Abstract
Because there is a lack of comparative studies assessing drug-coated balloon (DCB) and drug-eluting stent (DES) outcomes with respect to intraluminal (IL) and subintimal (SI) approaches in femoropopliteal (FP) total occlusive lesions, we compared the outcomes between DCB (including bailout stenting) and DES treatments for this lesion. A total of 487 limbs (434 patients) were divided into the IL (n = 344, DCB: n = 268, DES: n = 76) and SI (n = 143, DCB: n = 83, DES: n = 60) approach groups. The primary outcome was a major adverse limb event (MALE), defined as above-ankle amputation or repeat revascularization of the index limb. Secondary outcomes included clinically driven target lesion revascularization (TLR), loss of clinical patency, and all-cause death. After adjustment, in each IL and SI approach, the 2-year rates of MALE (p = 0.180 and p = 0.236, respectively), TLR, loss of clinical patency, and all-cause death were similar between the DCB and DES groups. In the DCB and DES groups, both primary and secondary outcomes were similar between the IL and SI approaches. DCB and DES strategies for patients presenting with FP total occlusive lesions demonstrated similar outcomes regardless of the IL or SI approach.
Clinical Trial Registration: NCT02748226.
Keywords: Chronic total occlusion, Drug-coated balloon, Drug-eluting stent, Femoropopliteal artery disease
Subject terms: Cardiology, Diseases
Introduction
Peripheral arterial disease (PAD) is the third most prevalent cause of atherosclerosis-related morbidity, after coronary heart disease and stroke1. Approximately 40% of individuals experiencing symptomatic PAD exhibit chronic total occlusion (CTO), which is defined by the presence of atherosclerotic plaques leading to complete artery blockage persisting for more than 3 months2,3. Traditional bypass surgery entails a more invasive procedure and a prolonged recovery period than percutaneous transluminal angioplasty (PTA)4. Furthermore, a supplement to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (Trans-Atlantic Inter-Society Consensus II, TASC II) emphasizes the increasing trend of adopting an endovascular approach for managing intricate femoropopliteal (FP) lesions, including those categorized as TASC II type D lesions5. While PTA effectively restores blood flow initially, more than 60% of patients experience restenosis within 1 year after the procedure because of vessel recoil and neointimal hyperplasia6. Drug-eluting stents (DES) have been observed to yield superior 2-year event-free survival (p = 0.01) and primary patency compared (p < 0.01) to bare-metal stents (BMS)7. A single-blind randomized controlled trial (RCT)8 showed higher 3-year primary patency (log-rank p < 0.001) and lower clinically driven target lesion revascularization (TLR, p = 0.002) in patients treated with a drug-coated balloon (DCB) than in those treated with PTA. A recent randomized controlled trial (RCT)9 including 150 patients with symptomatic femoropopliteal artery (FPA) disease suggested comparable effectiveness and safety of DES versus DCB plus bailout stenting in FPA interventions during a 3-year follow-up period. In that study9, more than half of the lesions were total occlusions, and the 3-year primary patency for DCB and DES in occluded lesions (n = 79) was similar (log-rank p = 0.93). In a small registry study10 involving a limited number of patients (41 patients, 43 lesions), DCB, even without bailout stents, demonstrated comparable 12-month primary patency to DES (92.0% vs. 87.2%, p = 0.47) for total occluded lesions in the superficial femoral artery (SFA). To date, there is limited research on the outcomes of DCB and DES in patients with FP total occlusive lesions; specifically, there is no comparative study between DCB and DES based on intraluminal (IL) and subintimal (SI) approaches. Hence, we aimed to compare the clinical outcomes of DCB and DES in patients with FP total occlusive lesions based on the IL and SI approaches.
Results
Baseline characteristics
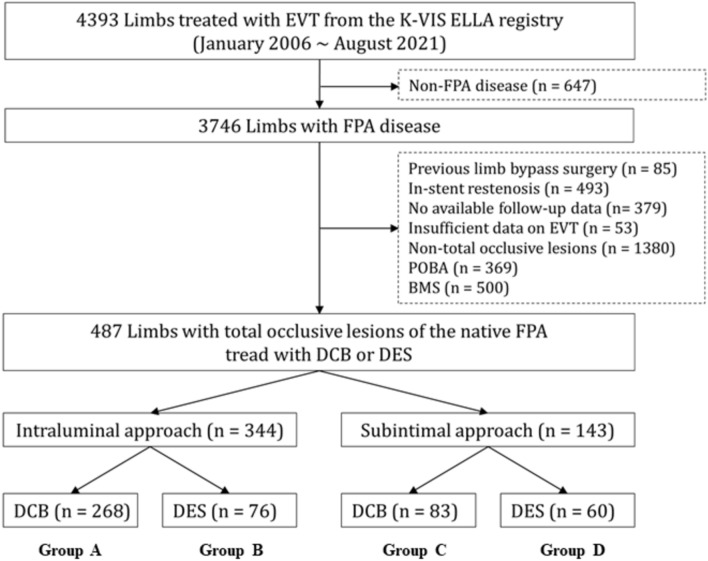
Figure 1 shows the flowchart of this study. Table 1 shows the baseline characteristics of the DCB (including the bailout stent group) and DES groups according to the IL or SI approaches. In the IL approach, the number of patients with a history of stroke, those who underwent rotational atherectomy, and the mean values of the total lesion length were higher in the DCB group than in the DES group. In contrast, postprocedural ABI was significantly higher in the DES group than in the DCB group. The mean values of the maximum device diameter and mean device diameter were higher in the DES group than in the DCB group. Using the SI approach, the number of male patients was higher in the DCB group than in the DES group. However, the postprocedural ABI and the mean values of the device maximum diameter and device mean diameter were larger in the DES group than in the DCB group. Supplementary Table S1 shows the baseline characteristics of IL and SI approaches in the DCB and DES groups. The number of patients with TASC II types A or B, those with concomitant infrapopliteal treatment, and those treated with Ranger (Boston Scientific, Marlborough, MA, USA) was higher with the IL approach than with the SI approach. The number of male patients, TASC-II types C or D, and the mean values of lesion length, maximum diameter of the device, mean diameter, and total device length were higher with the SI approach than with the IL approach. In the DES group, the Eluvia stent (Boston Scientific, Marlborough, MA, USA) was more frequently used for the IL approach, whereas the Zilver PTX stent (Cook Medical, IN, USA) was more frequently used for the SI approach in this study. The number of current smokers, the mean value of lesion length, and the number of those with a lesion length greater than or equal to 150 mm were higher in the SI approach than in the IL approach.
Fig. 1.
Flowchart. EVT endovascular therapy,K-VIS ELLA The Korean Vascular Intervention Society Endovascular therapy in Lower Limb Artery Diseases, FPA Femoropopliteal artery, POBA plain old balloon angioplasty, BMS bare-metal stent, DCB drug-coated balloon(including bailout stent group) group, DES drug-eluting stent group.
Table 1.
Baseline characteristics.
| Variables | Intraluminal approach (n = 344) | Subintimal approach (n = 143) | ||||
|---|---|---|---|---|---|---|
| DCB (n = 268, group A) | DES (n = 76, group B) | p value | DCB (n = 83, group C) | DES (n = 60, group D) | p value | |
| Age, years | 68.6 ± 11.9 | 69.7 ± 9.2 | 0.395 | 69.4 ± 11.7 | 71.9 ± 10.2 | 0.179 |
| Male, n (%) | 216 (80.6) | 61 (80.3) | 0.948 | 77 (92.8) | 47 (78.3) | 0.023 |
| BMI, kg/m2 | 23.5 ± 3.2 | 23.1 ± 3.9 | 0.455 | 23.1 ± 3.4 | 23.0 ± 4.0 | 0.968 |
| Hypertension, n (%) | 205 (76.5) | 52 (68.4) | 0.178 | 60 (72.3) | 41 (68.3) | 0.710 |
| Diabetes mellitus, n (%) | 160 (59.7) | 42 (55.3) | 0.511 | 41 (49.4) | 25 (41.7) | 0.398 |
| Dyslipidemia, n (%) | 169 (63.1) | 45 (59.2) | 0.592 | 45 (54.2) | 39 (65.0) | 0.230 |
| Coronary artery disease, n (%) | 99 (36.9) | 30 (39.5) | 0.689 | 31 (37.3) | 23 (38.3) | 0.905 |
| Heart failure, n (%) | 12 (4.5) | 4 (5.3) | 0.760 | 6 (7.2) | 2 (3.3) | 0.468 |
| Chronic kidney disease, n (%) | 70 (26.1) | 13 (17.1) | 0.129 | 22 (26.5) | 15 (25.0) | 0.839 |
| COPD, n (%) | 8 (3.0) | 4 (5.3) | 0.309 | 4 (4.8) | 4 (6.7) | 0.720 |
| Previous history of stroke, n (%) | 53 (19.8) | 6 (7.9) | 0.015 | 10 (12.0) | 8 (13.3) | 0.805 |
| Previous history of PTA, n (%) | 78 (29.1) | 18 (23.7) | 0.388 | 21 (25.3) | 18 (30.0) | 0.572 |
| Previous history of amputation, n (%) | 15 (5.6) | 2 (2.6) | 0.381 | 2 (2.4) | 1 (1.7) | 0.760 |
| Current smoker, n (%) | 78 (29.1) | 18 (23.7) | 0.388 | 23 (27.7) | 26 (43.3) | 0.074 |
| Clinical presentation | 0.342 | 0.735 | ||||
| Claudication, n (%) | 169 (63.1) | 53 (69.7) | 44 (53.0) | 34 (56.7) | ||
| CLTI, n (%) | 99 (36.9) | 23 (30.3) | 39 (47.0) | 26 (43.3) | ||
| Discharge medications | ||||||
| Aspirin, n (%) | 230 (85.8) | 70 (92.1) | 0.148 | 66 (79.5) | 53 (88.3) | 0.182 |
| Clopidogrel, n (%) | 235 (87.7) | 70 (92.1) | 0.411 | 71 (85.5) | 55 (91.7) | 0.306 |
| Cilostazol, n (%) | 80 (29.9) | 16 (21.1) | 0.149 | 33 (39.8) | 15 (25.0) | 0.075 |
| Statin, n (%) | 216 (80.6) | 58 (76.3) | 0.422 | 62 (74.7) | 49 (81.7) | 0.417 |
| Lesion length, mm | 220.1 ± 111.5 | 191.7 ± 102.9 | 0.039 | 263.9 ± 116.2 | 255.5 ± 91.9 | 0.630 |
| ≥ 150 mm, n (%) | 203 (75.7) | 47 (61.8) | 0.020 | 71 (85.5) | 53 (88.3) | 0.627 |
| TASC-II type | 0.143 | 0.928 | ||||
| A/B, n (%) | 76 (28.4) | 15 (19.7) | 12 (14.5) | 9 (15.0) | ||
| C/D, n (%) | 192 (71.6) | 61 (80.3) | 71 (85.5) | 51 (85.0) | ||
| Moderate/severe calcification, n (%) | 90 (33.6) | 29 (38.2) | 0.495 | 25 (30.1) | 16 (26.7) | 0.710 |
| DCB plus bailout stenting, n (%) | 55 (20.5) | 0 | 34 (41.0) | 0 | ||
| Device maximum diameter, mma | 6.03 ± 0.41 (n = 265) | 6.36 ± 0.51 (n = 75) | < 0.001 | 6.23 ± 0.42 (n = 81) | 6.48 ± 0.57 (n = 58) | 0.004 |
| Device mean diameter, mma | 5.92 ± 0.39 (n = 265) | 6.23 ± 0.37 (n = 75) | < 0.001 | 6.04 ± 0.45 (n = 81) | 6.24 ± 0.36 (n = 58) | 0.003 |
| Device total length, mma | 182.9 ± 59.0 (n = 265) | 187.0 ± 96.6 (n = 75) | 0.729 | 211.9 ± 79.2 (n = 81) | 200.7 ± 90.3 (n = 58) | 0.443 |
| Stent maximum diameter, mmb | 6.35 ± 0.39 (n = 53) | 6.36 ± 0.51 (n = 75) | 0.849 | 6.62 ± 0.50 (n = 33) | 6.48 ± 0.57 (n = 58) | 0.151 |
| Stent mean diameter, mmb | 6.28 ± 0.38 (n = 53) | 6.23 ± 0.37 (n = 75) | 0.271 | 6.43 ± 0.61 (n = 33) | 6.24 ± 0.36 (n = 58) | 0.019 |
| Stent total length, mmb | 139.4 ± 31.6 (n = 53) | 187.0 ± 96.6 (n = 75) | < 0.001 | 150.8 ± 47.0 (n = 33) | 200.7 ± 90.3 (n = 58) | < 0.001 |
| Pre-procedural ABIc | 0.56 ± 0.18 (n = 218) | 0.57 ± 0.17 (n = 67) | 0.831 | 0.55 ± 0.18 (n = 67) | 0.57 ± 0.19 (n = 53) | 0.644 |
| Post-procedural ABId | 0.86 ± 0.14 (n = 170) | 0.91 ± 0.14 (n = 48) | 0.010 | 0.86 ± 0.15 (n = 51) | 0.90 ± 0.13 (n = 43) | 0.049 |
| Atherectomy | ||||||
| Directional, n (%) | 19 (7.1) | 2 (2.6) | 0.184 | 1 (1.2) | 0 | 0.394 |
| Rotational, n (%) | 33 (12.3) | 2 (2.6) | 0.010 | 4 (4.8) | 0 | 0.139 |
| Concomitant treatment | ||||||
| Iliac lesion, n (%) | 41 (15.3) | 11 (14.5) | 0.859 | 17 (20.5) | 9 (15.0) | 0.511 |
| Infrapopliteal lesion, n (%) | 57 (21.3) | 9 (11.8) | 0.071 | 8 (9.6) | 7 (11.7) | 0.785 |
| Approach direction | ||||||
| Ipsilateral retrograde, n (%) | 130 (48.5) | 40 (52.6) | 0.603 | 45 (54.2) | 25 (41.7) | 0.175 |
| Contralateral antegrade, n (%) | 122 (45.5) | 33 (43.4) | 0.795 | 34 (41.0) | 27 (45.0) | 0.732 |
| Bidirectional, n (%) | 16 (6.0) | 3 (3.9) | 0.776 | 4 (4.8) | 8 (13.3) | 0.123 |
| Technical success, n (%) | 257 (95.9) | 73 (96.1) | 0.951 | 82 (98..8) | 58 (96.7) | 0.354 |
| Kinds of devicee | ||||||
| IN.PACT, n (%) | 216 (80.6) | 69 (83.1) | ||||
| Lutonix, n (%) | 27 (10.1) | 13 (15.7) | ||||
| Ranger, n (%) | 25 (9.3) | 1 (1.2) | ||||
| Eluvia, n (%) | 54 (71.1) | 30 (50.0) | ||||
| Zilver PTX, n (%) | 22 (28.9) | 30 (50.0) | ||||
| Complications | ||||||
| Distal embolization, n (%) | 0 | 1 (1.3) | 0.221 | 0 | 0 | – |
| Vascular rupture, n (%) | 3 (1.1) | 0 | 0.354 | 1 (1.2) | 0 | 0.694 |
| Bleeding, n (%) | 7 (2.6) | 0 | 0.355 | 2 (2.4) | 0 | 0.510 |
| Follow-up duration, days | 342 (166–680) | 240 (129–411) | 0.028 | 421 (183–693) | 212 (117–584) | 0.013 |
Values are means ± standard deviation or median (interquartile range) or numbers and percentages.
DCB drug-coated balloon group (including bailout stent group), DES drug-eluting stent group, BMI body mass index, COPD chronic obstructive pulmonary disease, PTA percutaneous transluminal angioplasty, CLTI chronic limb-threatening ischemia, TASC-II Trans-Atlantic Inter-Society Consensus-II.
The p values for continuous data were obtained from the unpaired t-test. The p values for categorical data from chi-square or Fisher’s exact test.
aData is available for 265, 75, 81, 58 limbs in Groups A, B, C, and D, respectively.
bData is available for 53, 75, 33, 58 limbs in Groups A, B, C, and D, respectively.
cData is available for 218, 67, 67, 53 limbs in Groups A, B, C, and D, respectively.
dData is available for 170, 48, 51, 43 limbs in Groups A, B, C, and D, respectively.
eIN.PACT (Medtronic Inc., Santa Rosa, CA, USA), Lutonix (Bard, Tempe, AZ, USA), Ranger (Boston Scientific, Marlborough, MA, USA), Eluvia (Boston Scientific, Marlborough, MA, USA), Zilver PTX (Cook Medical, IN, USA).
Clinical outcomes
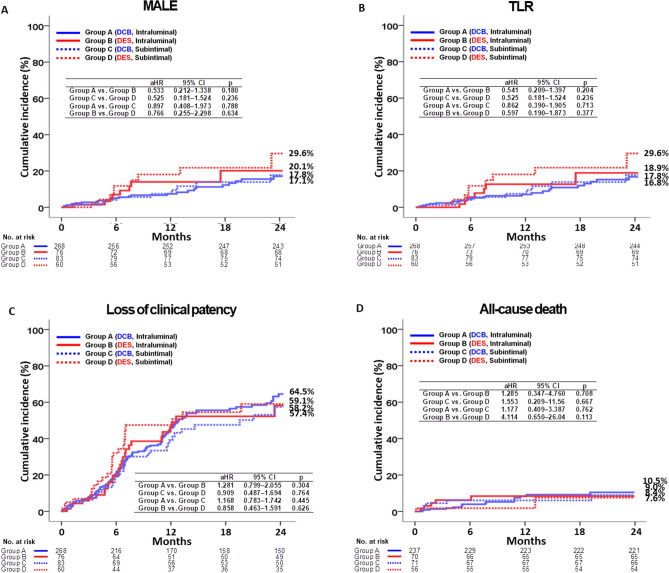
The primary and secondary outcomes at 2 years are shown in Tables 2 and 3, Fig. 2A–D, and Central Illustration. At 2 years, in the IL approach, MALE occurred in 17.1% of patients in the DCB group and in 20.1% of patients in the DES group (adjusted hazard ratio [aHR] 0.533; 95% confidence interval [CI] 0.212–1.338; p = 0.180, Fig. 2A). The adjusted clinically driven TLR (p = 0.204; Fig. 2B), loss of clinical patency (p = 0.304; Fig. 2C), and all-cause death (p = 0.708, Fig. 2D) rates were similar between the DCB and DES groups. In the SI approach, MALE occurred in 17.8% of patients in the DCB group and in 29.6% of patients in the DES group (aHR 0.525; 95% CI 0.181–1.524; p = 0.236; Fig. 2A). The adjusted clinically driven TLR (p = 0.236; Fig. 2B), loss of clinical patency (p = 0.764; Fig. 2C), and all-cause death (p = 0.667, Fig. 2D) rates were similar between the DCB and DES groups. These results were verified using a propensity (PS)-adjusted analysis. Supplementary Figure S1 shows the Kaplan–Meier curve analysis for MALE, TLR, loss of clinical patency, and all-cause death at 1 year and from 1 to 2 years after the index EVT. Supplementary Tables S2 and S3 show the results of the comparison of outcomes between the DCB and DES groups and between the IL and SI approaches at 1 year and from 1 to 2 years. As shown in Table 3, in the DCB and DES groups, the adjusted MALE rates at 2 years between the IL and SI groups were similar (p = 0.788 and 0.634, respectively). Adjusted TLR, loss of clinical patency, and all-cause mortality rates were similar between the IL and SI approaches. Independent predictors of MALE at the 2-year follow-up in the total study population were determined using multivariate Cox proportional hazards models, and the results are summarized in Supplementary Table S4. Hypertension (aHR 2.246; p = 0.013), diabetes mellitus (aHR, 2.097; p = 0.025), and current smoking status (aHR 2.219; p = 0.037) were significant independent predictors of MALE.
Table 2.
Clinical outcomes between the DCB and DES Groups according to different approaches at 2 years.
| Outcomes | Intraluminal, n = 344 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DCB (n = 268, group A) | DES (n = 76, group B) | Log-rank | Unadjusted | Multivariable-adjusteda | Propensity score-adjusted | ||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||||
| MALE | 25 (17.1) | 8 (20.1) | 0.369 | 0.695 (0.312–1.546) | 0.372 | 0.533 (0.212–1.338) | 0.180 | 0.654 (0.284–1.506) | 0.318 |
| TLR | 24 (16.8) | 7 (18.9) | 0.506 | 0.751 (0.323–1.750) | 0.508 | 0.541 (0.209–1.397) | 0.204 | 0.682 (0.286–1.627) | 0.388 |
| Loss of clinical patencyb | 118 (64.5) | 27 (58.2) | 0.863 | 1.038 (0.683–1.577) | 0.863 | 1.281 (0.799–2.055) | 0.304 | 1.037 (0.676–1.593) | 0.866 |
| All-cause deathc | 16 (10.5) | 5 (8.4) | 0.728 | 1.195 (0.437–3.265) | 0.728 | 1.285 (0.347–4.760) | 0.708 | 0.838 (0.294–2.393) | 0.742 |
| Outcomes | Subintimal, n = 143 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DCB (n = 83, group C) | DES (n = 60, group D) | Log-rank | Unadjusted | Multivariable-adjusteda | Propensity score-adjusted | ||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||||
| MALE | 9 (17.8) | 9 (29.6) | 0.197 | 0.549 (0.218–1.385) | 0.204 | 0.525 (0.181–1.524) | 0.236 | 0.432 (0.051–3.668) | 0.442 |
| TLR | 9 (17.8) | 9 (29.6) | 0.197 | 0.549 (0.218–1.385) | 0.204 | 0.525 (0.181–1.524) | 0.236 | 0.432 (0.051–3.668) | 0.442 |
| Loss of clinical patencyb | 33 (57.4) | 25 (59.1) | 0.263 | 0.744 (0.442–1.252) | 0.265 | 0.909 (0.487–1.694) | 0.764 | 0.891 (0.315–2.522) | 0.828 |
| All-cause deathc | 5 (9.0) | 2 (7.6), | 0.565 | 1.616 (0.310–8.418) | 0.569 | 1.553 (0.209–11.56) | 0.667 | 1.197 (0.225–6.381) | 0.833 |
| Outcomes | Total, n = 487 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DCB (n = 351, group A + C) | DES (n = 136, group B + D) | Log-rank | Unadjusted | Multivariable-adjusteda | Propensity score-adjusted | ||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||||
| MALE | 34 (17.2) | 17 (24.6) | 0.100 | 0.615 (0.343–1.103) | 0.103 | 0.549 (0.281–1.073) | 0.079 | 0.579 (0.309–1.086) | 0.089 |
| TLR | 33 (16.9) | 16 (24.0) | 0.126 | 0.629 (0.346–1.145) | 0.129 | 0.552 (0.280–1.089) | 0.087 | 0.582 (0.306–1.107) | 0.099 |
| Loss of clinical patencyb | 151 (62.8) | 52 (58.8) | 0.623 | 1.082 (0.789–1.484) | 0.624 | 1.256 (0.880–1.793) | 0.210 | 0.981 (0.513–1.877) | 0.953 |
| All-cause deathc | 21 (10.1) | 7 (8.2) | 0.923 | 1.043 (0.443–2.457) | 0.923 | 1.179 (0.403–3.447) | 0.764 | 0.942 (0.391–2.273) | 0.895 |
DCB drug-coated balloon group (including bailout stent group), DES drug-eluting stent group, MALE major adverse limb events, TLR clinically driven target lesion revascularization, HR hazard ratio, CI confidence interval, ABI ankle brachial index.
aAdjusted by age, male sex, previous history of stroke, current smoker, lesion length, stent mean diameter, stent total length, and post-procedural ABI (Supplementary Table S6).
bClinical patency was defined as freedom of symptom aggravation by at least 1 Rutherford category change accompanied by a decrease in ABI > 0.15 or absence of restenosis ≥ 50% on imaging studies such as duplex ultrasound, computed tomographic angiography, or intra-arterialangiography.
cAnalyzed on a per-patient basis, the number of patients was 237, 71, 70, and 56 in Groups A, B, C, and D, respectively.
Table 3.
Clinical outcomes between the intraluminal and subintimal groups after being treated with DCB or DES at 2 years.
| Outcomes | DCB, n = 344 | ||||||
|---|---|---|---|---|---|---|---|
| Intraluminal (n = 268, group A) | Subintiaml (n = 83, group C) | Log-rank | Unadjusted | Multivariable-adjusteda | |||
| HR (95% CI) | p | HR (95% CI) | p | ||||
| MALE | 25 (17.1) | 9 (17.8) | 0.947 | 0.974 (0.454–2.090) | 0.947 | 0.897 (0.408–1.973) | 0.788 |
| TLR | 24 (16.8) | 9 (17.8) | 0.871 | 0.939 (0.436–2.022) | 0.871 | 0.862 (0.390–1.905) | 0.713 |
| Loss of clinical patencyb | 118 (64.5) | 33 (57.4) | 0.354 | 1.200 (0.816–1.766) | 0.355 | 1.168 (0.783–1.742) | 0.445 |
| All-cause deathc | 16 (10.5) | 5 (9.0) | 0.917 | 1.055 (0.386–2.885) | 0.917 | 1.177 (0.409–3.387) | 0.762 |
| Outcomes | DES, n = 143 | ||||||
|---|---|---|---|---|---|---|---|
| Intraluminal (n = 76, group B) | Subintiaml (n = 60, group D) | Log-rank | Unadjusted | Multivariable-adjusteda | |||
| HR (95% CI) | p | HR (95% CI) | p | ||||
| MALE | 8 (20.1) | 9 (29.6) | 0.507 | 0.725 (0.279–1.885) | 0.509 | 0.766 (0.255–2.298) | 0.634 |
| TLR | 7 (18.9) | 9 (29.6) | 0.366 | 0.636 (0.236–1.713) | 0.370 | 0.597 (0.190–1.873) | 0.377 |
| Loss of clinical patencyb | 27 (58.2) | 25 (59.1) | 0.487 | 0.825 (0.479–1.422) | 0.488 | 0.858 (0.463–1.591) | 0.626 |
| All-cause deathc | 5 (8.4) | 2 (7.6) | 0.411 | 1.963 (0.381–10.13) | 0.420 | 4.114 (0.650–26.04) | 0.113 |
| Outcomes | Intraluminal (n = 344, group A + B) | Total, n = 487 | |||||
|---|---|---|---|---|---|---|---|
| Subintiaml (n = 143, group C + D) | Log-rank | Unadjusted | Multivariable-adjusteda | ||||
| HR (95% CI) | p | HR (95% CI) | p | ||||
| MALE | 33 (17.8) | 18 (22.0) | 0.455 | 0.803 (0.452–1.428) | 0.456 | 0.753 (0.415–1.366) | 0.351 |
| TLR | 31 (17.3) | 18 (22.0) | 0.345 | 0.756 (0.423–1.353) | 0.347 | 0.695 (0.380–1.271) | 0.237 |
| Loss of clinical patencyb | 145 (63.6) | 58 (58.1) | 0.770 | 1.046 (0.771–1.419) | 0.771 | 1.041 (0.759–1.418) | 0.803 |
| All-cause deathc | 21 (10.2) | 7 (8.1) | 0.600 | 1.257 (0.534–2.959) | 0.601 | 1.548 (0.636–3.765) | 0.335 |
DCB drug-coated balloon group (including bailout stent group), DES drug-eluting stent group, MALE major adverse limb events, TLR clinically driven target lesion revascularization, HR hazard ratio, CI confidence interval, ABI ankle brachial index.
aAdjusted by age, male sex, current smoker, lesion length, and device total length (Supplementary Table S7).
bClinical patency was defined as freedom of symptom aggravation by at least 1 Rutherford category change accompanied by a decrease in ABI > 0.15 or absence of restenosis ≥ 50% on imaging studies such as duplex ultrasound, computed tomographic angiography, or intra-arterialangiography.
cAnalyzed on a per-patient basis, the number of patients was 237, 71, 70, and 56 in Groups A, B, C, and D, respectively.
Fig. 2.
Kaplan–Meier curve analysis for MALE (A), TLR (B), loss of clinical patency (C), and all-cause death (D).
Discussion
The main findings of this nonrandomized, multicenter cohort study are as follows: First, after adjusting for each IL and SI approach, the MALE, TLR, loss of clinical patency, and all-cause death rates at 2 years were not significantly different between the DCB and DES groups. Second, in the DCB and DES groups, the MALE, TLR, loss of clinical patency, and all-cause death rates at 2 years were not significantly different between the IL and SI approaches. Third, hypertension, diabetes mellitus, and current smoking status were significant independent predictors of MALE in the total study population.
Previously, in patients with FPA disease, a BMS was deployed after PTA to overcome elastic recoil and neointimal hyperplasia11. Restenosis, a concern associated with repeat revascularization after BMS deployment, has decreased with the introduction of modern stents12 and DCBs13. The Zilver PTX stent was safe and clinically durable compared to standard endovascular treatment during the 5-year follow-up period14. In an RCT, DCBs were safe and effective in delaying restenosis, even in long, complex lesions and restenosis of the FP tract15. However, in the treatment of patients with FPA disease, there is still insufficient research on the relative superiority of DCB and DES in the treatment of patients with FPA, leading to ongoing debate9,10,16,17. Recently, Mohapatra et al.18 suggested that DCBs are most frequently applied for medium-length lesions with minimal calcification, whereas DESs are predominantly used for the treatment of heavily calcified lesions. In our study, the presence of lesions with moderate/severe calcification was similar between the DCB and DES groups in both the IL and SI approaches. However, in the IL approach, despite a higher mean lesion length in the DCB group than that in the DES group (p = 0.039; Table 1), the primary and secondary outcomes were similar between the DCB and DES groups (Tables 2, 3).
Regarding lesions with FPA CTO, the cumulative 1-year restenosis rate was comparable between the IL and SI approaches, regardless of whether intravascular ultrasound (IVUS) was used (p = 0.40)19 or not (p = 0.710)20 during EVT. In their study19, the 1-year restenosis rate, defined as either a peak systolic velocity ratio exceeding 2.4 according to duplex ultrasound, or the reappearance of stenosis equal to or greater than 50% of the arterial diameter, as identified through angiography, was 43.4% in the IL approach and 41.0% in the SI approach. In our study, 1-year loss in clinical patency rate was 47.0% for the IL approach and 45.0% for the SI approach (Supplementary Table S5). Another study21 showed that the 1-year restenosis rate in the SI group was 45%. One possible consideration for the observed frequency differences could be variations in the definition of restenosis or loss of clinical patency, as well as differences in the inclusion criteria. Although there are lingering concerns regarding the safety and efficacy of the SI approach for CTO lesions of the FP region19, the outcomes of SI have been enhanced through numerous advancements in techniques and devices5,22. Therefore, to date, there is no evidence favoring either the IL or SI route in terms of midterm results for BMS or drug-eluting devices5,19.
After the first year, the drug coating on the self-expandable nitinol DES was no longer present23. Hence, we compared the primary and secondary outcomes between the DCB and DES groups, and between the IL and SI groups at 1 year and between 1 and 2 years after the index EVT. The major clinical outcomes were similar between the DCB and DES groups and between the IL and SI approaches, except for variables with an extremely low number of events that precluded the calculation of adjusted hazard ratios (Supplementary Tables S2 and S3). In the IL and SI groups, there was a trend toward higher MALE and TLR rates in the DES group than in the DCB group at one year; however, the difference between the two groups was not statistically significant (Supplementary Table S2).
In a previous report24, restenosis appeared to plateau over time in the BMS group, in contrast to the ongoing restenosis observed in the DCB group during the specified time interval within a one-year period. However, Bausback et al.9 found that the introduction of BMS along with DCB did not result in signs of persistent restenosis over time, showing results similar to those with DCB alone. In our study, the rates of lesions treated with DCB and bailout stenting were low in the IL group compared to those in the SI group (20.5% vs. 41.0%, p < 0.001; Table 1). Furthermore, average total stent length (150.8 ± 47.0 mm) was more than half of the total lesion length (263.9 ± 116.2 mm). According to a recent report25, treating FP CTO lesions with a DCB showed that the 1-year freedom from restenosis in the IL approach was significantly lower compared to the SI approach (77.0% vs. 84.2%, respectively, p = 0.024) when wire passage (IL vs. SI) was monitored by IVUS during the PTA. In their study25, bail-out stenting was 9.7% in the IL group and 10.5% in the IL group, with no significant difference between the two groups (p = 0.69). We included the variables of maximum diameter, mean diameter, and total length of the device in our analysis to include DES without bailout lesions. Following the multicollinearity test, we incorporated variables showing noncollinearity into a multivariate Cox regression analysis (Supplementary Tables S6, S7). However, in the Toyoshima et al. study25, while the IL group in their study clearly demonstrated excellent 1-year freedom from restenosis after DCB treatment compared to the SI group, there are some differences when compared to our study. First, as seen in Fig. 1, we focused on native FPA CTO lesions and excluded patients who had undergone previous bypass grafting or had in-stent restenosis, whereas they included patients with in-stent restenosis in their study population. Second, in bail-out stenting lesions, unlike our study, they included the presence or absence of device use and bail-out stenting as variables but did not present variables such as the maximum and average diameter and total length of the device or stent, which were not included in their PSM. Furthermore, we compared the IL and SI approaches not only in the DCB treatment group but also in the DES treatment group for the same study population. Third, in Republic of Korea, the use of IVUS during PTA is limited due to restrictions on medical insurance coverage, which makes its use practically limited. However, their use of IVUS during PTA to precisely determine the path of wire tracking in CTO lesions likely provided a relative advantage26. IVUS allowed for more accurate assessment of lesion location, plaque characteristics, the need for bail-out stenting, and the size, position, and length of bail-out stents27. This capability likely helped avoid unnecessary bail-out stenting and allowed for the selection of shorter stent lengths when bail-out stenting was required. The differences between our study and theirs25 may be due to variations in study populations, the variables used to compare the two groups, and the use of IVUS, which may have led to different results. However, the high rate of bail-out stenting and the long average total stent length observed in group C of our study should be validated through further research.
As anticipated, the maximum and mean diameters of the devices were higher in the DES group than in the DCB group (Table 1, Supplementary Tables S1, S5). Similar to previous RCT results9,16, the TLR rate was similar between the DCB and DES groups. Therefore, the DES group did not exhibit a lower restenosis rate despite the larger device diameter. In the DES group, sustained external pressure from the oversized nitinol stents led to arterial injury and neointimal hyperplasia, especially as the effectiveness of anti-restenosis drugs diminished28.
As no comparative study exists between DCB and DES using IL and SI approaches, we compared the clinical outcomes of DCB and DES in patients with total occlusive FP lesions, based on the IL and SI approaches. We hope that our study results will provide valuable information to interventional cardiologists, radiologists, and vascular surgeons involved in EVT for total occlusive FP lesions.
This study had some limitations. First, this was a non-randomized registry study, and despite applying multivariable and PS-adjusted analyses, there still exists a significant potential for confounding bias and the influence of unmeasured variables. Second, because the choice of each device was at the discretion of the operator, controlling indication bias proved to be challenging. Third, the passage of the IL or SI through the wires was not confirmed by intravascular ultrasound during the procedure. Fourth, the number of enrolled limbs was small, making it difficult to draw definitive conclusions. Fifth, because both the DCB and DES treatment groups included various types of devices, the potential for heterogeneous treatment effects related to the specific devices used remains. Sixth, as this study did not include an evaluation of vessel patency through imaging studies for all participants, this is a major weakness. Finally, despite the reference vessel diameter being a crucial criterion for device selection and treatment strategies, the K-VIS ELLA, as a registry database, contains many missing values for reference vessel diameter, making it insufficient to evaluate the statistical significance between groups. We consider this a significant limitation of our study.
In conclusion, in this prospective observational multicenter registry study, both the DCB and DES strategies for patients presenting with FP total occlusive lesions demonstrated similar outcomes, regardless of the IL or SI approach.
Methods
Study population
Between January 2006 and August 2021, The Korean Vascular Intervention Society Endovascular Therapy in Lower Limb Artery Diseases (K-VIS ELLA) registry (ClinicalTrials.gov NCT02748226)29, examined a total of 4393 limbs (2951 patients). Of these, 3746 limbs (2564 patients) underwent endovascular therapy (EVT) for FP arterial (FPA) disease. This dedicated registry compiled information on patients who underwent EVT across 19 medical institutions in the Republic of Korea. The exclusion criteria were as follows: (1) limbs with prior bypass surgery (n = 85, 2.3%); (2) in-stent restenosis (n = 493, 13.2%); (3) cases with no available follow-up data (n = 379, 10.1%) or insufficient data on the EVT devices used (n = 53, 1.4%); (4) non-total occlusive lesions (n = 1380, 36.8%); (5) lesions where plain old balloon angioplasty (POBA) was performed (n = 369, 9.9%) or those where BMS were deployed (n = 500, 13.3%). Finally, 487 limbs (434 patients) with total occlusive lesions of the native FPA treated with DCB or DES remained (Fig. 1). They were divided into IL (n = 344, 70.6%) and SI (n = 143, 29.4%) approach groups. In each group, lesions were subdivided into the DCB group (including the bailout stent group; groups A [n = 268] and C [n = 83]) and the DES group (groups B [n = 76] and D [n = 60]) (Fig. 1). The study protocol was approved by the Institutional Review Board (IRB) of each hospital and the Severance Hospital IRB ethics committee (approval number: 4-2013-0463) and was conducted according to the principles of the 2004 Declaration of Helsinki. All prospectively enrolled participants provided informed consent, whereas participants in the retrospective cohort were exempt from providing informed consent. The enrolled data were collected from all participating centers using a web-based system. Initially, all patients underwent a thorough clinical assessment. Clinical and imaging data along with patient demographics and comorbidities were collected and retrospectively assessed. Follow-up evaluations were scheduled at 6, 12, and 24 months after the initial procedure. The median follow-up duration was 338 days (interquartile range 155–673 days), with 203 patients (46.4%) being followed for more than 1 year after the procedure.
Endovascular procedure and medical treatment
Experienced interventional cardiologists conducted all the endovascular procedures, and device selection for each case was at the operator’s discretion. Typically, IL wiring with either a 0.018-inch or a 0.035-inch guidewire was preferred. If the wire passage was unsuccessful, an SI approach was employed to reenter the distal true lumen. The guidewire tip was maneuvered into the SI channel, forming a loop, and then advanced distally with the aid of a 4–5Fr catheter or a microcatheter until it re-entered the true lumen at the distal stump. We classified the wire passage as SI when the wire tip looped, and linear or spiral dissections were evident at the proximal and distal stumps20. Predilatation using a plain balloon was the standard procedure, except when the limbs were initially treated with atherectomy. In cases with calcified or long-segment lesions, atherectomy devices were selectively employed for pretreatment following successful IL wire passage. The operator determined the dilatation pressure for the DCB, consistently setting it above the nominal pressure during a 180-s dilatation after achieving proper vessel preparation. Post-DCB treatment, if angiography revealed flow-limiting dissection or residual stenosis of 30% or more, provisional stenting with BMS was undertaken due to Korean medical insurance considerations, assigning these limbs to the DCB group. After implanting a bare-metal stent or DES, a non-compliant balloon was used for post-dilation in order to attain residual stenosis below 30%. Dual antiplatelet therapy with aspirin and clopidogrel was maintained for at least 6 months after the procedure unless contraindicated. Cilostazol was administered at the surgeon’s discretion.
Study definitions and clinical outcomes
Technical success was ascertained with target vessel vascularization, maintaining residual stenosis below 30%, and when no flow-limiting dissection was observed. CTO is characterized by the presence of atherosclerotic plaques, resulting in complete arterial blockage that endures for a period exceeding 3 months2. The primary outcome was the incidence of major adverse limb events (MALE)29. MALE was defined as an above-ankle amputation or repeat revascularization of the index limb. Secondary outcomes included clinically driven target lesion revascularization (TLR), loss of clinical patency, and all-cause death. Clinically driven TLR was characterized by the need for reintervention within a 5 mm range proximal or distal to the initial treatment segment. This intervention is prompted by the presence of more than 50% angiographic diameter stenosis coupled with a simultaneous deterioration of symptoms or a reduction in the ankle-brachial index (ABI) exceeding 0.15 compared with the immediate post-procedural ABI28. Clinical patency was defined as the absence of symptom exacerbation, indicated by a minimum of one Rutherford category change, coupled with a reduction in the ABI greater than 0.15. Alternatively, it was determined by the absence of restenosis equal to or exceeding 50% on imaging modalities, including duplex ultrasound, computed tomographic angiography, or intra-arterial angiography30. Limbs (or patients) were tracked from the procedure date until the occurrence of an outcome event. The principal investigators at each participating center identified and formally adjudicated all clinical events.
Statistical analyses
Statistical Package for the Social Sciences (SPSS) software version 20 (IBM, Armonk, NY, USA) was used to perform statistical analyses. All data were analyzed on a per-limb basis, unless otherwise indicated. Values are means ± standard deviation or numbers and percentages. We established a dedicated ‘missing’ category28 for variables exhibiting more than a 4% absence of values. For variables with less than a 4% absence, we performed a singular imputation utilizing the group median and mode for continuous and categorical variables, respectively. The p values for continuous data were obtained using unpaired t-tests. p values for categorical data were obtained using the chi-square test or Fisher’s exact test. Univariate analyses were performed for all variables in the DCB or DES groups and in the IL or SI groups; a p-value of < 0.05 was considered statistically significant. Subsequently, a multicollinearity test31 was performed for the included variables to confirm noncollinearity among them (Supplementary Table S6). We assessed the variance inflation factor values to gauge the extent of multicollinearity among variables. A variance inflation factor measurement greater than 5 was deemed to represent a high correlation32. To ascertain the presence of multicollinearity, we considered indicators such as a tolerance value below 0.1 or a condition index exceeding 1033. Finally, the following variables were included in the multivariate Cox regression analysis: adjusted for age, male sex, previous history of stroke, current smoking, lesion length, mean stent diameter, total stent length, and postprocedural ABI (Supplementary Table S6). To address confounding variables, we conducted a PS-adjusted analysis using a logistic regression model. All the baseline characteristics outlined in Table 1 were considered in the PS-adjusted analysis (Supplementary Table S5). The c-statistic for the PS-matched (PSM) analysis in this study was 0.894. Using the nearest available pair-matching method in a 1:1 ratio, patients in the DES group were matched to those in the DCB group. The caliper width was set as 0.05. The results of the collinearity test for MALE between the IL and SI groups are presented in Supplementary Table S7. Different clinical outcomes were assessed using the Kaplan–Meier curve analysis, and differences between the groups were examined using the log-rank test. Statistical significance was defined as p < 0.05.
Supplementary Information
Author contributions
Yong Hoon Kim and Ae-Young Her researched data and wrote the manuscript. Yong Hoon Kim, Ae-Young Her, Young-Guk Ko, Chul-Min Ahn, Seung-Jun Lee, Myeong-Ki Hong, Cheol Woong Yu, Jae-Hwan Lee, Seung Whan Lee, Young Jin Youn, Chang-Hwan Yoon, Seung-Woon Rha, Pil-Ki Min, Seung-Hyuk Choi, In-Ho Chae, and Donghoon Choi contributed to study design. Yong Hoon Kim, Ae-Young Her, Young-Guk Ko, Chul-Min Ahn, Seung-Jun Lee, Myeong-Ki Hong, Cheol Woong Yu, Jae-Hwan Lee, Seung Whan Lee, Young Jin Youn, Chang-Hwan Yoon, Seung-Woon Rha, Pil-Ki Min, Seung-Hyuk Choi, In-Ho Chae, and Donghoon Choi contributed to the collection research data. Yong Hoon Kim, Young-Guk Ko, Myeong-Ki Hong, Cheol Woong Yu, Jae-Hwan Lee, Seung Whan Lee, Seung-Woon Rha, Pil-Ki Min, Seung-Hyuk Choi, In-Ho Chae, and Donghoon Choi contributed to provide intellectual inputs for the discussion. Yong Hoon Kim, Ae-Young Her, Chul-Min Ahn, Seung-Jun Lee, Young Jin Youn, Chang-Hwan Yoon, and Pil-Ki Min contributed to data analysis and edited the manuscript. Yong Hoon Kim, Young-Guk Ko, Myeong-Ki Hong, Cheol Woong Yu, Jae-Hwan Lee, Seung Whan Lee, Seung-Woon Rha, Pil-Ki Min, Seung-Hyuk Choi, In-Ho Chae, and Donghoon Choi contributed to provide supervisor role during the full processes of manuscript submitting and editing. All authors have read and approved the manuscript, and all authors take full responsibility for this work.
Data availability
Data is contained with the article or Supplementary Material.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yong Hoon Kim and Ae-Young Her.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Yong Hoon Kim, Email: yhkim02@kangwon.ac.kr.
Young-Guk Ko, Email: ygko@yuhs.ac.
The K-VIS ELLA Investigators:
Woong Chol Kang, Sung-Ho Her, Yoon Seok Koh, Byung-Hee Hwang, Weon Kim, Sang Cheol Jo, Sanghoon Shin, Yun Hyeong Cho, Woo-Young Chung, Jung Kyu Han, Young Jin Choi, Su Hyun Kim, Sang-Ho Park, Jung-Hee Lee, Yu Jeong Choi, Sung Kee Ryu, Ju Han Kim, Sang-Rok Lee, Hoyoun Won, Ju Yeol Baek, Jang-Hwan Bae, and Hyun-Sook Kim
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-71745-0.
References
- 1.Criqui, M. H. et al. Lower extremity peripheral artery disease: Contemporary epidemiology, management gaps, and future directions: A scientific statement from the American Heart Association. Circulation144, e171–e191 (2021). 10.1161/CIR.0000000000001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers, J. H. et al. Overview of new technologies for lower extremity revascularization. Circulation116, 2072–2085 (2007). 10.1161/CIRCULATIONAHA.107.715433 [DOI] [PubMed] [Google Scholar]
- 3.Banerjee, S. et al. A percutaneous crossing algorithm for femoropopliteal and tibial artery chronic total occlusions (PCTO algorithm). J. Invas. Cardiol.31, 111–119 (2019). [PubMed] [Google Scholar]
- 4.Bisdas, T. et al. Tips and tricks to cross chronic total occlusions in the superficial femoral artery. J. Cardiovasc. Surg.60, 567–571 (2019). 10.23736/S0021-9509.19.11040-3 [DOI] [PubMed] [Google Scholar]
- 5.Jaff, M. R. et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: A supplement to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Endovasc. Ther.22, 663–677 (2015). 10.1177/1526602815592206 [DOI] [PubMed] [Google Scholar]
- 6.Rocha-Singh, K. J. et al. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter. Cardiovasc. Interv.69, 910–919 (2007). 10.1002/ccd.21104 [DOI] [PubMed] [Google Scholar]
- 7.Dake, M. D. et al. Sustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomized and single-arm clinical studies. J. Am. Coll. Cardiol.61, 2417–2427 (2013). 10.1016/j.jacc.2013.03.034 [DOI] [PubMed] [Google Scholar]
- 8.Schneider, P. A. et al. Treatment effect of drug-coated balloons is durable to 3 years in the femoropopliteal arteries: Long-term results of the IN.PACT SFA randomized trial. Circ. Cardiovasc. Interv.11, e005891 (2018). 10.1161/CIRCINTERVENTIONS.117.005891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bausback, Y. et al. Drug-eluting stent versus drug-coated balloon revascularization in patients with femoropopliteal arterial disease. J. Am. Coll. Cardiol.73, 667–679 (2019). 10.1016/j.jacc.2018.11.039 [DOI] [PubMed] [Google Scholar]
- 10.Hayakawa, N. et al. Optimal intraluminal drug-coated balloon versus drug-eluting stent in patients with chronic total occlusion of the superficial femoral artery: A retrospective analysis. Cardiovasc. Revasc. Med.43, 87–96 (2022). 10.1016/j.carrev.2022.04.002 [DOI] [PubMed] [Google Scholar]
- 11.Schillinger, M. et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N. Engl. J. Med.354, 1879–1888 (2006). 10.1056/NEJMoa051303 [DOI] [PubMed] [Google Scholar]
- 12.Dake, M. D. et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: Twelve-month Zilver PTX randomized study results. Circ. Cardiovasc. Interv.4, 495–504 (2011). 10.1161/CIRCINTERVENTIONS.111.962324 [DOI] [PubMed] [Google Scholar]
- 13.Rosenfield, K. et al. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N. Engl. J. Med.373, 145–153 (2015). 10.1056/NEJMoa1406235 [DOI] [PubMed] [Google Scholar]
- 14.Dake, M. D. et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the zilver PTX randomized trial. Circulation133, 1472–1483 (2016). 10.1161/CIRCULATIONAHA.115.016900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt, A. et al. Drug-coated balloons for complex femoropopliteal lesions: 2-year results of a real-world registry. JACC Cardiovasc. Interv.9, 715–724 (2016). 10.1016/j.jcin.2015.12.267 [DOI] [PubMed] [Google Scholar]
- 16.Liistro, F. et al. Drug-eluting balloon versus drug-eluting stent for complex femoropopliteal arterial lesions: The DRASTICO study. J. Am. Coll. Cardiol.74, 205–215 (2019). 10.1016/j.jacc.2019.04.057 [DOI] [PubMed] [Google Scholar]
- 17.Zenunaj, G. et al. Primary drug-coated balloon versus drug-eluting stent for native atherosclerotic femoropopliteal lesions: A systematic review and meta-analysis. Ann. Vasc. Surg.92, 294–303 (2023). 10.1016/j.avsg.2023.01.043 [DOI] [PubMed] [Google Scholar]
- 18.Mohapatra, A. et al. Nationwide trends in drug-coated balloon and drug-eluting stent utilization in the femoropopliteal arteries. J. Vasc. Surg.71, 560–566 (2020). 10.1016/j.jvs.2019.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomoi, Y. et al. Subintimal versus intraluminal approach for femoropopliteal chronic total occlusions treated with intravascular ultrasound guidance. J. Am. Heart Assoc.10, e021903 (2021). 10.1161/JAHA.121.021903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, K. et al. Clinical outcomes of subintimal vs intraluminal revascularization approaches for long femoropopliteal occlusions in a Korean multicenter retrospective registry cohort. Circ. J.82, 1900–1907 (2018). 10.1253/circj.CJ-17-1464 [DOI] [PubMed] [Google Scholar]
- 21.Ishihara, T. et al. Comparable 2-year restenosis rates following subintimal and intraluminal drug-eluting stent implantation for femoropopliteal chronic total occlusion. J. Endovasc. Ther.23, 889–895 (2016). 10.1177/1526602816666261 [DOI] [PubMed] [Google Scholar]
- 22.Bhatt, H. et al. Crossing techniques and devices in femoropopliteal chronic total occlusion intervention. Cardiovasc. Revasc. Med.18, 623–631 (2017). 10.1016/j.carrev.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 23.Dake, M. D. et al. Polymer-free paclitaxel-coated Zilver PTX stents—Evaluation of pharmacokinetics and comparative safety in porcine arteries. J. Vasc. Interv. Radiol.22, 603–610 (2011). 10.1016/j.jvir.2010.12.027 [DOI] [PubMed] [Google Scholar]
- 24.Steiner, S. et al. Midterm patency after femoropopliteal interventions: A comparison of standard and interwoven nitinol stents and drug-coated balloons in a single-center, propensity score-matched analysis. J. Endovasc. Ther.23, 347–355 (2016). 10.1177/1526602816628285 [DOI] [PubMed] [Google Scholar]
- 25.Toyoshima, T. et al. Intraluminal vs subintimal drug-coated balloon angioplasty for the treatment of femoropopliteal chronic total occlusions. JACC Cardiovasc. Interv.17, 608–618 (2024). 10.1016/j.jcin.2023.12.028 [DOI] [PubMed] [Google Scholar]
- 26.Song, L. et al. Intravascular ultrasound analysis of intraplaque versus subintimal tracking in percutaneous intervention for coronary chronic total occlusions and association with procedural outcomes. JACC Cardiovasc. Interv.10, 1011–1021 (2017). 10.1016/j.jcin.2017.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsukagoshi, J. The mid-term effect of intravascular ultrasound on endovascular interventions for lower extremity peripheral arterial disease: A systematic review and meta-analysis. J. Vasc. Surg.79, 963–972 (2024). 10.1016/j.jvs.2023.08.128 [DOI] [PubMed] [Google Scholar]
- 28.Lee, S. J. et al. Device effectiveness for femoropopliteal artery disease treatment: An analysis of K-VIS ELLA registry. JACC Cardiovasc. Interv.16, 1640–1650 (2023). 10.1016/j.jcin.2023.05.002 [DOI] [PubMed] [Google Scholar]
- 29.Ko, Y. G. et al. Baseline characteristics of a retrospective patient cohort in the Korean Vascular Intervention Society endovascular therapy in lower limb artery diseases (K-VIS ELLA) registry. Korean Circ. J.47, 469–476 (2017). 10.4070/kcj.2017.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, Y. J. et al. Drug eluting stent vs drug coated balloon for native femoropopliteal artery disease: A two centre experience. Eur. J. Vasc. Endovasc. Surg.61, 287–295 (2021). 10.1016/j.ejvs.2020.10.008 [DOI] [PubMed] [Google Scholar]
- 31.Vatcheva, K. P. et al. Multicollinearity in regression analyses conducted in epidemiologic studies. Epidemiology6, 227 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, J. H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol.72, 558–569 (2019). 10.4097/kja.19087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcoulides, K. M. et al. Evaluation of variance inflation factors in regression models using latent variable modeling methods. Educ. Psychol. Meas.79, 874–882 (2019). 10.1177/0013164418817803 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained with the article or Supplementary Material.