Abstract
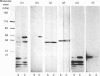
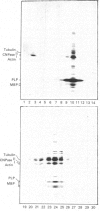
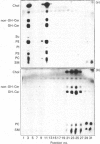
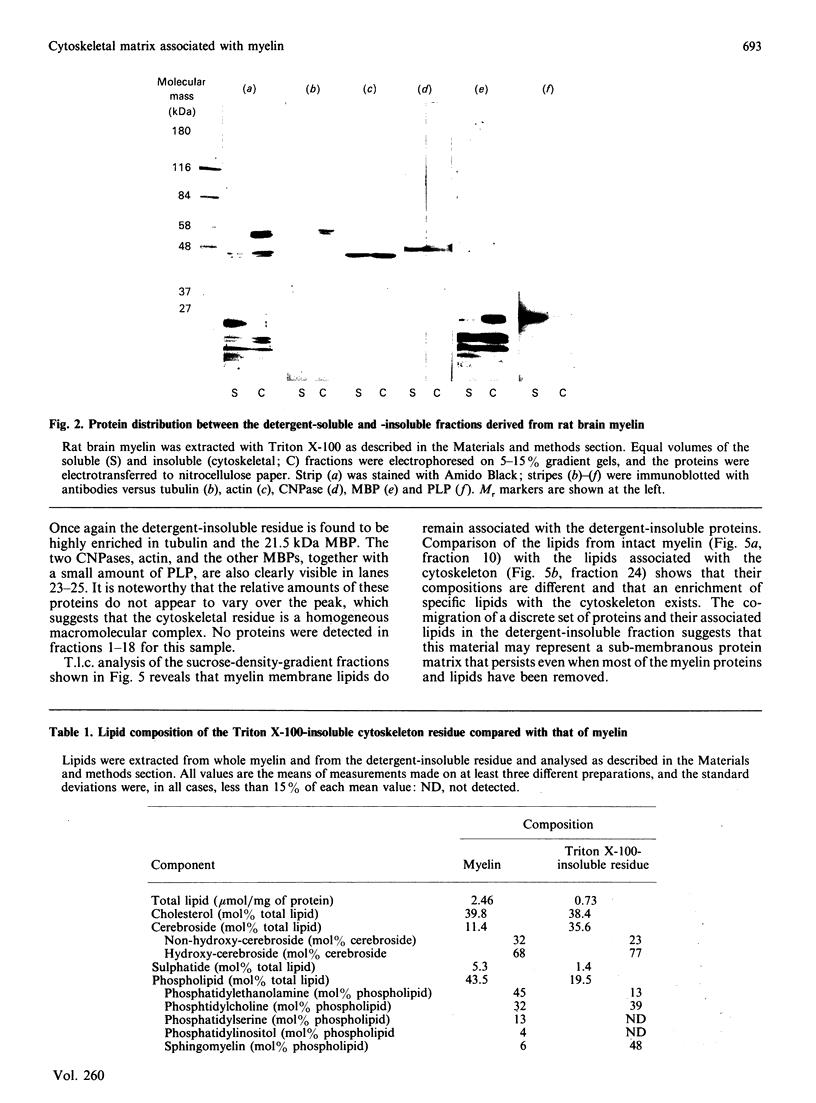
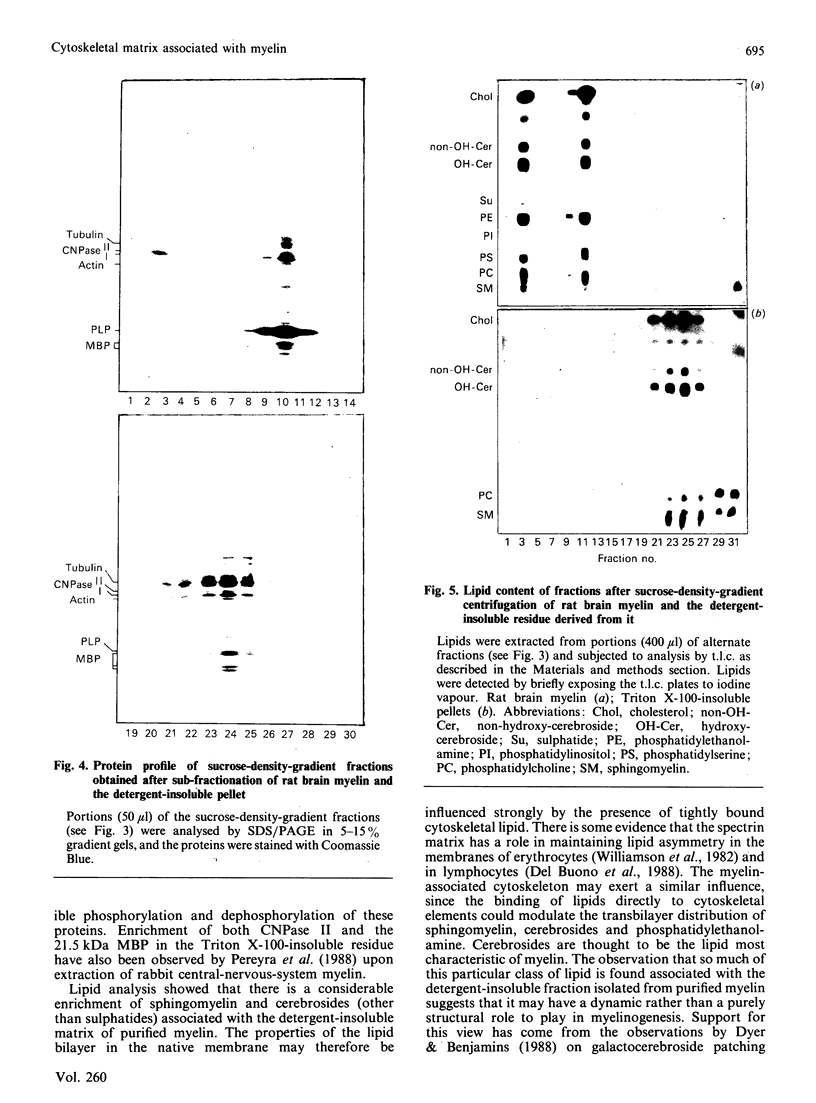
Extraction of rat brain myelin in a buffer containing Triton X-100 yielded a soluble fraction and an insoluble residue that was enriched in cytoskeletal elements. Immunoblot analysis of the detergent-soluble fraction and the insoluble cytoskeletal residue showed that all of the tubulin and more than half of the actin were found within the cytoskeletal fraction. The distribution of myelin-specific proteins was also examined, and revealed that 2',3'-cyclic nucleotide 3'-phosphohydrolase (CNPase) I and most of the myelin basic proteins (MBPs) were equally distributed between both fractions. By contrast, the large MBP (21.5 kDa) and CNPase II (50 kDa) were observed to partition almost entirely with the cytoskeletal fraction. Proteolipid protein was found predominantly in the detergent-soluble fraction, as was DM-20 protein. Analysis of the cytoskeletal fraction by sucrose-density-gradient centrifugation demonstrated that a distinct subset of lipids was tightly bound to the cytoskeletal protein residue. The cytoskeleton-associated lipid was considerably enriched in cerebroside and sphingomyelin by comparison with total myelin lipids. These results indicate that a cytoskeletal matrix is associated with multilamellar myelin, and suggest that this structure may play a fundamental role in myelinogenesis.
Full text
PDF


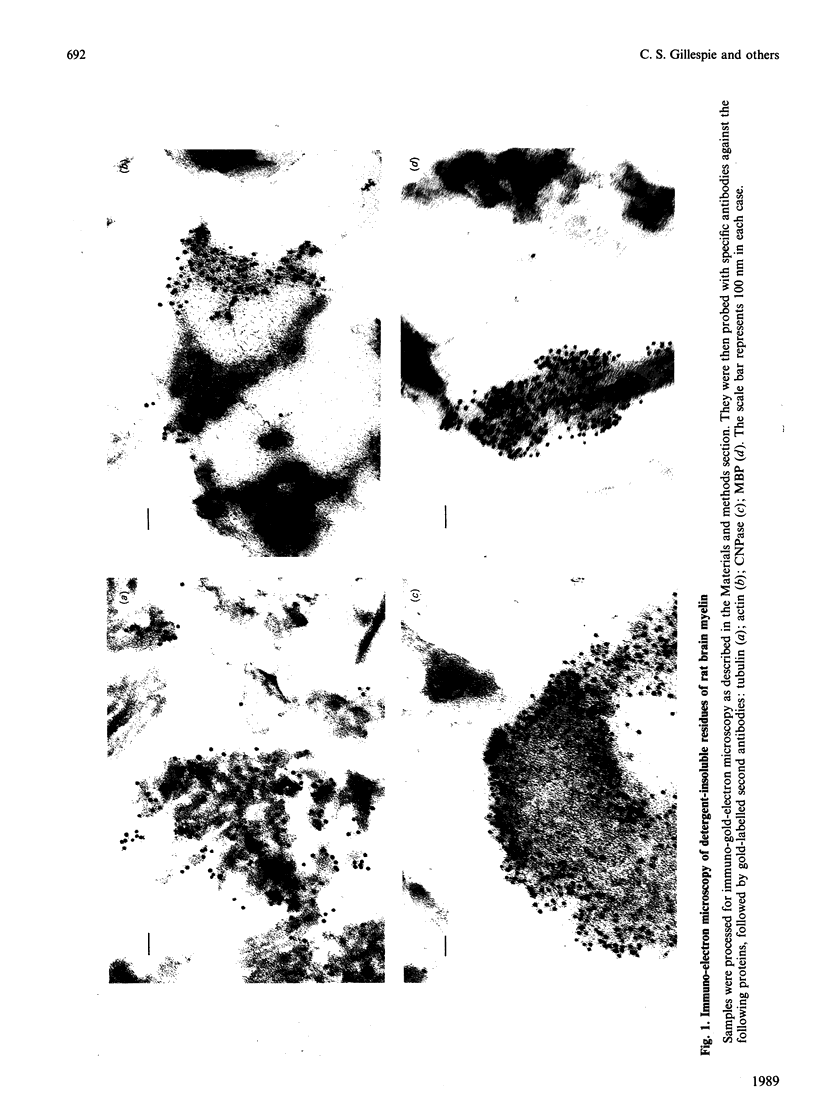


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal H. C., O'Connell K., Randle C. L., Agrawal D. Phosphorylation in vivo of four basic proteins of rat brain myelin. Biochem J. 1982 Jan 1;201(1):39–47. doi: 10.1042/bj2010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali I. U., Hynes R. O. Effects of cytochalasin B and colchicine on attachment of a major surface protein of fibroblasts. Biochim Biophys Acta. 1977 Nov 15;471(1):16–24. doi: 10.1016/0005-2736(77)90388-1. [DOI] [PubMed] [Google Scholar]
- Apgar J. R., Herrmann S. H., Robinson J. M., Mescher M. F. Triton X-100 extraction of P815 tumor cells: evidence for a plasma membrane skeleton structure. J Cell Biol. 1985 May;100(5):1369–1378. doi: 10.1083/jcb.100.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash J. F., Louvard D., Singer S. J. Antibody-induced linkages of plasma membrane proteins to intracellular actomyosin-containing filaments in cultured fibroblasts. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5584–5588. doi: 10.1073/pnas.74.12.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baryłko B., Dobrowolski Z. Ca2+-calmodulin-dependent regulation of F-actin-myelin basic protein interaction. Eur J Cell Biol. 1984 Nov;35(2):327–335. [PubMed] [Google Scholar]
- Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Annu Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Bradbury J. M., Campbell R. S., Thompson R. J. Endogenous cyclic AMP-stimulated phosphorylation of a Wolfgram protein component in rabbit central-nervous-system myelin. Biochem J. 1984 Jul 15;221(2):351–359. doi: 10.1042/bj2210351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürgisser P., Matthieu J. M., Jeserich G., Waehneldt T. V. Myelin lipids: a phylogenetic study. Neurochem Res. 1986 Sep;11(9):1261–1272. doi: 10.1007/BF00966121. [DOI] [PubMed] [Google Scholar]
- Carraway C. A., Jung G., Carraway K. L. Isolation of actin-containing transmembrane complexes from ascites adenocarcinoma sublines having mobile and immobile receptors. Proc Natl Acad Sci U S A. 1983 Jan;80(2):430–434. doi: 10.1073/pnas.80.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. A., Wigglesworth N. M., Allan D., Owens R. J., Crumpton M. J. Nonidet P-40 extraction of lymphocyte plasma membrane. Characterization of the insoluble residue. Biochem J. 1984 Apr 1;219(1):301–308. doi: 10.1042/bj2190301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Buono B. J., Williamson P. L., Schlegel R. A. Relation between the organization of spectrin and of membrane lipids in lymphocytes. J Cell Biol. 1988 Mar;106(3):697–703. doi: 10.1083/jcb.106.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer C. A., Benjamins J. A. Antibody to galactocerebroside alters organization of oligodendroglial membrane sheets in culture. J Neurosci. 1988 Nov;8(11):4307–4318. doi: 10.1523/JNEUROSCI.08-11-04307.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng L. F., Noble E. P. The maturation of rat brain myelin. Lipids. 1968 Mar;3(2):157–162. doi: 10.1007/BF02531734. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Boyles J. K., Berndt M. C., Steffen P. K., Anderson L. K. Identification of a membrane skeleton in platelets. J Cell Biol. 1988 May;106(5):1525–1538. doi: 10.1083/jcb.106.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzer W. B. The red cell membrane and its cytoskeleton. Biochem J. 1981 Jul 15;198(1):1–8. doi: 10.1042/bj1980001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves A. J., Wandosell F., Avila J. Phosphorylation of tubulin enhances its interaction with membranes. 1986 Oct 30-Nov 5Nature. 323(6091):827–828. doi: 10.1038/323827a0. [DOI] [PubMed] [Google Scholar]
- Hay E. D. Matrix-cytoskeletal interactions in the developing eye. J Cell Biochem. 1985;27(2):143–156. doi: 10.1002/jcb.240270208. [DOI] [PubMed] [Google Scholar]
- Kean E. L. Rapid, sensitive spectrophotometric method for quantitative determination of sulfatides. J Lipid Res. 1968 May;9(3):319–327. [PubMed] [Google Scholar]
- Kreibich G., Sabatini D. D. Selective release of content from microsomal vesicles without membrane disassembly. II. Electrophoretic and immunological characterization of microsomal subfractions. J Cell Biol. 1974 Jun;61(3):789–807. doi: 10.1083/jcb.61.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Macala L. J., Yu R. K., Ando S. Analysis of brain lipids by high performance thin-layer chromatography and densitometry. J Lipid Res. 1983 Sep;24(9):1243–1250. [PubMed] [Google Scholar]
- Matthieu J. M., Costantino-Ceccarini E., Bény M., Reigner J. Evidence for the association of 2',3'-cyclic-nucleotide 3'-phosphodiesterase with myelin-related membranes in peripheral nervous system. J Neurochem. 1980 Dec;35(6):1345–1350. doi: 10.1111/j.1471-4159.1980.tb09008.x. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Jose M. J., Balk S. P. Actin-containing matrix associated with the plasma membrane of murine tumour and lymphoid cells. Nature. 1981 Jan 15;289(5794):139–144. doi: 10.1038/289139a0. [DOI] [PubMed] [Google Scholar]
- Modesti N. M., Barra H. S. The interaction of myelin basic protein with tubulin and the inhibition of tubulin carboxypeptidase activity. Biochem Biophys Res Commun. 1986 Apr 29;136(2):482–489. doi: 10.1016/0006-291x(86)90466-3. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Lazarides E. Goblin (ankyrin) in striated muscle: identification of the potential membrane receptor for erythroid spectrin in muscle cells. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3292–3296. doi: 10.1073/pnas.81.11.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli V., Burger M. M. Interaction of the cytoskeleton with the plasma membrane. J Membr Biol. 1987;100(2):97–121. doi: 10.1007/BF02209144. [DOI] [PubMed] [Google Scholar]
- Norton W. T., Poduslo S. E. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973 Oct;21(4):749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Pereyra P. M., Horvath E., Braun P. E. Triton X-100 extractions of central nervous system myelin indicate a possible role for the minor myelin proteins in the stability in lamellae. Neurochem Res. 1988 Jun;13(6):583–595. doi: 10.1007/BF00973301. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. The structure of cortical cytoplasm. Philos Trans R Soc Lond B Biol Sci. 1982 Nov 4;299(1095):275–289. doi: 10.1098/rstb.1982.0132. [DOI] [PubMed] [Google Scholar]
- Timasheff S. N., Grisham L. M. In vitro assembly of cytoplasmic microtubules. Annu Rev Biochem. 1980;49:565–591. doi: 10.1146/annurev.bi.49.070180.003025. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waehneldt T. V. Density and protein profiles of myelin from two regions of young and adult rat CNS. Brain Res Bull. 1978 Jan-Feb;3(1):37–44. doi: 10.1016/0361-9230(78)90059-x. [DOI] [PubMed] [Google Scholar]
- Waxman S. G., Ritchie J. M. Organization of ion channels in the myelinated nerve fiber. Science. 1985 Jun 28;228(4707):1502–1507. doi: 10.1126/science.2409596. [DOI] [PubMed] [Google Scholar]
- Williamson P., Bateman J., Kozarsky K., Mattocks K., Hermanowicz N., Choe H. R., Schlegel R. A. Involvement of spectrin in the maintenance of phase-state asymmetry in the erythrocyte membrane. Cell. 1982 Oct;30(3):725–733. doi: 10.1016/0092-8674(82)90277-x. [DOI] [PubMed] [Google Scholar]
- de Néchaud B., Wolff A., Jeantet C., Bourre J. M. Characterization of tubulin in mouse brain myelin. J Neurochem. 1983 Dec;41(6):1538–1544. doi: 10.1111/j.1471-4159.1983.tb00861.x. [DOI] [PubMed] [Google Scholar]