Abstract
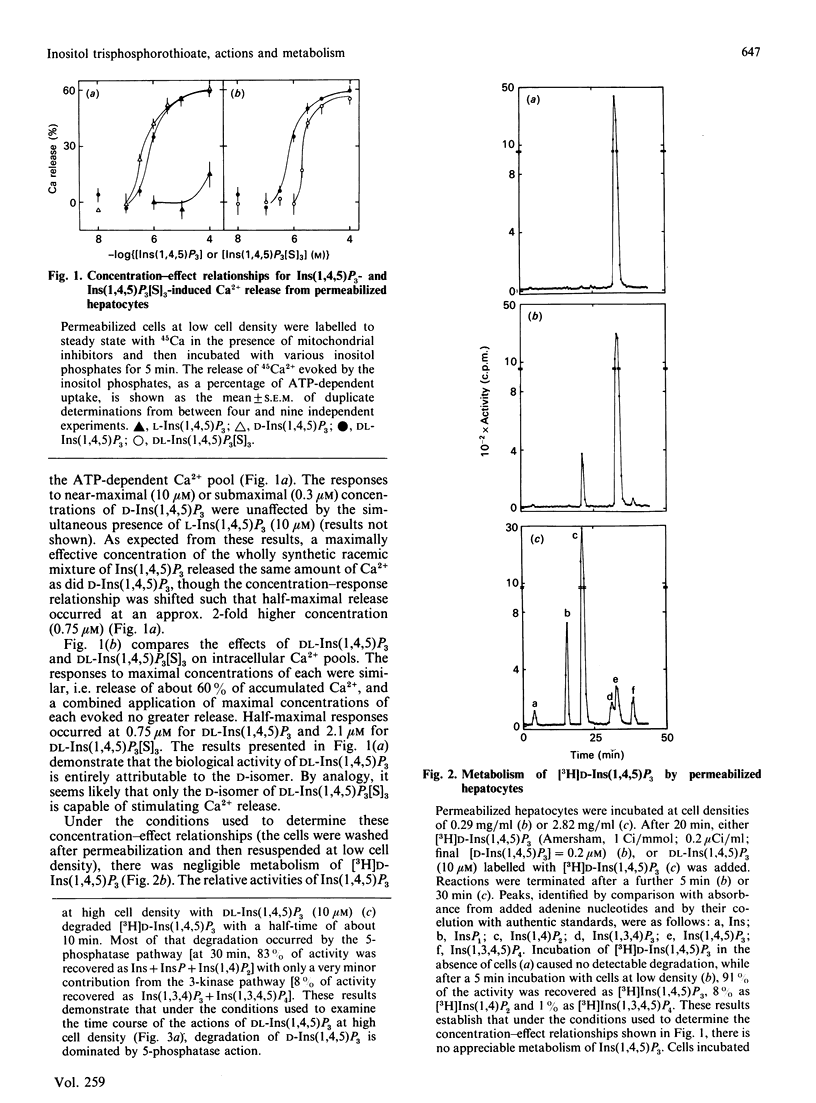
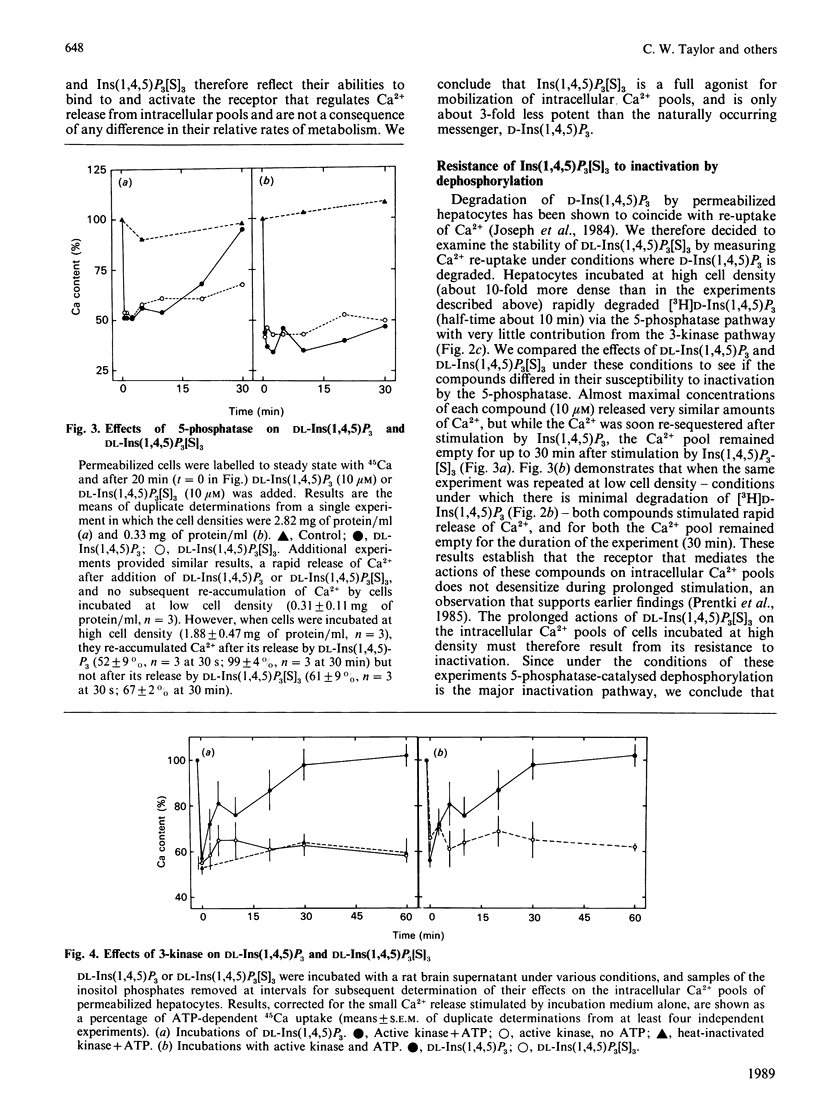
D-Ins(1,4,5)P3 is now recognized as an intracellular messenger that mediates the actions of many cell-surface receptors on intracellular Ca2+ pools, but its complex and rapid metabolism in intact cells has confused interpretation of its possible roles in oscillatory changes in intracellular [Ca2+] and in controlling Ca2+ entry at the plasma membrane. We now report the actions and metabolic stability of a synthetic analogue of Ins(1,4,5)P3, DL-inositol 1,4,5-trisphosphorothioate [DL-Ins(1,4,5)P3[S]3]. In permeabilized hepatocytes, DL-Ins(1,4,5)P3[S]3 and synthetic DL-Ins(1,4,5)P3 stimulated Ca2+ release from the same intracellular stores, though the concentration required for half-maximal release was 3-fold higher for DL-Ins(1,4,5)P3[S]3. Since L-Ins(1,4,5)P3 neither antagonized the effects of D-Ins(1,4,5)P3 nor itself stimulated appreciable Ca2+ release, the activity of the racemic mixture of Ins(1,4,5)P3, and presumably also of Ins(1,4,5)P3[S]3, is attributable to the D-isomer. Under conditions where there was negligible metabolism of D-[3H]Ins(1,4,5)P3, both DL-Ins(1,4,5)P3 and DL-Ins(1,4,5)P3[S]3 elicited rapid Ca2+ release from intracellular stores, and the stores remained empty during prolonged stimulation. When cells were incubated at high density, both compounds stimulated rapid Ca2+ release, but while the stores soon refilled as Ins(1,4,5)P3 was degraded to Ins(1,4)P2, there was no refilling of the pools after stimulation with DL-Ins(1,4,5)P3[S]3. When DL-Ins(1,4,5)P3 or DL-Ins(1,4,5)P3[S]3 was treated with a crude preparation of Ins(1,4,5)P3 3-kinase and ATP, and the Ca2+-releasing activity of the products subsequently assayed, DL-Ins(1,4,5)P3 was completely inactivated by phosphorylation, but there was no loss of activity of the phosphorothioate analogue. In additional experiments, DL-Ins(1,4,5)P3[S]3 (10 microM) did not affect the rate of phosphorylation of D-[3H]Ins(1,4,5)P3 (1 microM). We conclude that Ins(1,4,5)P3[S]3 is a full agonist and only 3-fold less potent than Ins(1,4,5)P3 in mobilizing intracellular Ca2+ stores, but unlike the natural messenger it is resistant to both phosphorylation and dephosphorylation. We propose that this stable analogue will allow the direct actions of Ins(1,4,5)P3 to be resolved from those that require its metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Cobbold P. H., Cuthbertson K. S. Spatial and temporal aspects of cell signalling. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):325–343. doi: 10.1098/rstb.1988.0080. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol trisphosphate formation and calcium mobilization in Swiss 3T3 cells in response to platelet-derived growth factor. Biochem J. 1984 Aug 15;222(1):195–201. doi: 10.1042/bj2220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Fabiato A., Leslie B. A., Putney J. W., Jr Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem. 1983 Dec 25;258(24):15336–15345. [PubMed] [Google Scholar]
- Cooke A. M., Nahorski S. R., Potter B. V. myo-inositol 1,4,5-trisphosphorothioate is a potent competitive inhibitor of human erythrocyte 5-phosphatase. FEBS Lett. 1989 Jan 2;242(2):373–377. doi: 10.1016/0014-5793(89)80504-6. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Bansal V. S., Inhorn R. C., Ross T. S., Lips D. L. Inositol phosphates: synthesis and degradation. J Biol Chem. 1988 Mar 5;263(7):3051–3054. [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Prentki M., Corkey B. E., Matschinsky F. M. Inositol 1,4,5-trisphosphate and the endoplasmic reticulum Ca2+ cycle of a rat insulinoma cell line. J Biol Chem. 1985 Aug 5;260(16):9185–9190. [PubMed] [Google Scholar]
- Sharps E. S., McCarl R. L. A high-performance liquid chromatographic method to measure 32P incorporation into phosphorylated metabolites in cultured cells. Anal Biochem. 1982 Aug;124(2):421–424. doi: 10.1016/0003-2697(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Strupish J., Cooke A. M., Potter B. V., Gigg R., Nahorski S. R. Stereospecific mobilization of intracellular Ca2+ by inositol 1,4,5-triphosphate. Comparison with inositol 1,4,5-trisphosphorothioate and inositol 1,3,4-trisphosphate. Biochem J. 1988 Aug 1;253(3):901–905. doi: 10.1042/bj2530901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W., Berridge M. J., Brown K. D., Cooke A. M., Potter B. V. DL-myo-inositol 1,4,5-trisphosphorothioate mobilizes intracellular calcium in Swiss 3T3 cells and Xenopus oocytes. Biochem Biophys Res Commun. 1988 Jan 29;150(2):626–632. doi: 10.1016/0006-291x(88)90438-x. [DOI] [PubMed] [Google Scholar]
- Taylor C. W., Putney J. W., Jr Size of the inositol 1,4,5-trisphosphate-sensitive calcium pool in guinea-pig hepatocytes. Biochem J. 1985 Dec 1;232(2):435–438. doi: 10.1042/bj2320435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennes K. A., McKinney J. S., Putney J. W., Jr Metabolism of inositol 1,4,5-trisphosphate in guinea-pig hepatocytes. Biochem J. 1987 Mar 15;242(3):797–802. doi: 10.1042/bj2420797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks A. L., Cooke A. M., Potter B. V., Nahorski S. R. Stereospecific recognition sites for [3H]inositol(1,4,5)-triphosphate in particulate preparations of rat cerebellum. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1071–1078. doi: 10.1016/0006-291x(87)90756-x. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- Wreggett K. A., Irvine R. F. A rapid separation method for inositol phosphates and their isomers. Biochem J. 1987 Aug 1;245(3):655–660. doi: 10.1042/bj2450655. [DOI] [PMC free article] [PubMed] [Google Scholar]


