Abstract
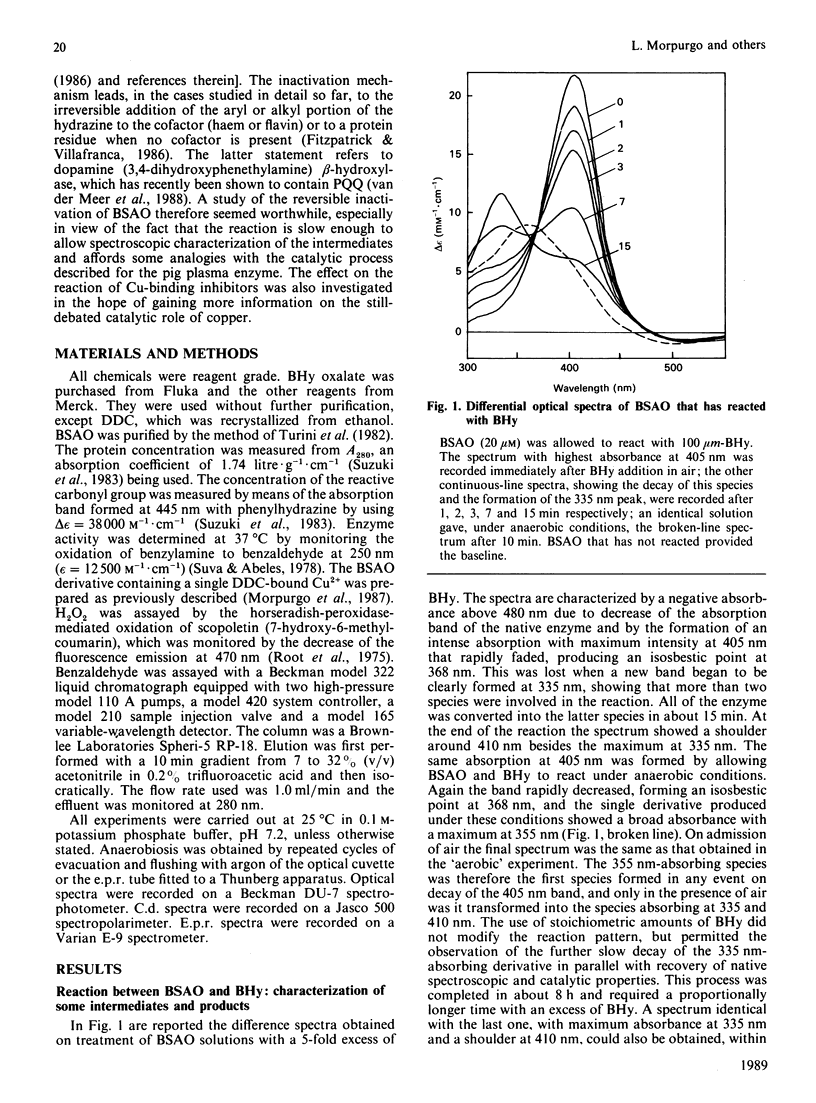
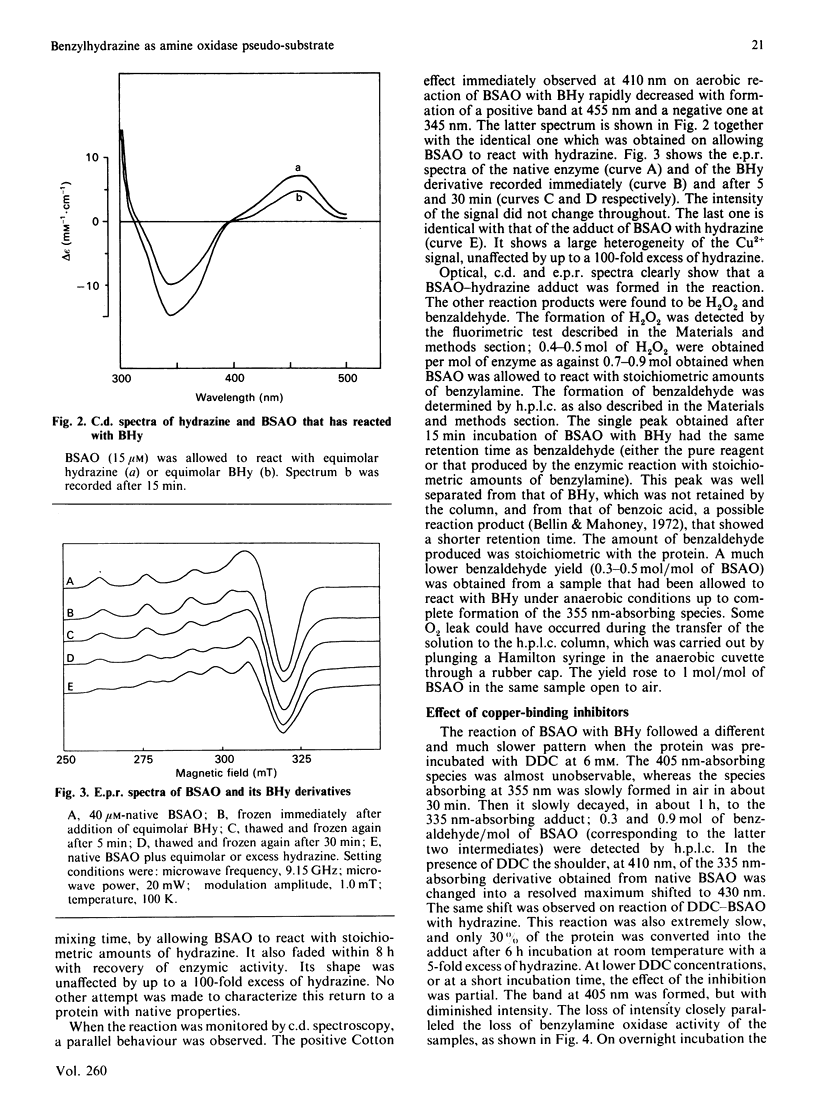
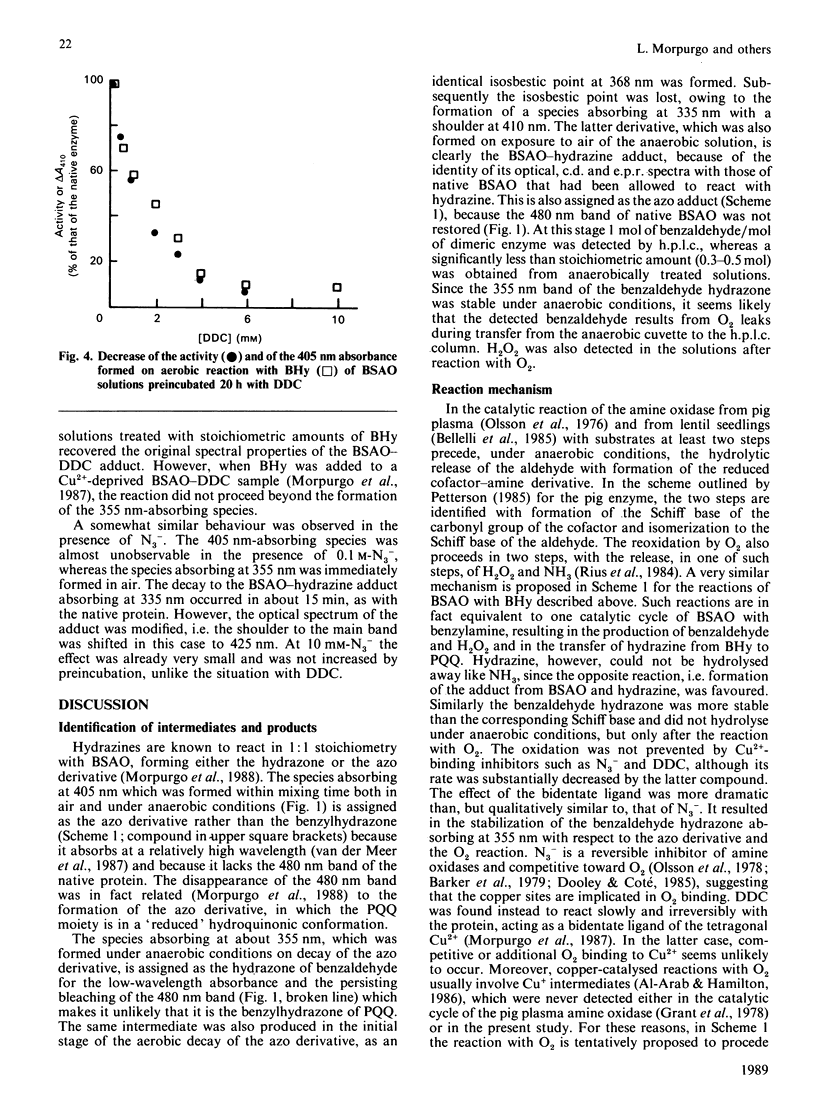
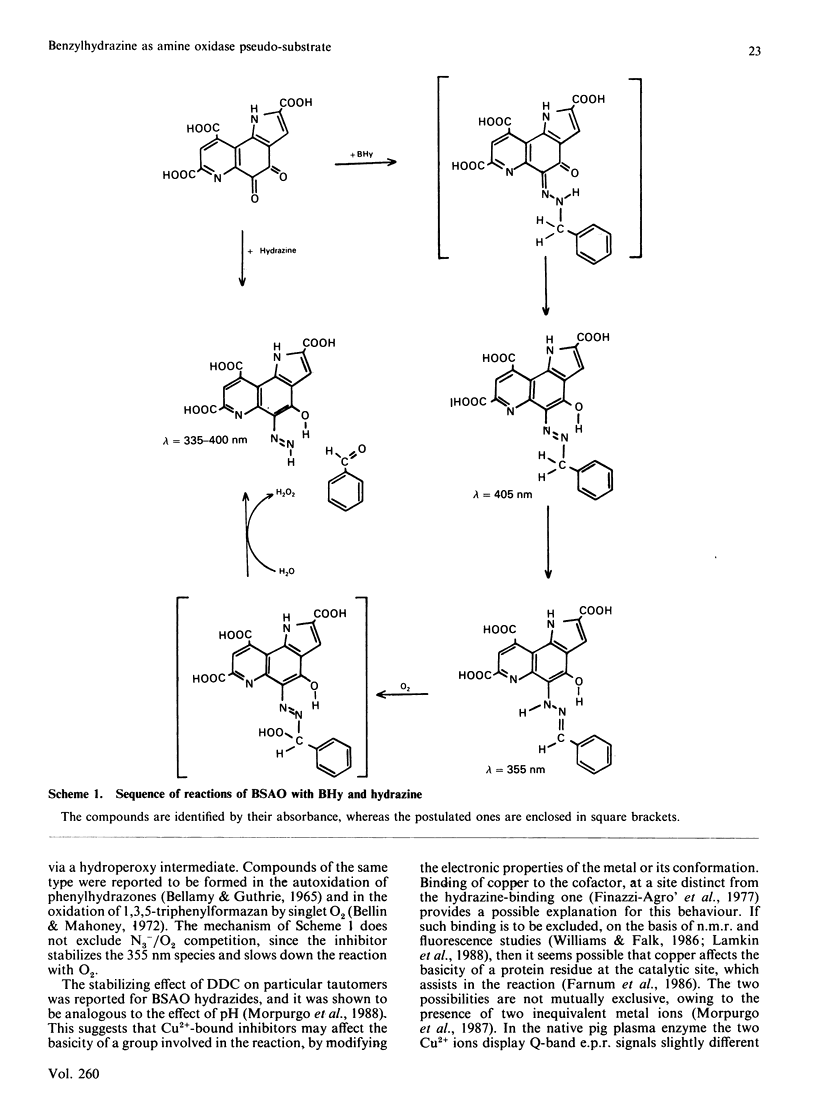
Bovine serum amine oxidase is inhibited by benzylhydrazine (BHy), but recovers full activity after a few hours incubation [Hucko-Haas & Reed (1970) Biochem. Biophys. Res. Commun. 38, 396-400]. The first phase of the process, requiring about 15 min, was found to consist of a mechanism-based hydrazine-transfer reaction leading to formation of the hydrazine-bound enzyme, benzaldehyde and H2O2. At variance with the enzymic process, the reaction with O2 preceded the benzaldehyde release. Two reaction intermediates could be characterized by optical spectroscopy and were assigned as the azo derivative and the benzaldehyde hydrazone, the latter one probably being involved in the reaction with O2. No reduction of Cu was detected at any stage. The hydrazine adduct could also be obtained by stoichiometric reaction of hydrazine with the native enzyme. The decay of this species occurred in about 8 h and was not studied in detail. The Cu-binding inhibitor NN-diethyldithiocarbamate affected the BHy reaction by stabilizing the benzaldehyde hydrazone form as against the azo derivative and the reaction with O2. However, under these same conditions the initial spectroscopic properties of the diethyldithiocarbamate adduct were recovered if the oxidase was left overnight. The reaction with O2 was abolished only upon removal of at least one Cu atom from the enzyme. On the basis of the failure to detect any change of Cu redox state and the enzyme behaviour in the presence of inhibitors, a reaction mechanism involving the formation of a hydroperoxy intermediate, as in the FAD-containing enzymes, is tentatively proposed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrò A. F., Guerrieri P., Costa M. T., Mondovì B. On the nature of chromophore in pig kidney diamine oxidase. Eur J Biochem. 1977 Apr 15;74(3):435–440. doi: 10.1111/j.1432-1033.1977.tb11409.x. [DOI] [PubMed] [Google Scholar]
- Barker R., Boden N., Cayley G., Charlton S. C., Henson R., Holmes M. C., Kelly I. D., Knowles P. F. Properties of cupric ions in benzylamine oxidase from pig plasma as studied by magnetic-resonance and kinetic methods. Biochem J. 1979 Jan 1;177(1):289–302. doi: 10.1042/bj1770289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellelli A., Brunori M., Finazzi-Agró A., Floris G., Giartosi A., Rinaldi A. Transient kinetics of copper-containing lentil (Lens culinaris) seedling amine oxidase. Biochem J. 1985 Dec 15;232(3):923–926. doi: 10.1042/bj2320923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnum M., Palcic M., Klinman J. P. pH dependence of deuterium isotope effects and tritium exchange in the bovine plasma amine oxidase reaction: a role for single-base catalysis in amine oxidation and imine exchange. Biochemistry. 1986 Apr 22;25(8):1898–1904. doi: 10.1021/bi00356a010. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick P. F., Villafranca J. J. The mechanism of inactivation of dopamine beta-hydroxylase by hydrazines. J Biol Chem. 1986 Apr 5;261(10):4510–4518. [PubMed] [Google Scholar]
- Grant J., Kelly I., Knowles P., Olsson J., Pettersson G. Changes in the copper centres of benzylamine oxidase from pig plasma during the catalytic cycle. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1216–1224. doi: 10.1016/0006-291x(78)91524-3. [DOI] [PubMed] [Google Scholar]
- Húcko-Haas J. E., Reed D. J. Hydrazines as substrates for bovine plasma amine oxidase (PAO). Biochem Biophys Res Commun. 1970 May 11;39(3):396–400. doi: 10.1016/0006-291x(70)90590-5. [DOI] [PubMed] [Google Scholar]
- Kluetz M. D., Schmidt P. G. Proton relaxation study of the hog kidney diamine oxidase active center. Biochemistry. 1977 Nov 29;16(24):5191–5199. doi: 10.1021/bi00643a006. [DOI] [PubMed] [Google Scholar]
- Lamkin M. S., Williams T. J., Falk M. C. Excitation energy transfer study of the spatial relationship between the carbonyl and metal cofactors in pig plasma amine oxidase. Arch Biochem Biophys. 1988 Feb 15;261(1):72–79. doi: 10.1016/0003-9861(88)90105-1. [DOI] [PubMed] [Google Scholar]
- Lobenstein-Verbeek C. L., Jongejan J. A., Frank J., Duine J. A. Bovine serum amine oxidase: a mammalian enzyme having covalently bound PQQ as prosthetic group. FEBS Lett. 1984 May 21;170(2):305–309. doi: 10.1016/0014-5793(84)81333-2. [DOI] [PubMed] [Google Scholar]
- Mondovi B., Morpurgo L., Agostinelli E., Befani O., McCracken J., Peisach J. A comparison of the local environment of Cu(II) in native and half-Cu-depleted bovine serum amine oxidase. Eur J Biochem. 1987 Nov 2;168(3):503–507. doi: 10.1111/j.1432-1033.1987.tb13446.x. [DOI] [PubMed] [Google Scholar]
- Morpurgo L., Agostinelli E., Befani O., Mondovì B. Reactions of bovine serum amine oxidase with NN-diethyldithiocarbamate. Selective removal of one copper ion. Biochem J. 1987 Dec 15;248(3):865–870. doi: 10.1042/bj2480865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J., Kenney W. C., Singer T. P. The reaction of phenylhydrazine with trimethylamine dehydrogenase and with free flavins. J Biol Chem. 1979 Apr 25;254(8):2684–2688. [PubMed] [Google Scholar]
- Olsson B., Olsson J., Pettersson G. Effects on enzyme activity of ligand-binding to copper in pig-plasma benzylamine oxidase. Eur J Biochem. 1978 Jun 1;87(1):1–8. doi: 10.1111/j.1432-1033.1978.tb12345.x. [DOI] [PubMed] [Google Scholar]
- Olsson B., Olsson J., Pettersson G. Stopped-flow spectrophotometric characterization of enzymic reaction intermediates in the anaerobic reduction of pig-plasma benzylamine oxidase by amine substrates. Eur J Biochem. 1976 Dec 11;71(2):375–382. doi: 10.1111/j.1432-1033.1976.tb11124.x. [DOI] [PubMed] [Google Scholar]
- Olsson B., Olsson J., Pettersson G. The kinetics of reoxidation of reduced benzylamine oxidase. Eur J Biochem. 1977 Apr 1;74(2):329–335. doi: 10.1111/j.1432-1033.1977.tb11397.x. [DOI] [PubMed] [Google Scholar]
- Rinaldi A., Giartosio A., Floris G., Medda R., Finazzi Agrò A. Lentil seedlings amine oxidase: preparation and properties of the copper-free enzyme. Biochem Biophys Res Commun. 1984 Apr 16;120(1):242–249. doi: 10.1016/0006-291x(84)91440-2. [DOI] [PubMed] [Google Scholar]
- Rius F. X., Knowles P. F., Pettersson G. The kinetics of ammonia release during the catalytic cycle of pig plasma amine oxidase. Biochem J. 1984 Jun 15;220(3):767–772. doi: 10.1042/bj2200767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suva R. H., Abeles R. H. Studies on the mechanism of action of plasma amine oxidase. Biochemistry. 1978 Aug 22;17(17):3538–3545. doi: 10.1021/bi00610a018. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Sakurai T., Nakahara A., Manabe T., Okuyama T. Effect of metal substitution on the chromophore of bovine serum amine oxidase. Biochemistry. 1983 Mar 29;22(7):1630–1635. doi: 10.1021/bi00276a016. [DOI] [PubMed] [Google Scholar]
- Turini P., Sabatini S., Befani O., Chimenti F., Casanova C., Riccio P. L., Mondovì B. Purification of bovine plasma amine oxidase. Anal Biochem. 1982 Sep 15;125(2):294–298. doi: 10.1016/0003-2697(82)90009-4. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Falk M. C. Spatial relationship between the copper and carbonyl cofactors in the active site of pig plasma amine oxidase. J Biol Chem. 1986 Dec 5;261(34):15949–15954. [PubMed] [Google Scholar]
- van der Meer R. A., Jongejan J. A., Duine J. A. Dopamine beta-hydroxylase from bovine adrenal medulla contains covalently-bound pyrroloquinoline quinone. FEBS Lett. 1988 Apr 25;231(2):303–307. doi: 10.1016/0014-5793(88)80838-x. [DOI] [PubMed] [Google Scholar]


