Abstract
IMPORTANCE
Combination therapy with cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i: palbociclib, ribociclib, abemaciclib) and endocrine therapy (ET) has been a major advance for the treatment of hormone receptor–positive (HR+), ERBB2 (formerly HER2)–negative (ERBB2−) advanced or metastatic breast cancer.
OBSERVATIONS
Randomized phase 3 studies demonstrated that the addition of CDK4/6i reduced the hazard risk of disease progression by approximately half compared with hormonal monotherapy (an aromatase inhibitor, tamoxifen, or fulvestrant) in the first-line (1L) and/or second-line (2L) setting. Hence, the US Food and Drug Administration and European Medicines Agency approved 3 CDK4/6i, in both 1L and 2L settings. However, differences among the CDK4/6i regarding mechanisms of action, adverse effect profiles, and overall survival (OS) are emerging. Both abemaciclib and ribociclib have demonstrated efficacy in high-risk HR+ early breast cancer. While ET with or without CDK4/6i is accepted as standard treatment for persons with advanced HR+ ERBB2− metastatic breast cancer, several key issues remain. First, why are there discordances in OS in the metastatic setting and efficacy differences in the adjuvant setting? Additionally, apart from HR status, there are few biomarkers predictive of response to CDK4/6i plus ET, and these are not used routinely. Despite the clear OS advantage noted in the 1L and 2L metastatic setting with some CDK4/6i, a subset of patients with highly endocrine-sensitive disease do well with ET alone. Therefore, an unanswered question is whether some patients can postpone CDK4/6i until the 2L setting, particularly if financial toxicity is a concern. Finally, given the lack of endocrine responsiveness following progression on some CDK4/6i, strategies to optimally sequence treatment are needed.
CONCLUSIONS AND RELEVANCE
Future research should focus on defining the role of each CDK4/6i in HR+ breast cancer and developing a biomarker-directed integration of these agents.
Breast cancer (BC) is the most prevalent malignant neoplasm worldwide,1 and estrogen receptor (ER)–positive (ER+) BC is the most common subtype (approximately 70%). As approximately 350 000 women die from hormone receptor–positive (HR+), human epidermal growth factor receptor 2 (ERBB2, formerly HER2)–negative (ERBB2−) metastatic breast cancer (MBC) annually worldwide,2 better treatments are needed. For approximately 50 years, treatment of HR+ BC focused on targeting ER signaling either directly (antiestrogens) or indirectly (aromatase inhibitors [AIs]). More recent efforts have focused on cotargeting ER and other cell signaling pathways, such as the phosphatidylinositol 3-kinase–Akt–mammalian target ofrapamycin (PI3K/Akt/mTOR) and cyclin-dependent kinase (CDK) 4 and 6.2,3
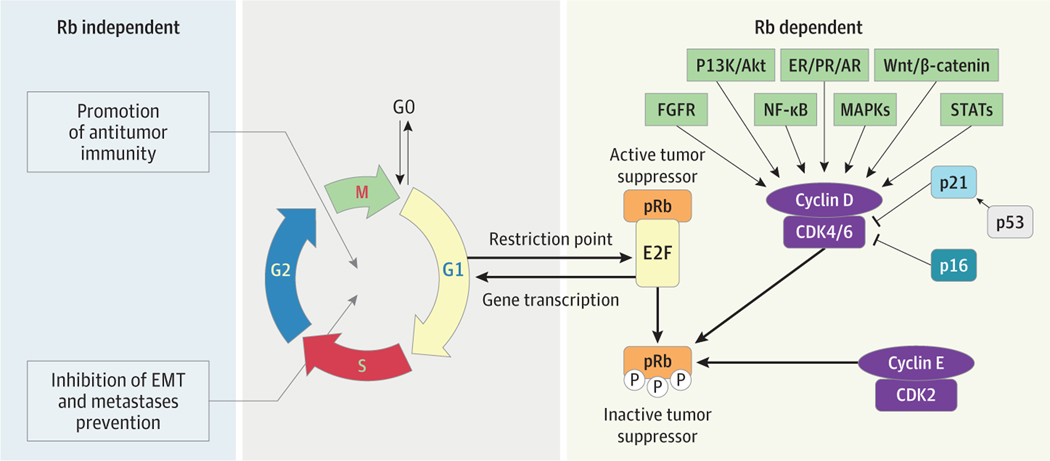
Cyclin-dependent kinase 4 and 6 are key mediators of cell growth and division, regulating the restriction point and transition through the G1 to S phase of the cell cycle (Figure). High cyclin D1 expression is a dominant feature of ER+ BC4 and is associated with a worse prognosis and endocrine resistance.5 Cyclin-dependent kinase 4 and 6 are critical regulators of ER+ BC cell proliferation. Initial clinical development of pan-CDK inhibitors was limited by myelosuppression, gastrointestinal, and hepatic toxic effects. However, palbociclib (Ibrance), ribociclib (Kisqali), and abemaciclib (Verzenio) exhibited favorable toxicity profiles in phase 1 trials. While palbociclib has comparable potency against cyclin D1/CDK4 and cyclin D2/CDK6,6 abemaciclib and ribociclib have greater potency against CDK4 than CDK6. Abemaciclib also inhibits multiple other closely related kinases,7 including CDK1, CDK2, and CDK5.8
Figure. Mechanism of Action of CDK4/6 Inhibitors (CDK4/6i).
Activation of cyclin-dependent kinases (CDKs) promotes cell cycle progression, a fundamental step in oncogenesis. The main drivers of cell cycle proliferation are regulated by CDK4/6. Cross talk between cyclin D, CDK4/6, retinoblastoma-associated protein 1 (Rb1), and the estrogen receptor (ER) is a dynamic process, which can lead to cellular proliferation. Estrogen receptor signaling also induces cyclin D messenger RNA upregulation and protein expression. Further, cyclin D can activate both CDK4 and CDK6, which leads to Rb phosphorylation and release of E2F, a transcription factor, which in turn triggers cell cycle progression from G1 to S phase, and subsequently DNA replication. Release of E2F initiates a positive feedback loop, inducing transcription of E-cyclins, which triggers activation of CDK2 and other proteins, as well as Rb phosphorylation, which also promotes DNA synthesis. The cyclin D–CDK4/6 axis is regulated by other protein families, ie, CDK inhibitors. An INK4 protein, p16, may be activated by tumor growth factor-β (TGFβ) signaling and can bind to CDK4 and CDK6, inhibiting G1 to S phase progression and suppressing tumor growth by opposing CDK4/6i and ER signaling pathways. The Rb-independent mechanisms of CDK4/6i include promotion of antitumor immunity, effects on epithelial-mesenchymal transition (EMT), and metastases prevention. Preclinical data suggest that CDK4/6i may promote antitumor immune responses by helping T cells survive longer and function better, while also facilitating antigen presentation by tumor cells, so that CDK4/6i may exert proimmune effects that cancel out anti-immune effects. In keeping with these preclinical observations, data from clinical studies infer that CDK4/6i may upregulate genes typically implicated in promoting antitumor immune responses. E2F indicates E2F transcription factor 1; FGFR, fibroblast growth factor receptor; G1, G1 phase; G2, G2 phase; M, mitosis; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; P, phosphorylated; PI3K/Akt, phosphatidylinositol 3-kinase; S, S phase; STATs, signal transducer and activation of transcription protein family.
PALOMA-1 (NCT00721409), a phase 1/2 study, evaluated palbociclib plus letrozole in the first-line (1L) treatment of postmenopausal ER+ ERBB2− MBC. The combination prolonged progression-free survival (PFS), resulting in accelerated US Food and Drug Administration (FDA) approval.3 Consequently, palbociclib, abemaciclib, and ribociclib in combination with endocrine therapy (ET) were studied in the 1L and second-line (2L) settings (Tables 1 and 2), resulting in FDA approvals. Abemaciclib is also approved as monotherapy in pretreated patients with HR+ ERBB2− MBC9; phase 3 trials are ongoing in ERBB2+ BC.10
Table 1.
PFS and OS Results From First-Line Trials of CDK4/6i
| Trial | Patients, No. | CDK4/6i | ET | Design and study population | PFS | OS | Comments |
|---|---|---|---|---|---|---|---|
| PALOMA-1 (NCT00721409) | 165 | Palbociclib (P) | Letrozole (L) | Phase 2, postmenopausal women | 10.2 mo (L) vs 20.2 mo (L + P), HR, 0.488; 95% CI, 0.319–0.748; P < .001 | OS in the P + L vs L alone arm (37.5 mo vs 33.3 mo, respectively); HR, 0.813; 95% CI, 0.492–1. 345; P = .42 | No statistically significant OS advantage noted |
| PALOMA-2 (NCT01740427) | 666 | Palbociclib (P) | Letrozole (L) | Phase 3, postmenopausal women | 14.5 mo (L) vs 24.8 mo (L + P); HR, 0.58; 95% CI, 0.46–0.72; P < .001 | Median OS in the P + L vs L alone arm, 53.9 (95% CI, 49.8–60.8) mo vs 51.2 (95% CI, 43.7–58.9) mo; HR, 0.956; 95% CI, 0.777–1.177; stratified 1-sided P = .34 | No statistically significant OS advantage noted |
| MONARCH 3 (NCT02246621) | 493 | Abemaciclib (A) | NSAI | Phase 3, postmenopausal women | 14.76 mo (A) vs 28.18 mo (ET + A), HR, 0.54; 95% CI, 0.418–0.698; P < .001 | Mature OS data pending | NA |
| MONALEESA-2 (NCT01958021) | 668 | Ribociclib (R) | Letrozole (L) | Phase 3, postmenopausal women | 16.0 (95% CI, 13.4–18.2) mo (L) vs 25.3 (95% CI, 23.0–30.3) mo (L + R); HR, 0.568; 95% CI, 0.457–0.704; log-rank P = 9.63 × 10−8 | Median OS, 63.9 mo (R + L) vs 51.4 mo (L); HR, 0.76 | OS advantage seen |
| MONALEESA-7 (NCT02278120) | 672 | Ribociclib (R) | ET (Tam or AI) and OFS | Phase 3, pre- and perimenopausal women, previous ET permitted in (neo) adjuvant setting (chemotherapy also permitted in the [neo]adjuvant setting or for advanced BC) | Median PFS with R + ET was 23.8 mo vs 13.0 mo with ET + placebo (HR, 0.55; 95% CI, 0.44–0.69; P < .001) | OS at 42 mo: 70.2% (95% CI, 63.5%–76.0%) in R group and 46.0% (95% CI, 32.0%–58.9%) in placebo group (HR for death, 0.71; 95% CI, 0.54–0.95; P = .01 by log-rank test) | OS advantage seen |
| DAWNA-2 (NCT03966898) | 456 | Dalpiciclib | Letrozole, anastrozole | Phase 3, pre- and postmenopausal women, 2:1 randomization | Median PFS was significantly improved in the dalpiciclib arm (30.6 mo vs 18.2 mo; HR, 0.51; 1-sided P < .001) | OS data immature | NA |
Abbreviations: AI, aromatase inhibitor; BC, breast cancer; CDK4/6i, cyclin-dependent kinase 4/6 inhibitors; ET, endocrine therapy; HR, hazard ratio; NA, not applicable; NSAI, nonsteroidal aromatase inhibitor; OFS, ovarian function suppression; OS, overall survival; PFS, progression-free survival; Tam, tamoxifen.
Table 2.
PFS and OS Results From Second-Line Trials of CDK4/6i
| Trial | Patients, No. | CDK4/6i | ET | Design and study population | PFS | OS | Comments |
|---|---|---|---|---|---|---|---|
| PALOMA-3 (NCT01942135) | 521 | Palbociclib (P) | Fulvestrant (F) | Phase 3, pre- and postmenopausal women, 2:1 randomization | 5.6 mo (F) vs 9.5 mo (F + P); HR, 0.50; 95% CI, 0.29–0.87; P < .001 | Median OS, 39.7 mo; 95% CI, 34.8–45.7 (F + P); vs 29.7 mo; 95% CI, 23.8–37.9 (F alone) | No statistically significant OS advantage noteda |
| MONARCH 2 (NCT02107703) | 669 | Abemaciclib (A) | Fulvestrant (F) | Phase 3, pre- and postmenopausal women, 2:1 randomization | 9.3 mo (F) vs 16.4 mo (F + A); HR, 0.553; 95% CI, 0.449–0.681; P < .001 | Median OS, 46.7 mo (F + A) and 37.3 mo for F + placebo (HR, 0.757; 95% CI, 0.606–0.945; P = .01) | OS advantage seen |
| MONALEESA-3 (NCT02422615) | 726 | Ribociclib (R) | Fulvestrant (F) | Phase 3, men and postmenopausal women, 2:1 randomization | PFS: 20.5 mo (F + R) vs 12.8 mo (F alone); HR, 0.593; 95% CI, 0.480–0.732; P < .001 | OS at 42 mo, 57.8%; 95% CI, 52.0%–63.2% (F + R) vs 45.9%; 95% CI, 36.9%–54.5% (F + placebo); 28% difference in the relative risk of death (HR, 0.72; 95% CI, 0.57–0.92; P = .005) | OS advantage seenb |
| DAWNA-1 (NCT03927456) | 361 | Dalpiciclib | Fulvestrant | Phase 3, pre- and postmenopausal women, 2:1 randomization | PFS outcomes were superior in the dalpiciclib arm (median, 15.7 mo vs 7.2 mo; HR, 0.42; 1-sided P < .001) | OS data immature | NA |
Abbreviations: CDK4/6i, cyclin-dependent kinase 4/6 inhibitors;ET, endocrine therapy; HR, hazard ratio; NA, not applicable; OS, overall survival; PFS, progression-free survival.
An OS advantage was seen in the ET-sensitive group.
This trial included both first-line and second-line patients.
While OS was not a primary end point of the metastatic trials, no OS benefit has been reported with palbociclib either in the 1L (PALOMA-1 and PALOMA-2 [NCT01740427]) or 2L (PALOMA-3 [NCT01942135]) setting. In contrast, an OS benefit has been consistently reported for ribociclib (1L and 2L trials) and abemaciclib (2L). The OS advantage reported in MONALEESA-2 (NCT01958021) supports 1L therapy with a CDK4/6i plus ET. Although some patients with endocrine-sensitive disease may dowell on ET alone, it is unclear whether similar or greater benefit would result from addition of CDK4/6i at disease progression. However, this approach may be an option for patients with financial constraints who cannot afford CDK4/6i, for whom 1 or more of the following apply: (1) long treatment-free interval (TFI) between original BC diagnosis and metastatic relapse, (2) bony and/or oligometastatic disease, and (3) limited life expectancy (eg, due to comorbidities and/or inferior performance status). Determining optimal sequencing of CDK4/6i is critical, as recent data suggest loss of endocrine sensitivity following progression on CDK4/6i, with PFS of 2 to 3 months and response rates less than 5%.11–13 Most patients received prior palbociclib, and preliminary data from the pooled 2L data from MONALEESA-2, MONALEESA-3, and MONALEESA-7 suggest this may not be the case with ribociclib.14 Given limited therapeutic options in the post-CDK4/6i setting, it is vital to address existing knowledge gaps. Currently, we know the following:
Palbociclib did not improve OS in either the 1L (PALOMA-1 and PALOMA-2) or 2L metastatic setting. A statistically significant difference in efficacy was not noted with adjuvant palbociclib plus ET compared with ET alone.
Abemaciclib improves OS in the 2L metastatic setting and invasive disease–free survival (IDFS) in the adjuvant setting. Final OS data from the 1L (MONARCH 3 [NCT02246621]) are awaited.
Ribociclib improves OS in the 1L metastatic setting in pre- and postmenopausal patients (MONALEESA-7 and MONALEESA-2, respectively) and combined 1L and 2L settings (MONALEESA-3). The NATALEE trial (NCT03701334) testing ribociclib in the adjuvant setting recently met its primary end point, achieving a statistically significant improvement in IDFS in women and men with HR+, ERBB2− early breast cancer (EBC) when compared with ET alone.
The adverse effect profiles of the CDK4/6i are distinctly different.
Overall Survival
Until recently, clinicians have used CDK4/6i interchangeably. However, the final survival analysis of PALOMA-2 was recently reported.15,16 With a median follow-up of 90 months, median OS (mOS) was 53.9 months in the palbociclib arm and 51.2 months in the placebo arm (hazard ratio, 0.956; 95% CI, 0.777–1.177; 1-sided P = .34).15 In contrast, in MONALEESA-2, ribociclib plus letrozole improved OS vs placebo plus letrozole (median, 63.9 vs 51.4 months; hazard ratio, 0.76; 95% CI, 0.63–0.93; P = .004).17 MONARCH 3 reported second interim OS results in 2022, with a greater than 12-month nonsignificant improvement in OS (median, 67.1 vs 54.5 months; hazard ratio, 0.75; 95% CI, 0.58–0.97; P = .03). Final OS data are expected in 2023.
There have been several attempts to reconcile the lack of an OS benefit in the PALOMA studies. One distinction is whether a greater number of patients with endocrine-resistant disease were enrolled onto PALOMA-2 vs MONALEESA-2. However, using the definition of “time from the end of (neo)adjuvant treatment to disease recurrence” (termed disease-free interval in PALOMA-2 and treatment-free interval in MONALEESA-2), no differences were seen comparing PALOMA-2 and MONALEESA-2. Furthermore, the extent of crossover was similar. A similar percentage of patients in both PALOMA-2 and MONALEESA-2 were lost to follow-up, and post hoc sensitivity analysis did not change the overall conclusions (hazard ratio, 0.956; 95% CI, 0.777–1.177).4,18 Finally, median OS in the placebo arm of PALOMA-2 was comparable to that in MONALEESA-2 (51.2 months vs 51.4 months), inferring that patient censoring from dropouts did not affect median OS. Thus, to date, ribociclib is the only CDK4/6i to report a significant OS benefit in the 1L setting in HR+ ERBB2− MBC, in both premenopausal (MONALEESA-7 [NCT02278120]) and postmenopausal women (MONALEESA-2 and MONALEESA-3 [NCT02422615]).17,19 eTable 1 in the Supplement outlines postprogression treatments received across the seminal CDK4/6i trials.
Some 2L CDK4/6i trials in HR+ ERBB2− MBC demonstrated an OS benefit (Table 2).9,10,19,20 In MONALEESA-3, compared with placebo plus fulvestrant, ribociclib plus fulvestrant improved PFS (20.5 vs 12.8 months; P < .001) and OS (mOS not reached vs 40.0 months; P = .005).19 The hazard ratio for death was similar for 1L (0.70; 95% CI, 0.48–1.02) and 2L (0.73; 95% CI, 0.53–1.00). In PALOMA-3 (NCT01942135), OS (prespecified secondary end point) was not significantly improved. Subgroup analysis of patients with sensitivity to prior ET showed an improvement in mOS from 29.7 to 39.7 months (hazard ratio, 0.72; 95% CI, 0.55–0.94). Exploratory analyses in other subgroups prespecified for stratification, such as visceral disease, found no significant OS improvement. In MONARCH 2 (NCT02107703), compared with fulvestrant alone, fulvestrant plus abemaciclib increased mOS by 9.4 months (hazard ratio, 0.757; 95% CI, 0.606–0.945; P = .01).20 In contrast to PALOMA-3, OS benefit was larger in MONARCH 2 patients with visceral disease (hazard ratio, 0.675; 95% CI, 0.511–0.891) and those with primary ET resistance (hazard ratio, 0.686; 95% CI, 0.451–1.043). Differences in OS among MONARCH 3, MONALEESA-2, and PALOMA-2 and PALOMA-3 infer fundamental differences in the respective mechanism(s) of actions of CDK4/6i.20 The reported benefit of palbociclib in the endocrine-sensitive subset of PALOMA-3 or in the PALOMA-1 and PALOMA-2 patients with a disease-free interval greater than 12 months may be chance results; therefore, these findings are hypothesis generating.
In summary, ribociclib and abemaciclib (plus ET) improve OS in HR+ ERBB2− MBC, whereas an OS benefit was not demonstrated for palbociclib. A major question remains why a drug that consistently improves PFS in the metastatic setting does not improve OS nor IDFS in the adjuvant setting. While a limitation of IDFS is inclusion of second nonbreast malignant neoplasms, palbociclib did not significantly improve distant disease–free survival. The finding with palbociclib is reminiscent of the vascular endothelial growth factor receptor inhibitor, bevacizumab,21,22 wherein improvements in PFS did not result in OS (metastatic) or IDFS (adjuvant) benefit. These data suggest that oncologists should not prescribe CDK4/6i interchangeably. Patients should be carefully counseled, and therapy individualized based on adverse effect profile and the consistent differences observed regarding OS.
Other CDK4/6i Under Evaluation: Dalpiciclib
Dalpiciclib is an orally administered, selective CDK4/6i given intermittently. Studies in both the 1L and 2L settings (DAWNA-1 and DAWNA-223,24) have demonstrated significant improvements in PFS in favor of the dalpiciclib arm (Tables 1 and 2). The PFS hazard ratios for both trials were similar to phase 3 trial findings for palbociclib, ribociclib, and abemaciclib. In both trials, the most common grade 3 or greater adverse events (AEs) in the dalpiciclib arm were neutropenia and leukopenia.
Endocrine Sensitivity After CDK4/6i
After progression on a CDK4/6i plus ET, there is no standard approach. Options include alpelisib (plus ET); exemestane plus the mTOR inhibitor, everolimus25; or chemotherapy.26 Multiple studies have evaluated endocrine monotherapy in patients with HR+ ERBB2− MBC following progression on a CDK4/6i (mainly palbociclib) and confirmed an alarmingly short median PFS (mPFS) in the ET-alone arm (approximately 2 months) (Table 3). Many of the oral selective estrogen receptor degraders that exhibited strong preclinical antitumor activity have demonstrated limited clinical activity after treatment with CDK4/6i. The EMERALD trial28 showed that elacestrant provided a statistical improvement in PFS vs standard ET (mPFS, 3.78 vs 1.87 months; hazard ratio, 0.546; P = .001), with recent FDA approval for the drug in the ESR1-mutant subset. One explanation for rapid progression on 2L endocrine monotherapy after palbociclib progression is pantumor cell release from G1/S blockade increasing proliferation following discontinuation of the CDK4/6i. Of note, this phenomenon was not observed in a pooled analysis of postprogression treatments after 1L ribociclib in the MONALEESA-2, MONALEESA-3, and MONALEESA-7 studies,14 wherein the mPFS for single-agent ET following progression on ribociclib was 8 months.11–13,27,29 In summary, these data suggest that improvements in PFS achieved while on palbociclib are not maintained during subsequent therapies, and this may contribute to the lack of an OS benefit. Such findings seemed to have been predicted in the neoadjuvant NeoPalAna study,30 wherein discontinuation of palbociclib plus ET prior to surgery resulted in marked Ki-67 activation. These data support completed and ongoing studies, such as MAINTAIN (NCT02632045), PACE (NCT03147287), and postMONARCH (NCT05169567), which are testing the role of continuing CDK4/6i in patients who experience disease progression on 1L CDK4/6i-based therapy.
Table 3.
Trials of ET+ Targeted/Experimental Therapy in Patients With HR+ ERBB2− MBC After Progression on a CDK4/6i
| Trial | Targeted/experimental agent | Study design | Study population | PFS (ET + experimental therapy vs ET alone) | Comments |
|---|---|---|---|---|---|
| Alliance A011203 (NCT02311933) | Z-endoxifen (TAM metabolite) | Randomized phase 2; TAM vs Z-endoxifen, stratification factor: prior CDK4/6i use | Postmenopausal women with HR+ ERBB2− MBC | Z-endoxifen did not improve mPFS vs TAM (4.3 mo vs 1.8 mo; HR, 0.77; P = .31) | Z-endoxifen improved mPFS in CDK4/6i-naive patients (7.2 mo vs 2.4 mo; HR, 0.42; 95% CI, 0.22–0.80; P = .002) |
| BYLieve (NCT03056755) | Alpelisib (PI3Ki) | Phase 2, open-label, noncomparative; alpelisib + different ET | PIK3CA mt+ HR+ ERBB2− MBC, prior CDK4/6i | Alpelisib + fulvestrant: mPFS, 7.3 mo; 95% CI, 5.6–8.3; with ORR of 17%; 95% CI, 11%–25% | No comparison to ET alone |
| SOLAR-1 (NCT02437318) | Alpelisib (PI3Ki) | Phase 3 alpelisib + fulvestrant vs placebo + fulvestrant | PIK3CA mt+ and PIK3CA mt− HR+ ERBB2− MBC, prior CDK4/6i in only 6% | In PIK3CA mt+ cohort who received prior CDK4/6i (n = 20, 5.9%), mPFS was only 1.8 mo in placebo arm vs 5.5 mo in alpelisib arm | Results led to FDA approval of alpelisib + fulvestrant in PIK3CA mt HR+ ERBB2− MBC |
| VERONICA (NCT03584009) | Venetoclax (selective BCL2i) | Randomized phase 2; venetoclax + fulvestrant vs fulvestrant alone (second vs third line) | Pre- and postmenopausal women with HR+ ERBB2− MBC, ≤2 prior lines of ET and no prior chemotherapy, prior CDK4/6i | mPFS was 2.69 (95% CI, 1.94–3.71) mo in the venetoclax + fulvestrant arm vs 1.9 (1.84–3.55) mo in the fulvestrant arm (HR, 0.94; 95% CI, 0.61–1.45) | NA |
| Retrospective review; Dhakal et al27 | mTORi (everolimus) | NA | Pre- and postmenopausal women with HR+ ERBB2− MBC, received everolimus + ET after progression on CDK4/6i | mPFS, 4.2 mo; 95% CI, 3.2–6.2 | No comparison to ET alone |
Abbreviations: BCL2i, BCL2 inhibitor; CDK4/6i, cyclin-dependent kinase 4/6 inhibitors; ET, endocrine therapy; FDA, US Food and Drug Administration; HR+, hormone receptor positive; MBC, metastatic breast cancer; mt, mutation; mPFS, median progression-free survival; mTORi, mammalian target of rapamycin inhibitor; NA, not applicable; ORR, overall response rate; PI3Ki, PI3K/Akt/mTOR inhibitor; Tam, tamoxifen.
New strategies to target de novo and acquired CDK4/6i resistance (eg, cyclin E and CDK2 inhibitors) and drivers of ET resistance (ESR1 mutations, AKT, FGFR, AURKA) are under evaluation. A phase 2 study of the novel selective estrogen receptor modulator lasofoxifene plus abemaciclib following progression on CDK4/6i showed a response rate of 50% and an mPFS of 55.7 weeks, leading to an FDA registration trial comparing abemaciclib plus fulvestrant with abemaciclib plus lasofoxifene for patients with ESR1 mutations and progression on a CDK4/6i.31
Resistance Mechanisms and Biomarkers
Intrinsic and acquired endocrine resistance remain a major challenge32 (Figure). Preclinical and clinical data suggest that CDK4/6i efficacy is restricted to luminal/Rb-proficient tumors, whereas cellular models with Rb loss exhibit de novo resistance to CDK4/6i. Furthermore, 5% or greater tumors and/or circulating tumor DNA exhibit Rb1 alterations at progression on CDK4/6i.16 In preclinical models, CDK6 and cyclin E1 (CCNE1) overexpression contribute to CDK4/6i resistance.33,34 In PALOMA-3, increased tumor expression of CCNE1 was associated with inferior response to palbociclib.34 Activation of the PI3K/AKT/mTOR pathway may also confer resistance to CDK4/6i, but clinical studies of everolimus/PI3Ki plus CDK4/6i are not moving forward because of toxicity.35 Additional biomarkers of palbociclib response may include the tumor suppressor function of Hippo signaling, with FAT1 loss associated with CDK4/6i resistance.36 Wander et al37 sequenced CDK4/6i-exposed tumors and identified potential resistance mechanisms, although it is unclear whether all are predictive of CDK4/6i response and whether they differ depending on the type of CDK4/6i exposure. Further studies evaluating these biomarkers in patients treated with abemaciclib and ribociclib are needed.
Treatment with CDK4/6 inhibition can enhance antitumor immunity by promoting tumor antigen presentation and clearance of tumor cells regulated by T cells,38 increasing immune infiltration and triggering T-cell activation to promote antitumor immunity.39 Dysregulation of these immune pathways may contribute to CDK4/6i resistance.38 Interferon (IFN) signaling is associated with intrinsic resistance to CDK4/6i, and acquired resistance to palbociclib is associated with IFN pathway activation.40 Pandey et al41 used genomic and transcriptomic screening to identify genes associated with palbociclib resistance in preclinical BC models. Annotation of differentially expressed genes correlated with activation of the type I IFN and immune checkpoint inhibitory pathway, and suppression of the latter with palbociclib resistance. Additional mechanisms of resistance, including differentially altered DNA damage repair pathways, may also be potential therapeutic targets.42
Intrinsic Subtypes
Data are emerging regarding the prognostic and predictive value of BC intrinsic subtypes in patients with HR+ ERBB2− MBC on CDK4/6i plus ET. In PALOMA-2 and PALOMA-3,43 messenger RNA profiling using the EdgeSeq Oncology Biomarker Panel showed that both luminal subtypes (A and B) benefited from addition of palbociclib to letrozole,44 although the number of patients with nonluminal intrinsic subtypes was small. Prat et al45 performed a retrospective biomarker analysis of the PAM50 intrinsic subtypes across the MONALEESA trials and identified differential response to ribociclib. The ERBB2-enriched subtype exhibited the worst prognosis with ET alone but had the greatest relative reduction in the risk of progression or death with ribociclib plus ET (hazard ratio, 0.39; P < .001): luminal A and B subtypes had a significant PFS advantage, with no benefit in the basal-like subtype. These findings may be broadly clinically applicable; validation studies are planned.46
Two caveats prevent a more personalized approach to prescribing CDK4/6i in HR+ MBC. First, there is an incomplete understanding of the biologic basis for both primary and secondary resistance. Second, accurate predictive biomarkers are lacking.47 Only ER expression and Rb mutations are predictive of CDK4/6i responsiveness.47 Despite the OS advantage seen in seminal trials of CDK4/6i plus ET in HR+ ERBB2− MBC, level 1 evidence regarding when a patient should optimally receive a CDK4/6i is lacking. The phase 3 SONIA study (NCT03425838) is comparing 1Lvs 2L CDK4/6i use in HR+ ERBB2− MBC, with a primary end point of PFS2 (PFS after 2 lines of treatment). As palbociclib will have been prescribed most frequently in this trial, PFS2 may not be the best end point, given that palbociclib-induced PFS gains do not translate into improvement in OS. Finally, given the emerging benefit of continuation of some CDK4/6i in the 2L setting, results from completed and ongoing studies such as MAINTAIN (NCT02632045), PACE (NCT03147287), and postMONARCH (NCT05169567) will be necessary to interpret results from the SONIA trial.
Regarding ET sequencing in the setting of CDK4/6, the phase 2 PARSIFAL trial48 sought to determine the optimal ET to combine with palbociclib in this setting. No PFS advantage was observed for fulvestrant over letrozole (27.9 vs 32.8 months; hazard ratio, 1.13; P = .32). In contrast, in the phase 3 PADA-1 trial,49 at ESR1 mutation detection, PFS doubled for patients who switched from palbociclib plus AI to palbociclib plus fulvestrant (11.9 vs 5.7 months; stratified hazard ratio, 0.61; P = .005). Randomized trials are under way testing whether oral selective estrogen receptor degraders and selective estrogen receptor modulators should be combined with CDK4/6i either up front or on emergence of AI resistance (eg, ESR1 mutations) as measured by minimal residual disease or radiographic/clinical resistance.
Finally, disease biology and/or sites of metastatic disease may assist with determining the incremental benefit of a CDK4/6i added to ET. For ET monotherapy, meta-analysis demonstrated that postmenopausal patients with HR+ ERBB2− MBC with visceral disease had significantly worse outcomes in the setting of liver vs nonliver metastases.50 An exploratory combined analysis of MONARCH 2 and 3 showed a larger benefit for the addition of abemaciclib to ET for subsets of patients with aggressive clinical and biological features, such as liver metastases.51 In contrast, in MONALEESA-2, patients with de novo HR+ MBC derived a greater OS benefit from ribociclib plus letrozole (hazard ratio, 0.52) vs other participants (hazard ratio, 0.91). Final OS data from the phase 3 trials will be critical to understanding whether these biological characteristics alter the survival benefit of either ribociclib or abemaciclib (Table 4).
Table 4.
Clinical Efficacy of CDK4/6i in HR+ ERBB2− EBC and MBC
| CDK4/6i | Palbociclib | Abemaciclib | Ribociclib | Comment |
|---|---|---|---|---|
| HR+ ERBB2− MBC | ||||
| Improved PFS (first line) | Yes | Yes | Yes | NA |
| Improved PFS (second line) | Yes | Yes | Yesa | NA |
| Improved OS (first line) | No | Unknown | Yes | NA |
| Improved OS (second line) | No | Yes | Yesa | NA |
| HR+ ERBB2− EBC | ||||
| Improved IDFS | No | Yes | Yes | OS data immature |
Abbreviations:
CDK4/6i, cyclin-dependent kinase 4/6 inhibitors; EBC, early breast cancer; HR+, hormone receptor positive; IDFS, invasive disease–free survival; MBC, metastatic breast cancer; NA, not applicable; OS, overall survival; PFS, progression-free survival.
No study of ribociclib in HR+ ERBB2− MBC enrolled exclusively second-line patients.
Toxic Effects
While combination therapy with CDK4/6i plus ET may increase toxicity vs ET alone, global quality-of-life reductions have not been observed.19,20,52,53 With palbociclib and ribociclib, the most common grade 3 and 4 AEs are neutropenia and leukopenia (approximately 50%−60%).16 Ribociclib can cause QTcF interval prolongation (approximately 16% in patients receiving ribociclib plus tamoxifen vs 7% in patients receiving ribociclib plus an nonsteroidal AI54) and elevated serum transaminases, a common reason for therapy interruption.9,19,54,55 Abemaciclib has a different pharmacologic and toxicity profile from palbociclib and ribociclib,56 ie, less neutropenia but more diarrhea, nausea, and, less commonly, venous thromboembolic events (5%).9 The diarrhea is generally low grade and infrequently leads to dose reductions or hospitalizations. However, approximately 81% of patients reported diarrhea (grade 3/4 in 9.5%) in MONARCH 3; grade 1 and 2 AEs can adversely affect quality of life.57 Despite the high incidence of neutropenia with CDK4/6i, febrile neutropenia is rare, and dose modifications for grade 3 to 4 neutropenia have not negatively affected PFS.58,59 Other uncommon but severe adverse effects include interstitial lung disease/pneumonitis (1.6%60) and venous thromboembolic events (0.6%−5%61).
Adjuvant Trials
Prospective trials evaluating adjuvant CDK4/6i in HR+ EBC have shown conflicting results. At the time of writing, 3 adjuvant trials had reported efficacy data. The PALLAS trial (n = 4600) assessed whether addition of 2 years of palbociclib to ET improved IDFS in stage2 and3 ER+ ERBB2− EBC. At the second interim analysis, the study was stopped for futility.62 Specifically, 3-year IDFS was 88.2% (95% CI, 85.2%−90.6%) for palbociclib plus ET and 88.5% (85.8%−90.7%) for ET alone (hazard ratio, 0.93 [95% CI, 0.76–1.15]; log-rank P = .51). PENELOPE-B (n = 1250) was a double-blind, placebo-controlled, phase 3 study that evaluated the benefit of 1 year of palbociclib plus ET for women with high-risk HR+ ERBB2− EBC without a pathological complete response after neoadjuvant systemic therapy.63 Like PALLAS, palbociclib did not improve IDFS vs placebo plus ET (hazard ratio, 0.93; 95% CI, 0.74–1.17; P = .52).
The monarchE (n = 5637) trial was a phase 3 study randomizing high-risk patients with HR+ ERBB2− EBC64 to ET with or without abemaciclib for 2 years. At a preplanned efficacy interim analysis, abemaciclib plus ET improved IDFS vs ET alone (hazard ratio, 0.713; 95% CI, 0.583–0.871; P = .001), with 2-year IDFS rates of 92.3% vs 89.3%. Since then, there have been 2 published updates, at 27 and 42 months. At the 42-month follow-up, all patients had discontinued abemaciclib, and the IDFS benefit increased: hazard ratio, 0.664 (95% CI, 0.578–0.762). At 4 years, the absolute difference in IDFS increased to 6.4% (79.4% vs 85.8%) compared with 2- and 3-year IDFS (2.8% and 4.8%, respectively).65 While Ki-67 was prognostic, abemaciclib benefit was observed regardless. While the FDA approved abemaciclib plus ET in node-positive HR+ ERBB2− EBC with a Ki-67 of 20% or greater, American Society of Clinical Oncology and National Comprehensive Cancer Network guidelines recommend dosing based on the intent-to-treat population.66,67 Results from the phase 3 NATALEE trial (NCT03701334) that randomized participants with HR+ ERBB2− EBC to ET plus 3 years of ribociclib vs ET alone showed a statistically significant IDFS advantage favoring the ribociclib arm. Full results will be presented in June 2023.
Pharmacogenomics and Ethnicity
Inhibitors of CDK4/6 exhibit distinct differences in their pharmacology, kinase targets, central nervous system penetration, and clinical activity as monotherapy.7 Further, variances in drug metabolism, genetic, nutritional, and clinicopathologic features between Asian patients and White patients may affect CDK4/6i responsiveness.68,69 In the landmark CDK4/6i trials, approximately 8%, 30%, and 30%, respectively, of participants were Asian.16,54,57 The 1L randomized CDK4/6i trials reported a significant difference in the pooled PFS hazard ratio for Asian and non-Asian patients (0.39 vs 0.62; P = .002) (eTable 2 in the Supplement). While toxicity data by ethnic subgroup were only available from 2 trials, Asian patients had a significantly higher prevalence of selected AEs. Analysis of Asian PALOMA-3 participants noted that palbociclib plus fulvestrant was safe and effective, but the incidence of grade 3 and 4 neutropenia was higher.70 PALOMA-4 (n = 340) confirmed efficacy and safety of palbociclib plus letrozole as 1L therapy in postmenopausal Asian women with HR+ ERBB2− MBC vs placebo plus letrozole (mPFS, 21.5 vs 13.9 months; hazard ratio, 0.68; 95% CI, 0.53–0.87; P = .001). The most common grade 3 and 4 AE with palbociclib plus letrozole was neutropenia.71
Conclusions
Treatment with CDK4/6i plus ET is standard of care for patients with HR+ ERBB2− MBC in the 1L and 2L settings, with seminal trials reflecting improved PFS, OS, and preserved quality of life. However, a consistent lack of improvement in OS (metastatic setting) and IDFS (adjuvant) with palbociclib suggests that CDK4/6i should not be prescribed interchangeably. Continued efforts to identify patients with HR+ EBC and MBC most likely to benefit from CDK4/6i, and to optimally sequence treatment, should afford greater insights into the discordant results from the metastatic and adjuvant CDK4/6i trials. Treatment with CDK4/6i is a fundamental part of the HR+ ERBB2− MBC therapy paradigm; the onus is on researchers to discover the optimal regimens for clinical utility, while prescribing in the most evidence-based manner.
Supplementary Material
Key Questions.
Although all CDK4/6i improve PFS, a survival (metastatic) and IDFS (adjuvant) benefit was not observed with palbociclib. What explains these discrepancies?
Can we personalize therapy by selecting different CDK4/6i for different patient populations and clinical scenarios? Are there biomarkers to identify when 1L CDK4/6i should always be used (eg, anticipated primary endocrine resistance) vs after progression on 1L ET (highly endocrine-sensitive MBC)?
Do tumors that progress on CDK4/6i retain endocrine sensitivity? Are there differences among CDK4/6i?
What are the mechanisms of resistance to each CDK4/6i, and how do they differ?
How does the tumor microenvironment affect response to CDK4/6i, and are there differences?
Can endocrine sensitivity be restored, and if so, how?
Funding/Support:
This work was supported by the George M. Eisenberg Foundation, and National Cancer Institute funding to the Mayo Clinic Breast Specialized Program of Research Excellence (SPORE) (P50CA116201) and the Mayo Clinic Cancer Center (P30CA15083) (Dr Goetz).
Role of the Funder/Sponsor:
The funders had no role in the preparation, review, or approval of the manuscript and decision to submit the manuscript for publication.
Conflict of Interest Disclosures:
Dr O’Sullivan reported research funding to institution from Lilly, Seagen, Minneamrita Therapeutics, Biovica, nference, ACCRU, Bavarian Nordic, Sermonix Pharmaceuticals, and AstraZeneca, and serving as an adviser for AstraZeneca outside the submitted work. Dr Clarke reported relationships with American Gene Technologies, Inc, and Bayer Pharmaceuticals outside the submitted work.
Dr Goetz reported grants from National Cancer Institute (P50CA116201) during the conduct of the study; consulting fees to Mayo from ARC Therapeutics, AstraZeneca, Biotheranostics, Blueprint Medicines, Lilly, RNA Diagnostics, Sanofi Genzyme, and Seattle Genetics; and grant funding to Mayo from Lilly, Pfizer, Sermonix Pharmaceuticals, and Atossa outside the submitted work. Dr Robertson reported grants from AstraZeneca to institution for clinical trials and personal fees from AstraZeneca for consultancy outside the submitted work.
Footnotes
Additional Contributions: The authors would like to thank Mark Korinek, BS, Advanced Image Processing at Mayo Clinic, Rochester, Minnesota, for his assistance with the Figure. No compensation was received for this contribution.
Additional Information: Search strategy and selection criteria: References for this Review were identified through searches of PubMed with the search terms “Cyclin dependent kinase 4 and 6 inhibitors (CDK4/6i); abemaciclib; ribociclib; palbociclib; endocrine resistance; metastatic hormone receptor positive (HR+), HER2-negative (−) advanced or metastatic breast cancer (MBC)” from 2012 until February 2022. Articles were also identified through searches of the authors’ own files. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this Review.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 3.Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet. 2021;397 (10286):1750–1769. doi: 10.1016/S0140-6736(20)32381-3 [DOI] [PubMed] [Google Scholar]
- 4.Ahlin C, Lundgren C, Embretsén-Varro E, Jirström K, Blomqvist C, Fjällskog M. High expression of cyclin D1 is associated to high proliferation rate and increased risk of mortality in women with ER-positive but not in ER-negative breast cancers. Breast Cancer Res Treat. 2017;164 (3):667–678. doi: 10.1007/s10549-017-4294-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenny FS, Hui R, Musgrove EA, et al. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin Cancer Res. 1999;5(8):2069–2076. [PubMed] [Google Scholar]
- 6.Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3 (11):1427–1438. doi: 10.1158/1535-7163.1427.3.11 [DOI] [PubMed] [Google Scholar]
- 7.George MA, Qureshi S, Omene C, Toppmeyer DL, Ganesan S. Clinical and pharmacologic differences of CDK4/6 inhibitors in breast cancer. Front Oncol. 2021;11:693104. doi: 10.3389/fonc.2021.693104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32(5):825–837. doi: 10.1007/s10637-014-0120-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancer. Clin Cancer Res. 2017;23(17):5218–5224. doi: 10.1158/1078-0432.CCR-17-0754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Sullivan CC, Suman VJ, Goetz MP. The emerging role of CDK4/6i in HER2-positive breast cancer. Ther Adv Med Oncol. 2019;11: 1758835919887665. doi: 10.1177/1758835919887665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetz MP, Suman VJ, Reid JM, et al. Abstract PD7–06: a randomized phase II trial of tamoxifen versus Z-endoxifen HCL in postmenopausal women with metastatic estrogen receptor positive, HER2 negative breast cancer. Cancer Res. 2020;80(4) (suppl):PD7–06. doi: 10.1158/1538-7445.SABCS19-PD7-06 [DOI] [Google Scholar]
- 12.Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22(4):489–498. doi: 10.1016/S1470-2045(21)00034-6 [DOI] [PubMed] [Google Scholar]
- 13.Lindeman GJ, Bowen R, Jerzak KJ, et al. Results from VERONICA: a randomized, phase II study of second-/third-line venetoclax (VEN) + fulvestrant (F) versus F alone in estrogen receptor (ER)-positive, HER2-negative, locally advanced, or metastatic breast cancer (LA/MBC). J Clin Oncol. 2021;39(15)(suppl):1004. doi: 10.1200/JCO.2021.39.15_suppl.1004 [DOI] [Google Scholar]
- 14.Hamilton E, Spring L, Fasching PA, et al. Pooled analysis of post-progression treatments after first-line ribociclib + endocrine therapy in patients with HR+/HER2− advanced breast cancer in the MONALEESA-2, −3 and −7 studies [P4–01-42]. Presented at: 45th Annual San Antonio Breast Cancer Symposium; December 6–10, 2022; San Antonio, Texas. [Google Scholar]
- 15.Finn RS, Rugo HS, Dieras VC, et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): analyses from PALOMA-2. J Clin Oncol. 2022;40(17) (suppl):LBA1003. doi: 10.1200/JCO.2022.40.17_suppl.LBA1003 [DOI] [Google Scholar]
- 16.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- 17.Hortobagyi GN, Stemmer SM, Burris HA, et al. LBA17: overall survival (OS) results from the phase III MONALEESA-2 (ML-2) trial of postmenopausal patients (pts) with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib (RIB). Ann Oncol. 2021;32(suppl 5): S1290–S1291. doi: 10.1016/j.annonc.2021.08.2090 [DOI] [Google Scholar]
- 18.Finn RS, Boer K, Bondarenko I, et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res Treat. 2020;183(2): 419–428. doi: 10.1007/s10549-020-05755-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24): 2465–2472. doi: 10.1200/JCO.2018.78.9909 [DOI] [PubMed] [Google Scholar]
- 20.Sledge GW Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116–124. doi: 10.1001/jamaoncol.2019.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossari JR, Metzger-Filho O, Paesmans M, et al. Bevacizumab and breast cancer: a meta-analysis of first-line phase III studies and a critical reappraisal of available evidence. J Oncol. 2012;2012:417673. doi: 10.1155/2012/417673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller KD, O’Neill A, Gradishar W, et al. Double-blind phase III trial of adjuvant chemotherapy with and without bevacizumab in patients with lymph node-positive and high-risk lymph node-negative breast cancer (E5103). J Clin Oncol. 2018;36(25):2621–2629. doi: 10.1200/JCO.2018.79.2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu B, Zhang Q, Zhang P, et al. ; DAWNA-1 Study Consortium. Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: a randomized, phase 3 trial. Nat Med. 2021;27(11):1904–1909. doi: 10.1038/s41591-021-01562-9 [DOI] [PubMed] [Google Scholar]
- 24.Xu B, Zhang QY, Zhang P, et al. LBA16: dalpiciclib plus letrozole or anastrozole as first-line treatment for HR+/HER2- advanced breast cancer (DAWNA-2): a phase III trial. Ann Oncol. 2022;33 (suppl 7):S1384–S1385. doi: 10.1016/j.annonc.2022.08.010 [DOI] [Google Scholar]
- 25.Giridhar K, Choong G, Leon-Ferre RA, et al. Clinical management of metastatic breast cancer after CDK4/6 inhibitors: a retrospective single-institution study. Paper presented at: San Antonio Breast Cancer Symposium; December 4–8, 2018; San Antonio, Texas. [Google Scholar]
- 26.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: breast cancer. Accessed August 21, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf
- 27.Dhakal A, Antony Thomas R, Levine EG, et al. Outcome of everolimus-based therapy in hormone-receptor-positive metastatic breast cancer patients after progression on palbociclib. Breast Cancer (Auckl). 2020;14:1178223420944864. doi: 10.1177/1178223420944864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bidard FC, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;40(28):3246–3256. doi: 10.1200/JCO.22.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.André F, Ciruelos EM, Juric D, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol. 2021;32(2):208–217. doi: 10.1016/j.annonc.2020.11.011 [DOI] [PubMed] [Google Scholar]
- 30.Ma CX, Gao F, Luo J, et al. NeoPalAna: neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res. 2017;23(15):4055–4065. doi: 10.1158/1078-0432.CCR-16-3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damodaran S, Plourde PV, Moore HCF, et al. Open-label, phase 2, multicenter study of lasofoxifene (LAS) combined with abemaciclib (Abema) for treating pre- and postmenopausal women with locally advanced or metastatic ER+/HER2− breast cancer and an ESR1 mutation after progression on prior therapies. J Clin Oncol. 2022;40(suppl 16):1022. doi: 10.1200/JCO.2022.40.16_suppl.1022 [DOI] [Google Scholar]
- 32.Spring LM, Wander SA, Andre F, Moy B, Turner NC, Bardia A. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet. 2020;395 (10226):817–827. doi: 10.1016/S0140-6736(20)30165-3 [DOI] [PubMed] [Google Scholar]
- 33.Yang C, Li Z, Bhatt T, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene. 2017;36(16):2255–2264. doi: 10.1038/onc.2016.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner NC, Liu Y, Zhu Z, et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor–positive metastatic breast cancer. J Clin Oncol. 2019;37(14):1169–1178. doi: 10.1200/JCO.18.00925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bardia A, Hurvitz SA, DeMichele A, et al. Phase I/II trial of exemestane, ribociclib, and everolimus in women with HR+/HER2− advanced breast cancer after progression on CDK4/6 inhibitors (TRINITI-1). Clin Cancer Res. 2021;27(15):4177–4185. doi: 10.1158/1078-0432.CCR-20-2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, Razavi P, Li Q, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cell. 2018; 34(6):893–905.e8. doi: 10.1016/j.ccell.2018.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wander SA, Cohen O, Gong X, et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov. 2020;10(8):1174–1193. doi: 10.1158/2159-8290.CD-19-1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471–475. doi: 10.1038/nature23465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng J, Wang ES, Jenkins RW, et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 2018;8 (2):216–233. doi: 10.1158/2159-8290.CD-17-0915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Angelis C, Fu X, Cataldo ML, et al. Activation of the IFN signaling pathway is associated with resistance to CDK4/6 inhibitors and immune checkpoint activation in ER-positive breast cancer. Clin Cancer Res. 2021;27(17):4870–4882. doi: 10.1158/1078-0432.CCR-19-4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandey K, Lee E, Park N, et al. Deregulated immune pathway associated with palbociclib resistance in preclinical breast cancer models: integrative genomics and transcriptomics. Genes (Basel). 2021;12(2):159. doi: 10.3390/genes12020159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navarro-Yepes J, Chen X, Bui T, Kettner NM, Hunt KK, Keyomarsi K. Abstract PD2–05: differential mechanisms of acquired resistance to abemaciclib versus palbociclib reveal novel therapeutic strategies for CDK4/6 therapy-resistant breast cancers. Cancer Res. 2020;80(4)(suppl): PD2–05. doi: 10.1158/1538-7445.SABCS19-PD2-05 [DOI] [Google Scholar]
- 43.Finn RS, Cristofanilli M, Ettl J, et al. Treatment effect of palbociclib plus endocrine therapy by prognostic and intrinsic subtype and biomarker analysis in patients with bone-only disease: a joint analysis of PALOMA-2 and PALOMA-3 clinical trials. Breast Cancer Res Treat. 2020;184(1):23–35. doi: 10.1007/s10549-020-05782-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finn RS, Liu Y, Zhu Z, et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naïve metastatic breast cancer. Clin Cancer Res. 2020;26(1):110–121. doi: 10.1158/1078-0432.CCR-19-0751 [DOI] [PubMed] [Google Scholar]
- 45.Prat A, Chaudhury A, Solovieff N, et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J Clin Oncol. 2021;39(13):1458–1467. doi: 10.1200/JCO.20.02977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tolosa P, Pascual T, Hernando C, et al. Abstract OT-26–04: Solti-1801. analysis of the efficacy of CDK4/6 inhibitors in combination with hormonal treatment in luminal breast cancer in relation to the intrinsic subtype and markers of immunity (CDK-PREDICT). Cancer Res. 2021;81(4)(suppl):OT-26–04. doi: 10.1158/1538-7445.SABCS20-OT-26-04 [DOI] [Google Scholar]
- 47.Schoninger SF, Blain SW. The ongoing search for biomarkers of CDK4/6 inhibitor responsiveness in breast cancer. Mol Cancer Ther. 2020;19(1):3–12. doi: 10.1158/1535-7163.MCT-19-0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Llombart-Cussac A, Pérez-García JM, Bellet M, et al. ; PARSIFAL Steering Committee and Trial Investigators. Fulvestrant-palbociclib vs letrozole-palbociclib as initial therapy for endocrine-sensitive, hormone receptor-positive, ERBB2-negative advanced breast cancer: a randomized clinical trial. JAMA Oncol. 2021;7(12):1791–1799. doi: 10.1001/jamaoncol.2021.4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bidard FC, Hardy-Bessard AC, Dalenc F, et al. ; PADA-1 investigators. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022;23(11):1367–1377. doi: 10.1016/S1470-2045(22)00555-1 [DOI] [PubMed] [Google Scholar]
- 50.Robertson JFR, Di Leo A, Johnston S, et al. Meta-analyses of visceral versus non-visceral metastatic hormone receptor-positive breast cancer treated by endocrine monotherapies. NPJ Breast Cancer. 2021;7(1):11. doi: 10.1038/s41523-021-00222-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goetz MP, O’Shaughnessy J, Sledge GW Jr, et al. Abstract GS6–02: the benefit of abemaciclib in prognostic subgroups: An exploratory analysis of combined data from the MONARCH 2 and 3 studies. Cancer Res. 2018;78(4)(suppl):GS6–02. doi: 10.1158/1538-7445.SABCS17-GS6-02 [DOI] [Google Scholar]
- 52.Im S-A, Lu Y-S, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–316. doi: 10.1056/NEJMoa1903765 [DOI] [PubMed] [Google Scholar]
- 53.Turner NC, Ro J, André F, et al. ; PALOMA3 Study Group. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209–219. doi: 10.1056/NEJMoa1505270 [DOI] [PubMed] [Google Scholar]
- 54.Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–915. doi: 10.1016/S1470-2045(18)30292-4 [DOI] [PubMed] [Google Scholar]
- 55.Rossi V, Berchialla P, Giannarelli D, et al. Should all patients with HR-positive HER2-negative metastatic breast cancer receive CDK 4/6 inhibitor as first-line based therapy? a network meta-analysis of data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT trials. Cancers (Basel). 2019;11(11):1661. doi: 10.3390/cancers11111661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin M, Garcia-Saenz JA, Manso L, et al. Abemaciclib, a CDK4 and CDK6 inhibitor for the treatment of metastatic breast cancer. Future Oncol. 2020;16(33):2763–2778. doi: 10.2217/fon-2020-0604 [DOI] [PubMed] [Google Scholar]
- 57.Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–3646. doi: 10.1200/JCO.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- 58.Verma S, Bartlett CH, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist. 2016;21(10):1165–1175. doi: 10.1634/theoncologist.2016-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kristensen KB, Thomsen IMN, Berg T, Kodahl AR, Jensen AB. Dose modifications of ribociclib and endocrine therapy for treatment of ER+ HER2- metastatic breast cancer. Breast Cancer Res Treat. 2021;188(3):799–809. doi: 10.1007/s10549-021-06215-6 [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Ma Z, Sun X, Feng X, An Z. Interstitial lung disease in patients treated with cyclin-dependent kinase 4/6 inhibitors: a systematic review and meta-analysis of randomized controlled trials. Breast. 2022;62: 162–169. doi: 10.1016/j.breast.2022.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gervaso L, Montero AJ, Jia X, Khorana AA. Venous thromboembolism in breast cancer patients receiving cyclin-dependent kinase inhibitors. J Thromb Haemost. 2020;18(1):162–168. doi: 10.1111/jth.14630 [DOI] [PubMed] [Google Scholar]
- 62.Mayer EL, Dueck AC, Martin M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22(2):212–222. doi: 10.1016/S1470-2045(20)30642–2 [DOI] [PubMed] [Google Scholar]
- 63.Loibl S, Marmé F, Martin M, et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer—the Penelope-B trial. J Clin Oncol. 2021;39(14):1518–1530. doi: 10.1200/JCO.20.03639 [DOI] [PubMed] [Google Scholar]
- 64.Johnston SRD, Harbeck N, Hegg R, et al. ; monarchE Committee Members and Investigators. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987–3998. doi: 10.1200/JCO.20.02514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnston SRD, Toi M, O’Shaughnessy J, et al. ; monarchE Committee Members. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24(1):77–90. doi: 10.1016/S1470-2045(22)00694-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: invasive breast cancer. Version 4.2022. Accessed January 23, 2023. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419
- 67.Freedman RA, Graff SL, Somerfield MR, Telli ML, Wolff AC, Giordano SH. Adjuvant abemaciclib plus endocrine therapy in the treatment of high-risk early breast cancer: ASCO guideline rapid recommendation update Q and A. JCO Oncol Pract. 2022;18(7):516–519. doi: 10.1200/OP.22.00140 [DOI] [PubMed] [Google Scholar]
- 68.Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev. 2003;35 (2–3):99–106. doi: 10.1081/DMR-120023681 [DOI] [PubMed] [Google Scholar]
- 69.Pala L, Conforti F, Goldhirsch A. Ethnicity-based differences in breast cancer features and responsiveness to CDK4/6 inhibitors combined with endocrine therapy. Lancet Oncol. 2020;21(3):e130. doi: 10.1016/S1470-2045(20)30072-3 [DOI] [PubMed] [Google Scholar]
- 70.Iwata H, Im SA, Masuda N, et al. PALOMA-3: phase III trial of fulvestrant with or without palbociclib in premenopausal and postmenopausal women with hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer that progressed on prior endocrine therapy—safety and efficacy in Asian patients. J Glob Oncol. 2017;3(4):289–303. doi: 10.1200/JGO.2016.008318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu B, Hu X, Li W, et al. 228MO PALOMA-4: primary results from a phase III trial of palbociclib (PAL)+ letrozole (LET) vs placebo (PBO)+ LET in Asian postmenopausal women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative (ER+/HER2–) advanced breast cancer (ABC). Ann Oncol. 2021;32(suppl 5):S457. doi: 10.1016/j.annonc.2021.08.511 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.