cancer (mCRPC) J. Clin. Oncol. 2019;37:5008. doi: 10.1200/JCO.2019.37.15_suppl.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruse M.L., Patel M., McManus J., Chung Y.M., Li X., Wei W., Bazeley P.S., Nakamura F., Hardaway A., Downs E., et al. Adrenal-permissive HSD3B1 genetic inheritance and risk of estrogen-driven postmenopausal breast cancer. JCI Insight. 2021;6 doi: 10.1172/jci.insight.150403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith M.R., Hussain M., Saad F., Fizazi K., Sternberg C.N., Crawford E.D., Kopyltsov E., Park C.H., Alekseev B., Montesa-Pino Á., et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N. Engl. J. Med. 2022;386:1132–1142. doi: 10.1056/NEJMoa2119115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fizazi K., Foulon S., Carles J., Roubaud G., McDermott R., Fléchon A., Tombal B., Supiot S., Berthold D., Ronchin P., et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399:1695–1707. doi: 10.1016/s0140-6736(22)00367-1. [DOI] [PubMed] [Google Scholar]
- 27.Mei Z., Yang T., Liu Y., Gao Y., Hou Z., Zhuang Q., He D., Zhang X., Tan Q., Zhu X., et al. Management of prostate cancer by targeting 3βHSD1 after enzalutamide and abiraterone treatment. Cell Rep. Med. 2022;3 doi: 10.1016/j.xcrm.2022.100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou Z., Huang S., Mei Z., Chen L., Guo J., Gao Y., Zhuang Q., Zhang X., Tan Q., Yang T., et al. Inhibiting 3βHSD1 to eliminate the oncogenic effects of progesterone in prostate cancer. Cell Rep. Med. 2022;3 doi: 10.1016/j.xcrm.2022.100561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X., Berk M., Goins C., Alyamani M., Chung Y.-M., Wang C., Patel M., Rathi N., Zhu Z., Willard B., et al. BMX controls 3βHSD1 and sex steroid biosynthesis in cancer. J. Clin. Invest. 2023;133 doi: 10.1172/JCI163498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S., Cai C., Sowalsky A.G., Ye H., Ma F., Yuan X., Simon N.I., Gray N.S., Balk S.P. BMX-mediated regulation of multiple tyrosine kinases contributes to castration resistance in prostate cancer. Cancer Res. 2018;78:5203–5215. doi: 10.1158/0008-5472.Can-17-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hearn J.W.D., AbuAli G., Reichard C.A., Reddy C.A., Magi-Galluzzi C., Chang K.-H., Carlson R., Rangel L., Reagan K., Davis B.J., et al. HSD3B1 and resistance to androgen-deprivation therapy in prostate cancer: a retrospective, multicohort study. Lancet Oncol. 2016;17:1435–1444. doi: 10.1016/S1470-2045(16)30227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alyamani M., McManus J., Patel M., Sharifi N. Approaches to assessing 3β-hydroxysteroid dehydrogenase-1. Methods Enzymol. 2023;689:89–119. doi: 10.1016/bs.mie.2023.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.