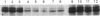Abstract
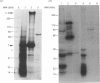
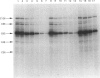
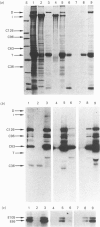
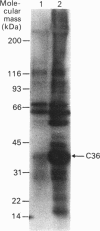
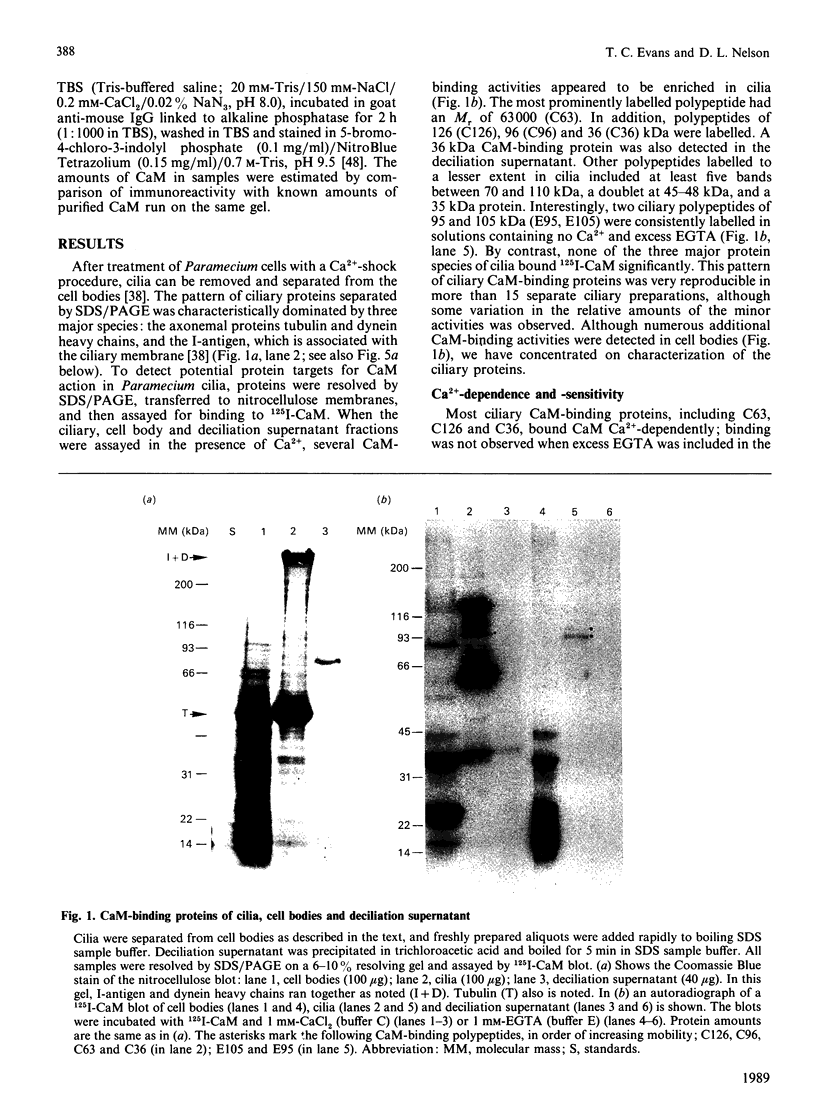
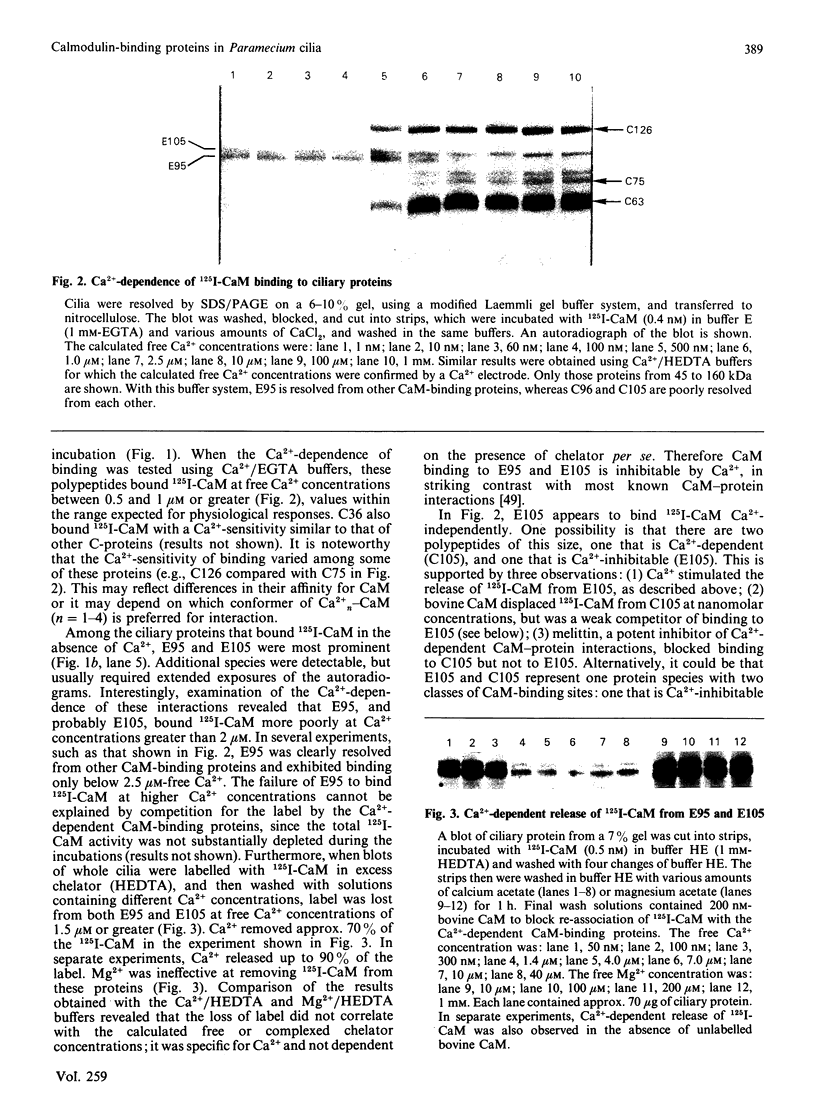
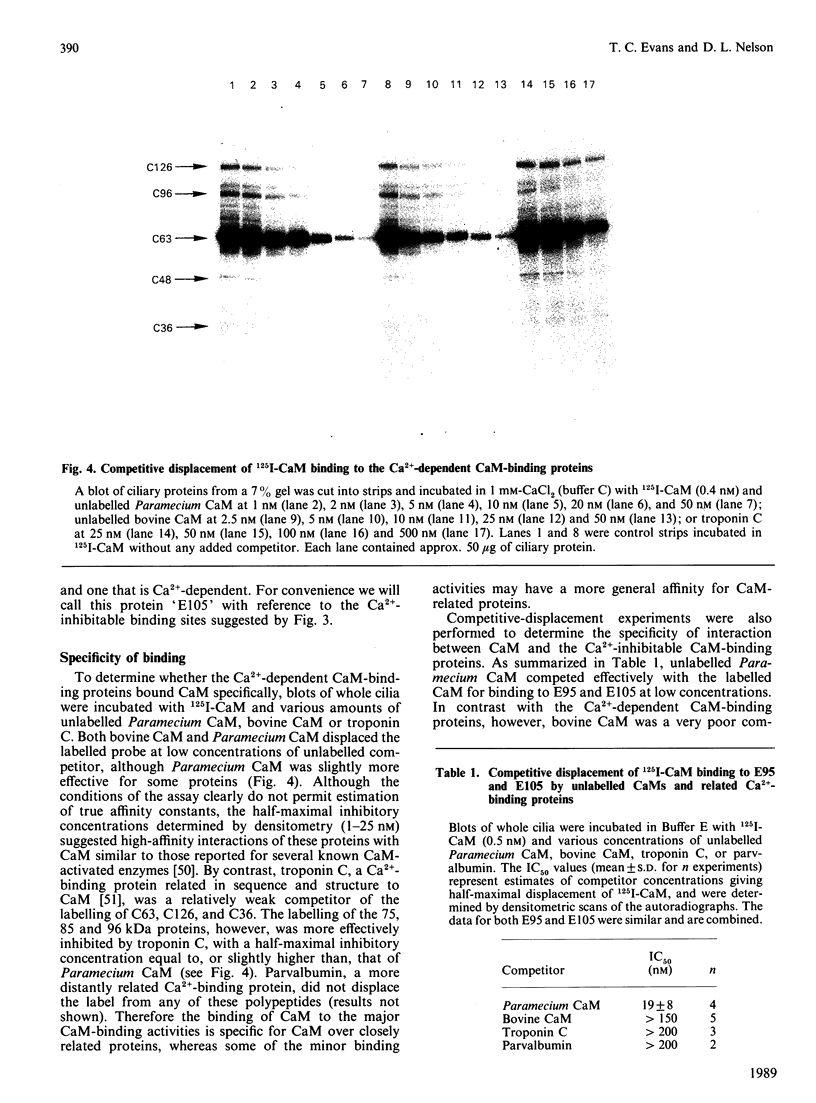
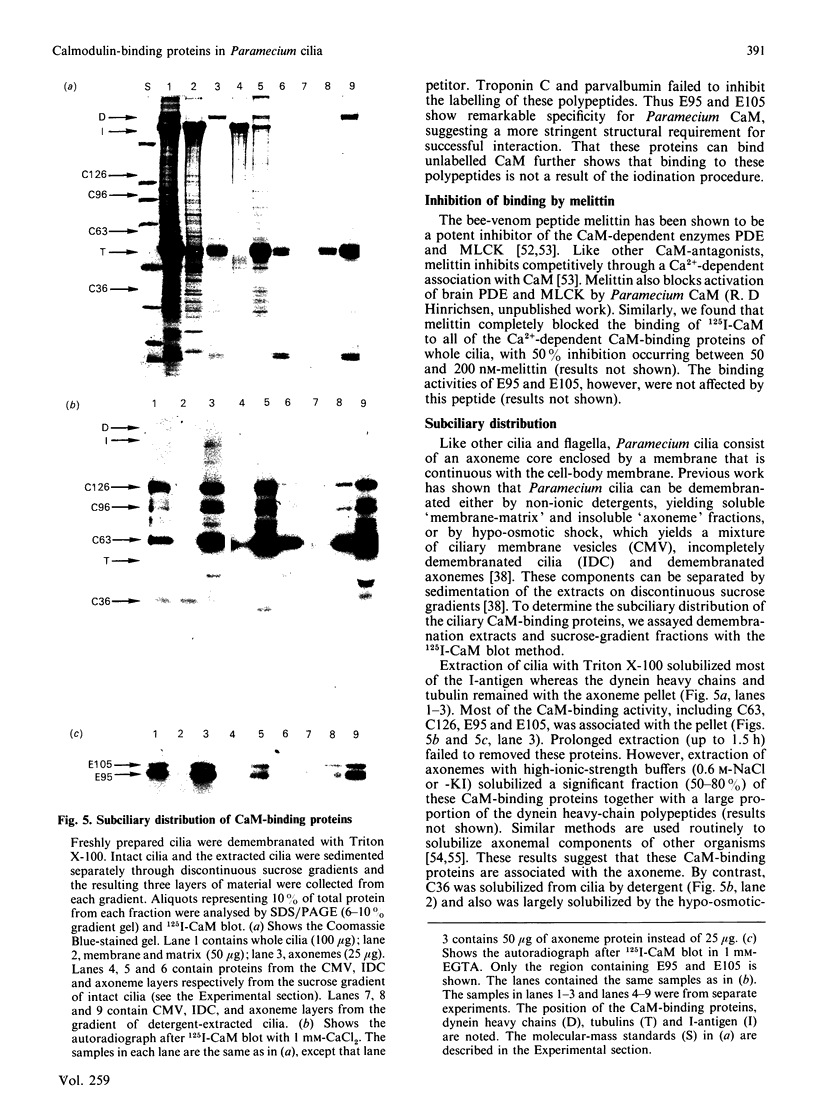
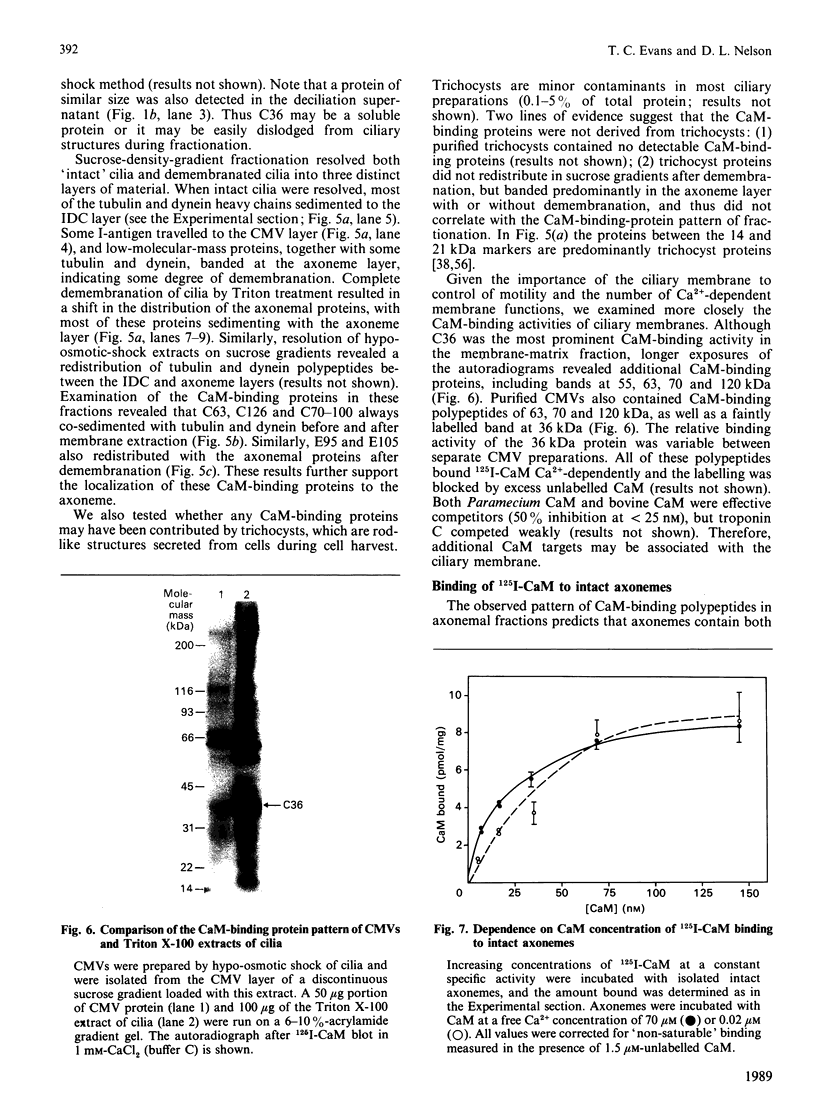
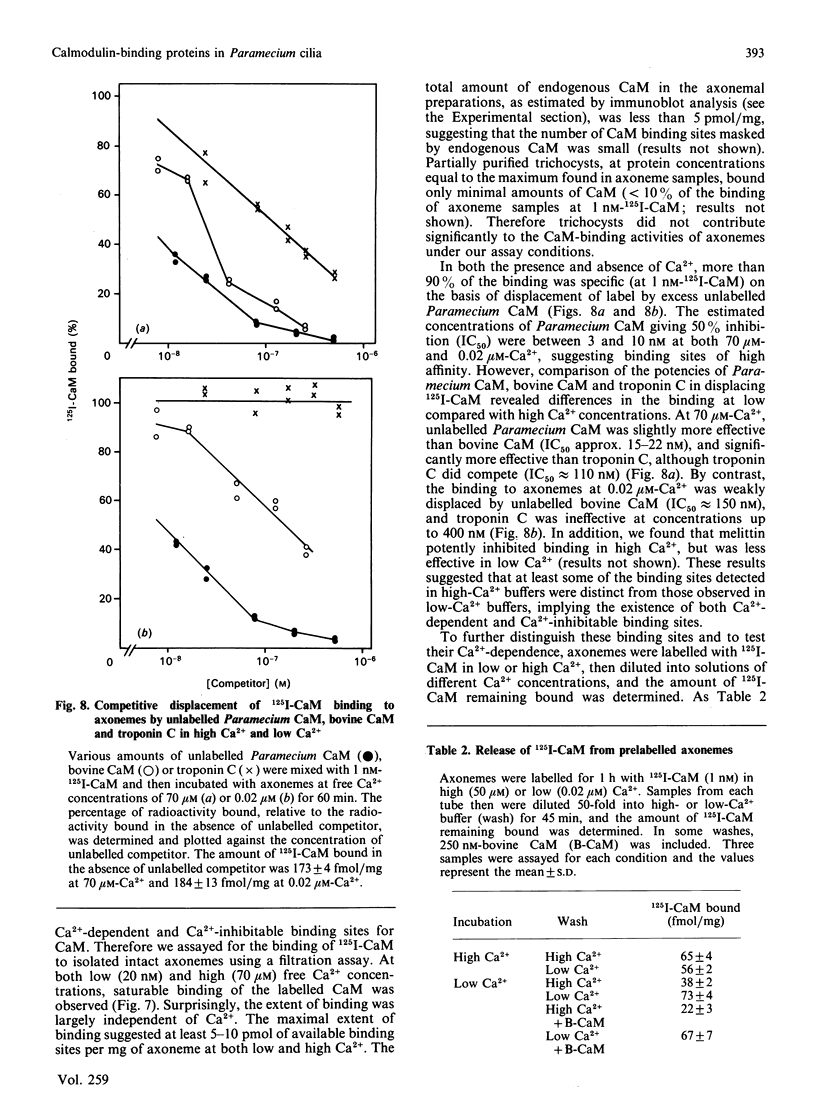
To identify protein targets for calmodulin (CaM) in the cilia of Paramecium tetraurelia, we employed a 125I-CaM blot assay after resolution of ciliary proteins on SDS/polyacrylamide gels. Two distinct types of CaM-binding proteins were detected. One group bound 125I-CaM at free Ca2+ concentrations above 0.5-1 microM and included a major binding activity of 63 kDa (C63) and activities of 126 kDa (C126), 96 kDa (C96), and 36 kDa (C36). CaM bound these proteins with high (nanomolar) affinity and specificity relative to related Ca2+ receptors. The second type of protein bound 125I-CaM only when the free Ca2+ concentration was below 1-2 microM and included polypeptides of 95 kDa (E95) and 105 kDa (E105). E105 may also contain Ca2+-dependent binding sites for CaM. Both E95 and E105 exhibited strong specificity for Paramecium CaM over bovine CaM. Ciliary subfractionation experiments suggested that C63, C126, C96, E95, and E105 are bound to the axoneme, whereas C36 is a soluble and/or membrane-associated protein. Additional Ca2+-dependent CaM-binding proteins of 63, 70, and 120 kDa were found associated with ciliary membrane vesicles. In support of these results, filtration binding assays also indicated high-affinity binding sites for CaM on isolated intact axonemes and suggested the presence of both Ca2+-dependent and Ca2+-inhibitable targets. Like E95 and E105, the Ca2+-inhibitable CaM-binding sites showed strong preference for Paramecium CaM over vertebrate CaM and troponin C. Together, these results suggest that CaM has multiple targets in the cilium and hence may regulate ciliary motility in a complex and pleiotropic fashion.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adoutte A., Ramanathan R., Lewis R. M., Dute R. R., Ling K. Y., Kung C., Nelson D. L. Biochemical studies of the excitable membrane of Paramecium tetraurelia. III. Proteins of cilia and ciliary membranes. J Cell Biol. 1980 Mar;84(3):717–738. doi: 10.1083/jcb.84.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen T. J., Luetje C. W., Heideman W., Storm D. R. Purification of a novel calmodulin binding protein from bovine cerebral cortex membranes. Biochemistry. 1983 Sep 27;22(20):4615–4618. doi: 10.1021/bi00289a001. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Blum J. J., Hayes A., Jamieson G. A., Jr, Vanaman T. C. Calmodulin confers calcium sensitivity on ciliary dynein ATPase. J Cell Biol. 1980 Nov;87(2 Pt 1):386–397. doi: 10.1083/jcb.87.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini N. M., Gustin M. C., Nelson D. L. Regulation of ciliary motility by membrane potential in Paramecium: a role for cyclic AMP. Cell Motil Cytoskeleton. 1986;6(3):256–272. doi: 10.1002/cm.970060303. [DOI] [PubMed] [Google Scholar]
- Bonini N. M., Nelson D. L. Differential regulation of Paramecium ciliary motility by cAMP and cGMP. J Cell Biol. 1988 May;106(5):1615–1623. doi: 10.1083/jcb.106.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978 Dec 15;202(4373):1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J., Nagayama S. M. Modulation of the asymmetry of sea urchin sperm flagellar bending by calmodulin. J Cell Biol. 1985 Jun;100(6):1875–1883. doi: 10.1083/jcb.100.6.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess-Cassler A., Hinrichsen R. D., Maley M. E., Kung C. Biochemical characterization of a genetically altered calmodulin in Paramecium. Biochim Biophys Acta. 1987 Jul 7;913(3):321–328. doi: 10.1016/0167-4838(87)90142-7. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Dedman J. R., Munjaal R. P., Means A. R. Calmodulin. Development and application of a sensitive radioimmunoassay. J Biol Chem. 1979 Oct 25;254(20):10262–10267. [PubMed] [Google Scholar]
- Cimler B. M., Andreasen T. J., Andreasen K. I., Storm D. R. P-57 is a neural specific calmodulin-binding protein. J Biol Chem. 1985 Sep 5;260(19):10784–10788. [PubMed] [Google Scholar]
- Comte M., Maulet Y., Cox J. A. Ca2+-dependent high-affinity complex formation between calmodulin and melittin. Biochem J. 1983 Jan 1;209(1):269–272. doi: 10.1042/bj2090269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T. N., Urdea M. S., Masiarz F. R., Thorner J. Isolation of the yeast calmodulin gene: calmodulin is an essential protein. Cell. 1986 Nov 7;47(3):423–431. doi: 10.1016/0092-8674(86)90599-4. [DOI] [PubMed] [Google Scholar]
- Flanagan S. D., Yost B. Calmodulin-binding proteins: visualization by 125I-calmodulin overlay on blots quenched with Tween 20 or bovine serum albumin and poly(ethylene oxide). Anal Biochem. 1984 Aug 1;140(2):510–519. doi: 10.1016/0003-2697(84)90202-1. [DOI] [PubMed] [Google Scholar]
- Fulton C., Cheng K. L., Lai E. Y. Two calmodulins in Naegleria flagellates: characterization, intracellular segregation, and programmed regulation of mRNA abundance during differentiation. J Cell Biol. 1986 May;102(5):1671–1678. doi: 10.1083/jcb.102.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS I. R. STUDIES ON THE PROTEIN COMPONENTS OF CILIA FROM TETRAHYMENA PYRIFORMIS. Proc Natl Acad Sci U S A. 1963 Nov;50:1002–1010. doi: 10.1073/pnas.50.5.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman S. E., Witman G. B. Purification of calmodulin from Chlamydomonas: calmodulin occurs in cell bodies and flagella. J Cell Biol. 1980 Dec;87(3 Pt 1):764–770. doi: 10.1083/jcb.87.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Calmodulin binding to platelet plasma membranes. Biochim Biophys Acta. 1982 Mar 23;686(1):55–64. doi: 10.1016/0005-2736(82)90151-1. [DOI] [PubMed] [Google Scholar]
- Gundersen R. E., Nelson D. L. A novel Ca2+-dependent protein kinase from Paramecium tetraurelia. J Biol Chem. 1987 Apr 5;262(10):4602–4609. [PubMed] [Google Scholar]
- Gustin M. C., Nelson D. L. Regulation of ciliary adenylate cyclase by Ca2+ in Paramecium. Biochem J. 1987 Sep 1;246(2):337–345. doi: 10.1042/bj2460337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga N., Forte M., Ramanathan R., Hennessey T., Takahashi M., Kung C. Characterization and purification of a soluble protein controlling Ca-channel activity in paramecium. Cell. 1984 Nov;39(1):71–78. doi: 10.1016/0092-8674(84)90192-2. [DOI] [PubMed] [Google Scholar]
- Hinrichsen R. D., Burgess-Cassler A., Soltvedt B. C., Hennessey T., Kung C. Restoration by calmodulin of a Ca2+-dependent K+ current missing in a mutant of Paramecium. Science. 1986 Apr 25;232(4749):503–506. doi: 10.1126/science.2421410. [DOI] [PubMed] [Google Scholar]
- Hirano J., Watanabe Y. Studies on calmodulin-binding proteins (CaMBPs) in the cilia of Tetrahymena. Exp Cell Res. 1985 Apr;157(2):441–450. doi: 10.1016/0014-4827(85)90129-6. [DOI] [PubMed] [Google Scholar]
- Hisanaga S., Pratt M. M. Calmodulin interaction with cytoplasmic and flagellar dynein: calcium-dependent binding and stimulation of adenosinetriphosphatase activity. Biochemistry. 1984 Jun 19;23(13):3032–3037. doi: 10.1021/bi00308a029. [DOI] [PubMed] [Google Scholar]
- Ikemoto N. Structure and function of the calcium pump protein of sarcoplasmic reticulum. Annu Rev Physiol. 1982;44:297–317. doi: 10.1146/annurev.ph.44.030182.001501. [DOI] [PubMed] [Google Scholar]
- Jamieson G. A., Jr, Vanaman T. C., Blum J. J. Presence of calmodulin in Tetrahymena. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6471–6475. doi: 10.1073/pnas.76.12.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh N., Raynor R. L., Wise B. C., Schatzman R. C., Turner R. S., Helfman D. M., Fain J. N., Kuo J. F. Inhibition by melittin of phospholipid-sensitive and calmodulin-sensitive Ca2+-dependent protein kinases. Biochem J. 1982 Jan 15;202(1):217–224. doi: 10.1042/bj2020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R. L., Coulson C. C. Rapid purification of calmodulin and S-100 protein by affinity chromatography with melittin immobilized to sepharose. Biochem Biophys Res Commun. 1985 Nov 27;133(1):256–264. doi: 10.1016/0006-291x(85)91869-8. [DOI] [PubMed] [Google Scholar]
- Klumpp S., Kleefeld G., Schultz J. E. Calcium/calmodulin-regulated guanylate cyclase of the excitable ciliary membrane from Paramecium. Dissociation of calmodulin by La3+: calmodulin specificity and properties of the reconstituted guanylate cyclase. J Biol Chem. 1983 Oct 25;258(20):12455–12459. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Maihle N. J., Dedman J. R., Means A. R., Chafouleas J. G., Satir B. H. Presence and indirect immunofluorescent localization of calmodulin in Paramecium tetraurelia. J Cell Biol. 1981 Jun;89(3):695–699. doi: 10.1083/jcb.89.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalan A. S., Klee C. B. Calmodulin. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:227–278. [PubMed] [Google Scholar]
- Masure H. R., Alexander K. A., Wakim B. T., Storm D. R. Physicochemical and hydrodynamic characterization of P-57, a neurospecific calmodulin binding protein. Biochemistry. 1986 Nov 18;25(23):7553–7560. doi: 10.1021/bi00371a044. [DOI] [PubMed] [Google Scholar]
- Naito Y., Kaneko H. Reactivated triton-extracted models o paramecium: modification of ciliary movement by calcium ions. Science. 1972 May 5;176(4034):523–524. doi: 10.1126/science.176.4034.523. [DOI] [PubMed] [Google Scholar]
- Nakaoka Y., Ooi H. Regulation of ciliary reversal in triton-extracted Paramecium by calcium and cyclic adenosine monophosphate. J Cell Sci. 1985 Aug;77:185–195. doi: 10.1242/jcs.77.1.185. [DOI] [PubMed] [Google Scholar]
- Nakaoka Y., Tanaka H., Oosawa F. Ca2+-dependent regulation of beat frequency of cilia in Paramecium. J Cell Sci. 1984 Jan;65:223–231. doi: 10.1242/jcs.65.1.223. [DOI] [PubMed] [Google Scholar]
- Ohnishi K., Watanabe Y. Purification and some properties of a new Ca2+-binding protein (TCBP-10) present in tetrahymena cilium. J Biol Chem. 1983 Nov 25;258(22):13978–13985. [PubMed] [Google Scholar]
- Otter T., Satir B. H., Satir P. Trifluoperazine-induced changes in swimming behavior of paramecium: evidence for two sites of drug action. Cell Motil. 1984;4(4):249–267. doi: 10.1002/cm.970040404. [DOI] [PubMed] [Google Scholar]
- Piperno G., Luck D. J. Axonemal adenosine triphosphatases from flagella of Chlamydomonas reinhardtii. Purification of two dyneins. J Biol Chem. 1979 Apr 25;254(8):3084–3090. [PubMed] [Google Scholar]
- Rasmussen H. The calcium messenger system (2). N Engl J Med. 1986 May 1;314(18):1164–1170. doi: 10.1056/NEJM198605013141807. [DOI] [PubMed] [Google Scholar]
- Saimi Y., Hinrichsen R. D., Forte M., Kung C. Mutant analysis shows that the Ca2+-induced K+ current shuts off one type of excitation in Paramecium. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5112–5116. doi: 10.1073/pnas.80.16.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saimi Y., Kung C. A Ca-induced Na-current in Paramecium. J Exp Biol. 1980 Oct;88:305–325. doi: 10.1242/jeb.88.1.305. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Schwartz J. H. Serotonin alters the subcellular distribution of a Ca2+/calmodulin-binding protein in neurons of Aplysia. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6708–6712. doi: 10.1073/pnas.80.21.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satow Y., Kung C. Ca-induced K+-outward current in Paramecium tetraurelia. J Exp Biol. 1980 Oct;88:293–303. doi: 10.1242/jeb.88.1.293. [DOI] [PubMed] [Google Scholar]
- Schaefer W. H., Hinrichsen R. D., Burgess-Cassler A., Kung C., Blair I. A., Watterson D. M. A mutant Paramecium with a defective calcium-dependent potassium conductance has an altered calmodulin: a nonlethal selective alteration in calmodulin regulation. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3931–3935. doi: 10.1073/pnas.84.11.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer W. H., Lukas T. J., Blair I. A., Schultz J. E., Watterson D. M. Amino acid sequence of a novel calmodulin from Paramecium tetraurelia that contains dimethyllysine in the first domain. J Biol Chem. 1987 Jan 25;262(3):1025–1029. [PubMed] [Google Scholar]
- Steers E., Jr, Beisson J., Marchesi V. T. A structural protein extracted from the trichocyst of Paramecium aurelia. Exp Cell Res. 1969 Oct;57(2):392–396. doi: 10.1016/0014-4827(69)90165-7. [DOI] [PubMed] [Google Scholar]
- Stommel E. W., Stephens R. E., Alkon D. L. Motile statocyst cilia transmit rather than directly transduce mechanical stimuli. J Cell Biol. 1980 Dec;87(3 Pt 1):652–662. doi: 10.1083/jcb.87.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stommel E. W., Stephens R. E. Cyclic AMP and calcium in the differential control of Mytilus gill cilia. J Comp Physiol A. 1985 Oct;157(4):451–459. doi: 10.1007/BF00615145. [DOI] [PubMed] [Google Scholar]
- Tash J. S., Means A. R. Cyclic adenosine 3',5' monophosphate, calcium and protein phosphorylation in flagellar motility. Biol Reprod. 1983 Feb;28(1):75–104. doi: 10.1095/biolreprod28.1.75. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis S. M., Nelson D. L. Regulation of axonemal Mg2+-ATPase from Paramecium cilia: effects of Ca2+ and cyclic nucleotides. Biochim Biophys Acta. 1988 Jul 14;966(1):84–93. doi: 10.1016/0304-4165(88)90131-6. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Van Eldik L. J., Piperno G., Watterson D. M. Similarities and dissimilarities between calmodulin and a Chlamydomonas flagellar protein. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4779–4783. doi: 10.1073/pnas.77.8.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. F., Schultz J. E. Calcium receptor protein calmodulin isolated from cilia and cells of Paramecium tetraurelia. Eur J Cell Biol. 1981 Apr;24(1):97–100. [PubMed] [Google Scholar]
- Watterson D. M., Sharief F., Vanaman T. C. The complete amino acid sequence of the Ca2+-dependent modulator protein (calmodulin) of bovine brain. J Biol Chem. 1980 Feb 10;255(3):962–975. [PubMed] [Google Scholar]