Abstract
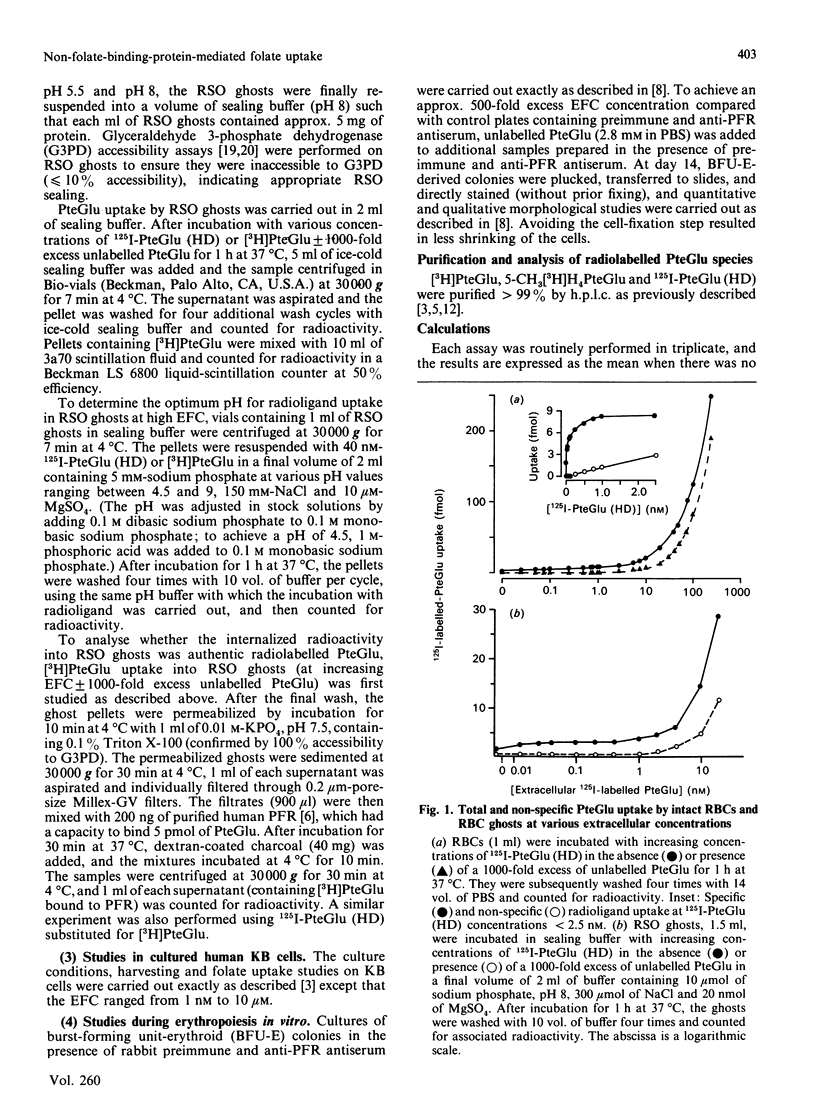
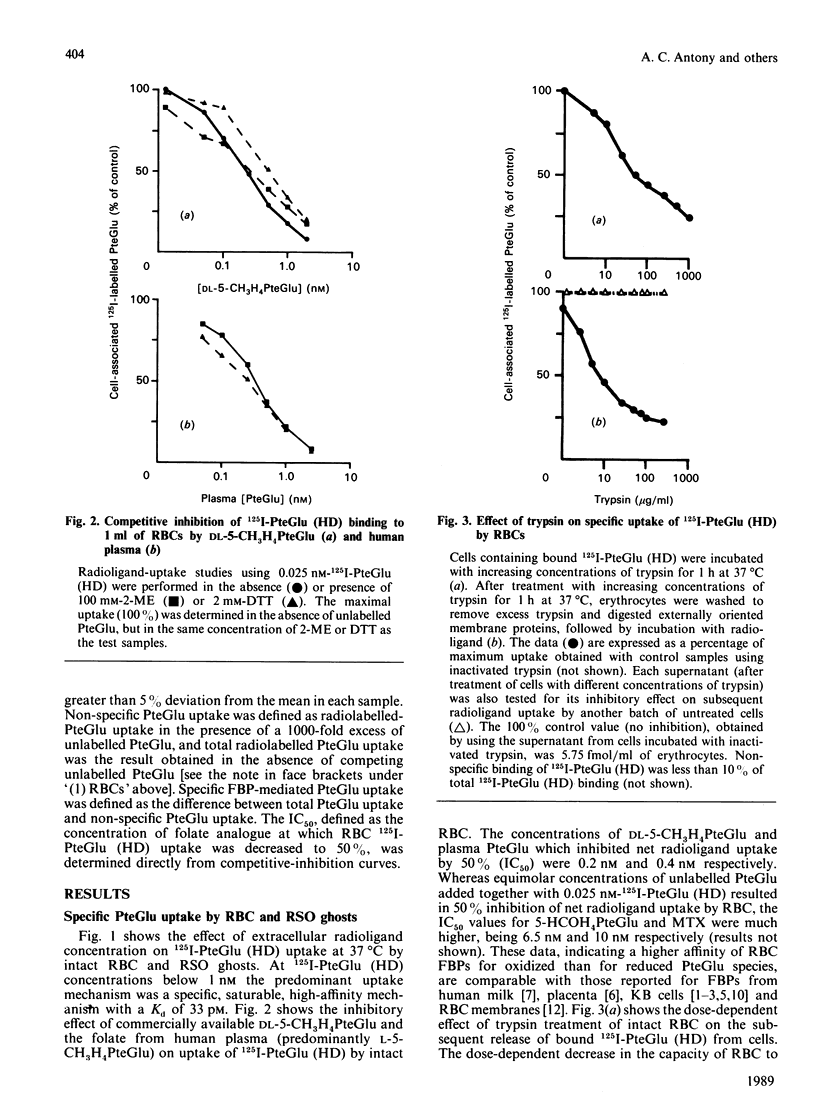
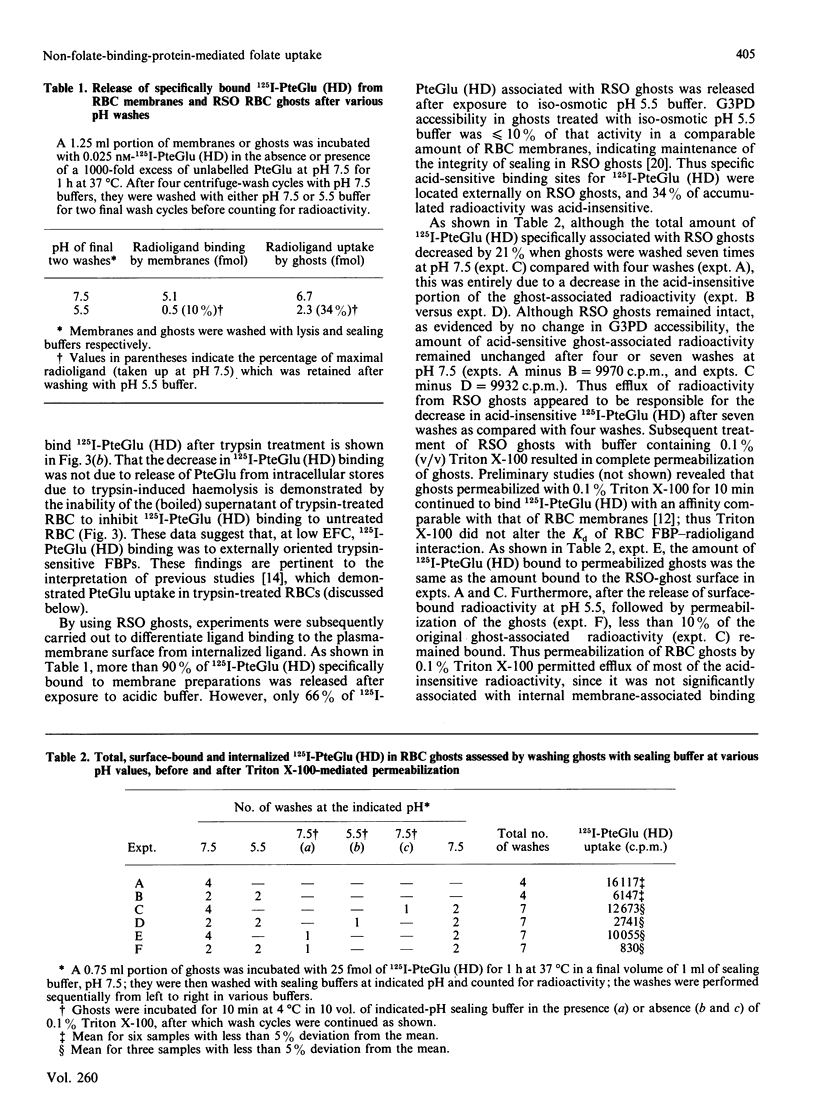
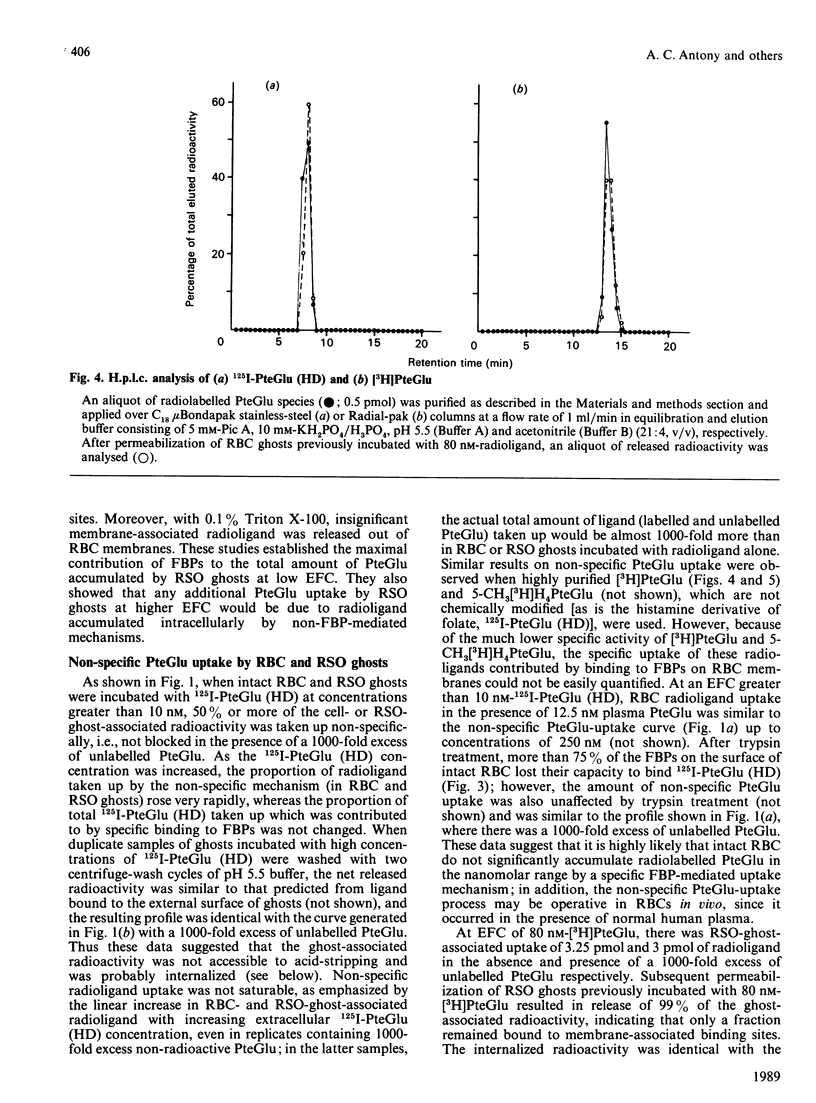
Membrane-associated folate (pteroylglutamate, PteGlu)-binding proteins (FBPs) play an important role as PteGlu-transport proteins in malignant and normal human cells. Since high extracellular folate (PteGlu) concentrations (EFC) profoundly influenced uptake and toxicity of the anti-PteGlu methotrexate in malignant KB cells, we studied human cells to determine additional mechanisms for PteGlu uptake when the EFC was varied. At low EFC (less than 10 nM), the predominant mechanism for folate uptake in mature erythrocytes was through binding to externally oriented FBPs which were quantitatively insignificant (4-6 orders of magnitude lower) and of no apparent physiological relevance when compared with KB cells. However, the predominant mechanism of PteGlu accumulation at high EFC [10-250 nM] in intact erythrocytes and sealed right-side-out (RSO) ghosts was not FBP-mediated and non-specific. This conclusion was based on the findings that radiolabelled PteGlu uptake: (i) continued even in the presence of a 1000-fold excess of unlabelled PteGlu and was linear and not saturable up to 250 nM; (ii) was two-fold higher at pH 4.5 than 7.5; (iii) was less than 2-fold increased at 37 degrees C compared with 4 degrees C; and (iv) was unaffected after trypsin-mediated proteolysis of greater than 75% FBPs. The [3H]PteGlu and 125I-PteGlu (histamine derivative) accumulated intracellularly through the non-specific PteGlu-uptake mechanism was unaltered biochemically and in a soluble compartment. Raising the EFC 500-fold higher than controls during erythropoiesis in vitro resulted in reversal of the expected anti-(placental folate-receptor)-antiserum-induced megaloblastic changes in orthochromatic normoblasts derived from burst-forming unit-erythroid colonies. Furthermore, at EFC greater than 0.1 microM, KB-cell accumulation of [3H]PteGlu was also predominantly through a mechanism that did not involve specific FBPs. Thus, at high EFC, a major component of PteGlu transport in human cells is not mediated through FBPs and is likely to be a passive diffusion process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antony A. C., Bruno E., Briddell R. A., Brandt J. E., Verma R. S., Hoffman R. Effect of perturbation of specific folate receptors during in vitro erythropoiesis. J Clin Invest. 1987 Dec;80(6):1618–1623. doi: 10.1172/JCI113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony A. C., Kane M. A., Portillo R. M., Elwood P. C., Kolhouse J. F. Studies of the role of a particulate folate-binding protein in the uptake of 5-methyltetrahydrofolate by cultured human KB cells. J Biol Chem. 1985 Dec 5;260(28):14911–14917. [PubMed] [Google Scholar]
- Antony A. C., Kincade R. S., Verma R. S., Krishnan S. R. Identification of high affinity folate binding proteins in human erythrocyte membranes. J Clin Invest. 1987 Sep;80(3):711–723. doi: 10.1172/JCI113126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony A. C., Utley C. S., Marcell P. D., Kolhouse J. F. Isolation, characterization, and comparison of the solubilized particulate and soluble folate binding proteins from human milk. J Biol Chem. 1982 Sep 10;257(17):10081–10089. [PubMed] [Google Scholar]
- Antony A. C., Utley C., Van Horne K. C., Kolhouse J. F. Isolation and characterization of a folate receptor from human placenta. J Biol Chem. 1981 Sep 25;256(18):9684–9692. [PubMed] [Google Scholar]
- Antony A. C., Verma R. S., Kincade R. S. Development of a specific radioimmunoassay for the placental folate receptor and related high-affinity folate binding proteins in human tissues. Anal Biochem. 1987 Apr;162(1):224–235. doi: 10.1016/0003-2697(87)90031-5. [DOI] [PubMed] [Google Scholar]
- Bender B. S., Quinn T. C., Spivak J. L. Homosexual men with thrombocytopenia have impaired reticuloendothelial system Fc receptor-specific clearance. Blood. 1987 Aug;70(2):392–395. [PubMed] [Google Scholar]
- Benesch R. E., Kwong S., Benesch R., Baugh C. M. The binding of folyl- and antifolylpolyglutamates to hemoglobin. J Biol Chem. 1985 Nov 25;260(27):14653–14658. [PubMed] [Google Scholar]
- Benesch R., Waxman S., Benesch R., Baugh C. The binding of folyl polyglutamates by hemoglobin. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1359–1363. doi: 10.1016/0006-291x(82)91263-3. [DOI] [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Bobzien W. F., 3rd, Goldman I. D. The mechanism of folate transport in rabbit reticulocytes. J Clin Invest. 1972 Jul;51(7):1688–1696. doi: 10.1172/JCI106970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda R. F., Anthony B. K. Evidence for transfer of folate compounds by a specialized erythrocyte membrane system. J Lab Clin Med. 1979 Aug;94(2):354–360. [PubMed] [Google Scholar]
- Branda R. F., Anthony B. K., Jacob H. S. The mechanism of 5-methyltetrahydrofolate transport by human erythrocytes. J Clin Invest. 1978 May;61(5):1270–1275. doi: 10.1172/JCI109043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda R. F., Moldow C. F., MacArthur J. R., Wintrobe M. M., Anthony B. K., Jacob H. S. Folate-induced remission in aplastic anemia with familial defect of cellular folate uptake. N Engl J Med. 1978 Mar 2;298(9):469–475. doi: 10.1056/NEJM197803022980901. [DOI] [PubMed] [Google Scholar]
- Cooper B. A., Peyman J. Folic acid and its pentaglutamate leak from human erythrocyte ghosts but not from liposomes. Biochim Biophys Acta. 1982 Oct 22;692(1):161–164. doi: 10.1016/0005-2736(82)90514-4. [DOI] [PubMed] [Google Scholar]
- Corbeel L., Van den Berghe G., Jaeken J., Van Tornout J., Eeckels R. Congenital folate malabsorption. Eur J Pediatr. 1985 Mar;143(4):284–290. doi: 10.1007/BF00442302. [DOI] [PubMed] [Google Scholar]
- Corcino J. J., Waxman S., Herbert V. Uptake of tritiated folates by human bone marrow cells in vitro. Br J Haematol. 1971 May;20(5):503–509. doi: 10.1111/j.1365-2141.1971.tb07064.x. [DOI] [PubMed] [Google Scholar]
- Das K. C., Hoffbrand A. V. Studies of folate uptake by phytohaemagglutinin-stimulated lymphocytes. Br J Haematol. 1970 Aug;19(2):203–221. doi: 10.1111/j.1365-2141.1970.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Elwood P. C., Kane M. A., Portillo R. M., Kolhouse J. F. The isolation, characterization, and comparison of the membrane-associated and soluble folate-binding proteins from human KB cells. J Biol Chem. 1986 Nov 25;261(33):15416–15423. [PubMed] [Google Scholar]
- Evans W. E., Crom W. R., Abromowitch M., Dodge R., Look A. T., Bowman W. P., George S. L., Pui C. H. Clinical pharmacodynamics of high-dose methotrexate in acute lymphocytic leukemia. Identification of a relation between concentration and effect. N Engl J Med. 1986 Feb 20;314(8):471–477. doi: 10.1056/NEJM198602203140803. [DOI] [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Granuloerythropoietic colonies in human bone marrow, peripheral blood, and cord blood. Blood. 1978 Dec;52(6):1243–1248. [PubMed] [Google Scholar]
- Goldman I. D., Matherly L. H. The cellular pharmacology of methotrexate. Pharmacol Ther. 1985;28(1):77–102. doi: 10.1016/0163-7258(85)90083-x. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Grzelakowska-Sztabert B., Zevely E. M., Huennekens F. M. Binding properties of the 5-methyltetrahydrofolate/methotrexate transport system in L1210 cells. Arch Biochem Biophys. 1980 Jun;202(1):144–149. doi: 10.1016/0003-9861(80)90416-6. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M. Transport routes utilized by L1210 cells for the influx and efflux of methotrexate. J Biol Chem. 1984 Feb 10;259(3):1526–1531. [PubMed] [Google Scholar]
- Hoffbrand V., Tripp E., Catovsky D., Das K. C. Transport of methotrexate into normal haemopoietic cells and into leukaemic cells and its effects on DNA synthesis. Br J Haematol. 1973 Oct;25(4):497–511. doi: 10.1111/j.1365-2141.1973.tb01762.x. [DOI] [PubMed] [Google Scholar]
- Jolivet J., Chabner B. A. Intracellular pharmacokinetics of methotrexate polyglutamates in human breast cancer cells. Selective retention and less dissociable binding of 4-NH2-10-CH3-pteroylglutamate4 and 4-NH2-10-CH3-pteroylglutamate5 to dihydrofolate reductase. J Clin Invest. 1983 Sep;72(3):773–778. doi: 10.1172/JCI111048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivet J., Schilsky R. L., Bailey B. D., Drake J. C., Chabner B. A. Synthesis, retention, and biological activity of methotrexate polyglutamates in cultured human breast cancer cells. J Clin Invest. 1982 Aug;70(2):351–360. doi: 10.1172/JCI110624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen B. A., Capdevila A. Receptor-mediated folate accumulation is regulated by the cellular folate content. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5983–5987. doi: 10.1073/pnas.83.16.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M. A., Elwood P. C., Portillo R. M., Antony A. C., Kolhouse J. F. The interrelationship of the soluble and membrane-associated folate-binding proteins in human KB cells. J Biol Chem. 1986 Nov 25;261(33):15625–15631. [PubMed] [Google Scholar]
- Kane M. A., Elwood P. C., Portillo R. M., Antony A. C., Najfeld V., Finley A., Waxman S., Kolhouse J. F. Influence on immunoreactive folate-binding proteins of extracellular folate concentration in cultured human cells. J Clin Invest. 1988 May;81(5):1398–1406. doi: 10.1172/JCI113469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M. A., Portillo R. M., Elwood P. C., Antony A. C., Kolhouse J. F. The influence of extracellular folate concentration on methotrexate uptake by human KB cells. Partial characterization of a membrane-associated methotrexate binding protein. J Biol Chem. 1986 Jan 5;261(1):44–49. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Steck T. L. A description of the holes in human erythrocyte membrane ghosts. J Biol Chem. 1982 Oct 10;257(19):11651–11659. [PubMed] [Google Scholar]
- Lodish H. F., Braell W. A. Methods for study of the synthesis and maturation of the erythrocyte anion transport protein. Methods Enzymol. 1983;96:257–267. doi: 10.1016/s0076-6879(83)96024-x. [DOI] [PubMed] [Google Scholar]
- McHugh M., Cheng Y. C. Demonstration of a high affinity folate binder in human cell membranes and its characterization in cultured human KB cells. J Biol Chem. 1979 Nov 25;254(22):11312–11318. [PubMed] [Google Scholar]
- Rosenblatt D. S., Whitehead V. M., Vera N., Pottier A., Dupont M., Vuchich M. J. Prolonged inhibition of DNA synthesis associated with the accumulation of methotrexate polyglutamates by cultured human cells. Mol Pharmacol. 1978 Nov;14(6):1143–1147. [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Wagner C. Cellular folate binding proteins; function and significance. Annu Rev Nutr. 1982;2:229–248. doi: 10.1146/annurev.nu.02.070182.001305. [DOI] [PubMed] [Google Scholar]
- Zamierowski M. M., Wagner C. Identification of folate binding proteins in rat liver. J Biol Chem. 1977 Feb 10;252(3):933–938. [PubMed] [Google Scholar]
- Zettner A., Boss G. R., Seegmiller J. E. A long-term study of the absorption of large oral doses of folic acid. Ann Clin Lab Sci. 1981 Nov-Dec;11(6):516–524. [PubMed] [Google Scholar]
- da Costa M., Iqbal M. P. The transport and accumulation of methotrexate in human erythrocytes. Cancer. 1981 Dec 1;48(11):2427–2432. doi: 10.1002/1097-0142(19811201)48:11<2427::aid-cncr2820481115>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]


