Abstract
Chromogranin A is a major component of storage granules in many different secretory cell types. After [35S]methionine labelling of proteins from cultured bovine chromaffin cells, chromogranin A was immunoprecipitated with specific antibodies, and the radioactivity incorporated into chromogranin A was determined and used as an index of its synthesis rate. Depolarization of cells with nicotine or high K+ evoked a Ca2+-dependent increase in chromogranin A synthesis, whereas muscarine, which does not evoke significant Ca2+ influx from bovine chromaffin cells, had no effect on chromogranin A synthesis. Forskolin, an activator of adenylate cyclase, affected neither the basal nor the nicotine-stimulated rate of chromogranin A synthesis. In contrast, 12-O-tetradecanoylphorbol 13-acetate (TPA), an activator of protein kinase C, significantly enhanced the incorporation of radioactivity into chromogranin A. Sphingosine, an inhibitor of protein kinase C, abolished both nicotine-stimulated and TPA-induced chromogranin A synthesis. In addition, long-term treatment of chromaffin cells with TPA decreased protein kinase C activity and inhibited the nicotine-stimulated chromogranin A synthesis. These results suggest that protein kinase C may play an important role in the control of chromogranin A synthesis.
Full text
PDF

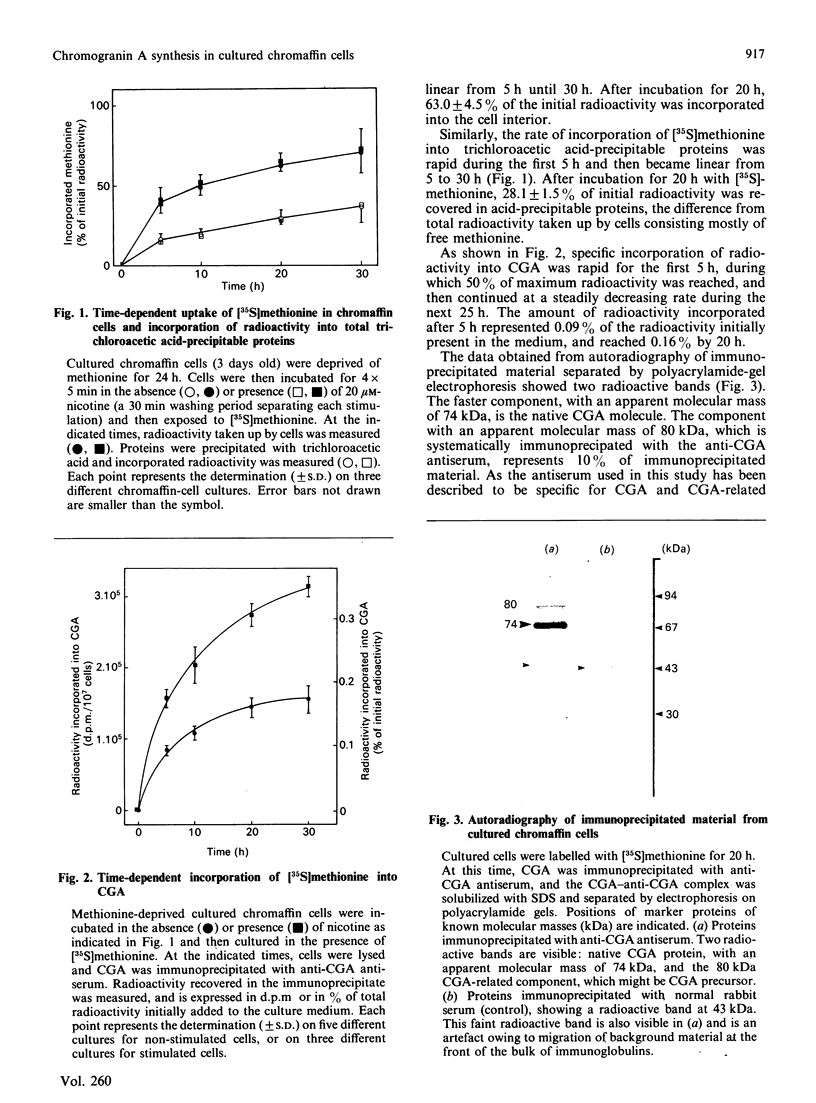
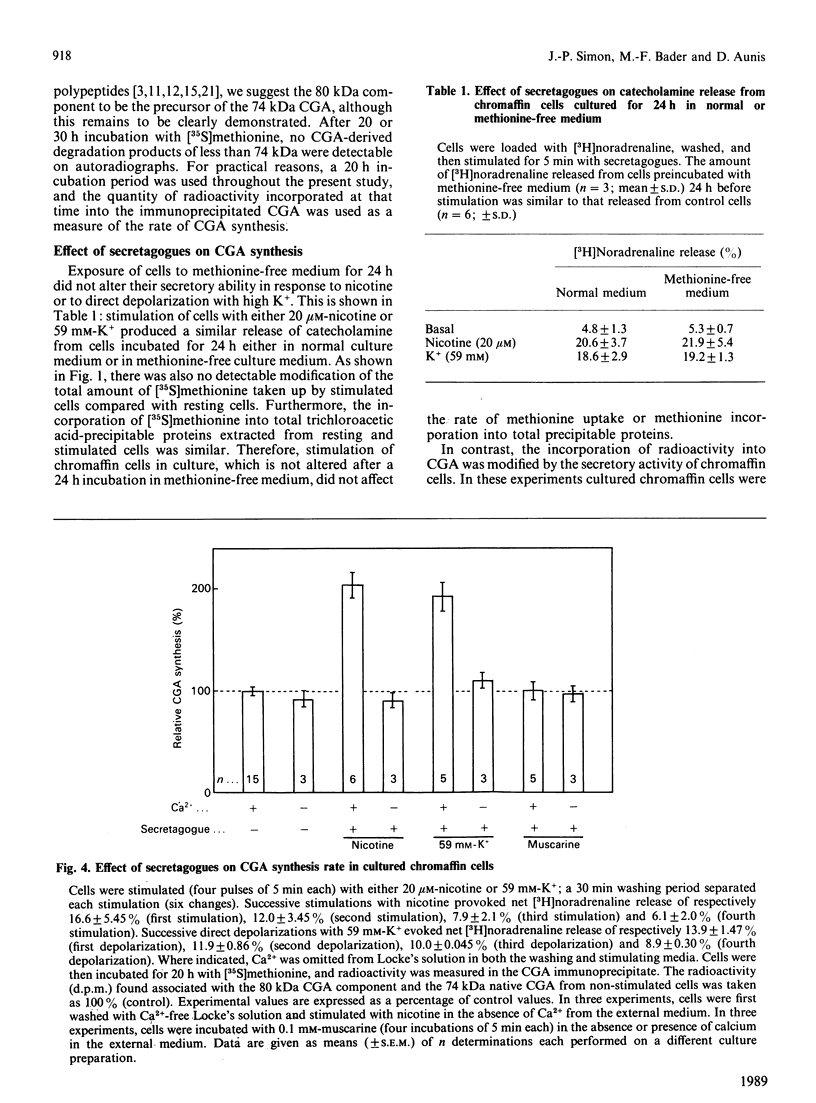
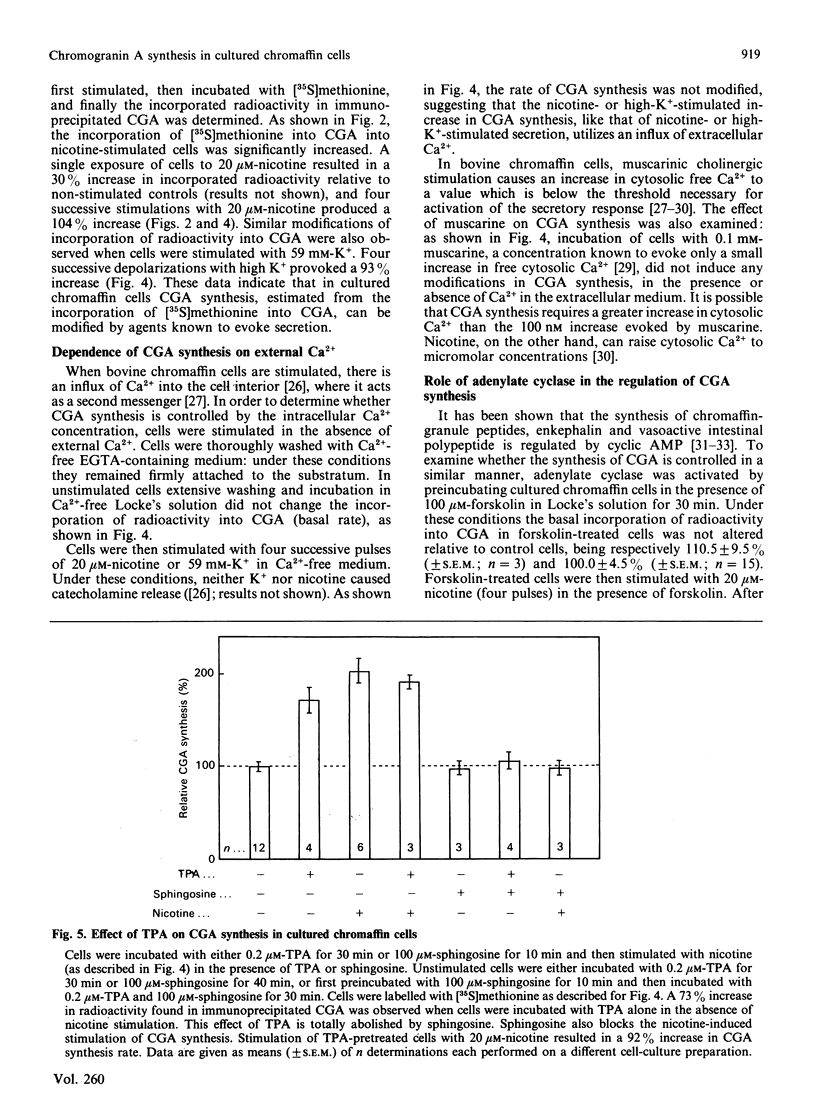


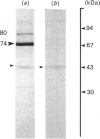
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artalejo C. R., García A. G., Aunis D. Chromaffin cell calcium channel kinetics measured isotopically through fast calcium, strontium, and barium fluxes. J Biol Chem. 1987 Jan 15;262(2):915–926. [PubMed] [Google Scholar]
- Bader M. F., Ciesielski-Treska J., Thierse D., Hesketh J. E., Aunis D. Immunocytochemical study of microtubules in chromaffin cells in culture and evidence that tubulin is not an integral protein of the chromaffin granule membrane. J Neurochem. 1981 Oct;37(4):917–933. doi: 10.1111/j.1471-4159.1981.tb04479.x. [DOI] [PubMed] [Google Scholar]
- Bader M. F., Georges E., Mushynski W. E., Trifaró J. M. Neurofilament proteins in cultured chromaffin cells. J Neurochem. 1984 Oct;43(4):1180–1193. doi: 10.1111/j.1471-4159.1984.tb12859.x. [DOI] [PubMed] [Google Scholar]
- Bader M. F., Thiersé D., Aunis D., Ahnert-Hilger G., Gratzl M. Characterization of hormone and protein release from alpha-toxin-permeabilized chromaffin cells in primary culture. J Biol Chem. 1986 May 5;261(13):5777–5783. [PubMed] [Google Scholar]
- Benedum U. M., Baeuerle P. A., Konecki D. S., Frank R., Powell J., Mallet J., Huttner W. B. The primary structure of bovine chromogranin A: a representative of a class of acidic secretory proteins common to a variety of peptidergic cells. EMBO J. 1986 Jul;5(7):1495–1502. doi: 10.1002/j.1460-2075.1986.tb04388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschko H., Comline R. S., Schneider F. H., Silver M., Smith A. D. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature. 1967 Jul 1;215(5096):58–59. doi: 10.1038/215058a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K. W., Morita K., Pollard H. B. Characterization of protein kinase C and its role in catecholamine secretion from bovine adrenal-medullary cells. Biochem J. 1985 May 15;228(1):35–42. doi: 10.1042/bj2280035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cohn D. V., Zangerle R., Fischer-Colbrie R., Chu L. L., Elting J. J., Hamilton J. W., Winkler H. Similarity of secretory protein I from parathyroid gland to chromogranin A from adrenal medulla. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6056–6059. doi: 10.1073/pnas.79.19.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deftos L. J., Björnsson B. T., Burton D. W., O'Connor D. T., Copp D. H. Chromogranin A is present in and released by fish endocrine tissue. Life Sci. 1987 Jun 1;40(22):2133–2136. doi: 10.1016/0024-3205(87)90002-6. [DOI] [PubMed] [Google Scholar]
- Ehrhart M., Grube D., Bader M. F., Aunis D., Gratzl M. Chromogranin A in the pancreatic islet: cellular and subcellular distribution. J Histochem Cytochem. 1986 Dec;34(12):1673–1682. doi: 10.1177/34.12.2878021. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Giraud P., Affolter H. U., Herbert E., Hotchkiss A. J. Alternative modes of enkephalin biosynthesis regulation by reserpine and cyclic AMP in cultured chromaffin cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3949–3953. doi: 10.1073/pnas.81.13.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden L. E., Giraud P., Dave J. R., Hotchkiss A. J., Affolter H. U. Nicotinic receptor stimulation activates enkephalin release and biosynthesis in adrenal chromaffin cells. Nature. 1984 Dec 13;312(5995):661–663. doi: 10.1038/312661a0. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Hotchkiss A. J. Cyclic adenosine monophosphate regulates vasoactive intestinal polypeptide and enkephalin biosynthesis in cultured bovine chromaffin cells. Neuropeptides. 1983 Dec;4(1):1–9. doi: 10.1016/0143-4179(83)90002-1. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Iacangelo A., Hsu C. M., Hotchkiss A. J., Bader M. F., Aunis D. Chromogranin A synthesis and secretion in chromaffin cells. J Neurochem. 1987 Jul;49(1):65–74. doi: 10.1111/j.1471-4159.1987.tb03395.x. [DOI] [PubMed] [Google Scholar]
- Falkensammer G., Fischer-Colbrie R., Richter K., Winkler H. Cell-free and cellular synthesis of chromogranin A and B of bovine adrenal medulla. Neuroscience. 1985 Feb;14(2):735–746. doi: 10.1016/0306-4522(85)90323-9. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie R., Iacangelo A., Eiden L. E. Neural and humoral factors separately regulate neuropeptide Y, enkephalin, and chromogranin A and B mRNA levels in rat adrenal medulla. Proc Natl Acad Sci U S A. 1988 May;85(9):3240–3244. doi: 10.1073/pnas.85.9.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube D., Aunis D., Bader F., Cetin Y., Jörns A., Yoshie S. Chromogranin A (CGA) in the gastro-entero-pancreatic (GEP) endocrine system. I. CGA in the mammalian endocrine pancreas. Histochemistry. 1986;85(6):441–452. doi: 10.1007/BF00508425. [DOI] [PubMed] [Google Scholar]
- Hagn C., Schmid K. W., Fischer-Colbrie R., Winkler H. Chromogranin A, B, and C in human adrenal medulla and endocrine tissues. Lab Invest. 1986 Oct;55(4):405–411. [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Phorbol ester binding and activation of protein kinase C on triton X-100 mixed micelles containing phosphatidylserine. J Biol Chem. 1986 Jul 15;261(20):9341–9347. [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Protein kinase C activation in mixed micelles. Mechanistic implications of phospholipid, diacylglycerol, and calcium interdependencies. J Biol Chem. 1986 Jun 5;261(16):7184–7190. [PubMed] [Google Scholar]
- Haycock J. W., Browning M. D., Greengard P. Cholinergic regulation of protein phosphorylation in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1677–1681. doi: 10.1073/pnas.85.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock J. W., Meligeni J. A., Bennett W. F., Waymire J. C. Phosphorylation and activation of tyrosine hydroxylase mediate the acetylcholine-induced increase in catecholamine biosynthesis in adrenal chromaffin cells. J Biol Chem. 1982 Nov 10;257(21):12641–12648. [PubMed] [Google Scholar]
- Hii C. S., Jones P. M., Persaud S. J., Howell S. L. A re-assessment of the role of protein kinase C in glucose-stimulated insulin secretion. Biochem J. 1987 Sep 1;246(2):489–493. doi: 10.1042/bj2460489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchi H., Nakanishi A., Uddin M. M., Ohuchi T., Oka M. Phorbol ester stimulates catecholamine synthesis in isolated bovine adrenal medullary cells. FEBS Lett. 1985 Sep 2;188(2):205–208. doi: 10.1016/0014-5793(85)80372-0. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Davidson H. W., Grimaldi K. A., Peshavaria M. Biosynthesis of betagranin in pancreatic beta-cells. Identification of a chromogranin A-like precursor and its parallel processing with proinsulin. Biochem J. 1987 Jun 1;244(2):449–456. doi: 10.1042/bj2440449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacangelo A., Affolter H. U., Eiden L. E., Herbert E., Grimes M. Bovine chromogranin A sequence and distribution of its messenger RNA in endocrine tissues. Nature. 1986 Sep 4;323(6083):82–86. doi: 10.1038/323082a0. [DOI] [PubMed] [Google Scholar]
- Kao L. S., Schneider A. S. Calcium mobilization and catecholamine secretion in adrenal chromaffin cells. A Quin-2 fluorescence study. J Biol Chem. 1986 Apr 15;261(11):4881–4888. [PubMed] [Google Scholar]
- Kao L. S., Schneider A. S. Muscarinic receptors on bovine chromaffin cells mediate a rise in cytosolic calcium that is independent of extracellular calcium. J Biol Chem. 1985 Feb 25;260(4):2019–2022. [PubMed] [Google Scholar]
- Kilpatrick L., Gavine F., Apps D., Phillips J. Biosynthetic relationship between the major matrix proteins of adrenal chromaffin granules. FEBS Lett. 1983 Dec 12;164(2):383–388. doi: 10.1016/0014-5793(83)80322-6. [DOI] [PubMed] [Google Scholar]
- Kley N. Multiple regulation of proenkephalin gene expression by protein kinase C. J Biol Chem. 1988 Feb 5;263(4):2003–2008. [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. The phorbol ester TPA increases the affinity of exocytosis for calcium in 'leaky' adrenal medullary cells. FEBS Lett. 1983 Aug 22;160(1-2):98–100. doi: 10.1016/0014-5793(83)80944-2. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Dean D. M., Whelan L. G., Udenfriend S., Rossier J. Co-release of enkephalin and catecholamines from cultured adrenal chromaffin cells. Nature. 1981 Jan 22;289(5795):317–319. doi: 10.1038/289317a0. [DOI] [PubMed] [Google Scholar]
- Meligeni J. A., Haycock J. W., Bennett W. F., Waymire J. C. Phosphorylation and activation of tyrosine hydroxylase mediate the cAMP-induced increase in catecholamine biosynthesis in adrenal chromaffin cells. J Biol Chem. 1982 Nov 10;257(21):12632–12640. [PubMed] [Google Scholar]
- Murray S. S., Burton D. W., Deftos L. J. The coregulation of secretion and cytoplasmic ribonucleic acid of chromogranin-A and calcitonin by phorbol ester in cells that produce both substances. Endocrinology. 1988 Feb;122(2):495–499. doi: 10.1210/endo-122-2-495. [DOI] [PubMed] [Google Scholar]
- Nolan J. A., Trojanowski J. Q., Hogue-Angeletti R. Neurons and neuroendocrine cells contain chromogranin: detection of the molecule in normal bovine tissues by immunochemical and immunohistochemical methods. J Histochem Cytochem. 1985 Aug;33(8):791–798. doi: 10.1177/33.8.3894497. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Burton D., Deftos L. J. Chromogranin A: immunohistology reveals its universal occurrence in normal polypeptide hormone producing endocrine glands. Life Sci. 1983 Oct 24;33(17):1657–1663. doi: 10.1016/0024-3205(83)90721-x. [DOI] [PubMed] [Google Scholar]
- Schmid K. W., Fischer-Colbrie R., Hagn C., Jasani B., Williams E. D., Winkler H. Chromogranin A and B and secretogranin II in medullary carcinomas of the thyroid. Am J Surg Pathol. 1987 Jul;11(7):551–556. doi: 10.1097/00000478-198707000-00007. [DOI] [PubMed] [Google Scholar]
- Sietzen M., Schober M., Fischer-Colbrie R., Scherman D., Sperk G., Winkler H. Rat adrenal medulla: levels of chromogranins, enkephalins, dopamine beta-hydroxylase and of the amine transporter are changed by nervous activity and hypophysectomy. Neuroscience. 1987 Jul;22(1):131–139. doi: 10.1016/0306-4522(87)90203-x. [DOI] [PubMed] [Google Scholar]
- Simon J. P., Bader M. F., Aunis D. Secretion from chromaffin cells is controlled by chromogranin A-derived peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1712–1716. doi: 10.1073/pnas.85.5.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P., Hodgson A. J., DePotter R. W., Fischer-Colbrie R., Schober M., Winkler H., Chubb I. W. Chromogranin immunoreactivity in the central nervous system. Immunochemical characterisation, distribution and relationship to catecholamine and enkephalin pathways. Brain Res. 1984 Dec;320(2-3):193–230. doi: 10.1016/0165-0173(84)90007-9. [DOI] [PubMed] [Google Scholar]
- TerBush D. R., Holz R. W. Effects of phorbol esters, diglyceride, and cholinergic agonists on the subcellular distribution of protein kinase C in intact or digitonin-permeabilized adrenal chromaffin cells. J Biol Chem. 1986 Dec 25;261(36):17099–17106. [PubMed] [Google Scholar]
- Viveros O. H., Diliberto E. J., Jr, Hazum E., Chang K. J. Opiate-like materials in the adrenal medulla: evidence for storage and secretion with catecholamines. Mol Pharmacol. 1979 Nov;16(3):1101–1108. [PubMed] [Google Scholar]
- Waschek J. A., Pruss R. M., Siegel R. E., Eiden L. E., Bader M. F., Aunis D. Regulation of enkephalin, VIP, and chromogranin biosynthesis in actively secreting chromaffin cells. Multiple strategies for multiple peptides. Ann N Y Acad Sci. 1987;493:308–323. doi: 10.1111/j.1749-6632.1987.tb27215.x. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Kirshner N. Calcium-evoked secretion from digitonin-permeabilized adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4994–5000. [PubMed] [Google Scholar]
- Winkler H., Apps D. K., Fischer-Colbrie R. The molecular function of adrenal chromaffin granules: established facts and unresolved topics. Neuroscience. 1986 Jun;18(2):261–290. doi: 10.1016/0306-4522(86)90154-5. [DOI] [PubMed] [Google Scholar]
- Winkler H. The composition of adrenal chromaffin granules: an assessment of controversial results. Neuroscience. 1976;1(2):65–80. doi: 10.1016/0306-4522(76)90001-4. [DOI] [PubMed] [Google Scholar]
- Zwiller J., Revel M. O., Malviya A. N. Protein kinase C catalyzes phosphorylation of guanylate cyclase in vitro. J Biol Chem. 1985 Feb 10;260(3):1350–1353. [PubMed] [Google Scholar]