Abstract
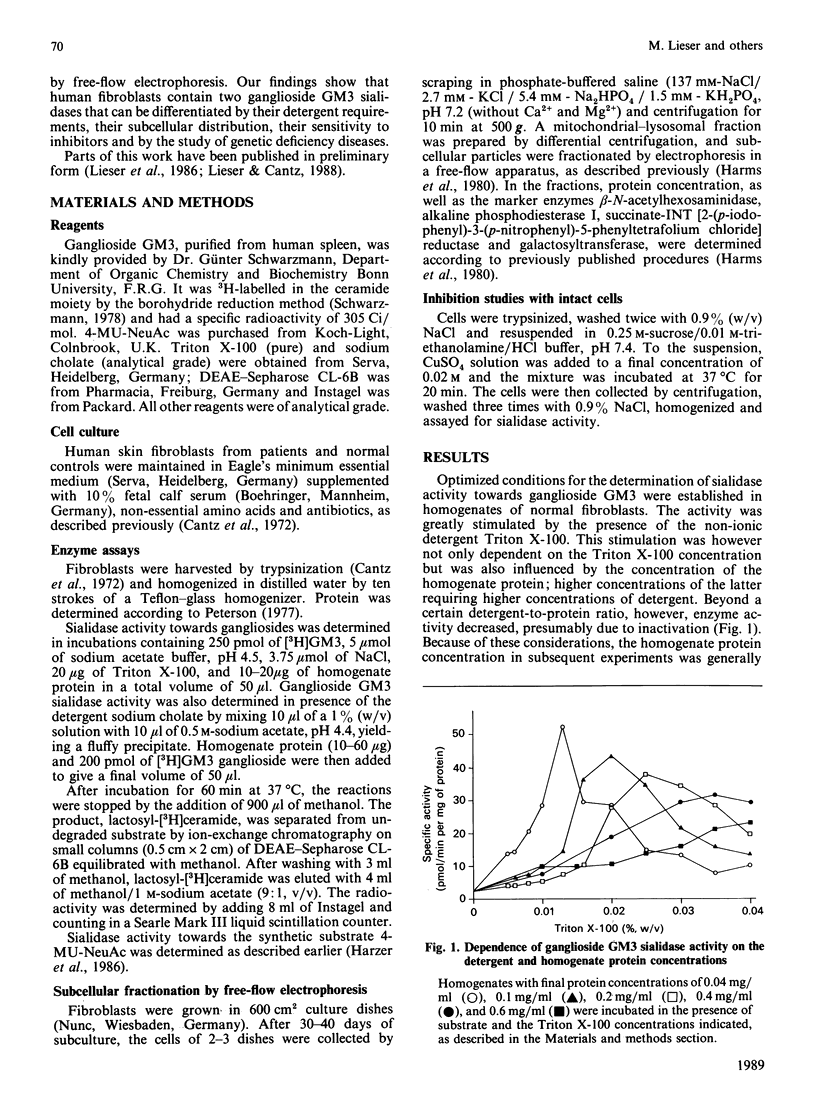
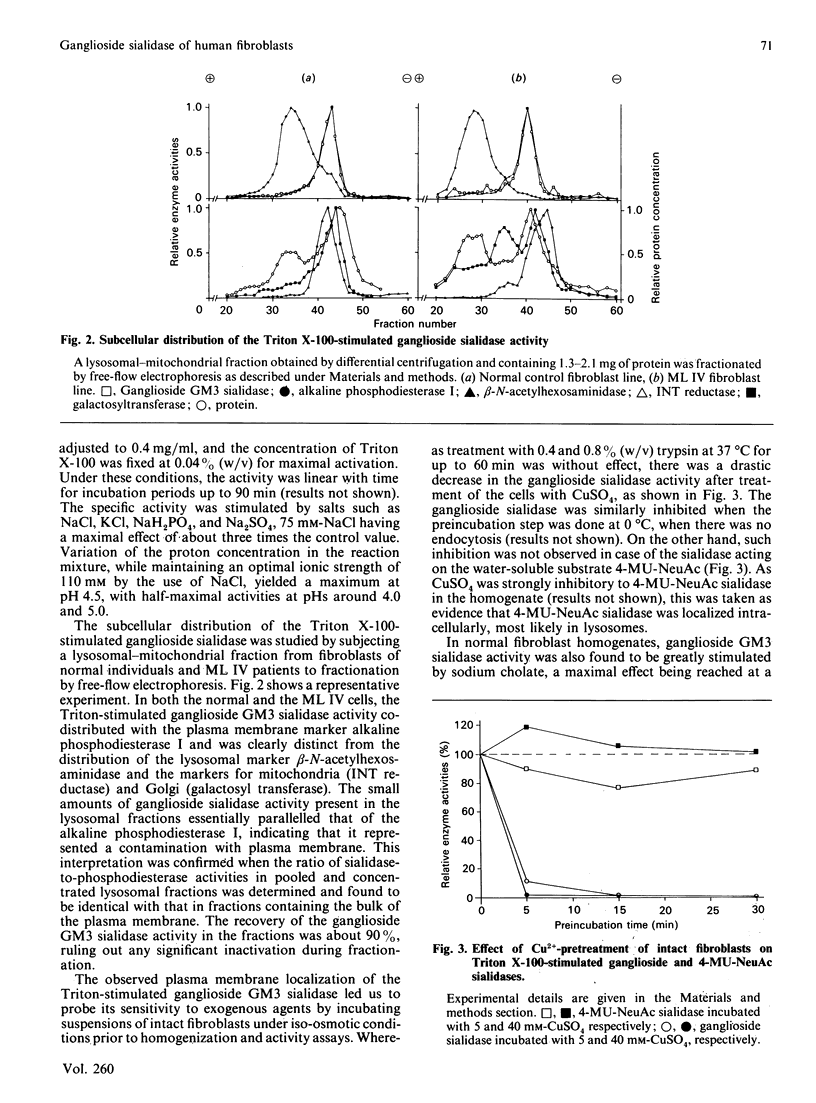
Sensitive assays for the determination of the ganglioside sialidase activity of fibroblast homogenates were established using ganglioside GM3, 3H-labelled in the sphingosine moiety, as a substrate. Ganglioside GM3 sialidase activity was greatly stimulated by the presence of the non-ionic detergent Triton X-100 and was further enhanced by salts such as NaCl; the optimal pH was 4.5. The subcellular localization of this activity was determined by fractionation using free-flow electrophoresis and found to be exclusively associated with the marker for the plasma membrane, but not with that for lysosomes. This Triton-stimulated ganglioside sialidase activity was selectively inhibited by preincubating intact cells in the presence of millimolar concentrations of Cu2+, suggesting that the activity resides on the external surface of the plasma membrane. In normal fibroblasts homogenates, ganglioside GM3 sialidase was also greatly stimulated by sodium cholate. In contrast to the Triton X-100-activated reaction, however, it was not diminished by prior incubation of intact cells in the presence of Cu2+. Only after cell lysis was Cu2+ inhibitory. the cholate-stimulated ganglioside sialidase activity thus paralleled the behaviour of the lysosomal 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid (4-MU-NeuAc) sialidase. In fibroblasts from sialidosis patients, the cholate-stimulated ganglioside GM3 sialidase activity, but not that of the Triton-activated enzyme, was profoundly diminished. In fibroblasts from patients with mucolipidosis IV (ML IV), both the Triton X-100- and the cholate-stimulated ganglioside GM3 sialidase activities were in the range of normal controls. The Triton-activated enzyme was associated with the plasma membrane in the same manner as in normal cells. Our findings suggest that, in human fibroblasts, there exist two sialidases that degrade ganglioside GM3: one on the external surface of the plasma membrane, and another that is localized in lysosomes and seems identical with the activity that acts on sialyloligosaccharides and 4-MU-NeuAc. As neither activity was found to be deficient in ML IV fibroblasts, our results argue against the hypothesis of a primary involvement of a ganglioside GM3 sialidase in the pathogenesis of ML IV.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach G., Cohen M. M., Kohn G. Abnormal ganglioside accumulation in cultured fibroblasts from patients with mucolipidosis IV. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1483–1490. doi: 10.1016/0006-291x(75)90526-4. [DOI] [PubMed] [Google Scholar]
- Bach G., Zeigler M., Schaap T., Kohn G. Mucolipidosis type IV: ganglioside sialidase deficiency. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1341–1347. doi: 10.1016/0006-291x(79)91183-5. [DOI] [PubMed] [Google Scholar]
- Bach G., Ziegler M., Kohn G., Cohen M. M. Mucopolysaccharide accumulation in cultured skin fibroblasts derived from patients with mucolipidosis IV. Am J Hum Genet. 1977 Nov;29(6):610–618. [PMC free article] [PubMed] [Google Scholar]
- Baumkötter J., Cantz M. Decreased ganglioside neuraminidase activity in fibroblasts from mucopolysaccharidosis patients. Inhibition of the activity in vitro by sulfated glycosaminoglycans and other compounds. Biochim Biophys Acta. 1983 Dec 13;761(2):163–170. doi: 10.1016/0304-4165(83)90225-8. [DOI] [PubMed] [Google Scholar]
- Ben-Yoseph Y., Momoi T., Hahn L. C., Nadler H. L. Catalytically defective ganglioside neuraminidase in mucolipidosis IV. Clin Genet. 1982 Jun;21(6):374–381. doi: 10.1111/j.1399-0004.1982.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Berman E. R., Livni N., Shapira E., Merin S., Levij I. S. Congenital corneal clouding with abnormal systemic storage bodies: a new variant of mucolipidosis. J Pediatr. 1974 Apr;84(4):519–526. doi: 10.1016/s0022-3476(74)80671-2. [DOI] [PubMed] [Google Scholar]
- Caimi L., Lombardo A., Preti A., Wiesmann U., Tettamanti G. Optimal conditions for the assay of fibroblast neuraminidase with different natural substrates. Biochim Biophys Acta. 1979 Nov 9;571(1):137–146. doi: 10.1016/0005-2744(79)90234-1. [DOI] [PubMed] [Google Scholar]
- Caimi L., Tettamanti G., Berra B., Omodeo Sale F., Borrone C., Gatti R., Durand P., Martin J. J. Mucolipidosis IV, a sialolipidosis due to ganglioside sialidase deficiency. J Inherit Metab Dis. 1982;5(4):218–224. doi: 10.1007/BF02179146. [DOI] [PubMed] [Google Scholar]
- Cantz M., Messer H. Oligosaccharide and ganglioside neuraminidase activities of mucolipidosis I (sialidosis) and mucolipidosis II (I-cell disease) fibroblasts. Eur J Biochem. 1979 Jun;97(1):113–118. doi: 10.1111/j.1432-1033.1979.tb13091.x. [DOI] [PubMed] [Google Scholar]
- Chigorno V., Cardace G., Pitto M., Sonnino S., Ghidoni R., Tettamanti G. A radiometric assay for ganglioside sialidase applied to the determination of the enzyme subcellular location in cultured human fibroblasts. Anal Biochem. 1986 Mar;153(2):283–294. doi: 10.1016/0003-2697(86)90094-1. [DOI] [PubMed] [Google Scholar]
- Crandall B. F., Philippart M., Brown W. J., Bluestone D. A. Review article: mucolipidosis IV. Am J Med Genet. 1982 Jul;12(3):301–308. doi: 10.1002/ajmg.1320120308. [DOI] [PubMed] [Google Scholar]
- Draye J. P., Courtoy P. J., Quintart J., Baudhuin P. Relations between plasma membrane and lysosomal membrane. 2. Quantitative evaluation of plasma membrane marker enzymes in the lysosomes. Eur J Biochem. 1987 Dec 30;170(1-2):405–411. doi: 10.1111/j.1432-1033.1987.tb13714.x. [DOI] [PubMed] [Google Scholar]
- Harms E., Kern H., Schneider J. A. Human lysosomes can be purified from diploid skin fibroblasts by free-flow electrophoresis. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6139–6143. doi: 10.1073/pnas.77.10.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzer K., Cantz M., Sewell A. C., Dhareshwar S. S., Roggendorf W., Heckl R. W., Schofer O., Thumler R., Peiffer J., Schlote W. Normomorphic sialidosis in two female adults with severe neurologic disease and without sialyl oligosacchariduria. Hum Genet. 1986 Nov;74(3):209–214. doi: 10.1007/BF00282535. [DOI] [PubMed] [Google Scholar]
- Hiraiwa M., Nishizawa M., Uda Y., Nakajima T., Miyatake T. Human placental sialidase: further purification and characterization. J Biochem. 1988 Jan;103(1):86–90. doi: 10.1093/oxfordjournals.jbchem.a122245. [DOI] [PubMed] [Google Scholar]
- Michalski J. C., Corfield A. P., Schauer R. Properties of human liver lysosomal sialidase. Biol Chem Hoppe Seyler. 1986 Aug;367(8):715–722. doi: 10.1515/bchm3.1986.367.2.715. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Warner T. G. Sialidosis: delineation of subtypes by neuraminidase assay. Clin Genet. 1980 Jan;17(1):35–38. doi: 10.1111/j.1399-0004.1980.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Schengrund C. L., Lausch R. N., Rosenberg A. Sialidase activity in transformed cells. J Biol Chem. 1973 Jun 25;248(12):4424–4428. [PubMed] [Google Scholar]
- Schengrund C. L., Rosenberg A., Repman M. A. Ecto-ganglioside-sialidase activity of herpes simplex virus-transformed hamster embryo fibroblasts. J Cell Biol. 1976 Sep;70(3):555–561. doi: 10.1083/jcb.70.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann G. A simple and novel method for tritium labeling of gangliosides and other sphingolipids. Biochim Biophys Acta. 1978 Apr 28;529(1):106–114. doi: 10.1016/0005-2760(78)90108-x. [DOI] [PubMed] [Google Scholar]
- Ulrich-Bott B., Klem B., Kaiser R., Spranger J., Cantz M. Lysosomal sialidase deficiency: increased ganglioside content in autopsy tissues of a sialidosis patient. Enzyme. 1987;38(1-4):262–266. doi: 10.1159/000469214. [DOI] [PubMed] [Google Scholar]
- Usuki S., Lyu S. C., Sweeley C. C. Sialidase activities of cultured human fibroblasts and the metabolism of GM3 ganglioside. J Biol Chem. 1988 May 15;263(14):6847–6853. [PubMed] [Google Scholar]
- Verheijen F. W., Janse H. C., van Diggelen O. P., Bakker H. D., Loonen M. C., Durand P., Galjaard H. Two genetically different MU-NANA neuraminidases in human leucocytes. Biochem Biophys Res Commun. 1983 Dec 16;117(2):470–478. doi: 10.1016/0006-291x(83)91224-x. [DOI] [PubMed] [Google Scholar]
- Verheijen F. W., Palmeri S., Galjaard H. Purification and partial characterization of lysosomal neuraminidase from human placenta. Eur J Biochem. 1987 Jan 2;162(1):63–67. doi: 10.1111/j.1432-1033.1987.tb10542.x. [DOI] [PubMed] [Google Scholar]
- Zeigler M., Bach G. Cellular localization of neuraminidases in cultured human fibroblasts. Biochem J. 1981 Sep 15;198(3):505–508. doi: 10.1042/bj1980505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler M., Bach G. Ganglioside sialidase distribution in mucolipidosis type IV cultured fibroblasts. Arch Biochem Biophys. 1985 Sep;241(2):602–607. doi: 10.1016/0003-9861(85)90586-7. [DOI] [PubMed] [Google Scholar]


