Abstract
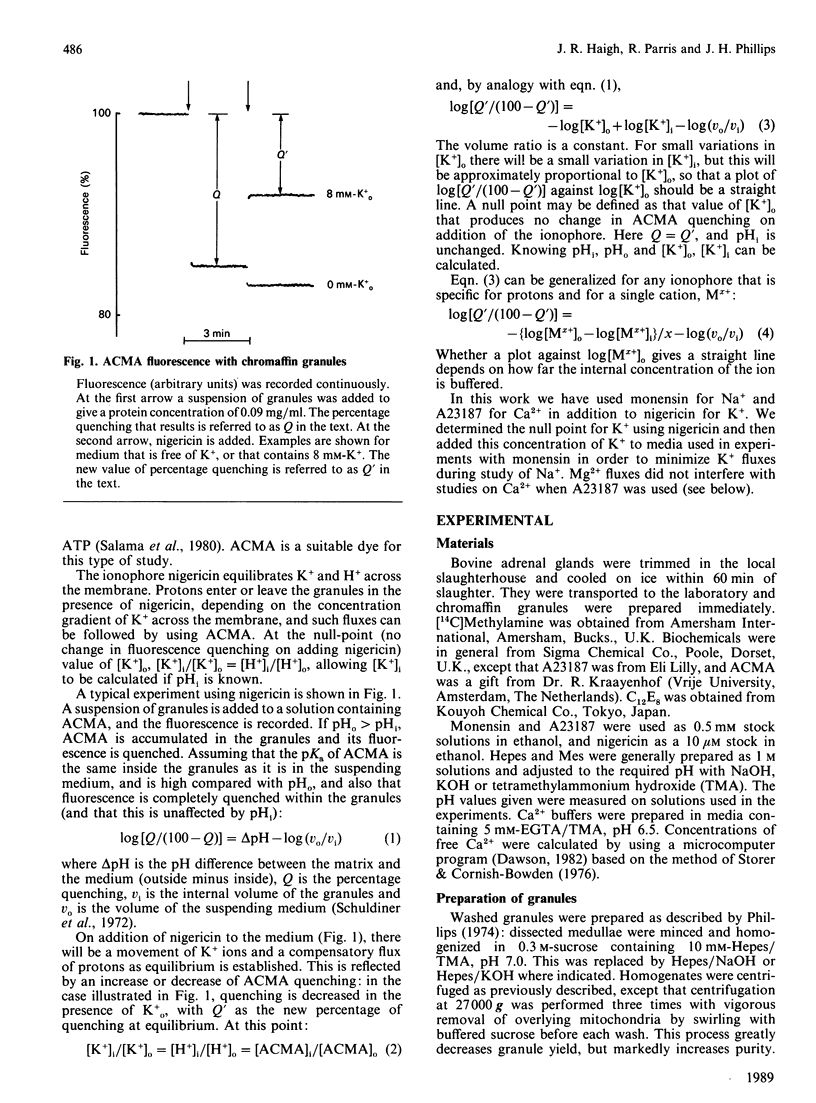
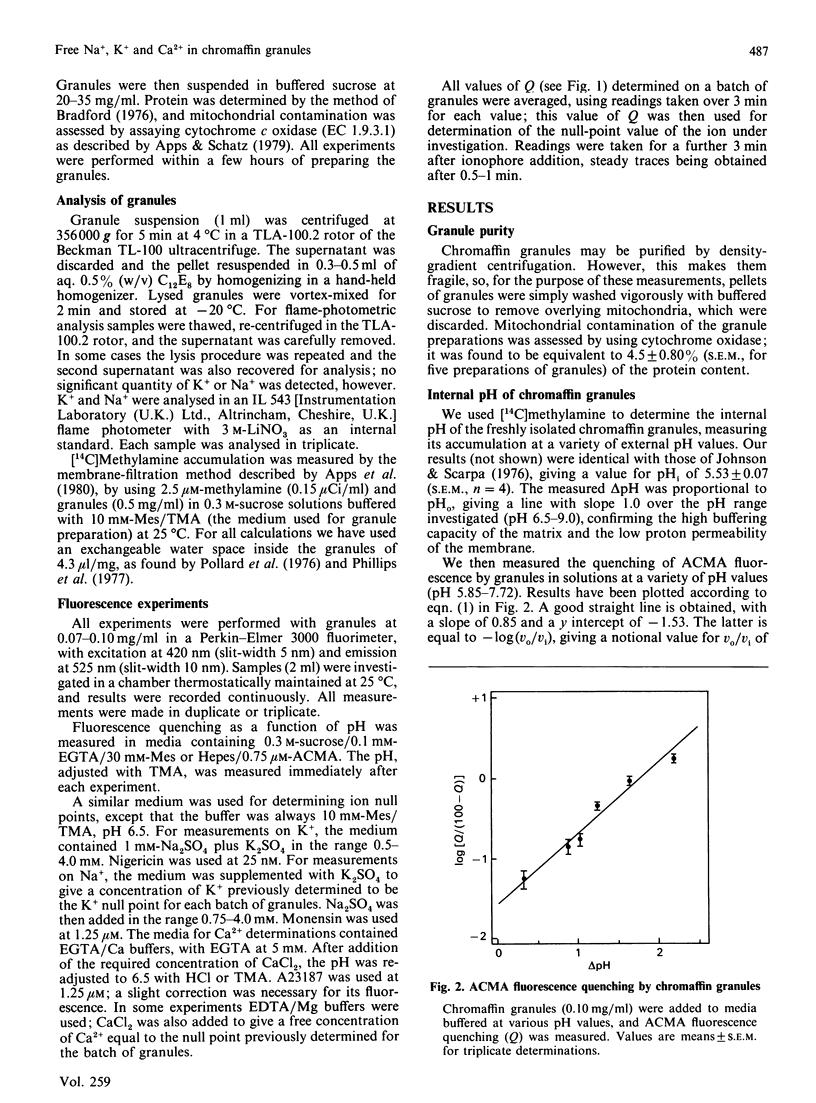
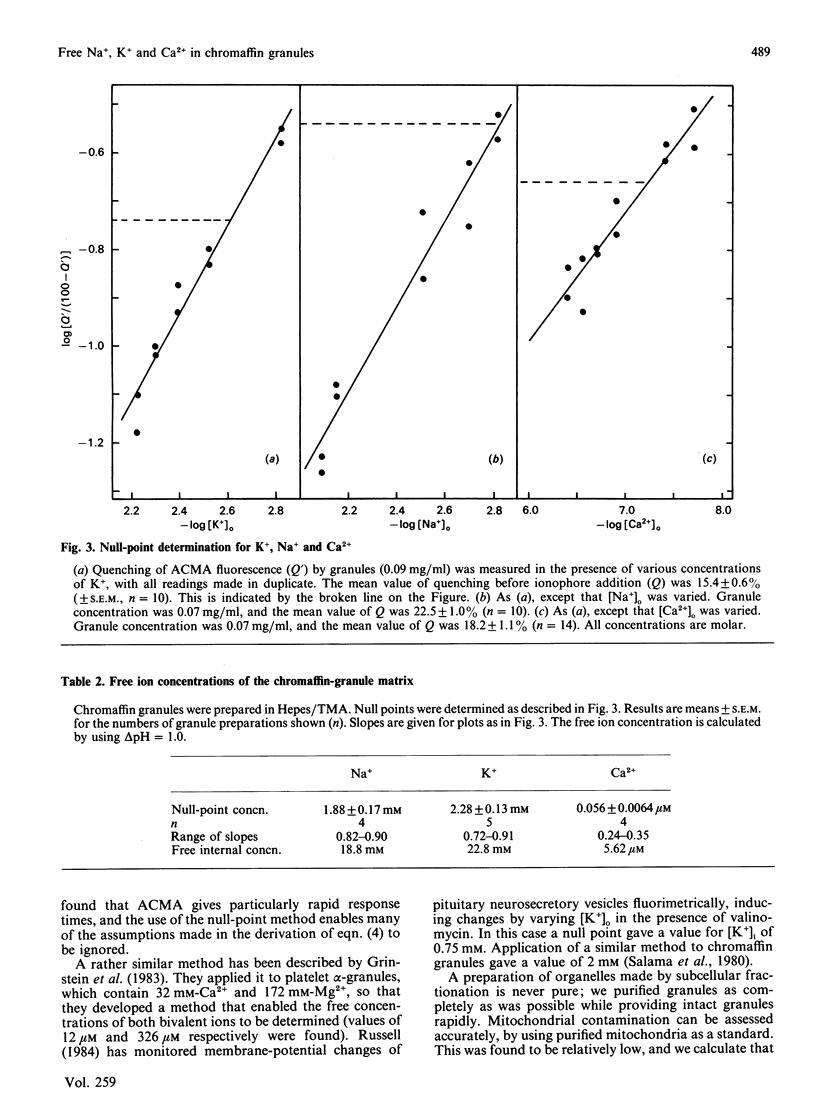
We have measured the contents of Na+ and K+ in isolated chromaffin granules. Total contents varied between 227 and 283 nmol/mg of protein, equivalent to matrix concentrations of 53-66 mM. The value found depended on the isolation buffer used, and the ratio of the two ions reflected the composition of the buffer. We then measured the free concentration of each of these ions, and of Ca2+, in the matrix, by using a null-point method with acridine-fluorescence quenching. This monitored H+ fluxes induced by an ionophore in the presence of known concentrations of the ion in the supporting medium. In contrast with organic constituents of the matrix, which have low activity coefficients, Na+ and K+ were found to have activity coefficients around 0.8 Ca2+, however, was strongly bound: its free concentration was only 0.03% of the total.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apps D. K., Pryde J. G., Sutton R., Phillips J. H. Inhibition of adenosine triphosphatase, 5-hydroxytryptamine transport and proton-translocation activities of resealed chromaffin-granule 'ghosts'. Biochem J. 1980 Aug 15;190(2):273–282. doi: 10.1042/bj1900273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps D. K., Schatz G. An adenosine triphosphatase isolated from chromaffin-granulate membranes is closely similar to F1-adenosine triphosphatase of mitochondria. Eur J Biochem. 1979 Oct 15;100(2):411–419. doi: 10.1111/j.1432-1033.1979.tb04184.x. [DOI] [PubMed] [Google Scholar]
- Borowitz J. L. Calcium binding by subcellular fractions of bovine adrenal medulla. J Cell Physiol. 1967 Jun;69(3):311–319. doi: 10.1002/jcp.1040690307. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bulenda D., Gratzl M. Matrix free Ca2+ in isolated chromaffin vesicles. Biochemistry. 1985 Dec 17;24(26):7760–7765. doi: 10.1021/bi00347a039. [DOI] [PubMed] [Google Scholar]
- Castle J. D., Cameron R. S., Arvan P., von Zastrow M., Rudnick G. Similarities and differences among neuroendocrine, exocrine, and endocytic vesicles. Ann N Y Acad Sci. 1987;493:448–460. doi: 10.1111/j.1749-6632.1987.tb27230.x. [DOI] [PubMed] [Google Scholar]
- Dawson A. P. Kinetic properties of the Ca2+-accumulation system of a rat liver microsomal fraction. Biochem J. 1982 Jul 15;206(1):73–79. doi: 10.1042/bj2060073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W., Vander Meulen J., Hancock R. G. The total and free concentrations of Ca2+ and Mg2+ inside platelet secretory granules. Measurements employing a novel double null point technique. J Biol Chem. 1983 Dec 25;258(24):14774–14777. [PubMed] [Google Scholar]
- Haigh J. R., Phillips J. H. A sodium/proton antiporter in chromaffin-granule membranes. Biochem J. 1989 Jan 15;257(2):499–507. doi: 10.1042/bj2570499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Peshavaria M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983 Feb 15;210(2):297–305. doi: 10.1042/bj2100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A. Internal pH of isolated chromaffin vesicles. J Biol Chem. 1976 Apr 10;251(7):2189–2191. [PubMed] [Google Scholar]
- Kopell W. N., Westhead E. W. Osmotic pressures of solutions of ATP and catecholamines relating to storage in chromaffin granules. J Biol Chem. 1982 May 25;257(10):5707–5710. [PubMed] [Google Scholar]
- Krieger-Brauer H., Gratzl M. Uptake of Ca2+ by isolated secretory vesicles from adrenal medulla. Biochim Biophys Acta. 1982 Sep 24;691(1):61–70. doi: 10.1016/0005-2736(82)90214-0. [DOI] [PubMed] [Google Scholar]
- Njus D., Kelley P. M., Harnadek G. J. Bioenergetics of secretory vesicles. Biochim Biophys Acta. 1986;853(3-4):237–265. doi: 10.1016/0304-4173(87)90003-6. [DOI] [PubMed] [Google Scholar]
- Ornberg R. L., Kuijpers G. A., Leapman R. D. Electron probe microanalysis of the subcellular compartments of bovine adrenal chromaffin cells. Comparison of chromaffin granules in situ and in vitro. J Biol Chem. 1988 Jan 25;263(3):1488–1493. [PubMed] [Google Scholar]
- Phillips J. H., Allison Y. P., Morris S. J. The distribution of calcium, magnesium, copper and iron in the bovine adrenal medulla. Neuroscience. 1977;2(1):147–152. doi: 10.1016/0306-4522(77)90075-6. [DOI] [PubMed] [Google Scholar]
- Phillips J. H. Dynamic aspects of chromaffin granule structure. Neuroscience. 1982 Jul;7(7):1595–1609. doi: 10.1016/0306-4522(82)90017-3. [DOI] [PubMed] [Google Scholar]
- Phillips J. H. Phosphatidylinositol kinase. A component of the chromaffin-granule membrane. Biochem J. 1973 Nov;136(3):579–587. doi: 10.1042/bj1360579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H. Transport of Ca2+ and Na+ across the chromaffin-granule membrane. Biochem J. 1981 Oct 15;200(1):99–107. doi: 10.1042/bj2000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H. Transport of catecholamines by resealed chromaffin-grnaule "ghosts". Biochem J. 1974 Nov;144(2):311–318. doi: 10.1042/bj1440311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard H. B., Zinder O., Hoffman P. G., Nikodejevic O. Regulation of the transmembrane potential of isolated chromaffin granules by ATP, ATP analogs, and external pH. J Biol Chem. 1976 Aug 10;251(15):4544–4550. [PubMed] [Google Scholar]
- Reiffen F. U., Gratzl M. Ca2+ binding to chromaffin vesicle matrix proteins: effect of pH, Mg2+, and ionic strength. Biochemistry. 1986 Jul 29;25(15):4402–4406. doi: 10.1021/bi00363a034. [DOI] [PubMed] [Google Scholar]
- Russell J. T. Delta pH, H+ diffusion potentials, and Mg2+ ATPase in neurosecretory vesicles isolated from bovine neurohypophyses. J Biol Chem. 1984 Aug 10;259(15):9496–9507. [PubMed] [Google Scholar]
- Salama G., Johnson R. G., Scarpa A. Spectrophotometric measurements of transmembrane potential and pH gradients in chromaffin granules. J Gen Physiol. 1980 Feb;75(2):109–140. doi: 10.1085/jgp.75.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Rottenberg H., Avron M. Determination of pH in chloroplasts. 2. Fluorescent amines as a probe for the determination of pH in chloroplasts. Eur J Biochem. 1972 Jan 31;25(1):64–70. doi: 10.1111/j.1432-1033.1972.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. The localization of lysosomal enzymes in chromaffin tissue. J Physiol. 1966 Mar;183(1):179–188. doi: 10.1113/jphysiol.1966.sp007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C. Evidence for a divalent cation dependent catecholamine storage complex in chromaffin granules. Biochem Biophys Res Commun. 1983 Oct 31;116(2):663–668. doi: 10.1016/0006-291x(83)90576-4. [DOI] [PubMed] [Google Scholar]
- Warnock D. G., Reenstra W. W., Yee V. J. Na+/H+ antiporter of brush border vesicles: studies with acridine orange uptake. Am J Physiol. 1982 Jun;242(6):F733–F739. doi: 10.1152/ajprenal.1982.242.6.F733. [DOI] [PubMed] [Google Scholar]
- Winkler H. The biogenesis of adrenal chromaffin granules. Neuroscience. 1977;2(5):657–683. doi: 10.1016/0306-4522(77)90022-7. [DOI] [PubMed] [Google Scholar]
- Winkler H. The composition of adrenal chromaffin granules: an assessment of controversial results. Neuroscience. 1976;1(2):65–80. doi: 10.1016/0306-4522(76)90001-4. [DOI] [PubMed] [Google Scholar]
- Winkler H., Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5(11):1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]


