Abstract
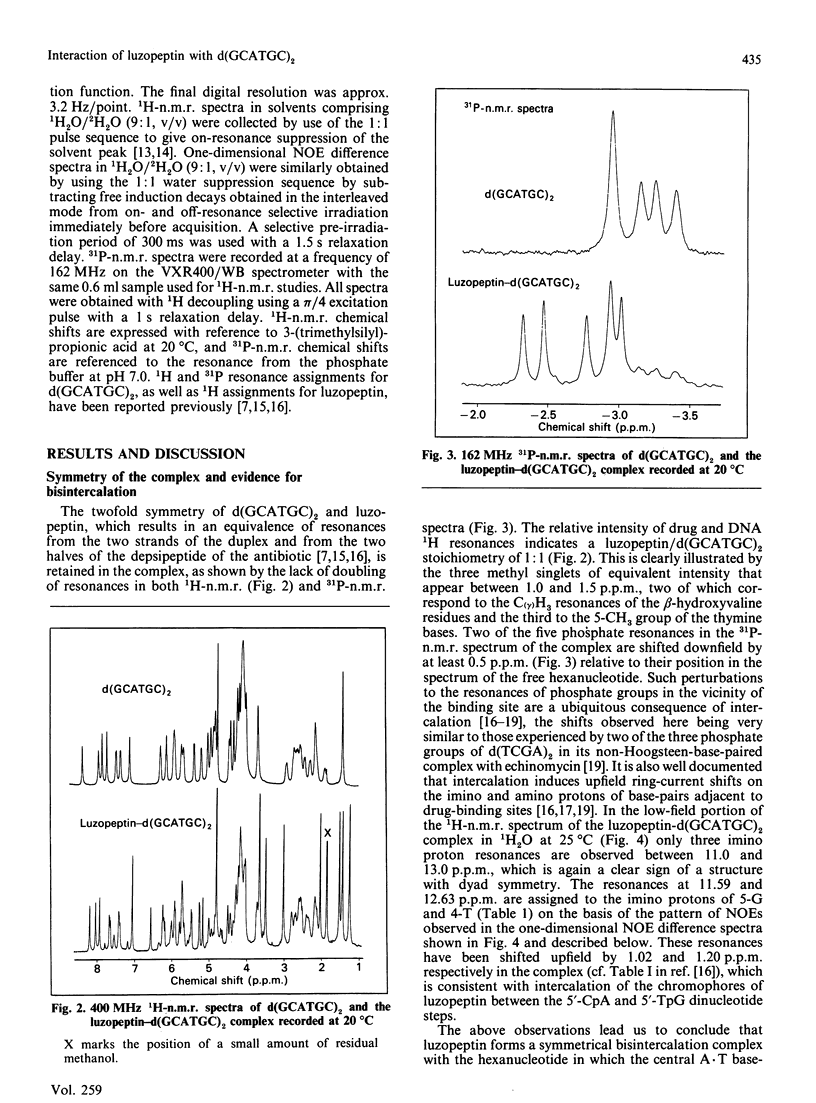
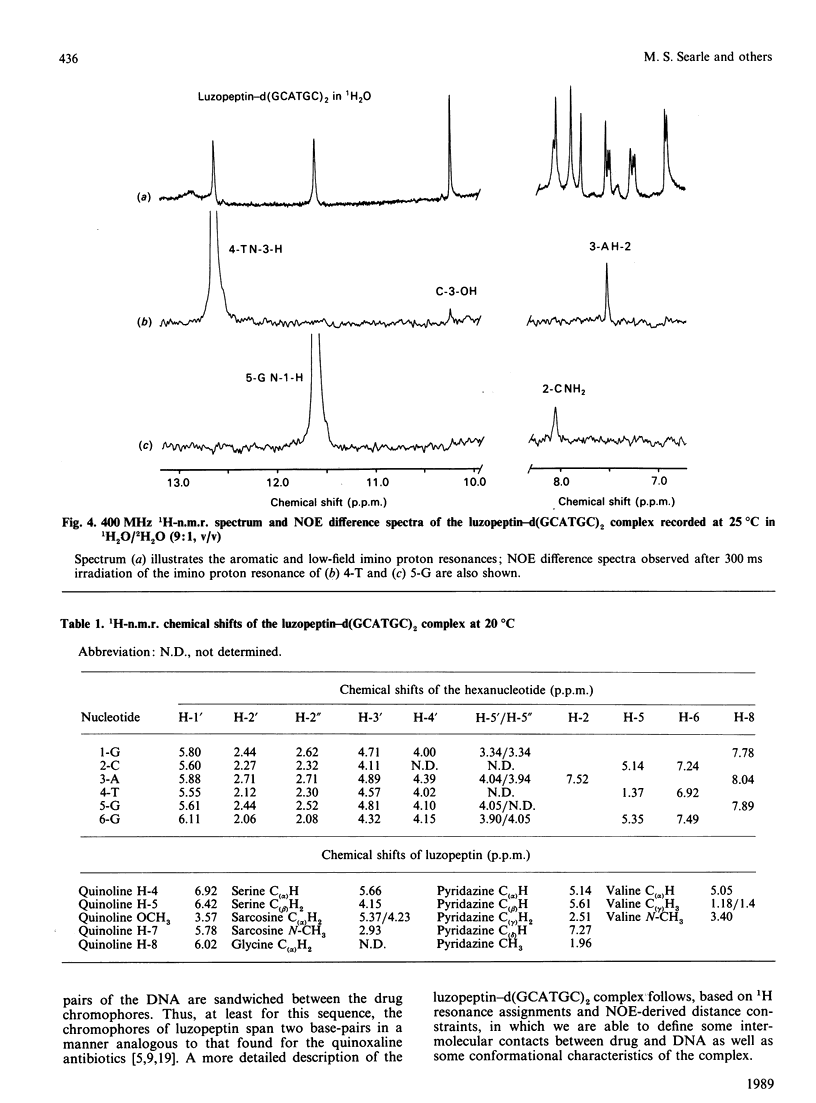
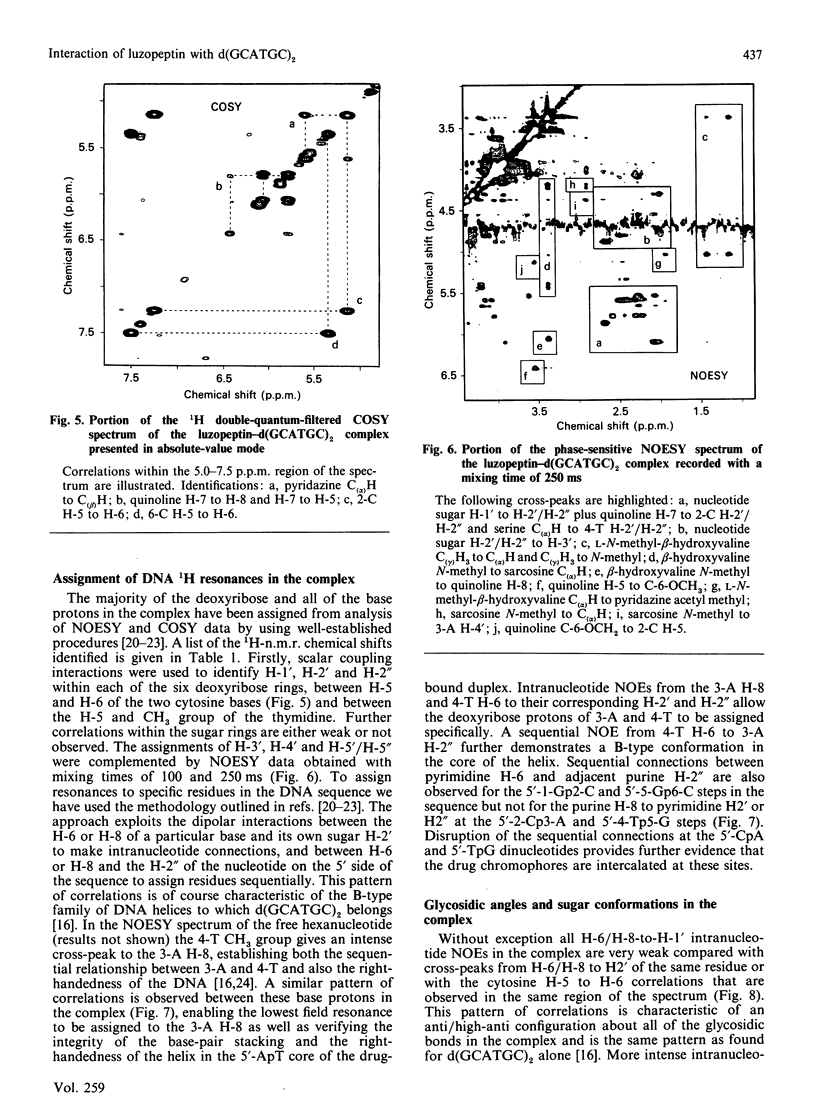
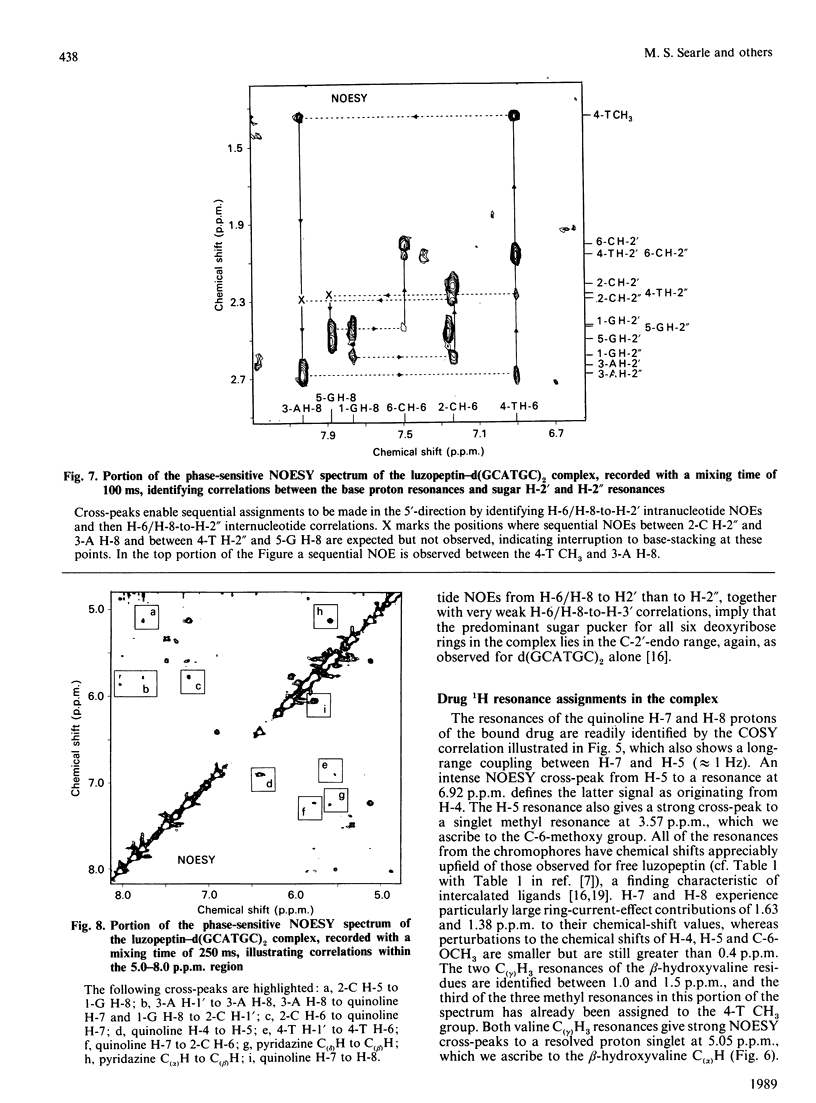
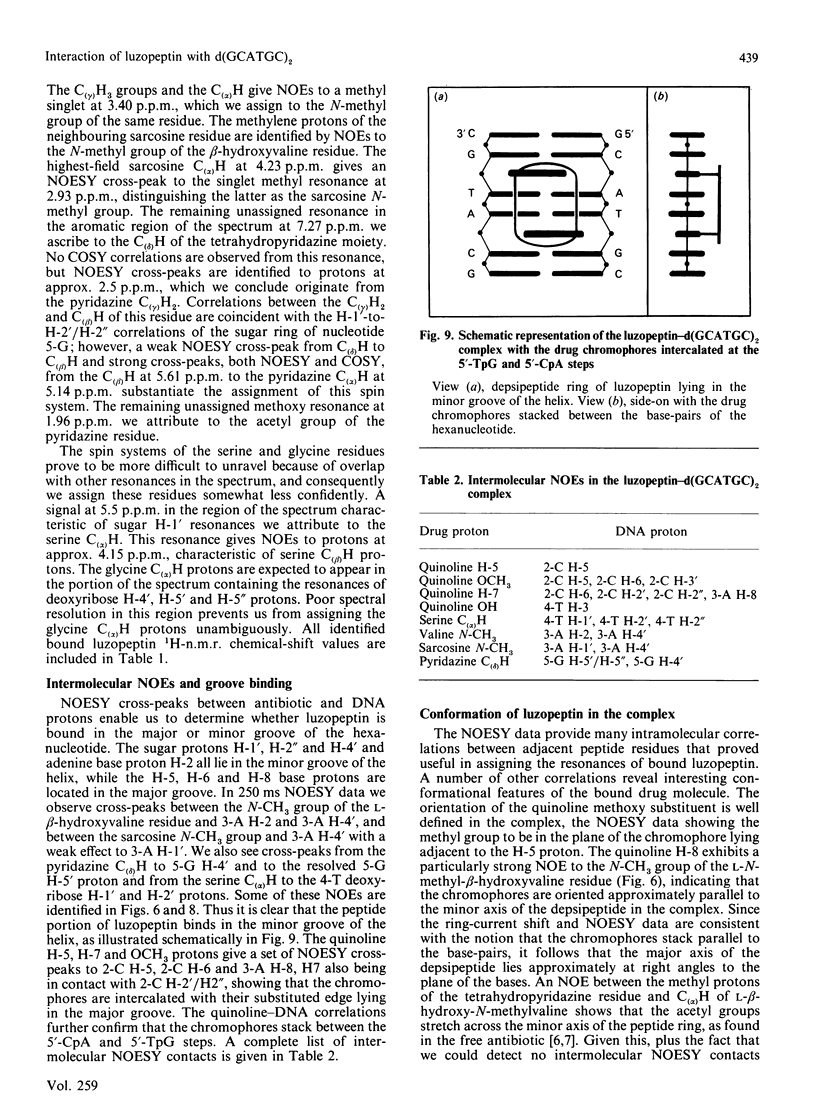
1H- and 31P-n.m.r. spectroscopy were used to characterize the solution structure of the 1:1 complex formed between the antitumour antibiotic luzopeptin and the self-complementary hexanucleotide d(5'-GCATGC)2. Eighteen nuclear Overhauser effects between antibiotic and nucleotide protons, together with ring-current-induced perturbations to base-pair and quinoline 1H resonances, define the position and orientation of the bound drug molecule. Luzopeptin binds in the minor groove of the DNA with full retention of dyad symmetry, its quinoline chromophores intercalating at the 5'-CpA and 5'-TpG steps and its depsipeptide ring spanning the central two A.T base-pairs. The chromophores stack principally on the adenine base with their carbocyclic rings pointing towards the deoxyribose of the cytosine. There is no evidence for Hoogsteen base-pairing in the complex, all glycosidic bond angles and sugar puckers being typical of B-DNA as found for the free hexanucleotide. The 'breathing' motions of the A.T and internal G.C base-pairs are substantially slowed in the complex compared with the free DNA, and the observation that two phosphate resonances are shifted downfield by at least 0.5 p.p.m. in the 31P-n.m.r. spectrum of the complex suggests pronounced local helix unwinding at the intercalation sites. The data are consistent with a model of the complex in which luzopeptin bisintercalates with its depsipeptide essentially in the conformation found in the crystal of the free antibiotic [Arnold & Clardy (1981) J. Am. Chem. Soc. 103, 1243-1244]. We postulate only one conformational change within the peptide ring, which involves rotation of the pyridazine-glycine amide group linkage by 90 degrees towards the DNA surface. This manoeuvre breaks the glycine-to-glycine transannular hydrogen bonds and enables the glycine NH groups to bond to the thymine O-2 atoms of the sandwiched A.T base-pairs. It also shortens the major axis of the depsipeptide so that the interchromophore distance is more suitable for spanning two base-pairs. The model further implies that the carboxy and hydroxy groups of the L-beta-hydroxyvaline residue are appropriately positioned for hydrogen-bonding to the 2-amino group of guanine and the O-2 atom of cytosine of the adjacent G.C base-pair.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chazin W. J., Wüthrich K., Hyberts S., Rance M., Denny W. A., Leupin W. 1H nuclear magnetic resonance assignments for d-(GCATTAATGC)2 using experimental refinements of established procedures. J Mol Biol. 1986 Aug 5;190(3):439–453. doi: 10.1016/0022-2836(86)90014-8. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Gronenborn A. M. Sequence-dependent structural variations in two right-handed alternating pyrimidine-purine DNA oligomers in solution determined by nuclear Overhauser enhancement measurements. EMBO J. 1983;2(12):2109–2115. doi: 10.1002/j.1460-2075.1983.tb01710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. R., Davies H., Adams G. R., Portugal J., Waring M. J. Sequence-specific binding of luzopeptin to DNA. Nucleic Acids Res. 1988 Mar 25;16(6):2489–2507. doi: 10.1093/nar/16.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey M. H., Leupin W., Sørensen O. W., Denny W. A., Ernst R. R. Sequence-specific assignment of the backbone 1H-and 31P-NMR lines in a short DNA duplex with homo- and heteronuclear correlated spectroscopy. Biopolymers. 1985 Dec;24(12):2371–2380. doi: 10.1002/bip.360241214. [DOI] [PubMed] [Google Scholar]
- Gao X. L., Patel D. J. NMR studies of echinomycin bisintercalation complexes with d(A1-C2-G3-T4) and d(T1-C2-G3-A4) duplexes in aqueous solution: sequence-dependent formation of Hoogsteen A1.T4 and Watson--Crick T1.A4 base pairs flanking the bisintercalation site. Biochemistry. 1988 Mar 8;27(5):1744–1751. doi: 10.1021/bi00405a054. [DOI] [PubMed] [Google Scholar]
- Hare D. R., Wemmer D. E., Chou S. H., Drobny G., Reid B. R. Assignment of the non-exchangeable proton resonances of d(C-G-C-G-A-A-T-T-C-G-C-G) using two-dimensional nuclear magnetic resonance methods. J Mol Biol. 1983 Dec 15;171(3):319–336. doi: 10.1016/0022-2836(83)90096-7. [DOI] [PubMed] [Google Scholar]
- Huang C. H., Mong S., Crooke S. T. Interactions of a new antitumor antibiotic BBM-928A with deoxyribonucleic acid. Bifunctional intercalative binding studied by fluorometry and viscometry. Biochemistry. 1980 Nov 25;19(24):5537–5542. doi: 10.1021/bi00565a012. [DOI] [PubMed] [Google Scholar]
- Ohkuma H., Sakai F., Nishiyama Y., Ohbayashi M., Imanishi H., Konishi M., Miyaki T., Koshiyama H., Kawaguchi H. BBM-928, a new antitumor antibiotic complex. I. Production, isolation, characterization and antitumor activity. J Antibiot (Tokyo) 1980 Oct;33(10):1087–1097. doi: 10.7164/antibiotics.33.1087. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Nordheim A., Rich A. Right-handed and left-handed DNA: studies of B- and Z-DNA by using proton nuclear Overhauser effect and P NMR. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1413–1417. doi: 10.1073/pnas.79.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Shapiro L., Hare D. Sequence-dependent conformations of DNA duplexes: the TATA segment of the d(G-G-T-A-T-A-C-C) duplex in aqueous solution. Biopolymers. 1986 Apr;25(4):693–706. doi: 10.1002/bip.360250412. [DOI] [PubMed] [Google Scholar]
- Scheek R. M., Boelens R., Russo N., van Boom J. H., Kaptein R. Sequential resonance assignments in 1H NMR spectra of oligonucleotides by two-dimensional NMR spectroscopy. Biochemistry. 1984 Mar 27;23(7):1371–1376. doi: 10.1021/bi00302a006. [DOI] [PubMed] [Google Scholar]
- Searle M. S., Hall J. G., Denny W. A., Wakelin L. P. NMR studies of the interaction of the antibiotic nogalamycin with the hexadeoxyribonucleotide duplex d(5'-GCATGC)2. Biochemistry. 1988 Jun 14;27(12):4340–4349. doi: 10.1021/bi00412a022. [DOI] [PubMed] [Google Scholar]
- Searle M. S., Hall J. G., Wakelin P. G. 1H- and 13C-n.m.r. studies of the antitumour antibiotic luzopeptin. Resonance assignments, conformation and flexibility in solution. Biochem J. 1988 Nov 15;256(1):271–278. doi: 10.1042/bj2560271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K., Hoshino Y., Sasahira T., Kawaguchi H. BBM-928, a new antitumor antibiotic complex. II. Taxonomic studies on the producing organism. J Antibiot (Tokyo) 1980 Oct;33(10):1098–1102. doi: 10.7164/antibiotics.33.1098. [DOI] [PubMed] [Google Scholar]
- Ughetto G., Wang A. H., Quigley G. J., van der Marel G. A., van Boom J. H., Rich A. A comparison of the structure of echinomycin and triostin A complexed to a DNA fragment. Nucleic Acids Res. 1985 Apr 11;13(7):2305–2323. doi: 10.1093/nar/13.7.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelin L. P. Polyfunctional DNA intercalating agents. Med Res Rev. 1986 Jul-Sep;6(3):275–340. doi: 10.1002/med.2610060303. [DOI] [PubMed] [Google Scholar]


