Abstract
Background
Tirbanibulin is approved for actinic keratosis (AK) field treatment up to 25 cm2. However, AK often affects larger areas; thus, AK treatments for larger fields are needed.
Objective
Evaluate the safety and tolerability of tirbanibulin when applied to a field of approximately 100 cm2.
Methods
Phase 3, multicenter, open-label, single-arm study among adult patients having a treatment field on the face or balding scalp of approximately 100 cm2 with 4-12 AKs. Patients received tirbanibulin to cover the treatment field once daily (5 consecutive days). Safety was assessed by evaluating treatment emergent adverse events and tolerability by composite score of 6 local tolerability signs (LTS).
Results
A total of 105 patients were included. The most common LTS were erythema (96.1%) and flaking/scaling (84.4%), being mostly mild-to-moderate severity, and resolved/returned to or close to baseline by Day 29. The only severe LTS were erythema (5.8%) and flaking/scaling (8.7%). Most frequent treatment emergent adverse events were application site pruritus (10.5%) and application site pain (8.6%). Mean total number of AKs decreased from 7.7 AKs at baseline to 1.8 AKs at Day 57. Mean percent of change (reduction) from baseline in lesion count was 77.8% at Day 57.
Limitations
No control group. No long-term follow-up.
Conclusion
Safety and tolerability profiles in patients treated with tirbanibulin up to 100 cm2 were consistent with those previously reported over smaller field. Tirbanibulin could be used on a larger field (>25 cm2).
Key words: actinic keratosis, face, precancerous skin disease, safety, scalp, tirbanibulin, tolerability, treatment field, UV light-damaged skin
Capsule Summary.
-
•
Tirbanibulin is approved for the field treatment of actinic keratosis up to 25 cm2. However, actinic keratosis often affects larger areas requiring the need for treatments for larger fields.
-
•
Findings from this study support the use of tirbanibulin over a treatment field up to 100 cm2.
Introduction
Actinic keratosis (AK) is an ultraviolet light-induced precancerous skin disease resulting from the atypical proliferation of keratinocytes, which may progress to squamous cell carcinoma (SCC).1, 2, 3 Given the potential for progression to invasive SCC, dermatologists encourage early and effective treatment of AK, as recommended in current national and international treatment guidelines.4, 5, 6, 7, 8, 9, 10
Tirbanibulin ointment 1% is a synthetic, first-in-class, reversible tubulin polymerization inhibitor with potent antiproliferative and antitumoral effects.11,12 This novel drug has demonstrated efficacy, tolerability, and safety over the phase 1 (NCT02337205), phase 2 (NCT02838628), and phase 3 (NCT03285477 and NCT03285490) trials, as well as in real-world studies.13, 14, 15, 16 Tirbanibulin is approved for topical treatment of AK on the face or scalp over a field up to 25 cm2.17,18 However, AK often appears in larger areas of photo-damaged skin. Moreover, the number of AK field-directed therapies targeting areas >25 cm2 currently available is limited, and does not address all the patient needs.19,20 Therefore, there is a need for AK treatments to encompass fields >25 cm2, with an optimal efficacy-tolerability profile and patient convenience. Pharmacokinetics under maximal use conditions has been evaluated in 100 cm2 followed a successful phase 1 study.21 Here, we report the safety, tolerability, and exploratory efficacy of tirbanibulin applied to a field of approximately 100 cm2 on the face or balding scalp.
Methods
Trial design
Phase 3, multicenter, open-label, single-arm trial (NCT05279131) to evaluate safety and local tolerability of tirbanibulin ointment 1% administered topically once daily for 5 consecutive days over a field of approximately 100 cm2 on the face or balding scalp in adult patients with AK. The trial was conducted from June to December 2022 in the United States in accordance with the Declaration of Helsinki and the International Council for Harmonization Good Clinical Practice guidelines. Ethical approval was obtained from the Institutional Ethics Committee, Institutional Review Board, and Regulatory Authority before study initiation. All patients signed the written informed consent form (including consent to use the photographs of the treatment fields) before participating in the trial.
Participants
Eligible participants were adult patients with AKs on their face or balding scalp (excluding lips, eyelids, and inside nostrils and ears) measuring approximately 100 cm2 and containing 4-12 clinically typical, visible, and discrete AKs within the treatment field. Exclusion criteria included the following: history of invasive SCC, Bowen’s disease, basal cell carcinoma, or other malignant tumors in the treatment field; hyperkeratotic or hypertrophic lesions, recalcitrant disease and/or cutaneous horn; and any other dermatologic disease or condition that could interfere with the evaluations. Moreover, patients who had received any of the following treatments within the treatment field or within 2 cm of the treatment field, within 8 weeks prior to the screening visit were excluded: 5-fluorouracil, imiquimod, ingenol mebutate, diclofenac, or photodynamic therapy. Other therapeutic procedures such cryotherapy or curettage were excluded 4 weeks prior to screening.
Enrollment was controlled such that the trial population was representative of the population expected to use the product in terms of age and area treated. Therefore, it was designed to include a minimum of 50% of patients older than 65 year-old and approximately two-thirds of the patients treated on the face and one-third on the scalp.
Interventions
The study consisted of a 4-week screening period, a 5-day treatment period, and a response evaluation period of approximately 7 weeks (57 days). The total duration of each patient’s participation in the trial was approximately 3 months. During the treatment period, patients applied tirbanibulin once daily (enough to cover the treatment field from a sachet with 350 mg) for 5 days starting on Day 1. On Day 1, treatment application was performed at the investigation site under the supervision of clinical trial site staff. Between days 2 and 5, the treatment was self-administered once daily at home. Safety, tolerability, and the presence of AKs in the treatment field were evaluated in all patients until the completion of the response assessment period on Day 57. These assessments were done on Days 5, 8, 15, 29, and 57.
Outcomes and measures
The primary objective of the trial was to evaluate the tolerability and safety of tirbanibulin when applied to a field of approximately 100 cm2 on the face or balding scalp. The local tolerability endpoints were as follows: local tolerability signs (LTS) score by visit (0 absent, 1 mild, 2 moderate, and 3 severe) for each individual sign (erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration); maximum local tolerability score postbaseline through all the visits for each individual sign; time to maximum local tolerability score for each individual sign; local tolerability signs composite score (0-18) by visit, defined as the sum of the scores graded 0-3 on all 6 individual tolerability sign categories; maximum LTS composite score postbaseline through all the visits; time to maximum local tolerability composite score; and pigmentation and scarring in the treatment field through all the visits.
Safety endpoints were the assessment of treatment-emergent adverse events (TEAEs), treatment-emergent serious adverse events, adverse events of special interest, clinical laboratory data, vital signs, physical examinations, and electrocardiograms.
Additionally, the treatment effect was explored by the absolute number, change from baseline, and percent change from baseline in AK lesion count from total lesions in the treatment field at each visit (for new lesions only the absolute number and percent change).
Statistical analysis
It was calculated that a sample size of 100 patients would provide a precision (defined as the width of the 95% confidence interval [CI]) in the estimation of the percentage of LTS of approximately 11% (assuming an expected percentage of patients with at least 1 LTS of approximately 90%). Furthermore, 100 patients would provide approximately 10% accuracy in estimating the percentage of patients with vesiculation/pustulation (expected percentage: 8%) and 13% with erosion/ulceration (expected percentage: 12%).
No imputation for missing data was done. All variables were analyzed descriptively with appropriate statistical methods. Categorical variables were summarized with counts and percentages. For continuous variables, the number of nonmissing observations, mean, standard deviation (SD), standard error of the mean, 95% CI of the mean, median, first and third quartiles, minimum and maximum were used. Subgroup descriptive statistics were performed for the exploratory endpoints according to age (<65 vs ≥65), sex (male vs female), number of AK lesions in the treatment field at baseline (≤8 vs >8), treatment area (face vs scalp), and Fitzpatrick skin type (I/II vs III/IV/V/VI). All analyses were performed using SAS (SAS Institute) version 9.4.
Results
Patient characteristics
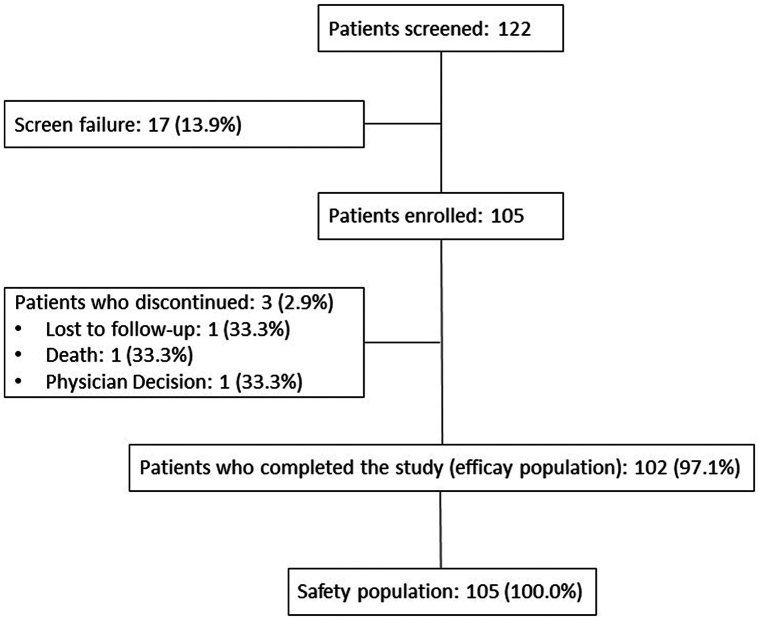
A total of 105 patients were enrolled in the study and 102 patients completed the study. There was one withdrawal due to investigator’s decision, one loss to follow-up, and one death (nonrelated, because of a diffuse metastatic cancer). The safety analysis set included all enrolled patients who received at least one dose of tirbanibulin (N = 105), and the exploratory efficacy analysis set included all patients who completed the study (N = 102) (Fig 1). Baseline demographics and disease characteristics are shown on Table I.
Fig 1.
Actinic keratosis. Patient flow diagram.
Table I.
Baseline demographics and disease characteristics
| Characteristic | Tirbanibulin patients on 100 cm2 (N = 105) |
|---|---|
| Sex, n (%) | |
| Male | 72 (68.6) |
| Female | 33 (31.4) |
| Age, mean (SD), years | 69.1 (8.9) |
| Age group, n (%) | |
| <65 years | 37 (35.2) |
| ≥65 years | 68 (64.8) |
| White, n (%) | 105 (100.0) |
| Hispanic or Latino | 7 (6.7) |
| Not Hispanic or Latino | 98 (93.3) |
| Fitzpatrick skin type, n (%) | |
| Type I | 8 (7.6) |
| Type II | 66 (62.9) |
| Type III | 30 (28.6) |
| Type IV | 1 (1.0) |
| AK location, n (%) | |
| Face | 71 (67.6) |
| Scalp | 34 (32.4) |
| Number of baseline AK lesions, mean (SD) | 7.7 (2.7) |
| Number of baseline AK lesions group, n (%) | |
| ≤8 lesions | 65 (61.9) |
| >8 lesions | 40 (38.1) |
AK, Actinic keratosis; SD, standard deviation.
Study treatment exposure
Among the safety population (N = 105), the mean (SD) exposure period was 4.9 (0.6) days and the mean (SD) cumulative amount of tirbanibulin applied was 1.6 (0.3) g.
Local tolerability outcomes
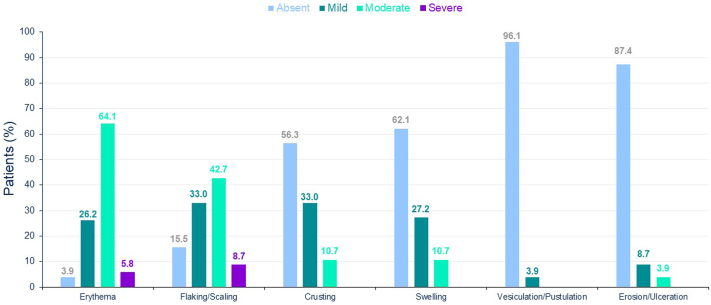
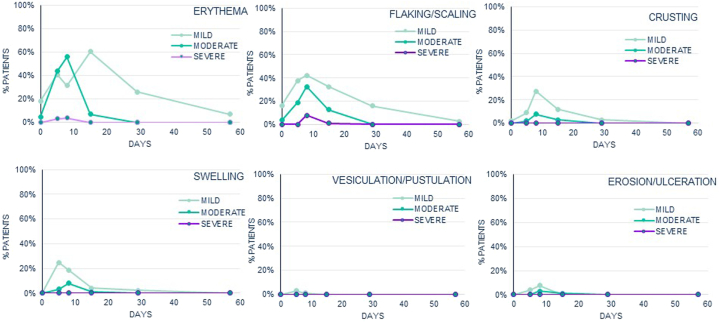
At baseline, few patients had mild or moderate erythema (22.9%), flaking/scaling (20.0%), or crusting (1.9%). The most common LTS after 100 cm2 tirbanibulin administration were erythema (99 patients [96.1%]) and flaking/scaling (87 patients [84.4%]). In most of the patients, maximum local tolerability score postbaseline was moderate for erythema (66 patients [64.1%]) and flaking/scaling (44 patients [42.7%]) and absent for vesiculation/postulation (99 patients [96.1%]), erosion/ulceration (90 patients [87.4%]), swelling (64 patients [62.1%]), and crusting (58 patients [56.3%]). Erythema and flaking/scaling were the only LTS in which severe cases were reported; 5.8% of patients (n = 6) and 8.7% of patients (n = 9), respectively (Fig 2). LTS peaked around Days 5 to 8 and resolved or returned to or close to baseline by Day 29 (Fig 3). At Day 57, the percentage of patients with erythema (6.9%), flaking/scaling (2.9%) or crusting (0.0%) was lower than that recorded at baseline (22.9%, 20.0%, and 1.9%, respectively).
Fig 2.
Actinic keratosis. Maximum local tolerability score throughout study (safety population, N = 105).
Fig 3.
Actinic keratosis. Evolution of the local tolerability signs across the study (safety population, N = 105).
The mean (SD) maximum local tolerability composite score was 4.1 (2.1) out of 18 with median (95% CI) time to the maximum total composite score from Kaplan–Meier estimation of 7.0 (6.0-7.0) days. The composite score followed the same trend as individual signs, with a mean (SD) composite score increasing over time up to Day 8 (mean composite score of 3.8 [2.3]) and then decreased at Day 15 (mean composite score of 1.6 [1.5]) to return to values close to baseline by Day 29 (mean composite score of 0.5 [0.8]).
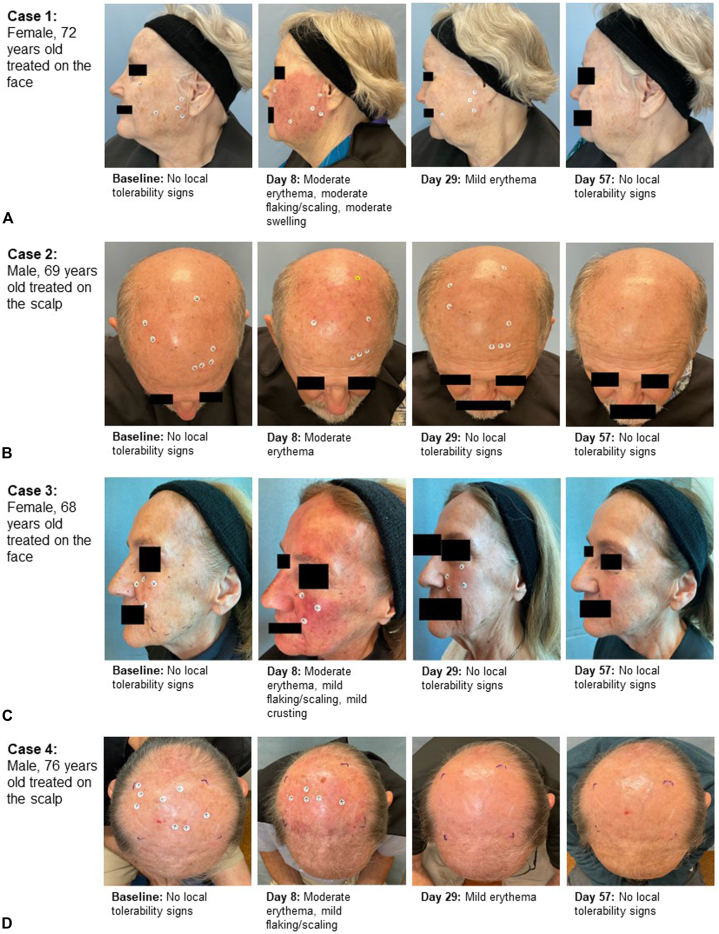
At baseline, most patients had absence of scarring (93 patients [88.6%]), hypopigmentation (83 patients [79.0%]), or hyperpigmentation (77 patients [73.3%]) in the treatment field. Overall, the number of patients without scarring; hypopigmentation, and hyperpigmentation in the treatment field was slightly higher at Day 57 compared to baseline (without scarring: 93 patients [88.6%] at Day 57 vs 93 patients [88.6] at baseline; without hypopigmentation: 84 patients [80.0%] at Day 57 vs 83 patients [79.0%] at baseline; and without hyperpigmentation: 86 patients [81.9%] at Day 57 vs 77 patients [73.3%] at baseline). Fig 4 shows photographs of the evolution of the AK lesions during the study in selected patients.
Fig 4.
Actinic keratosis. A-D, Pre- and post-treatment appearance and changes during the study in 4 cases of the treated sample.
Safety outcomes
A total of 29 (27.6%) patients reported at least one TEAE; in 19 (18.1%) of these patients, the TEAEs were considered treatment-related by the investigator. All treatment-related TEAEs were resolved or resolving at the end of the study, and none required treatment with a concomitant medication. Most TEAEs were rated as mild (22 patients [21.0%]) intensity. The most frequent TEAEs were application site pruritus (11 patients [10.5%]) and application site pain (9 patients [8.6%]). There were 2 (1.9%) patients with at least one serious TEAE, both serious events were considered nonrelated to the drug: tachycardia reported in a 78-year-old patient that required hospitalization with discharge the next day; and diffuse metastatic cancer in an 86-year-old patient with history of prostatic cancer culminating in death. No TEAEs leading to treatment discontinuation were reported. Two adverse events of special interest were reported: 1 SCC diagnosed 4 days after treatment start classified as moderate, outside the treatment field; and 1 SCC in situ diagnosed 59 days after treatment start classified as mild. Both considered not related to tirbanibulin by the investigator (Table II). There were no clinically significant treatment-related changes in laboratory data, vital signs, physical examinations, or electrocardiograms.
Table II.
Summary of adverse events
| Adverse events, n (%) | Tirbanibulin patients on 100 cm2 (N = 105) |
|---|---|
| Patients with at least one TEAE | 29 (27.6) |
| Patients with at least one treatment-related TEAE | 19 (18.1) |
| Patients with at least one TEAE by worst severity: | |
| Mild | 22 (21.0) |
| Moderate | 6 (5.7) |
| Severe | 1 (1.0) |
| Patients with at least one serious TEAE | 2∗ (1.9) |
| Patients with at least one TEAE leading to death | 1† (1.0) |
| Patients with at least one adverse event of special interest | 2‡ (1.9) |
| Patients with at least one application site TEAE | 18 (17.1) |
| Patients with at least one TEAE leading to treatment discontinuation | 0 (0) |
| TEAEs reported in >1% | |
| Application site pruritus | 11 (10.5) |
| Application site pain | 9 (8.6) |
| Dizziness | 2 (1.9) |
| Paraesthesia | 2 (1.9) |
| SCC | 2‡ (1.9) |
SCC, Squamous cell carcinoma; TEAE, treatment emergent adverse event.
Tachycardia and diffuse metastatic cancer; both events considered non-related to the drug as per investigator criteria.
Diffuse metastatic cancer considered serious and non-related to the drug as per investigator criteria that led to death.
Two SCCs; both events considered non-related to the drug as per investigator criteria. A 75-year-old patient with SCC outside the treatment field, 4 days after treatment start, moderate, non-serious, considered nonrelated to the treatment. And an 81-year-old patient with SCC inside the treatment field, 59 days after treatment start, mild, nonserious, considered nonrelated to the treatment.
Exploratory efficacy outcomes
At baseline, patients had a mean (SD) total number of 7.7 (2.7) AKs. The total number of AKs in the treatment field progressively decreased over the study with a mean (SD) number of 5.9 (3.4) at Day 5, 5.4 (4.0) at Day 8, 3.8 (3.9) at Day 15, 2.3 (2.6) at Day 29, and 1.8 (2.2) at the end of the study (Day 57).
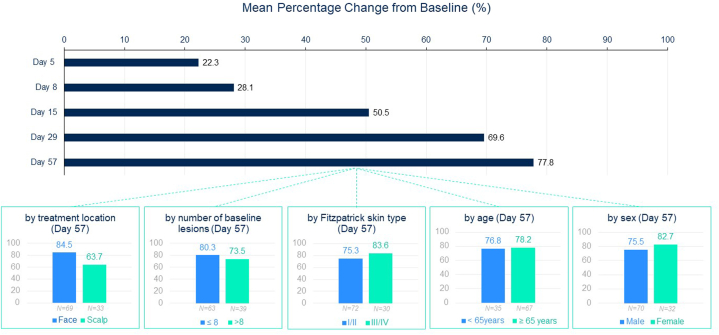
Mean (SD) percent of change (reduction) from baseline in lesion count by visit was 22.3% (36.4) at Day 5, 28.1% (47.3) at Day 8, 50.5% (45.2) at Day 15, 69.6% (35.0) at Day 29, and 77.8% (26.8) at Day 57. When analyzing the percent change from baseline in the total number of AKs by subgroups of patients at Day 57, mean percentage of change was similar by age (<65 years vs ≥65 years), by sex (male vs female), by number of AKs at baseline (≤8 AKs vs >8 AKs), and by Fitzpatrick skin type (type I/II vs type III/IV) while small differences were observed by treatment area (face vs scalp) (Fig 5).
Fig 5.
Actinic keratosis. Mean percentage change from baseline in lesion count by visit and by subgroups of patients at Day 57 (N = 102).
Overall, the mean number of new AKs that appeared in the treatment field remained generally low from Day 5 up to Day 57 (mean ranging between 0.2 and 0.6 new AKs), and at least 75% of patients did not have any new lesions at each visit. The maximum number of new AKs was observed at Day 8 with a mean percent change of 8.3% of baseline AK lesion count.
Discussion
The results of this study revealed that tirbanibulin 1% was well tolerated and showed a good overall safety profile after application to a larger field of 100 cm2 on the face or scalp. As seen in the previous phase 3 studies with a smaller treatment field,14 LTS were mostly mild to moderate in severity and most frequently consisted of erythema (96.1% in this study and 90.9% in the pooled analysis of the 2 small field studies) and flaking/scaling (84.4% vs 81.9%). As observed in clinical trials with tirbanibulin over a 25 cm2 treatment field14 and in contrast to most topical treatments for AK,19,20,22,23 severe LTS were infrequent with tirbanibulin; few patients had severe transient erythema (5.8% vs 6.2% in the small field studies) and/or severe transient flaking/scaling (8.7% vs 8.8% in the small field studies). The individual LTS scores increased to peak around Day 5 to Day 8 and then decreased to return to or close to baseline score by Day 29 without treatment. Tirbanibulin showed no detrimental cosmetic change to the treated skin area as concluded without relevant change in pigmentation and/or scarring in the treatment field.
The most common TEAEs reported in the study were application site reactions, mainly mild to moderate transient application site pruritus (10.5%) and application site pain (8.6%). Systemic TEAEs were below 2% (dizziness 1.9% and paresthesia 1.9%). No treatment-related serious AEs or treatment-related deaths occurred in in the study. Additionally, no clinically significant treatment-related changes in laboratory parameters, vital signs, or electrocardiogram were observed. SCCs were reported in less than 2% of the patients; one of them was outside the application area and the other one inside; both were considered not related to the study treatment. Similar results were obtained in the phase 3 clinical trials with tirbanibulin ointment over a 25 cm2 treatment field.14
The analysis of the exploratory efficacy data revealed that treatment with tirbanibulin 1% progressively reduced the total number of AK lesions in the treatment field over the course of the study with a mean percent reduction of 77.8% at Day 57 (similar to those observed in the small field clinical trials [76% in trial NCT03285477, and 82% in trial NCT03285490]).14 Furthermore, the mean number of new AK lesions that appeared in the treatment field remained generally low over time (ranging between 0.2 and 0.6 new AK lesions) and 3 quarters of patients did not have any new lesions.
Limitations
One of the potential limitations of this study is the lack of a placebo group. Additionally, there was no long-term follow-up to assess long-term safety events and persistent efficacy.
Conclusions
In this phase 3 clinical trial, local tolerability and safety profiles were well characterized in patients with 4-12 clinically typical, visible, and discrete AK lesions in a field of 100 cm2, and were consistent with those previously reported in patients with AK treated in pivotal trials with tirbanibulin over a smaller field (25 cm2).13,14 Moreover, efficacy was explored and showed a percent reduction of lesions from baseline (77.8%), consistent with the reduction observed in a treatment field of 25 cm2.14 These results support the safety of tirbanibulin ointment 1% for use in patients with AK of the face or scalp in disease fields up to 100 cm2.
Conflicts of interest
Dr Bhatia is a consulting honoraria from and investigator for Almirall, Biofrontera, Leo, Ortho, and Sun Pharma. Dr Blauvelt is a speaker (received honoraria) for AbbVie, Eli Lilly and Company, Pfizer, and UCB; scientific adviser (received honoraria) for AbbVie, Abcentra, Aclaris, Affibody, Aligos, Almirall, Alumis, Amgen, Anaptysbio, Apogee, Arcutis, Arena, Aslan, Athenex, Bluefin Biomedicine, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, CTI BioPharma, Dermavant, EcoR1, Eli Lilly and Company, Escient, Evelo, Evommune, Forte, Galderma, HighlightII Pharma, Incyte, InnoventBio, Janssen, Landos, Leo, Lipidio, Microbion, Merck, Monte Rosa Therapeutics, Nektar, Novartis, Overtone Therapeutics, Paragon, Pfizer, Q32 Bio, Rani, Rapt, Regeneron, Sanofi Genzyme, Spherix Global Insights, Sun Pharma, Takeda, TLL Pharmaceutical, TrialSpark, UCB Pharma, Union, Ventyx, Vibliome, and Xencor; and clinical study investigator (institution has received clinical study funds) for AbbVie, Acelyrin, Allakos, Almirall, Alumis, Amgen, Arcutis, Athenex, Boehringer Ingelheim, Bristol-Myers Squibb, Concert, Dermavant, Eli Lilly and Company, Evelo, Evommune, Galderma, Incyte, Janssen, Leo, Merck, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, UCB Pharma, and Ventyx. Dr Lain is a clinical investigator, consultant, advisor, and/or paid speaker for Almirall, Athenex, Gage Pharmaceuticals, UCB, Abbvie, Sanofi, Regeneron, Vyne Pharmaceuticals, Pfizer, Amgen, Novartis, Eli Lilly, Kadmon, Chemocentryx, Bausch Health, Galderma, Dermavant, Arcutis, Bristol Myers Squibb, Kiniksa, Mindera, Sebacia, Pulse BioSciences, Leo Pharmaceuticals, Aclaris, Biorasi, Brickell, Cassiopea, Dr Reddy, Endo Pharmaceuticals, and G&E Herbal Biotechnology. Dr Jarell is an investigator of or received grants/research funding from AbbVie, Almirall, Arcutis Biotherapeutics, Arcutis, Inc, Asana Biosciences, LLC, BMS, Castle Biosciences, Concert Pharmaceuticals, Dermira, Foamix Pharmaceuticals Ltd, Incyte Corporation, Leo Pharma Inc, Lilly ICOS LLC, Novartis, Sanofi/Regeneron, UCB, Vivex Biomedical, Inc. Dr DuBois is an investigator of or received grants/research funding from AbbVie, AiViva BioPharma, Allergan, Inc, Almirall, AnaptysBio, Arcutis Biotherapeutics, Bausch Health, Biofrontera, Bristol-Myers Squibb, Caliway Biopharmaceuticals Co, Ltd, Cara Therapeutics, Croma-Pharma GmbH Austria, Dermata Therapeutics, DermBiont, Dr Reddy, Endo Pharmaceuticals, Evommune, Inc., Galderma USA, Incyte Corporation, LEO Laboratories Ltd (LEO Pharma), Merck, Moberg Pharma, Palvella Therapeutics, RAPT Therapeutics, Scarless Laboratories, Therapeutics Inc, Veradermics Inc. Drs Tamarit, Falques, Kiyasova, Padulles, and Otero are employees of Almirall Spain.
Acknowledgments
The authors would like to thank Irene Mansilla Núñez, MSc, from TFS HealthScience, for editorial assistance and writing support.
Footnotes
Dr Blauvelt is currently affiliated with the Blauvelt Consulting, LLC, Lake Oswego, Oregon.
Funding sources: This study was funded by Almirall S.A.
Patient consent: The authors obtained written consent from patients for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available. Patient consent forms were not provided to the journal but are retained by the authors.
IRB approval status: Central Institutional Review Board (IRB) approving the study was Advarra, 6100 Merriweather Dr., Suite 600, Columbia, MD 21044, United States. Approval date IRB: July 3, 2022.
References
- 1.Siegel J.A., Korgavkar K., Weinstock M.A. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177(2):350–358. doi: 10.1111/bjd.14852. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez Figueras M.T. From actinic keratosis to squamous cell carcinoma: pathophysiology revisited. J Eur Acad Dermatol Venereol. 2017;31(Suppl 2):5–7. doi: 10.1111/jdv.14151. [DOI] [PubMed] [Google Scholar]
- 3.Balcere A., Rone Kupfere M., Čēma I., Krūmiņa A. Prevalence, discontinuation rate, and risk factors for severe local site reactions with topical field treatment options for actinic keratosis of the face and scalp. Medicina (Kaunas) 2019;55(4):92. doi: 10.3390/medicina55040092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisen D.B., Asgari M.M., Bennett D.D., et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85(4):e209–e233. doi: 10.1016/j.jaad.2021.02.082. [DOI] [PubMed] [Google Scholar]
- 5.Eisen D.B., Dellavalle R.P., Frazer-Green L., Schlesinger T.E., Shive M., Wu P.A. Focused update: guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2022;87(2):373–374.e5. doi: 10.1016/j.jaad.2022.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Massey P.R., Schmults C.D., Li S.J., et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients. JAMA Dermatol. 2021;157(10):1219–1226. doi: 10.1001/jamadermatol.2021.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Berker D., McGregor J.M., Mohd Mustapa M.F., Exton L.S., Hughes B.R. British Association of Dermatologists' guidelines for the care of patients with actinic keratosis 2017. Br J Dermatol. 2017;176(1):20–43. doi: 10.1111/bjd.15107. [DOI] [PubMed] [Google Scholar]
- 8.Hofbauer G., Anliker M., Boehncke W.H., et al. Swiss clinical practice guidelines on field cancerization of the skin. Swiss Med Wkly. 2014;144 doi: 10.4414/smw.2014.14026. [DOI] [PubMed] [Google Scholar]
- 9.Werner R.N., Stockfleth E., Connolly S.M., et al. Evidence- and consensus-based (S3) guidelines for the treatment of actinic keratosis - international league of dermatological societies in cooperation with the European Dermatology forum - short version. J Eur Acad Dermatol Venereol. 2015;29(11):2069–2079. doi: 10.1111/jdv.13180. [DOI] [PubMed] [Google Scholar]
- 10.Heppt M.V., Leiter U., Steeb T., et al. S3 guideline “actinic keratosis and cutaneous squamous cell carcinoma”- update 2023, part 1: treatment of actinic keratosis, actinic cheilitis, cutaneous squamous cell carcinoma in situ (Bowen's disease), occupational disease and structures of care. J Dtsch Dermatol Ges. 2023;21(10):1249–1262. doi: 10.1111/ddg.15231. [DOI] [PubMed] [Google Scholar]
- 11.Schlesinger T., Stockfleth E., Grada A., Berman B. Tirbanibulin for actinic keratosis: insights into the mechanism of action. Clin Cosmet Investig Dermatol. 2022;15:2495–2506. doi: 10.2147/CCID.S374122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smolinski M.P., Bu Y., Clements J., et al. Discovery of novel dual mechanism of action src signaling and tubulin polymerization inhibitors (KX2-391 and KX2-361) J Med Chem. 2018;61(11):4704–4719. doi: 10.1021/acs.jmedchem.8b00164. [DOI] [PubMed] [Google Scholar]
- 13.Kempers S., DuBois J., Forman S., et al. Tirbanibulin ointment 1% as a novel treatment for actinic keratosis: phase 1 and 2 results. J Drugs Dermatol. 2020;19(11):1093–1100. doi: 10.36849/JDD.2020.5576. [DOI] [PubMed] [Google Scholar]
- 14.Blauvelt A., Kempers S., Lain E., et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384(6):512–520. doi: 10.1056/NEJMoa2024040. [DOI] [PubMed] [Google Scholar]
- 15.Dao D.P.D., Sahni V.N., Sahni D.R., Balogh E.A., Grada A., Feldman S.R. 1% tirbanibulin ointment for the treatment of actinic keratoses. Ann Pharmacother. 2022;56(4):494–500. doi: 10.1177/10600280211031329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlesinger T., Kircik L., Del Rosso J., et al. Clinician- and patient-reported outcomes with tirbanibulin 1% treatment for actinic keratosis in routine clinical practice across the U.S. (PROAK study) J of Skin. 2023;7(3):771–787. doi: 10.25251/skin.7.3.3. [DOI] [Google Scholar]
- 17.Drugs@FDA: FDA-approved drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=213189
- 18.EMA. Klisyri. European Medicines Agency. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/klisyri
- 19.Del Rosso J.Q., Kircik L., Goldenberg G., Brian B. Comprehensive management of actinic keratoses. J Clin Aesthet Dermatol. 2014;7(9 Suppl S2-S12):S2–S12. [PMC free article] [PubMed] [Google Scholar]
- 20.Goldenberg G. Treatment considerations in actinic keratosis. J Eur Acad Dermatol Venereol. 2017;31(Suppl 2):12–16. doi: 10.1111/jdv.14152. [DOI] [PubMed] [Google Scholar]
- 21.DuBois J., Jones T.M., Lee M.S., et al. Pharmacokinetics, safety, and tolerability of a single 5-day treatment of tirbanibulin ointment 1% in 100 cm2: a phase 1 maximal-use trial in patients with actinic keratosis. Clin Pharmacol Drug Dev. 2024;13(2):208–218. doi: 10.1002/cpdd.1368. [DOI] [PubMed] [Google Scholar]
- 22.Khanna R., Bakshi A., Amir Y., Goldenberg G. Patient satisfaction and reported outcomes on the management of actinic keratosis. Clin Cosmet Investig Dermatol. 2017;10:179–184. doi: 10.2147/CCID.S121323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim S.F., Brown M.D. Actinic keratoses: a comprehensive update. J Clin Aesthet Dermatol. 2009;2(7):43–48. [PMC free article] [PubMed] [Google Scholar]