Abstract
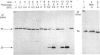
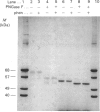
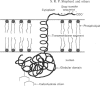
Bilirubin UDP-glucuronosyltransferase (UDPGT) activity in sealed hepatic microsomes from clofibrate-treated rats was highly latent and was fully expressed by disruption of vesicles with detergents. Antibodies raised against purified bilirubin UDPGT were used to study the transmembrane orientation of the protein to provide a molecular understanding of the UDPGT latency. Immunoblot analysis of sealed microsomes, and microsomes after treatment with proteinases, showed that only a small portion of the protein resides on the cytoplasmic side of the microsomal vesicles. Treatment of microsomes with sodium deoxycholate allowed subtilisin and proteinase K to cleave the transferase, causing loss of activity and the release of smaller immunodetectable peptides. Treatment of the purified bilirubin UDPGT with peptide N-glycosidase F indicated that the enzyme was a glycoprotein. A working model of the transmembrane topology of bilirubin UDPGT is described.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burchell A., Burchell B. Stabilization of partially-purified glucose 6-phosphatase by fluoride. Is enzyme inactivation caused by dephosphorylation? FEBS Lett. 1980 Sep 8;118(2):180–184. doi: 10.1016/0014-5793(80)80214-6. [DOI] [PubMed] [Google Scholar]
- Burchell B. Studies on the purification of rat liver uridine diphosphate glucuronyltransferase. Biochem J. 1977 Mar 1;161(3):543–549. doi: 10.1042/bj1610543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughtrie M. W., Burchell B., Shepherd I. M., Bend J. R. Defective induction of phenol glucuronidation by 3-methylcholanthrene in Gunn rats is due to the absence of a specific UDP-glucuronosyltransferase isoenzyme. Mol Pharmacol. 1987 Jun;31(6):585–591. [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Gartner L. M., Lee K. S., Vaisman S., Lane D., Zarafu I. Development of bilirubin transport and metabolism in the newborn rhesus monkey. J Pediatr. 1977 Apr;90(4):513–531. doi: 10.1016/s0022-3476(77)80360-0. [DOI] [PubMed] [Google Scholar]
- Graham A. B., Pechey D. T., Wood G. C. Responses of microsomal UDP-glucuronyltransferase to trypsin. Mol Pharmacol. 1979 Mar;15(2):375–385. [PubMed] [Google Scholar]
- Heirwegh K. P., Van de Vijver M., Fevery J. Assay and properties of dititonin-activated bilirubin uridine diphosphate glucuronyltransferase from rat liver. Biochem J. 1972 Sep;129(3):605–618. doi: 10.1042/bj1290605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyanagi T., Haniu M., Sogawa K., Fujii-Kuriyama Y., Watanabe S., Shively J. E., Anan K. F. Cloning and characterization of cDNA encoding 3-methylcholanthrene inducible rat mRNA for UDP-glucuronosyltransferase. J Biol Chem. 1986 Nov 25;261(33):15607–15614. [PubMed] [Google Scholar]
- Jackson M. R., Burchell B. The full length coding sequence of rat liver androsterone UDP-glucuronyltransferase cDNA and comparison with other members of this gene family. Nucleic Acids Res. 1986 Jan 24;14(2):779–795. doi: 10.1093/nar/14.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackenzie P. I. Rat liver UDP-glucuronosyltransferase. cDNA sequence and expression of a form glucuronidating 3-hydroxyandrogens. J Biol Chem. 1986 Oct 25;261(30):14112–14117. [PubMed] [Google Scholar]
- Päbo S., Bhat B. M., Wold W. S., Peterson P. A. A short sequence in the COOH-terminus makes an adenovirus membrane glycoprotein a resident of the endoplasmic reticulum. Cell. 1987 Jul 17;50(2):311–317. doi: 10.1016/0092-8674(87)90226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Chowdhury J., Roy Chowdhury N., Falany C. N., Tephly T. R., Arias I. M. Isolation and characterization of multiple forms of rat liver UDP-glucuronate glucuronosyltransferase. Biochem J. 1986 Feb 1;233(3):827–837. doi: 10.1042/bj2330827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstapel F., Blanckaert N. Topology and regulation of bilirubin UDP-glucuronyltransferase in sealed native microsomes from rat liver. Arch Biochem Biophys. 1988 May 15;263(1):216–225. doi: 10.1016/0003-9861(88)90630-3. [DOI] [PubMed] [Google Scholar]