Abstract
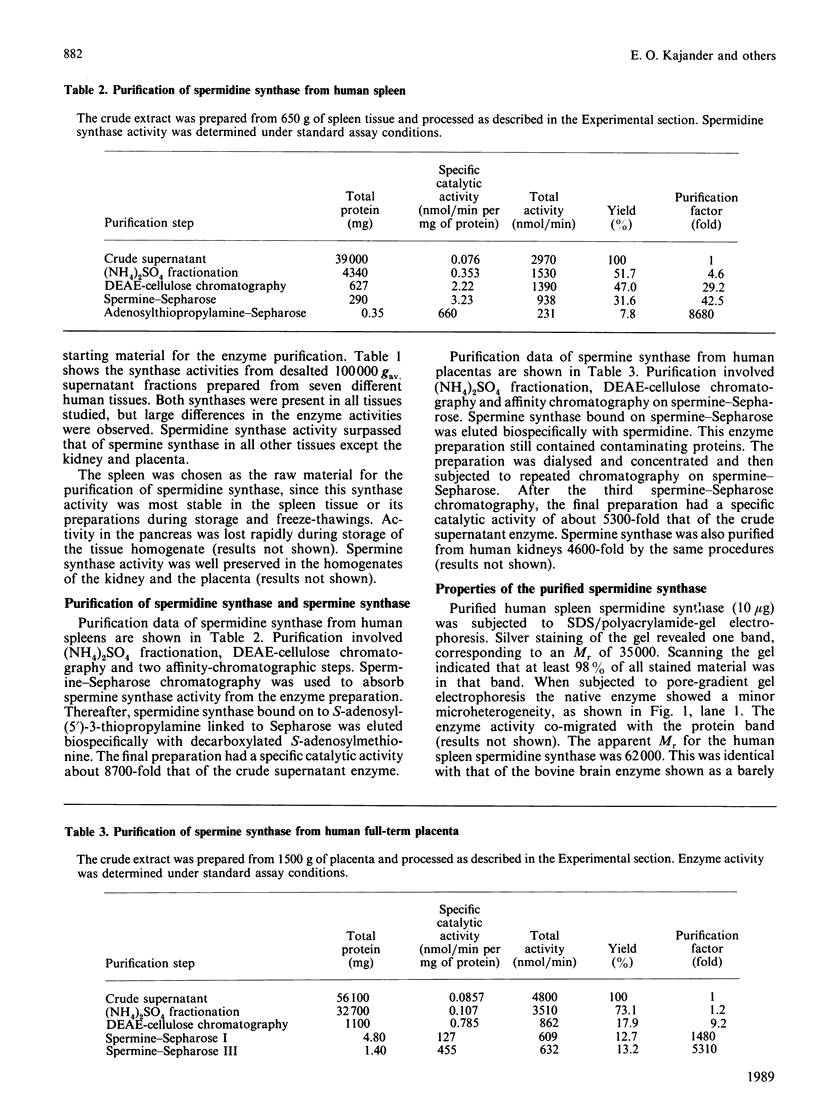
Spermidine synthase was purified to apparent homogeneity from human spleens (8700-fold) by affinity chromatography. The native enzyme was composed of two subunits of identical Mr (35,000) and showed an apparent Mr of 62,000 in pore-gradient gel electrophoresis. Its pI was 5.1, Spermine synthase was purified to apparent homogeneity from placenta (5300-fold) and from kidney (4600-fold). The native enzyme was composed of two subunits of identical Mr (45,000) and showed an apparent Mr of 78,000 in pore-gradient gel electrophoresis. In isoelectric focusing it revealed two bands, with pI values of 4.9 and 5.0. Both synthases were present in all human tissues studied, but revealed a clear tissue-specific pattern. Mouse antisera against spermidine synthase revealed only one band, of Mr 35,000, in all purified enzyme preparations and in crude human tissue extracts in immunoblotting. Antisera against spermine synthase showed an immunoreactive band corresponding to the Mr of the subunit of spermine synthase. These antisera did not indicate any cross-reactivity in immunoblotting. Thus spermine synthase and spermidine synthase do not share homologous antigenic sites and are totally different proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billings P. B., Hoch S. O., White P. J., Carson D. A., Vaughan J. H. Antibodies to the Epstein-Barr virus nuclear antigen and to rheumatoid arthritis nuclear antigen identify the same polypeptide. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7104–7108. doi: 10.1073/pnas.80.23.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Hannonen P., Jänne J., Raina A. Partial purification and characterization of spermine synthase from rat brain. Biochim Biophys Acta. 1972 Nov 10;289(1):225–231. doi: 10.1016/0005-2744(72)90125-8. [DOI] [PubMed] [Google Scholar]
- Hannonen P., Raina A., Jänne J. Polyamine synthesis in the regenerating rat liver: stimulation of S-adenosyl methionine decarboxylase, and spermidine and spermine synthases after partial hepatectomy. Biochim Biophys Acta. 1972 Jun 26;273(1):84–90. doi: 10.1016/0304-4165(72)90194-8. [DOI] [PubMed] [Google Scholar]
- Hickok N. J., Seppänen P. J., Gunsalus G. L., Jänne O. A. Complete amino acid sequence of human ornithine decarboxylase deduced from complementary DNA. DNA. 1987 Jun;6(3):179–187. doi: 10.1089/dna.1987.6.179. [DOI] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Kajander E. O., Raina A. M. Affinity-chromatographic purification of S-adenosyl-L-homocysteine hydrolase. Some properties of the enzyme from rat liver. Biochem J. 1981 Feb 1;193(2):503–512. doi: 10.1042/bj1930503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käpyaho K., Pösö H., Jänne J. Role of propylamine transferases in hormone-induced stimulation of polyamine biosynthesis. Biochem J. 1980 Oct 15;192(1):59–63. doi: 10.1042/bj1920059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markham G. D., Tabor C. W., Tabor H. S-adenosylmethionine decarboxylase (Escherichia coli). Methods Enzymol. 1983;94:228–230. doi: 10.1016/s0076-6879(83)94039-9. [DOI] [PubMed] [Google Scholar]
- Pajula R. L., Raina A., Eloranta T. Polyamine synthesis in mammalian tissues. Isolation and characterization of spermine synthase from bovine brain. Eur J Biochem. 1979 Nov;101(2):619–626. doi: 10.1111/j.1432-1033.1979.tb19756.x. [DOI] [PubMed] [Google Scholar]
- Pajula R. L., Raina A., Kekoni J. Purification of spermine synthase from bovine brain by spermine-Sepharose affinity chromatography. FEBS Lett. 1978 Jun 1;90(1):153–156. doi: 10.1016/0014-5793(78)80319-6. [DOI] [PubMed] [Google Scholar]
- Pajula R. L., Raina A. Methylthioadenosine, a potent inhibitor of spermine synthase from bovine brain. FEBS Lett. 1979 Mar 15;99(2):343–345. doi: 10.1016/0014-5793(79)80988-6. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Shuttleworth K., Hibasami H. Specificity of mammalian spermidine synthase and spermine synthase. Biochem J. 1981 Aug 1;197(2):315–320. doi: 10.1042/bj1970315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Porter C. W., Sufrin J. R. Interference with polyamine biosynthesis and/or function by analogs of polyamines or methionine as a potential anticancer chemotherapeutic strategy. Anticancer Res. 1986 Jul-Aug;6(4):525–542. [PubMed] [Google Scholar]
- Raina A., Eloranta T., Pajula R. L. Rapid assays for putrescine aminopropyltransferase (spermidine synthase) and spermidine aminopropyltransferase (spermine synthase). Methods Enzymol. 1983;94:257–260. doi: 10.1016/s0076-6879(83)94044-2. [DOI] [PubMed] [Google Scholar]
- Raina A., Hyvönen T., Eloranta T., Voutilainen M., Samejima K., Yamanoha B. Polyamine synthesis in mammalian tissues. Isolation and characterization of spermidine synthase from bovine brain. Biochem J. 1984 May 1;219(3):991–1000. doi: 10.1042/bj2190991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Pajula R. L., Eloranta T. A rapid assay method for spermidine and spermine synthases. Distribution of polyamine-synthesizing enzymes and methionine adenosyltransferase in rat tissues. FEBS Lett. 1976 Sep 1;67(3):252–255. doi: 10.1016/0014-5793(76)80540-6. [DOI] [PubMed] [Google Scholar]
- Samejima K., Raina A., Yamanoha B., Eloranta T. Purification of putrescine aminopropyltransferase (spermidine synthase) from eukaryotic tissues. Methods Enzymol. 1983;94:270–276. doi: 10.1016/s0076-6879(83)94047-8. [DOI] [PubMed] [Google Scholar]
- Samejima K., Yamanoha B. Purification of spermidine synthase from rat ventral prostate by affinity chromatography on immobilized S-adenosyl(5')-3-thiopropylamine. Arch Biochem Biophys. 1982 Jun;216(1):213–222. doi: 10.1016/0003-9861(82)90206-5. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. The speEspeD operon of Escherichia coli. Formation and processing of a proenzyme form of S-adenosylmethionine decarboxylase. J Biol Chem. 1987 Nov 25;262(33):16037–16040. [PubMed] [Google Scholar]
- Tierney D. F., Marton L. J., Hacker A. D., Lowe N. Polyamines in clinical disorders. West J Med. 1985 Jan;142(1):63–73. [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yamanoha B., Samejima K., Nakajima T., Yasuhara T. Differences between homogeneous spermidine synthases isolated from rat and pig liver. J Biochem. 1984 Oct;96(4):1273–1281. doi: 10.1093/oxfordjournals.jbchem.a134946. [DOI] [PubMed] [Google Scholar]






