Abstract
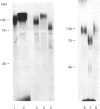
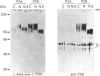
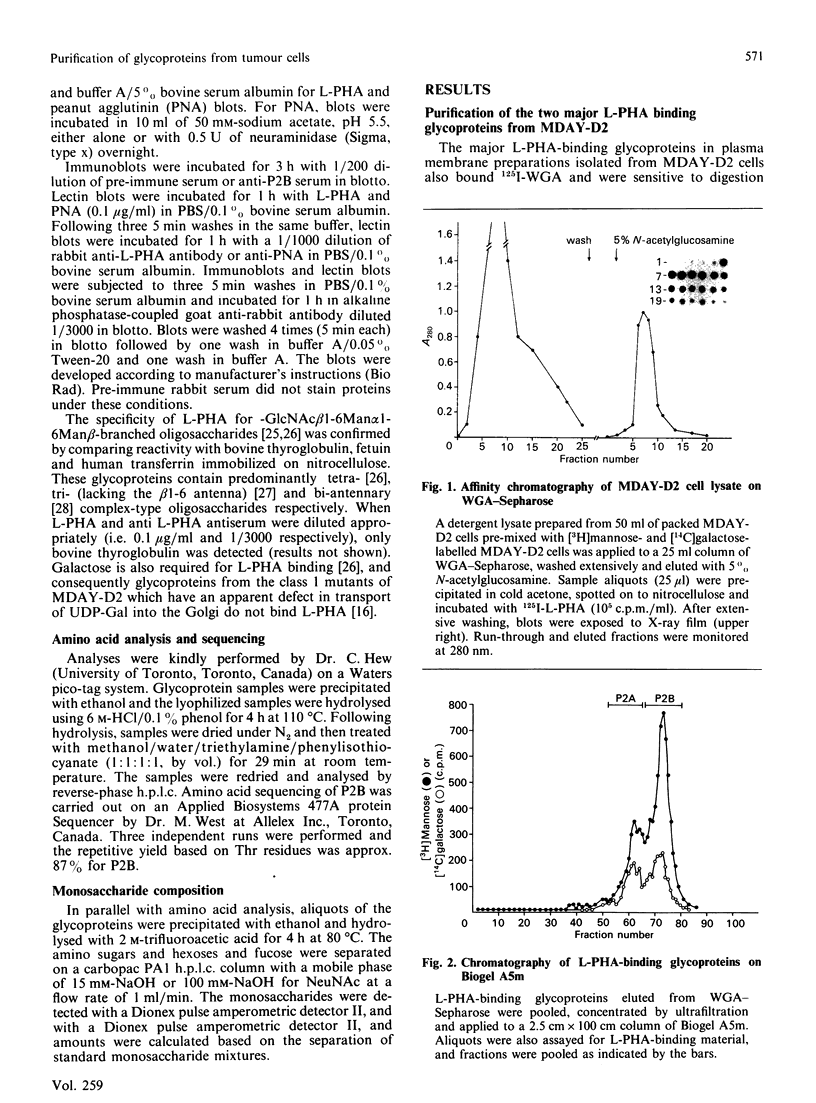
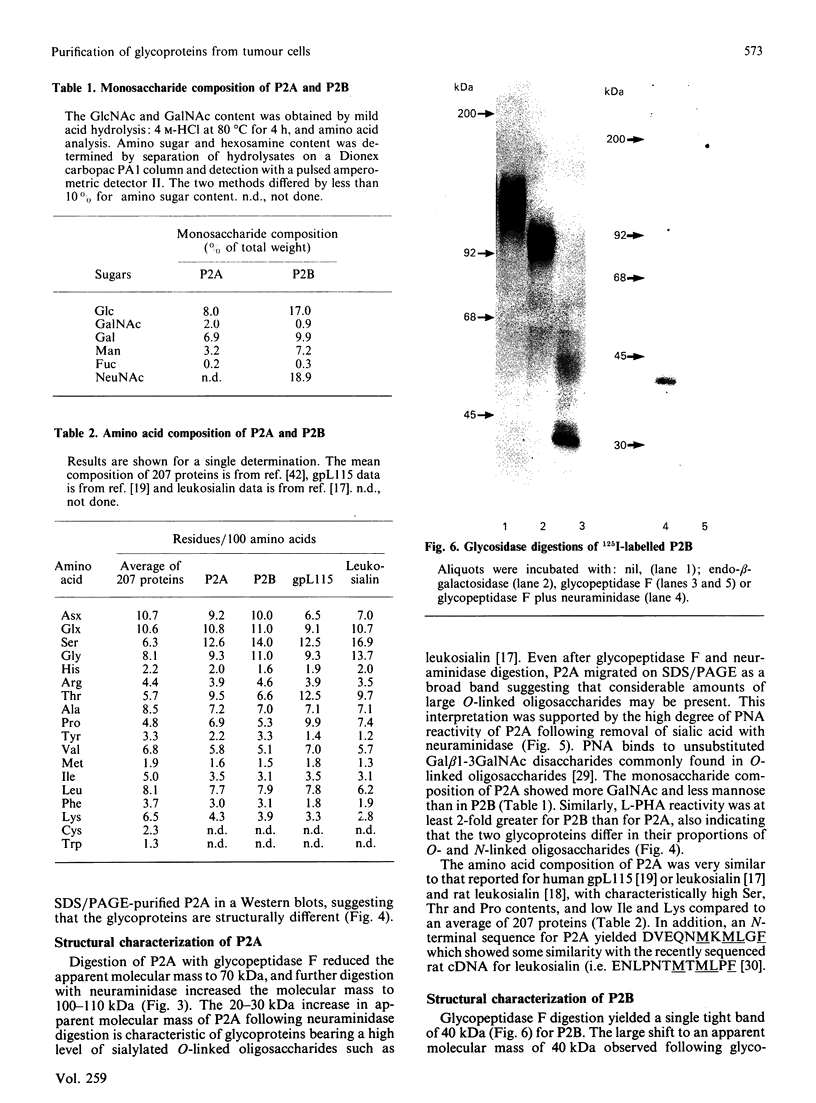
Increased branching at the trimannosyl core of 'complex-type' Asn-linked oligosaccharides has been observed in both human and murine tumour cells, and appears to be associated with enhanced metastatic potential in several murine tumour models [Dennis, Laferte, Waghorne, Breitman & Kerbel (1987), Science 236, 582-585]. The lectin leucoagglutinin (L-PHA) requires the-GlcNAc beta 1-6Man alpha 1-6Man-linked lactosamine antenna in complex-type oligosaccharides for high-affinity binding and can be used to detect these structures in glycoproteins separated on SDS/polyacrylamide-gel electrophoresis. The major L-PHA-binding glycoproteins in the highly metastatic lymphoid tumour cell line called MDAY-D2 were purified and resolved into two major species, termed P2A (110 kDa) and P2B (130 kDa). P2A had L-PHA-reactive Asn-linked oligosaccharides with polylactosamine sequences as well as a large component of sialylated O-linked carbohydrates. The glycoprotein showed structural characteristics similar to those of leukosialin (i.e. CD43), a glycoprotein previously identified on the surface of leukocytes. Based on monosaccharide compositional analysis and glycosidase digestions, P2B was found to be 50-60% Asn-linked oligosaccharide containing polylactosamine sequences and sialic acid. The N-terminal peptide sequence of P2B was determined to be very similar to that of murine lysosomal membrane glycoprotein (LAMP-1), a ubiquitous glycoprotein found largely in the lysosomal membranes but also in the plasma membrane of several murine and human tumour cell lines.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J. U., Fiete D. Structure of the complex oligosaccharides of fetuin. J Biol Chem. 1979 Feb 10;254(3):789–795. [PubMed] [Google Scholar]
- Bramwell M. E., Harris H. An abnormal membrane glycoprotein associated with malignancy in a wide range of different tumours. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):87–106. doi: 10.1098/rspb.1978.0034. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Barclay A. N., Sunderland C. A., Williams A. F. Identification of a glycophorin-like molecule at the cell surface of rat thymocytes. Nature. 1981 Feb 5;289(5797):456–460. doi: 10.1038/289456a0. [DOI] [PubMed] [Google Scholar]
- Carlsson S. R., Fukuda M. Isolation and characterization of leukosialin, a major sialoglycoprotein on human leukocytes. J Biol Chem. 1986 Sep 25;261(27):12779–12786. [PubMed] [Google Scholar]
- Chen J. W., Cha Y., Yuksel K. U., Gracy R. W., August J. T. Isolation and sequencing of a cDNA clone encoding lysosomal membrane glycoprotein mouse LAMP-1. Sequence similarity to proteins bearing onco-differentiation antigens. J Biol Chem. 1988 Jun 25;263(18):8754–8758. [PubMed] [Google Scholar]
- Chen J. W., Murphy T. L., Willingham M. C., Pastan I., August J. T. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985 Jul;101(1):85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. W., Pan W., D'Souza M. P., August J. T. Lysosome-associated membrane proteins: characterization of LAMP-1 of macrophage P388 and mouse embryo 3T3 cultured cells. Arch Biochem Biophys. 1985 Jun;239(2):574–586. doi: 10.1016/0003-9861(85)90727-1. [DOI] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982 Oct 10;257(19):11230–11234. [PubMed] [Google Scholar]
- Debray H., Qin Z., Delannoy P., Montreuil J., Duś D., Radzikowski C., Christensen B., Kieler J. Altered glycosylation of membrane glycoproteins in human uroepithelial cell lines. Int J Cancer. 1986 Apr 15;37(4):607–611. doi: 10.1002/ijc.2910370421. [DOI] [PubMed] [Google Scholar]
- Dennis J. W. Different metastatic phenotypes in two genetic classes of wheat germ agglutinin-resistant tumor cell mutants. Cancer Res. 1986 Sep;46(9):4594–4600. [PubMed] [Google Scholar]
- Dennis J. W. Effects of swainsonine and polyinosinic:polycytidylic acid on murine tumor cell growth and metastasis. Cancer Res. 1986 Oct;46(10):5131–5136. [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Fukuda M., Dell A., Carver J. P. Asn-linked oligosaccharides in lectin-resistant tumor-cell mutants with varying metastatic potential. Eur J Biochem. 1986 Dec 1;161(2):359–373. doi: 10.1111/j.1432-1033.1986.tb10455.x. [DOI] [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Dennis J. W. Partial reversion of the metastatic phenotype in a wheat germ agglutinin-resistant mutant of the murine tumor cell line MDAY-D2 selected with Bandeiraea simplicifolia seed lectin. J Natl Cancer Inst. 1985 May;74(5):1111–1120. [PubMed] [Google Scholar]
- Dennis J., Donaghue T., Florian M., Kerbel R. S. Apparent reversion of stable in vitro genetic markers detected in tumour cells from spontaneous metastases. Nature. 1981 Jul 16;292(5820):242–245. doi: 10.1038/292242a0. [DOI] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Hakomori S., Kannagi R. Glycosphingolipids as tumor-associated and differentiation markers. J Natl Cancer Inst. 1983 Aug;71(2):231–251. [PubMed] [Google Scholar]
- Hammarström S., Hammarström M. L., Sundblad G., Arnarp J., Lönngren J. Mitogenic leukoagglutinin from Phaseolus vulgaris binds to a pentasaccharide unit in N-acetyllactosamine-type glycoprotein glycans. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1611–1615. doi: 10.1073/pnas.79.5.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horejsí V., Bazil V. Surface proteins and glycoproteins of human leucocytes. Biochem J. 1988 Jul 1;253(1):1–26. doi: 10.1042/bj2530001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J., Matsumoto K., White S. L., Olden K. Inhibition of experimental metastasis by castanospermine in mice: blockage of two distinct stages of tumor colonization by oligosaccharide processing inhibitors. Cancer Res. 1986 Oct;46(10):5215–5222. [PubMed] [Google Scholar]
- Hunt L. A., Wright S. E. Both acidic-type and neutral-type asparaginyl-oligosaccharides of host-cell glycoproteins are altered in Rous-sarcoma-virus-transformed chick-embryo fibroblasts. Biochem J. 1985 Jul 15;229(2):441–451. doi: 10.1042/bj2290441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S. Immunologic studies of membrane mutants of a highly metastatic murine tumor. Am J Pathol. 1979 Dec;97(3):609–622. [PMC free article] [PubMed] [Google Scholar]
- Killeen N., Barclay A. N., Willis A. C., Williams A. F. The sequence of rat leukosialin (W3/13 antigen) reveals a molecule with O-linked glycosylation of one third of its extracellular amino acids. EMBO J. 1987 Dec 20;6(13):4029–4034. doi: 10.1002/j.1460-2075.1987.tb02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferté S., Dennis J. W. Glycosylation-dependent collagen-binding activities of two membrane glycoproteins in MDAY-D2 tumor cells. Cancer Res. 1988 Sep 1;48(17):4743–4748. [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Fambrough D. M. Lysosomal membrane dynamics: structure and interorganellar movement of a major lysosomal membrane glycoprotein. J Cell Biol. 1986 May;102(5):1593–1605. doi: 10.1083/jcb.102.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi I. R., Raz A. Loss of metastatic responsiveness to cell shape modulation in a newly characterized B16 melanoma adhesive cell variant. Cancer Res. 1988 Mar 1;48(5):1258–1264. [PubMed] [Google Scholar]
- Niedbala M. J., Madiyalakan R., Matta K., Crickard K., Sharma M., Bernacki R. J. Role of glycosidases in human ovarian carcinoma cell mediated degradation of subendothelial extracellular matrix. Cancer Res. 1987 Sep 1;47(17):4634–4641. [PubMed] [Google Scholar]
- Pierce M., Arango J. Rous sarcoma virus-transformed baby hamster kidney cells express higher levels of asparagine-linked tri- and tetraantennary glycopeptides containing [GlcNAc-beta (1,6)Man-alpha (1,6)Man] and poly-N-acetyllactosamine sequences than baby hamster kidney cells. J Biol Chem. 1986 Aug 15;261(23):10772–10777. [PubMed] [Google Scholar]
- Reading C. L., Hutchins J. T. Carbohydrate structure in tumor immunity. Cancer Metastasis Rev. 1985;4(3):221–260. doi: 10.1007/BF00048097. [DOI] [PubMed] [Google Scholar]
- Reeck G. R., Fisher L. A statistical analysis of the amino acid compositions of proteins. Int J Pept Protein Res. 1973;5(2):109–117. doi: 10.1111/j.1399-3011.1973.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Reisinger D., Parkman R. Molecular heterogeneity of a lymphocyte glycoprotein in immunodeficient patients. J Clin Invest. 1987 Feb;79(2):595–599. doi: 10.1172/JCI112852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Davis A. E., 3rd, Kenney D., Bhaskar K. R., Rosen F. S. Purification and chemical composition of gpL115, the human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Biol Chem. 1986 Jun 5;261(16):7526–7530. [PubMed] [Google Scholar]
- Remold-O'Donnell E., Kenney D. M., Parkman R., Cairns L., Savage B., Rosen F. S. Characterization of a human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Exp Med. 1984 Jun 1;159(6):1705–1723. doi: 10.1084/jem.159.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets L. A., Van Beek W. P. Carbohydrates of the tumor cell surface. Biochim Biophys Acta. 1984;738(4):237–249. doi: 10.1016/0304-419x(83)90006-9. [DOI] [PubMed] [Google Scholar]
- Spik G., Bayard B., Fournet B., Strecker G., Bouquelet S., Montreuil J. Studies on glycoconjugates. LXIV. Complete structure of two carbohydrate units of human serotransferrin. FEBS Lett. 1975 Feb 15;50(3):296–299. doi: 10.1016/0014-5793(75)80513-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Tsuji T., Tarutani O., Osawa T. Structural changes of carbohydrate chains of human thyroglobulin accompanying malignant transformations of thyroid glands. Eur J Biochem. 1984 Aug 15;143(1):133–144. doi: 10.1111/j.1432-1033.1984.tb08352.x. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Ohkura T., Tachibana Y., Takasaki S., Kobata A. Comparative study of the oligosaccharides released from baby hamster kidney cells and their polyoma transformant by hydrazinolysis. J Biol Chem. 1984 Sep 10;259(17):10834–10840. [PubMed] [Google Scholar]
- Yogeeswaran G., Salk P. L. Metastatic potential is positively correlated with cell surface sialylation of cultured murine tumor cell lines. Science. 1981 Jun 26;212(4502):1514–1516. doi: 10.1126/science.7233237. [DOI] [PubMed] [Google Scholar]
- Yuan M., Itzkowitz S. H., Palekar A., Shamsuddin A. M., Phelps P. C., Trump B. F., Kim Y. S. Distribution of blood group antigens A, B, H, Lewisa, and Lewisb in human normal, fetal, and malignant colonic tissue. Cancer Res. 1985 Sep;45(9):4499–4511. [PubMed] [Google Scholar]