Abstract
Purpose
To determine whether the Lrit3−/− mouse model of complete congenital stationary night blindness with an ON-pathway defect harbors myopic features and whether the genetic defect influences the recovery from lens-induced myopia.
Methods
Retinal levels of dopamine (DA) and 3,4 dihydroxyphenylacetic acid (DOPAC) from adult isolated Lrit3−/− retinas were quantified using ultra performance liquid chromatography after light adaptation. Natural refractive development of Lrit3−/− mice was measured from three weeks to nine weeks of age using an infrared photorefractometer. Susceptibility to myopia induction was assessed using a lens-induced myopia protocol with −25 D lenses placed in front of the right eye of the animals for three weeks; the mean interocular shift was measured with an infrared photorefractometer after two and three weeks of goggling and after one and two weeks after removal of goggles.
Results
Compared to wild-type littermates (Lrit3+/+), both DA and DOPAC were drastically reduced in Lrit3−/− retinas. Natural refractive development was normal but Lrit3−/− mice showed a higher myopic shift and a lower ability to recover from induced myopia.
Conclusions
Our data consolidate the link between ON pathway defect altered dopaminergic signaling and myopia. We document for the first time the role of ON pathway on the recovery from myopia induction.
Keywords: myopia, CSNB, refractometry, ON pathway, dopamine
Myopia, also known as nearsightedness, is a worldwide-spread ocular affliction with increasing prevalence, mostly in Southeast Asia.1–5 The axial form of myopia is characterized by an abnormal increase in the axial length occurring during emmetropization (e.g., the process during which the slightly hyperopic eye grows to place the retina onto the focal point), leading to a blurry far-sight.6 In humans, emmetropization occurs from birth to 12 years old, with the most active phase until six years old. Causes of myopia onset imply both environmental and genetic factors.6,7 In most cases, myopia occurs during school times because of reading habits and light environment.8 Genetic myopia, which is rarer, often causes earlier refractive error and faster myopia progression,9 leading to high myopia (HM, refractive error ≤−6 D). HM can lead to blindness through additional ocular signs such as retinal detachment, cataract, myopic macular degeneration, and glaucoma.10 Several studies highlighted a protective role of outdoor light upon onset and progression of myopia in humans11–13 and animal models.14–19 We and others unveiled many genes associated with syndromic7,20 (e.g., coexisting with other symptoms in a wider syndrome) and nonsyndromic7,21 (e.g., sole ocular symptom) myopia. The precise mechanisms implicated in physiological and abnormal eye growth still require further investigations. Therefore studying syndromic myopia can help decipher new pathways involved in emmetropization and myopia onset. The use of mouse models enables the modification of both environmental and genetic factors. Nevertheless, the small size of their eyes and their poor optics leads to difficulties in measuring myopia in murine models.22 A change of one diopter in refractive state correlates with a change of 5.4 to 6.5 µm in axial length of C57BL/6 mouse eyes23 compared to the 280 µm to 400 µm changes in human children and adults, respectively.24,25 Consequently, spontaneously occurring myopia is very rare in small animals, and previous studies focused on the induction of myopia through a lens-induction (LIM) or form deprivation (FDM) protocol to measure the sensitivity to myopia induction.22,26,27 When negative lenses (LIM) or diffusers (FDM) are removed, the eye of the animals can undergo a recovery process to return to a normal emmetropic state.28–30 Recovery was observed in several animal models: tree shrews,30 guinea pigs,28 chickens,31 non-human primates,32 and mice.29 Because the recovery from myopia requires a modification of the visual cue, one can hypothesize that the retinal signaling is primarily involved. The precise molecular mechanisms, however, necessitate that further examinations be unveiled.
Even though the molecular and cellular cascades implicated in emmetropization need additional investigations, many molecules acting in either promoting or inhibiting the eye growth have been discovered to date. Among them, the neurotransmitter dopamine (DA), its degradation metabolite 3,4 dihydroxyphenylacetic acid (DOPAC), and DA interactors have been extensively studied. DA, synthesized by dopaminergic amacrines cells (ACs) is thought to be a retinal stop signal for eye growth.33–36 Secretion of DA in the retina is mediated by light environment and circadian rhythm18,37–42 and requires an effective ON-pathway function.20,43–45 Furthermore, the retinal release of DA was found altered in several myopia models from multiple species and experimentally induced changes in retinal DA leads to consistent changes in myopia susceptibility.18,36,46–50
Studies focusing on syndromic forms of myopia can be helpful to decipher new pathophysiological components of abnormal eye growth. Among the inherited retinal diseases leading to syndromic myopia, complete congenital stationary night blindness (cCSNB) is of particular interest because cCSNB patients develop frequently other ocular signs such as nystagmus, strabismus but also high myopia with a median refractive error of −7.4 D.20,51–57 cCSNB is a group of clinically and genetically heterogenous inherited retinal diseases which main clinical feature consists in the loss of dim and night vision.54 Electroretinograms (ERG) from cCSNB patients display a normal a-wave but a severe or complete loss of the b-wave under scotopic conditions and altered b-waves under photopic conditions, reflecting an ON-pathway defect. Such abnormal ERGs are directly caused by the dysfunction of the ON-pathway,51,54,57,58 whereas the OFF pathway remains unaltered. To date, variants leading to cCSNB in humans were found in NYX, TRPM1, GRM6, GPR179, and LRIT3 genes.54,59–68 Animal models revealing a similar cCSNB phenotype harboring the same gene defects were studied to better understand the cCSNB phenotype, the pathophysiology and develop therapies: Nyx (also known as nob mice),69–71 Grm6,72–76 Trpm1,77–80 Gpr179,63,81–83 or Lrit3.84–88 The hypothesis of an impact of ON-pathway defects upon retinal dopamine metabolism and myopia raised attention as previous studies have shown that nob and Grm6−/− mice display a higher susceptibility to FDM and an altered retinal level of DOPAC.43,44 Using a LIM protocol with −25 D lenses, we strengthened this hypothesis by showing that the Gpr179−/− mice also have a higher susceptibility to myopia induction and reduced retinal levels of DA and DOPAC compared to wild-type littermates.45 The impact of ON-pathway defect in the development of myopia because of the loss of Lrit3 remains to be elucidated.
All genes mutated in cCSNB encode proteins implicated in the signal transmission at photoreceptors to ON-bipolar cells (ON-BCs) synapse.54 In this study, we focus on the model lacking the Leucine-rich repeat immunoglobulin transmembrane domain 3 (Lrit3−/−) gene. The LRIT3 protein is localized at the outer plexiform layer, similar as for other cCSNB molecules.51,54,87 As mentioned before, the consequences of Lrit3 depletion are similar to that of other cCSNB murine models: lack of the corresponding protein, reduced performances during the optomotor test in dark condition, and unmeasurable b-wave under scotopic conditions.85 Recently we demonstrated that LRIT3 is necessary for the correct localization of TRPM1 at the dendritic tip of ON-BCs.85,86
In this study, we hypothesized that the loss of LRIT3 would lead to altered dopamine metabolism and a higher susceptibility to myopia induction. In addition, we were interested to determine whether the recovery of experimentally induced myopia would be influenced by a genetic defect affecting the ON-bipolar cell function.
Methods
Animal Care and Ethical Statement
All animal procedures were performed according to the Council Directive 2010/63EU of the European Parliament and the Council of September 22, 2010, on the protection of animals used for scientific purposes, with the National Institutes of Health guidelines and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. They were approved by the French Minister of National Education, Superior Education and Research. Mouse lines and projects were registered as following: APAFIS #27474 2020100110251857 v5. Description of the generation of Lrit3−/− model can be found elsewhere.85 Mice were kept in 12-:12- hour light/dark cycles with mouse chow and water as desired.
PCR Genotyping
DNA was extracted from mouse tails with 50 mM NaOH after incubation at 95°C for 30 minutes. Wild-type and mutant alleles were amplified independently using a polymerase (HOT FIREPol; Solis Biodyne, Tartu, Estonia), a common forward primer: mLrit3_3F (5′- CTGTCACAAGACAAGCTATGC-3′) and two specific reverse primers: mLrit3_3R (5′- CCATGTCCTTGCATCCAATGA-3′) for the wild-type allele and mLrit3_casR (5′- CGACATTCAACAGACCTTGCA-3′) for the mutant allele. The following PCR program was used: 15 minutes at 95°C for denaturation, 35 cycles of 45 seconds at 95°C, one minute at 60°C, and 1.3 minutes at 72°C. A final extension for 10 minutes at 72°C was performed. This generates the following amplicons: PCR using mLrit3_3F and mLrit3_3R primers amplifies a product of 509 base pairs (bp) for the wild-type allele and no product for the mutant allele, PCR using mLrit3_3F and mLrit3_casR primers amplifies no product for the wild-type allele and a 377 bp product for the mutant allele. PCR products were separated by electrophoresis on 2% agarose gels, stained with ethidium bromide, and visualized using a documentation system (Molecular Imager Gel Doc XR+ System; Bio-Rad, Hercules, CA, USA).
DA and DOPAC Measurements
After four hours of light adaptation in a light-controlled room at 50 lux, adult Lrit3+/+ (n = 7) and Lrit3−/− (n = 7) mice were euthanized at 12 AM by CO2 inhalation followed by cervical dislocation. Retinas were isolated, frozen in liquid nitrogen, and stored at −80°C. Amounts of DA and DOPAC were quantified with ultra-performance liquid chromatography with coulometric detection as describe previously.45 Values are reported as mean ± standard error means (SEM). Data were tested for normality and analyzed using the nonparametric Mann-Whitney test to compare ranks. Statistical significance was obtained with P ≤ 0.05, P ≤ 0.01, P ≤ 0.001, and P ≤ 0.0001
Lens-Induced Myopia
Myopia was induced in mice from P21 to P42 using a −25 D lens placed in front of the right eye, according to a protocol previously published.45 Briefly, P21 Lrit3+/+ (n = 7–12) and Lrit3−/− (n = 8–13) mice were anesthetized by isoflurane inhalation (5% induction, 2% maintenance). The scalp was cut through the rostrocaudal axis to expose the skull. Two intracranial screws were implanted on both left and right sides of the skull at y = −2 mm from the bregma. A homemade goggle frame was placed on the skull and fixed using dental cement (FujiCEM, cat no. 900903; Phymep, Paris, France). The goggle frame, adapted from a previously validated protocol,48 was built in resin using a 3 D printer. Lenses of −25 D were stuck on the frame using surgical glue (vetbond, Phymep). Stitches were used to avoid displacement of lens by mice. The −25 D lens was always placed in front of the right eye for three weeks. The left eye was left untouched. The goggles were removed at least twice a week for cleaning.
Refractometry
The measurements of the refractive state were performed as previously described.45 Briefly, eye drops were used to dilate the pupils: 0.5% mydriaticum (Théa, Clermont Ferrand, France) and 5% neosynephrine (Europhta, Monaco), and mice were maintained in front of the photorefractometer in a restraining platform. Calibration of the infrared photorefractometer89 was verified using lenses of increasing power, from −10 D to 10 D placed in front of a mouse eye. Mice used for LIM differed from those used for the natural refractive development experiments but originated from the same breeding. For natural refractive development experiments, the measurements were performed once per week, every week from three to nine weeks old Lrit3+/+ (n = 20) and Lrit3−/− (n = 20) and the mean refractive state of both eyes was used for statistical analysis. To evaluate the sensitivity to myopia induction, the difference of refractive state between goggled and ungoggled eye (referred to as interocular shift) was measured at postnatal day 21 (P21, day of surgical procedure), P35 (14 days of goggling), P42 (21 days of goggling), P49 (seven days after lens removal), and P56 (14 days after lens removal) was used for statistical analysis. Only the mice displaying less than 2 D of interocular shift at P21 were used for the LIM protocol. Statistical analyses were performed using Prism 9.1.2 (GraphPad v7; GraphPad Software Inc., La Jolla, CA, USA). Statistical significance was measured with a two-way ANOVA test. Values are reported as mean ± SEM. Statistical significance was obtained with P ≤ 0.05, P ≤ 0.01, P ≤ 0.001, and P ≤ 0.0001.
Results
Quantification of Retinal Levels of DA and DOPAC
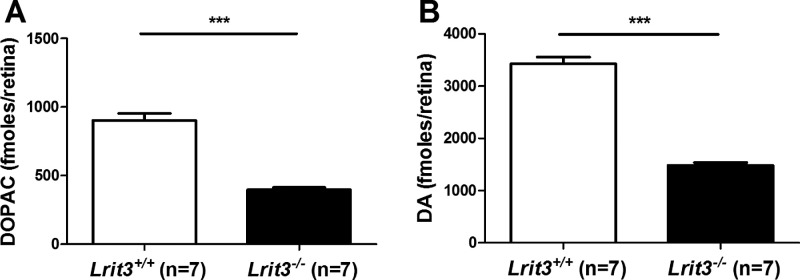
We first sought to determine whether the loss of ON-pathway function as observed in Lrit3−/− mice85 can cause an alteration of the dopaminergic activity. Thus, using ultra-performance liquid chromatography, we quantified retinal levels of DA and DOPAC in adult isolated retinas from Lrit3−/− and Lrit3+/+ mice after four hours of 50 lux light adaptation. Lrit3−/− retinas show a significant decrease in both DOPAC and DA levels compared to Lrit3+/+ retinas (Fig. 1). These findings were reviewed by us to validate that Lrit3−/− mice are good models to study myopia.20 Mean DOPAC levels were 396 ± 16 fmoles/retinas in Lrit3−/− animals compared with 902 ± 52 fmoles/retina in Lrit3+/+ animals (mean ± SEM). Similarly, mean levels of DA were 1482 ± 54 fmoles in Lrit3−/− animals compared with 3425 ± 132 fmoles in Lrit3+/+ animals (mean ± SEM). These findings were already included in a previous study.20
Figure 1.
Retinal levels of DA and DOPAC. Quantification of retinal levels of DOPAC (A) and DA (B) in 12 weeks old light adapted Lrit3+/+ and Lrit3−/− mice. Data are expressed as mean ± SEM. ns, not significant. ***P ˂ 0.001. This figure was published before in a review without describing the details.20 The authors obtained the permission to publish this figure herein.
Assessment of Natural Refractive Development
To decipher whether the loss of Lrit3 would induce changes in the refractive development of mice, we measured the refractive state of Lrit3−/− and Lrit3+/+ mice from three weeks old to nine weeks old using an infrared photorefractometer. At three weeks old, both genotypes revealed a similar refractive state. Both genotypes underwent a hyperopic shift from 0 to 5 D before reaching a plateau at nine weeks old. Lrit3+/+ mice reached the plateau at six weeks old whereas Lrit3−/− mice hit the maximum of refractive error at 4 weeks old (Fig. 2). This result indicates that the loss of Lrit3 causes a quicker refractive development with no change in the final refractive state.
Figure 2.
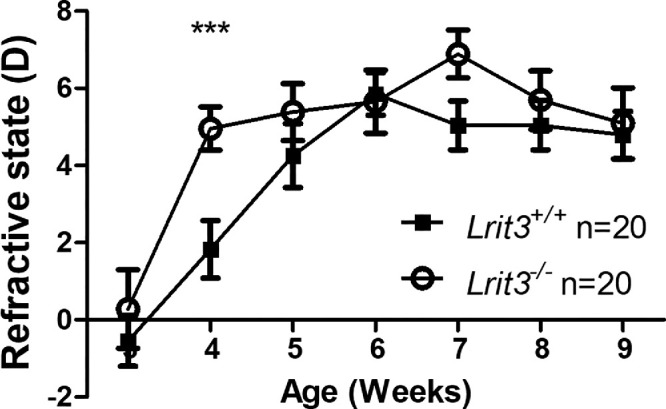
Natural refractive development of the Lrit3 mouse model. Measurement of the refractive development of Lrit+/+ and Lrit3−/− mice from three weeks to nine weeks old. Data are expressed as mean ± SEM. ***P ˂ 0.001.
Investigation of Susceptibility to Lens-Induced Myopia
We tested whether the lack of functional ON-pathway caused by the genetic inactivation of Lrit3 could affect the vulnerability to an environmentally induced myopia. Thus we measured the mean interocular shift (called myopic shift when negative) between the eyes of Lrit3−/− mice compared to their wild-type littermates, Lrit3+/+, after two and three weeks of goggling with a −25 D lens placed in front of the right eye and one and two weeks after removal of the lenses (Fig. 3). At two weeks after goggling, we observed a higher myopic shift in Lrit3−/− mice compared to Lrit3+/+. Lrit3−/− mice displayed a myopic shift of −9.12 ± 0.98 D compared to −5.46 ± 0.43 D in Lrit3+/+ mice (mean ± SEM). The difference between Lrit3−/− and Lrit3+/+ remained stable three weeks after goggling. Lrit3−/− mice displayed a myopic shift of −10.20 ± 1.19 D compared to −6.12 ± 0.54 D in Lrit3+/+ mice (mean ± SEM). Interestingly, one week after removal of the lenses, Lrit3−/− mice maintained a strong myopic shift whereas the mean interocular shift of Lrit3+/+ mice returned to a value similar to before goggling. Lrit3−/− mice showed a myopic shift of −10.47 ± 1.18 D whereas Lrit3+/+ mice have a mean interocular shift of −0.72 ± 0.98 (mean ± SEM). Two weeks after removal of the lenses, Lrit3−/− mice kept showing a significant myopic shift, although lower compared to the previous time point, whereas Lrit3+/+ mice maintained a normal mean interocular shift. The myopic shift of Lrit3−/− mice was −7.53 ± 1.98 whereas Lrit3+/+ mice harbored a mean interocular shift −0.11 ± 0.77 D (mean ± SEM). Altogether, these data suggest that the loss of LRIT3 and the subsequent impairment of the ON-pathway cause an increase in the sensitivity to experimentally induced myopia and a reduction in the ability to recover from three weeks of induction.
Figure 3.
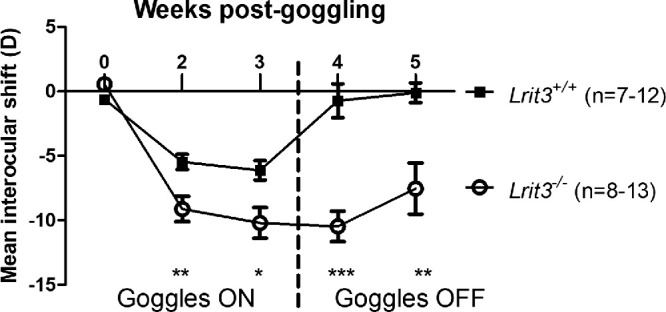
LIM in the Lrit3 mouse model. Assessment of the mean interocular shift of Lrit3+/+ and Lrit3−/− mice from zero weeks to three weeks of goggling with −25 D lenses (Goggles ON) and two weeks after removal of the lenses (Goggles OFF). Data are expressed as mean ± SEM. *P ≤ 0.1; **P ≤ 0.05; ***P ˂ 0.001.
Discussion
The main weakness of this work is the limited variety of parameters used to assess myopia. We used the refractive state as the sole indicator of the elongation of the eye. The refractive state is the most common hallmark used to measure myopia in humans and animal models. Nevertheless, axial elongation is not the only ocular biometric influencing the refractive state. For instance, the opacification of the lens as observed in patients with cataract can be a primary cause of index myopia.90 Thus it is relevant to propose different methods to evaluate myopia to overcome possible interactions between the different ocular biometrics and to gain a better insight of the implicated mechanisms, mainly for the recovery from myopia. In our case, our results remain in line with those of previously validated findings obtained in other cCSNB mouse models with similar methods.43–45
Emmetropization is a complex process occurring during the eye development. It involves most if not all the tissues of the eye and the precise cellular and molecular mechanisms by which the eye grows to reach emmetropia require extensive studies. It is well known that the eye growth observed during emmetropization arises from the remodeling of scleral extracellular matrix under retinal signaling and choroidal relay.91 The use of transgenic mice models enables the modification of both genetic and environmental factors, which is of particular interest when it comes to myopia.6,13,20 Here, we focus on a genetic mouse model lacking Lrit3 (Lrit3−/−), a model of cCSNB.85 In humans, mutations in LRIT3 cause cCSNB (with the loss of ON-pathway activity) and high myopia.20,54,61 Lrit3−/− mice show similar ERG abnormalities as all mouse models and patients associated with cCSNB.85
To date, several retinal factors involved in eye growth, either by inhibiting it (“stop” signals) or enhancing it (“go” signals) have been identified. Among the signals, the dopaminergic signaling is the most characterized.18,34–36,41,43,44,92–98 DA is considered as a stop signal in most species and myopia induction protocols.18,36,41,43,44,50,93,94,97,99,100 Our data revealed that retinal levels of both DA and DOPAC in Lrit3−/− eyes were halved compared to controls eyes (Fig. 1). In the retina, DA is synthesized and released by dopaminergic amacrines cells. Dopaminergic system is influenced by environmental light,18,36,41,93,101 circadian rhythm,39,42,102,103 and the activation of ON-pathway.43–45 Our results are in line with our previous study performed in our lab on other cCSNB models: the Gpr179−/− and Grm6−/− mice20,45 and in another lab on nob mice44 but slightly different from findings on a different Grm6 knock-out model in which only DOPAC levels were affected.43 These observed differences can be due to age, lightning environment, and background differences, influencing the dopaminergic system45,104 Overall, our results strengthen the hypothesis of an impact of ON-pathway defect upon the dopaminergic system. Because retinal DA is considered as a stop signal for eye growth, reduction in retinal DA release can lead to an altered emmetropization.
Interestingly, a faster refractive development was observed in Lrit3−/− mice compared to Lrit3+/+ with no change in the maximum value of the refractive state (Fig. 2). This data slightly differs from those obtained from other cCSNB models.43–45 Other cCSNB models displayed the following: nob mice were found to be more hyperopic than wild-type littermates, whereas Grm6−/− mice displayed a more myopic development.43,44 Furthermore, both Lrit3+/+ and Lrit3−/− mice were more myopic in general than some genetic mouse models,43,44 but similar to others.18,105–107 Both genetic107,108 and environmental18,106 factors can influence refractive development. This difference does not seem to be caused by differences in retinal DA or DOPAC levels as our findings are similar in two cCSNB models performed by us (Fig. 1 and reference 45). Here we used mice from a different genetic background (129/SvEv-C57BL/6J) than other laboratories.43,107–109 This difference might impact the results. However, Lrit3−/− mice are not spontaneously myopic, which is in line with the idea of a required induction protocol in small animals.20,43–45
Lrit3 − /− mice showed a significant increase in myopic shift after two weeks of goggling compared to Lrit3+/+ littermates (Fig. 3). It is noteworthy that, between two and three weeks of goggling, only a very slight increase in the myopic shift (≈1 D) was observed for both Lrit3−/− and Lrit3+/+ mice. This finding is comparable to our previous results obtained with a similar LIM protocol on Gpr179+/+ mice but different from Gpr179−/−, which displayed a continuous increase in the myopic shift.45 FDM experiments performed upon nob and Grm6−/− mice showed a significant increase in the myopic shift as well,43,44 although lower than in the present study. We do not know how the myopic shift of those models would evolve after more than two weeks of goggling, but the wild-type mice tested in parallel to nob mice displayed a continuous increase of the myopic shift for at least eight weeks of goggling.44 To explain this discrepancy, one can hypothesize LIM protocols suffer from a ceiling effect dependent on the optical power of the tested lens. In a theoretically perfect optical system, a −25 D lens should not cause a myopic shift higher than −25 D because the eye ends up by matching its size with the imposed defocus. In contrast, it is possible that the blurry effect imposed by the FDM paradigm cannot be countered by the growing eye. Testing both FDM and LIM on the same cCSNB mouse model for a longer period is required to test this hypothesis. Presuming that this hypothesis is validated by future studies, it might mean that, if LIM is often considered as more similar to human myopia, FDM keeps its usefulness to unveil the mechanisms implicated in the time course and speed of myopization. Regarding the prevention and control of myopia, it is widely accepted that there is a critical period of time during the development at which the eye is more sensitive to both myopization and anti-myopization stimuli (i.e., six to 12 years old in humans, two- to six-week-old in mice).6,110 Thus, if cCSNB mouse models develop a faster myopic shift rather than a higher one, deciphering the mechanisms implicated remains of interest.
Previous works from our lab and others indicate that, in addition to the ON pathway defect, the genetic ablation of Lrit3 could cause slight changes in OFF pathway activity,84,87 a finding not reported for other genes implicated in cCSNB. This data can point out that addressing the impact of OFF pathway defect on our results can be of interest. In contrast to the other genes implicated in cCSNB, it was suggested that Lrit3 in mouse is expressed in the presynaptic part of the photoreceptor to BC synapse,111 which can at least partly explain the changes observed in OFF activity. Studies focused on OFF pathway defect and its implication on eye growth already exist. A recent study pinpointed that the loss of contrast sensitivity as observed in myopia mostly implies ON pathway.112 Mice with a pure OFF dysfunction because of a lack of Vsx1 (Vsx1−/− on a 129S1/Sv genetic background) showed no change in refractive development and did not develop myopia under FDM but had a higher basal retinal DOPAC level107,113 rather than lower as observed in most myopia models. Vsx1−/− mice also displayed reduced optomotor response even if the effect is much lower than in nob mice.92 In humans, but not in mice, pathogenic mutations in the VSX1 gene can cause myopia through keratoconus rather than through retinal mechanisms.92,107,114 Nevertheless, mutations in GJD2 (also known as Cx36) are associated to myopia through an OFF-cone pathway in patients. Whether these mutations can cause myopia in mice is still unknown, but a recent study reported that Cx36−/− αRGCs were not able to detect and transmit defocus.115 In addition, mice with dysfunctions in cone photoreceptors (Gnat2−/− model) show a higher sensitivity to FDM but a normal DA/DOPAC metabolism.116 Patients with pathogenic mutations in GNAT2 develop achromatopsia with variable refractive errors, among which both high myopia and high hyperopia can appear.117,118 Altogether, these findings suggest that OFF pathway defects can also impact the refractive development and myopia in humans, a hypothesis further to be validated in mice but whether OFF pathway defect can cause myopia in mice lacks evidence. Consequently, deciphering the potential impact of the small dysfunction of the OFF pathway in Lrit3−/− mice on myopia development requires further investigations.
If most previous studies focused on the mechanisms implicated in myopia onset and progression, addressing the recovery step (e.g. after removal of the diffuser/lens) can also be of interest when it comes to the control and treatment of myopia. Similarly to wild-type mice from different genetic backgrounds, Lrit3+/+ mice fully recovered from three weeks of LIM one week after lens removal (Fig. 3 and reference 29). Interestingly, Lrit3−/− mice kept showing a significant myopic shift at least two weeks after removal of the lenses (Fig. 3, Goggle OFF) compared to Lrit3+/+ mice. Whether the maintenance of the myopic shift after lens removal is a specific trait of Lrit3−/− mice or exists in all cCSNB mouse models needs to be confirmed in the future. In addition, further investigations are required to decipher whether the recovery is lacking or just delayed in Lrit3−/− mice. Many studies documenting gene signatures during myopia induction and recovery in tree shrews exist.30,119–122 If changes in scleral and choroidal gene expression seem to be long term changes,30,120–122 changes in retinal gene expression are likely more short term.122,123 A previous study from our laboratory reported several retinal genes associated with myopia to be differentially expressed in multiple cCSNB models.20 As the retina is the driver of the visual information toward sclera and choroid, one can hypothesize that some of the differentially expressed genes observed in cCSNB retinas, physiologically expressed in wild-type mice during recovery, cannot be properly expressed in cCSNB models due to ON-pathway defect or due to genetic alterations, leading to an impossible or delayed recovery. Furthermore, if the increased myopic shift and the lack of recovery observed in Lrit3−/− mice was due to the defect of the ON-pathway, one could think that the protective effect of violet light observed in previous studies124,125 would not be as efficient in cCSNB models. The protective effect of violet light is most effective during dusk times125 (e.g., the time at which a functional ON-pathway is the most critical) because cCSNB patients are mostly affected at dim and night vision. Furthermore, available and functional neuropsin, encoded by Opn5 and expressed in RGCs is required for violet light-mediated protection from myopia.125,126 As ON retinal ganglion cells receive excitatory inputs from ON-BCs, these data seem to indicate a functional ON-pathway is required for protection from myopia and recovery from myopizing stimuli. However, this assumption requires further studies to be confirmed.
Similarly, many questions regarding the crosstalk between gene expression, ON/OFF pathway activity and eye growth remain under debate. In humans, previous work showed that reading under normal contrast (i.e., black letters on white background) overactivates the OFF pathway and causes a thinning of the sclera, while reading under inverted contrast (i.e., white letters on black background) overactivates the ON pathway and causes the thickening of the sclera.127 This protective effect of inverted contrast (and the subsequent ON pathway activation) was found in both emmetropic and myopic patients.128 In cCSNB, which represents a complete ON-pathway dysfunction, this protection is absent, which may explain high myopia in cCSNB patients and mice. As previously described,20 this dysfunction of the ON pathway leads to differentially expressed genes, some of which need to be correctly expressed to prevent the development of myopia. In summary, we can hypothesize that the complete loss of ON pathway activity may keep the retina unresponsive to stimuli that typically prevent myopia, such as inverted contrast or outdoor light. Apart from changes in gene expression, the loss of ON pathway activity can modify retinal circuitry, such as dopamine-releasing neurons.
Assessing retinal levels of DA and its metabolite DOPAC during myopia induction and recovery is of significant scientific interest. This interest is twofold: first, because DA/DOPAC levels can serve as biomarkers for axial myopia, and second, because the findings could provide critical insights into the mechanisms underlying both the onset and recovery of myopia via the ON pathway. Our research has demonstrated alterations in dopaminergic metabolism in cCSNB, suggesting a potential involvement in myopia. Previous studies have shown that both FDM and LIM reduce retinal DA/DOPAC levels,36,49 but it is unclear whether these reductions are linked to myopiagenic changes in ON-pathway activity. Current knowledge on the precise kinetics of retinal dopamine release during experimental myopia development is limited, as most studies have focused on preinduction and postinduction stages rather than the dynamic changes during LIM or FDM. Gene expression studies in tree shrews indicate that retinal gene expression changes occurring during myopia induction are largely absent after 24 hours of induction,122 unlike those in the sclera and choroid.30,120,121 Dopaminergic amacrine cells receive indirect excitatory input from ON-BCs, which are silenced in cCSNB. Considering that (1) the retina functions as a detector of defocus or blur, (2) myopia-induced changes in retinal activity and gene expression are primarily short-term and only maintained during the entire induction period, and (3) the myopic shift only marginally increases between two and three weeks of induction in both Lrit3+/+ wild-type and Lrit3−/− mice in the present study, we hypothesize that retinal levels of DA/DOPAC would remain stable between two and three weeks of goggling. This hypothesis merits further investigation, because it is plausible that DA/DOPAC levels would decrease rapidly after the initiation of goggling, at least in wild-type Lrit3+/+ mice and potentially in cCSNB mice. Determining whether the rates of change in DA/DOPAC levels during the early stages of myopia differ between wild-type and cCSNB mice would provide valuable insights into the retinal signaling mechanisms involved in myopia onset. Similarly, the behavior of retinal DA/DOPAC levels during myopia recovery remains uncertain. It can be hypothesized that in wild-type mice, retinal DA/DOPAC levels would correlate with the myopic shift, exhibiting a significant decrease during LIM and returning to baseline levels on recovery. In contrast, for cCSNB mice, retinal DA/DOPAC levels might also follow the myopic shift but would start from a much lower baseline. It is possible that in cCSNB mice, these levels might remain unaffected or they could decrease during LIM without returning to baseline during the recovery phase. Further investigations are necessary to validate these hypotheses.
Altogether, the findings of the present study further confirm the impact of ON-pathway defects—such as those observed in cCSNB—on emmetropization and myopia onset. They also propose new possibilities to investigate the recovery steps after myopia induction. Finally, the absence or delay of recovery in myopic mice offers researchers a larger time frame to study myopia without the need for mice to wear goggles or diffusers.
Acknowledgments
This study was carried out in Paris, France.
The authors are grateful to Manuel Simonutti, Julie Dégardin, Marion Cornebois, Pauline Abgrall, Mathilde Lappe, Julie Geus, and Pedro Palma for animal care (animal facility at the Institut de la Vision).
Supported by Agence Nationale de la Recherche (ANR-22-CHIN-0006) (CZ); Retina France; Valentin Haüy and AFM (CZ), IRP-INSERM (CZ and RD); Prix Dalloz pour la recherche en ophtalmologie and Fondation Dalloz—Institut de France (CZ); Fondation Voir et Entendre (CZ); Fondation de l'oeil—Fondation de France (IA), Ville de Paris and Region Ile de France; Labex Lifesenses (reference ANR-10-LABX-65), supported by French state funds managed by the ANR within the Investissements d'Avenir programme (ANR-11-IDEX-0004-0); the Programme Investissements d'Avenir IHU FOReSIGHT (ANR-18-IAHU-01); National Institutes of Health grant EY029985 (RD) and Essilor (BW).
Disclosure: B. Wilmet, None; C. Michiels, None; J. Zhang, None; J. Callebert, None; J.A. Sahel, None; S. Picaud, None; I. Audo, None; C. Zeitz, None
References
- 1. Wu PC, Huang HM, Yu HJ, Fang PC, Chen CT.. Epidemiology of myopia. Asia Pac J Ophthalmol (Phila). 2016; 5: 386–393. [DOI] [PubMed] [Google Scholar]
- 2. Cooper J, Tkatchenko AV.. A review of current concepts of the etiology and treatment of myopia. Eye Contact Lens. 2018; 44: 231–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dolgin E. The myopia boom. Nat News. 2015; 519: 276. [DOI] [PubMed] [Google Scholar]
- 4. Baird PN, Saw SM, Lanca C, et al.. Myopia. Nat Rev Dis Primers. 2020; 6: 99. [DOI] [PubMed] [Google Scholar]
- 5. Holden BA, Fricke TR, Wilson DA, et al.. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016; 123: 1036–1042. [DOI] [PubMed] [Google Scholar]
- 6. Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012; 379: 1739–1748. [DOI] [PubMed] [Google Scholar]
- 7. Tedja MS, Haarman AEG, Meester-Smoor MA, et al.. IMI–Myopia genetics report. Invest Ophthalmol Vis Sci. 2019; 60: M89–M105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Szeps A, Dankert S, Saracco G, Iribarren R.. A pilot study of axial length changes associated with myopia control spectacles in subjects reading under mesopic conditions. J AAPOS. 2024; 28: 103857. [DOI] [PubMed] [Google Scholar]
- 9. Rozema J, Dankert S, Iribarren R.. Emmetropization and nonmyopic eye growth. Surv Ophthalmol. 2023; 68: 759–783. [DOI] [PubMed] [Google Scholar]
- 10. Ikuno Y. Overview of the complications of high myopia. Retina. 2017; 37: 2347–2351. [DOI] [PubMed] [Google Scholar]
- 11. Wu PC, Chen CT, Lin KK, et al.. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology. 2018; 125: 1239–1250. [DOI] [PubMed] [Google Scholar]
- 12. Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK.. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013; 120: 1080–1085. [DOI] [PubMed] [Google Scholar]
- 13. French AN, Ashby RS, Morgan IG, Rose KA.. Time outdoors and the prevention of myopia. Exp Eye Res. 2013; 114: 58–68. [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Ding H, Stell WK, et al.. Exposure to sunlight reduces the risk of myopia in rhesus monkeys. PloS One. 2015; 10: e0127863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith EL 3rd, Hung LF, Arumugam B, Huang J.. Negative lens-induced myopia in infant monkeys: effects of high ambient lighting. Invest Ophthalmol Vis Sci. 2013; 54: 2959–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith EL 3rd, Hung LF, Huang J.. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012; 53: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li W, Lan W, Yang S, et al.. The effect of spectral property and intensity of light on natural refractive development and compensation to negative lenses in guinea pigs. Invest Ophthalmol Vis Sci. 2014; 55: 6324–6332. [DOI] [PubMed] [Google Scholar]
- 18. Landis EG, Park HN, Chrenek M, et al.. Ambient light regulates retinal dopamine signaling and myopia susceptibility. Invest Ophthalmol Vis Sci. 2021; 62: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Norton TT, Amedo AO, Siegwart JT Jr. Darkness causes myopia in visually experienced tree shrews. Invest Ophthalmol Vis Sci. 2006; 47: 4700–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zeitz C, Roger JE, Audo I, et al.. Shedding light on myopia by studying complete congenital stationary night blindness. Prog Retinal Eye Res. 2023; 93: 101155. [DOI] [PubMed] [Google Scholar]
- 21. Hysi PG, Choquet H, Khawaja AP, et al.. Meta-analysis of 542,934 subjects of European ancestry identifies new genes and mechanisms predisposing to refractive error and myopia. Nat Genet. 2020; 52: 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pardue MT, Stone RA, Iuvone PM.. Investigating mechanisms of myopia in mice. Exp Eye Res. 2013; 114: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmucker C, Schaeffel F.. In vivo biometry in the mouse eye with low coherence interferometry. Vis Res. 2004; 44: 2445–2456. [DOI] [PubMed] [Google Scholar]
- 24. Cruickshank FE, Logan NS.. Optical “dampening” of the refractive error to axial length ratio: implications for outcome measures in myopia control studies. Ophthalmic Physiol Opt. 2018; 38: 290–297. [DOI] [PubMed] [Google Scholar]
- 25. Atchison DA, Jones CE, Schmid KL, et al.. Eye shape in emmetropia and myopia. Invest Ophthalmol Vis Sci. 2004; 45: 3380–3386. [DOI] [PubMed] [Google Scholar]
- 26. Zhou X, Pardue MT, Iuvone PM, Qu J.. Dopamine signaling and myopia development: what are the key challenges. Prog Retin Eye Res. 2017; 61: 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schaeffel F, Feldkaemper M.. Animal models in myopia research. Clin Exp Optom. 2015; 98: 507–517. [DOI] [PubMed] [Google Scholar]
- 28. Xu H, Dong Y, He F, Qin B.. Changes of Melanopsin Expression in the Retina of Guinea Pig during Experimental Myopia and Recovery Period. Curr Eye Res. 2023; 48: 674–682. [DOI] [PubMed] [Google Scholar]
- 29. Ma Z, Jeong H, Yang Y, et al.. Contralateral effect in progression and recovery of lens-induced myopia in mice. Ophthalmic Physiol Opt. 2023; 43: 558–565. [DOI] [PubMed] [Google Scholar]
- 30. He L, Frost MR, Siegwart JT Jr., Norton TT. Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Exp Eye Res. 2014; 123: 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muralidharan AR, Low SWY, Lee YC, et al.. Recovery from form-deprivation myopia in chicks is dependent upon the fullness and correlated color temperature of the light spectrum. Invest Ophthalmol Vis Sci. 2022; 63: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL. Recovery from form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004; 45: 3361–3372. [DOI] [PubMed] [Google Scholar]
- 33. Wu XH, Qian KW, Xu GZ, et al.. The role of retinal dopamine in C57BL/6 mouse refractive development as revealed by intravitreal administration of 6-hydroxydopamine. Invest Ophthalmol Vis Sci. 2016; 57: 5393–5404. [DOI] [PubMed] [Google Scholar]
- 34. Bergen MA, Park HN, Chakraborty R, et al.. Altered refractive development in mice with reduced levels of retinal dopamine. Invest Ophthalmol Vis Sci. 2016; 57: 4412–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown DM, Mazade R, Clarkson-Townsend D, Hogan K, Datta Roy PM, Pardue MT. Candidate pathways for retina to scleral signaling in refractive eye growth. Exp Eye Res. 2022; 219: 109071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feldkaemper M, Schaeffel F.. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013; 114: 106–119. [DOI] [PubMed] [Google Scholar]
- 37. Boatright JH, Hoel MJ, Iuvone PM.. Stimulation of endogenous dopamine release and metabolism in amphibian retina by light- and K+-evoked depolarization. Brain Res. 1989; 482: 164–168. [DOI] [PubMed] [Google Scholar]
- 38. Chakraborty R, Landis EG, Mazade R, et al.. Melanopsin modulates refractive development and myopia. Exp Eye Res. 2022; 214: 108866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chakraborty R, Ostrin LA, Nickla DL, Iuvone PM, Pardue MT, Stone RA.. Circadian rhythms, refractive development, and myopia. Ophthalmic Physiol Opt. 2018; 38: 217–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chakraborty R, Pardue MT.. Molecular and biochemical aspects of the retina on refraction. Prog Mol Biol Transl Sci. 2015; 134: 249–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cohen Y, Peleg E, Belkin M, Polat U, Solomon AS.. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012; 103: 33–40. [DOI] [PubMed] [Google Scholar]
- 42. Ko GY. Circadian regulation in the retina: from molecules to network. Eur J Neurosci. 2020; 51: 194–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chakraborty R, Park HN, Hanif AM, Sidhu CS, Iuvone PM, Pardue MT.. ON pathway mutations increase susceptibility to form-deprivation myopia. Exp Eye Res. 2015; 137: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pardue MT, Faulkner AE, Fernandes A, et al.. High susceptibility to experimental myopia in a mouse model with a retinal ON pathway defect. Invest Ophthalmol Vis Sci. 2008; 49: 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilmet B, Callebert J, Duvoisin R, et al.. Mice lacking Gpr179 with complete congenital stationary night blindness are a good model for myopia. Int J Mol Sci. 2022; 24: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thomson K, Karouta C, Ashby R.. Form-deprivation and lens-induced myopia are similarly affected by pharmacological manipulation of the dopaminergic system in chicks. Invest Ophthalmol Vis Sci. 2020; 61: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thomson K, Morgan I, Karouta C, Ashby R.. Levodopa inhibits the development of lens-induced myopia in chicks. Sci Rep. 2020; 10: 13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jiang X, Kurihara T, Kunimi H, et al.. A highly efficient murine model of experimental myopia. Sci Rep. 2018; 8: 2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morgan IG, Ashby RS, Nickla DL, Guggenheim JA.. Form deprivation and lens-induced myopia: are they different? Ophthalmic Physiol Opt. 2013; 33: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Landis EG, Chrenek MA, Chakraborty R, et al.. Increased endogenous dopamine prevents myopia in mice. Exp Eye Res. 2020; 193: 107956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zeitz C. Molecular genetics and protein function involved in nocturnal vision. Expert Rev Ophthalmol. 2007; 2: 467–485. [Google Scholar]
- 52. Dry KL, Van Dorp DB, Aldred MA, Brown J, Hardwick LJ, Wright AF.. Linkage analysis in a family with complete type congenital stationary night blindness with and without myopia. Clin Genet. 1993; 43: 250–254. [DOI] [PubMed] [Google Scholar]
- 53. Zeitz C, Labs S, Lorenz B, et al.. Genotyping microarray for CSNB-associated genes. Invest Ophthalmol Vis Sci. 2009; 50: 5919–5926. [DOI] [PubMed] [Google Scholar]
- 54. Zeitz C, Robson AG, Audo I.. Congenital stationary night blindness: an analysis and update of genotype–phenotype correlations and pathogenic mechanisms. Prog Retinal Eye Res. 2015; 45: 58–110. [DOI] [PubMed] [Google Scholar]
- 55. Miyake Y, Yagasaki K, Horiguchi M, Kawase Y, Kanda T.. Congenital stationary night blindness with negative electroretinogram. A new classification. Arch Ophthalmol. 1986; 104: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 56. Bijveld MM, Florijn RJ, Bergen AA, et al.. Genotype and phenotype of 101 dutch patients with congenital stationary night blindness. Ophthalmology. 2013; 120: 2072–2081. [DOI] [PubMed] [Google Scholar]
- 57. Bijveld MM, van Genderen MM, Hoeben FP, et al.. Assessment of night vision problems in patients with congenital stationary night blindness. PloS One. 2013; 8: e62927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Audo I, Robson AG, Holder GE, Moore AT.. The negative ERG: clinical phenotypes and disease mechanisms of inner retinal dysfunction. Surv Ophthalmol. 2008; 53: 16–40. [DOI] [PubMed] [Google Scholar]
- 59. Audo I, Bujakowska K, Orhan E, et al.. Whole-exome sequencing identifies mutations in GPR179 leading to autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2012; 90: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Audo I, Kohl S, Leroy BP, et al.. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2009; 85: 720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zeitz C, Jacobson SG, Hamel CP, et al.. Whole-exome sequencing identifies LRIT3 mutations as a cause of autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2013; 92: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bech-Hansen NT, Naylor MJ, Maybaum TA, et al.. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet. 2000; 26: 319–323. [DOI] [PubMed] [Google Scholar]
- 63. Peachey NS, Ray TA, Florijn R, et al.. GPR179 is required for depolarizing bipolar cell function and is mutated in autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2012; 90: 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li Z, Sergouniotis PI, Michaelides M, et al.. Recessive mutations of the gene TRPM1 abrogate ON bipolar cell function and cause complete congenital stationary night blindness in humans. Am J Hum Genet. 2009; 85: 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van Genderen MM, Bijveld MM, Claassen YB, et al.. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet. 2009; 85: 730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dryja TP, McGee TL, Berson EL, et al.. Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proc Natl Acad Sci USA. 2005; 102: 4884–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pusch CM, Zeitz C, Brandau O, et al.. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nat Genet. 2000; 26: 324–327. [DOI] [PubMed] [Google Scholar]
- 68. Zeitz C, van Genderen M, Neidhardt J, et al.. Mutations in GRM6 cause autosomal recessive congenital stationary night blindness with a distinctive scotopic 15-Hz flicker electroretinogram. Invest Ophthalmol Vis Sci. 2005; 46: 4328–4335. [DOI] [PubMed] [Google Scholar]
- 69. Gregg RG, Kamermans M, Klooster J, et al.. Nyctalopin expression in retinal bipolar cells restores visual function in a mouse model of complete X-linked congenital stationary night blindness. J Neurophysiol. 2007; 98: 3023–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gregg RG, Mukhopadhyay S, Candille SI, et al.. Identification of the gene and the mutation responsible for the mouse nob phenotype. Invest Ophthalmol Vis Sci. 2003; 44: 378–384. [DOI] [PubMed] [Google Scholar]
- 71. Pardue MT, McCall MA, LaVail MM, Gregg RG, Peachey NS.. A naturally occurring mouse model of X-linked congenital stationary night blindness. Invest Ophthalmol Vis Sci. 1998; 39: 2443–2449. [PubMed] [Google Scholar]
- 72. Maddox DM, Vessey KA, Yarbrough GL, et al.. Allelic variance between GRM6 mutants, Grm6nob3 and Grm6nob4 results in differences in retinal ganglion cell visual responses. J Physiol. 2008; 586: 4409–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pinto LH, Vitaterna MH, Shimomura K, et al.. Generation, identification and functional characterization of the nob4 mutation of Grm6 in the mouse. Vis Neurosci. 2007; 24: 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Masu M, Iwakabe H, Tagawa Y, et al.. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995; 80: 757–765. [DOI] [PubMed] [Google Scholar]
- 75. Kinoshita J, Hasan N, Bell BA, Peachey NS.. Reduced expression of the nob8 gene does not normalize the distribution or function of mGluR6 in the mouse retina. Mol Vis. 2019; 25: 890–901. [PMC free article] [PubMed] [Google Scholar]
- 76. Varin J, Bouzidi N, Dias MMS, et al.. Restoration of mGluR6 localization following AAV-mediated delivery in a mouse model of congenital stationary night blindness. Invest Ophthalmol Vis Sci. 2021; 62: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Morgans CW, Brown RL, Duvoisin RM.. TRPM1: the endpoint of the mGluR6 signal transduction cascade in retinal ON-bipolar cells. BioEssays. 2010; 32: 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Morgans CW, Zhang J, Jeffrey BG, et al.. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci USA. 2009; 106: 19174–19178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Koike C, Obara T, Uriu Y, et al.. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci USA. 2010; 107: 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Peachey NS, Pearring JN, Bojang P Jr., et al.. Depolarizing bipolar cell dysfunction due to a Trpm1 point mutation. J Neurophysiol. 2012; 108: 2442–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Orlandi C, Omori Y, Wang Y, et al.. Transsynaptic binding of orphan receptor GPR179 to dystroglycan-pikachurin complex is essential for the synaptic organization of photoreceptors. Cell Rep. 2018; 25: 130–145.e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Orhan E, Neuillé M, de Sousa Dias M, et al.. A new mouse model for complete congenital stationary night blindness due to Gpr179 deficiency. Int J Mol Sci. 2021; 22: 4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Orhan E, Prézeau L, El Shamieh S, et al.. Further insights into GPR179: expression, localization, and associated pathogenic mechanisms leading to complete congenital stationary night blindness. Invest Ophthalmol Vis Sci. 2013; 54: 8041–8050. [DOI] [PubMed] [Google Scholar]
- 84. Neuillé M, Cao Y, Caplette R, et al.. LRIT3 differentially affects connectivity and synaptic transmission of cones to ON- and OFF-bipolar cells. Invest Ophthalmol Vis Sci. 2017; 58: 1768–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Neuillé M, El Shamieh S, Orhan E, et al.. Lrit3 deficient mouse (nob6): a novel model of complete congenital stationary night blindness (cCSNB). PloS One. 2014; 9(3): e90342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Neuillé M, Morgans CW, Cao Y, et al.. LRIT3 is essential to localize TRPM1 to the dendritic tips of depolarizing bipolar cells and may play a role in cone synapse formation. Eur J Neurosci. 2015; 42: 1966–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hasan N, Pangeni G, Ray TA, et al.. LRIT3 is required for nyctalopin expression and normal ON and OFF pathway signaling in the retina. eNeuro. 2020; 7:ENEURO.0002-0020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Varin J, Bouzidi N, Gauvain G, et al.. Substantial restoration of night vision in adult mice with congenital stationary night blindness. Mol Ther Methods Clin Dev. 2021; 22: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schaeffel F, Burkhardt E, Howland HC, Williams RW.. Measurement of refractive state and deprivation myopia in two strains of mice. Optom Vis Sci. 2004; 81: 99–110. [DOI] [PubMed] [Google Scholar]
- 90. Metge P, Donnadieu M.. Myopia and cataract. Rev Prat. 1993; 43: 1784–1786. [PubMed] [Google Scholar]
- 91. Summers JA. The choroid as a sclera growth regulator. Exp Eye Res. 2013; 114: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Aung MH, Hogan K, Mazade RE, et al.. ON than OFF pathway disruption leads to greater deficits in visual function and retinal dopamine signaling. Exp Eye Res. 2022; 220: 109091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen S, Zhi Z, Ruan Q, et al.. Bright light suppresses form-deprivation myopia development with activation of dopamine D1 receptor signaling in the ON pathway in retina. Invest Ophthalmol Vis Sci. 2017; 58: 2306–2316. [DOI] [PubMed] [Google Scholar]
- 94. Dong F, Zhi Z, Pan M, et al.. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol Vis. 2011; 17: 2824–2834. [PMC free article] [PubMed] [Google Scholar]
- 95. Luo X, Li B, Li T, et al.. Myopia induced by flickering light in guinea pig eyes is associated with increased rather than decreased dopamine release. Mol Vis. 2017; 23: 666–679. [PMC free article] [PubMed] [Google Scholar]
- 96. Popova E. Role of dopamine in retinal function. In: Kolb H, Fernandez E, Nelson R (eds). Webvision: The Organization of the Retina and Visual System . Salt Lake City: University of Utah Health Sciences Center. 1995. [Google Scholar]
- 97. Qian KW, Li YY, Wu XH, et al.. Altered retinal dopamine levels in a melatonin-proficient mouse model of form-deprivation myopia. Neurosci Bull. 2022; 38: 992–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Roy S, Field GD.. Dopaminergic modulation of retinal processing from starlight to sunlight. J Pharmacol Sci. 2019; 140: 86–93. [DOI] [PubMed] [Google Scholar]
- 99. Iuvone PM, Tigges M, Fernandes A, Tigges J.. Dopamine synthesis and metabolism in rhesus monkey retina: development, aging, and the effects of monocular visual deprivation. Vis Neurosci. 1989; 2: 465–471. [DOI] [PubMed] [Google Scholar]
- 100. Nickla DL, Totonelly K.. Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Exp Eye Res. 2011; 93: 782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bartmann M, Schaeffel F, Hagel G, Zrenner E.. Constant light affects retinal dopamine levels and blocks deprivation myopia but not lens-induced refractive errors in chickens. Vis Neurosci. 1994; 11: 199–208. [DOI] [PubMed] [Google Scholar]
- 102. Goel M, Mangel SC.. Dopamine-mediated circadian and light/dark-adaptive modulation of chemical and electrical synapses in the outer retina. Front Cell Neurosci. 2021; 15: 647541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nickla DL. Ocular diurnal rhythms and eye growth regulation: where we are 50 years after Lauber. Exp Eye Res. 2013; 114: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Park H, Jabbar SB, Tan CC, et al.. Visually-driven ocular growth in mice requires functional rod photoreceptors. Invest Ophthalmol Vis Sci. 2014; 55: 6272–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tkatchenko TV, Shen Y, Tkatchenko AV.. Analysis of postnatal eye development in the mouse with high-resolution small animal magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2010; 51: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yang J, Yang L, Chen R, et al.. A role of color vision in emmetropization in C57BL/6J mice. Sci Rep. 2020; 10: 14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chakraborty R, Park H, Aung MH, et al.. Comparison of refractive development and retinal dopamine in OFF pathway mutant and C57BL/6J wild-type mice. Mol Vis. 2014; 20: 1318–1327. [PMC free article] [PubMed] [Google Scholar]
- 108. Tkatchenko TV, Shah RL, Nagasaki T, Tkatchenko AV.. Analysis of genetic networks regulating refractive eye development in collaborative cross progenitor strain mice reveals new genes and pathways underlying human myopia. BMC Med Genomics. 2019; 12: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pardue MT, Faulkner AE, Fernandes A, et al.. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest Ophthalmol Vis Sci. 2008; 49: 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wang J, Han Y, Musch DC, et al.. Evaluation and follow-up of myopia prevalence among school-aged children subsequent to the COVID-19 home confinement in Feicheng, China. JAMA Ophthalmol. 2023; 141: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hasan N, Pangeni G, Cobb CA, et al.. Presynaptic expression of LRIT3 transsynaptically organizes the postsynaptic glutamate signaling complex containing TRPM1. Cell Rep. 2019; 27: 3107–3116.e3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Poudel S, Jin J, Rahimi-Nasrabadi H, et al.. Contrast Sensitivity of ON and OFF Human Retinal Pathways in Myopia. J Neurosci. 2024; 44(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chang B, Dacey MS, Hawes NL, et al.. Cone photoreceptor function loss-3, a novel mouse model of achromatopsia due to a mutation in Gnat2. Invest Ophthalmol Vis Sci. 2006; 47: 5017–5021. [DOI] [PubMed] [Google Scholar]
- 114. Zhang J, Cai B, Ma L, et al.. Clinical and genetic analysis VSX1 variants among families with keratoconus in northwest China. Front Genet. 2023; 14: 1145426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. So C, Zhang T, Wang Q, Qiu C, Elie LA, Pan F.. The response of retinal ganglion cells to optical defocused visual stimuli in mouse retinas. Exp Eye Res. 2024; 241: 109834. [DOI] [PubMed] [Google Scholar]
- 116. Chakraborty R, Yang V, Park HN, et al.. Lack of cone mediated retinal function increases susceptibility to form-deprivation myopia in mice. Exp Eye Res. 2019; 180: 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Michaelides M, Aligianis IA, Holder GE, et al.. Cone dystrophy phenotype associated with a frameshift mutation (M280fsX291) in the alpha-subunit of cone specific transducin (GNAT2). Br J Ophthalmol. 2003; 87: 1317–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Haegerstrom-Portnoy G, Schneck ME, Verdon WA, Hewlett SE.. Clinical vision characteristics of the congenital achromatopsias. II. Color vision. Optom Vis Sci. 1996; 73: 457–465. [DOI] [PubMed] [Google Scholar]
- 119. Zhou X, Ye J, Willcox MDP, et al.. Changes in protein profiles of guinea pig sclera during development of form deprivation myopia and recovery. Mol Vis. 2010; 16: 2163–2174. [PMC free article] [PubMed] [Google Scholar]
- 120. Guo L, Frost MR, Siegwart JT Jr., Norton TT. Scleral gene expression during recovery from myopia compared with expression during myopia development in tree shrew. Mol Vis. 2014; 20: 1643–1659. [PMC free article] [PubMed] [Google Scholar]
- 121. Guo L, Frost MR, Siegwart JT Jr., Norton TT. Gene expression signatures in tree shrew sclera during recovery from minus-lens wear and during plus-lens wear. Mol Vis. 2019; 25: 311–328. [PMC free article] [PubMed] [Google Scholar]
- 122. He L, Frost MR, Siegwart JT Jr., Norton TT. Altered gene expression in tree shrew retina and retinal pigment epithelium produced by short periods of minus-lens wear. Exp Eye Res. 2018; 168: 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Brand C, Schaeffel F, Feldkaemper MP.. A microarray analysis of retinal transcripts that are controlled by image contrast in mice. Mol Vis. 2007; 13: 920–932. [PMC free article] [PubMed] [Google Scholar]
- 124. Jeong H, Kurihara T, Jiang X, et al.. Suppressive effects of violet light transmission on myopia progression in a mouse model of lens-induced myopia. Exp Eye Res. 2023; 228: 109414. [DOI] [PubMed] [Google Scholar]
- 125. Jiang X, Pardue MT, Mori K, et al.. Violet light suppresses lens-induced myopia via neuropsin (OPN5) in mice. Proc Natl Acad Sci USA. 2021; 118(22): e2018840118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jeong H, Lee D, Jiang X, Negishi K, Tsubota K, Kurihara T.. Opsin 5 mediates violet light-induced early growth response-1 expression in the mouse retina. Sci Rep. 2023; 13: 17861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Aleman AC, Wang M, Schaeffel F. Reading and myopia: contrast polarity matters. Sci Rep. 2018; 8: 10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Swiatczak B, Schaeffel F.. Transient eye shortening during reading text with inverted contrast: effects of refractive error and letter size. Transl Vis Sci Technol. 2022; 11: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]