Abstract
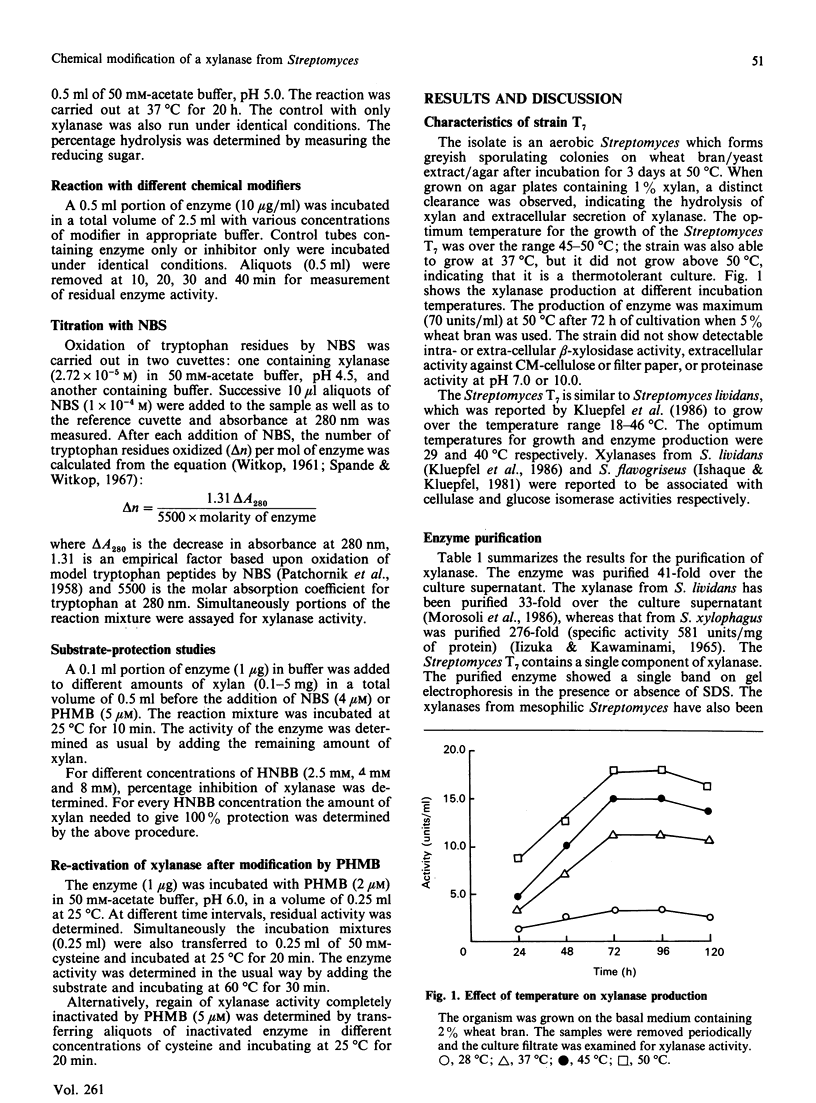
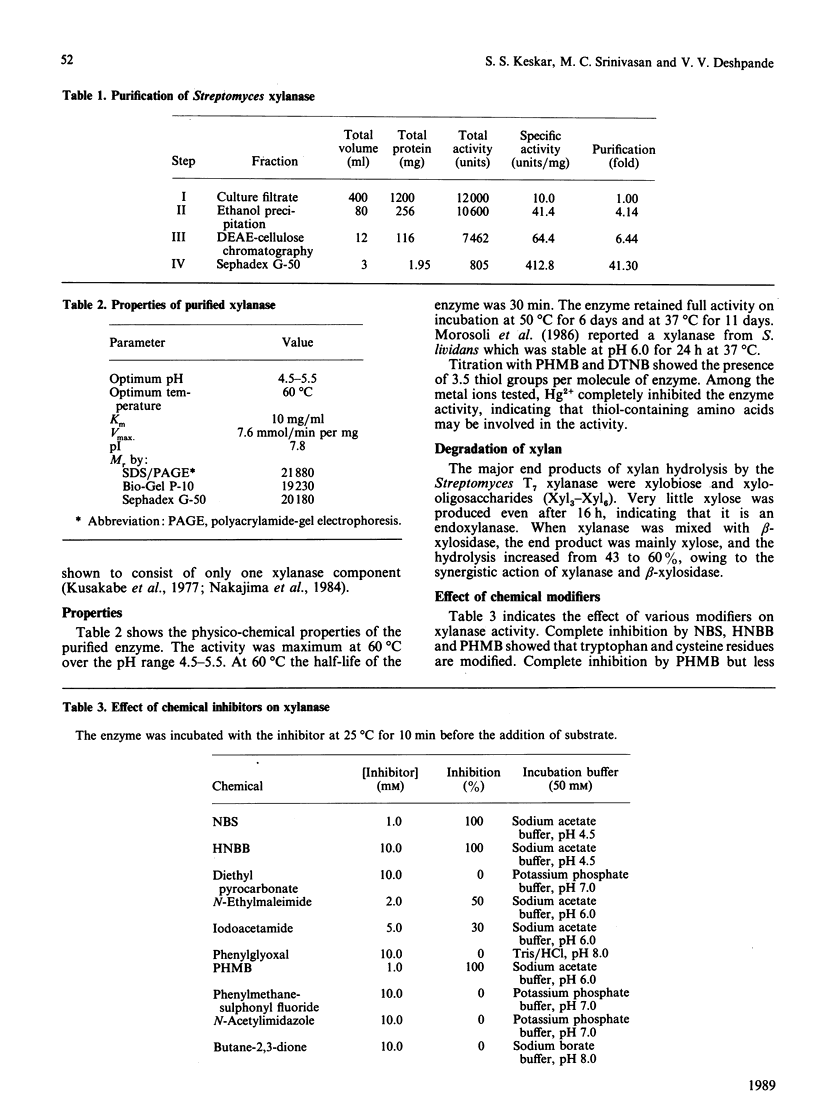
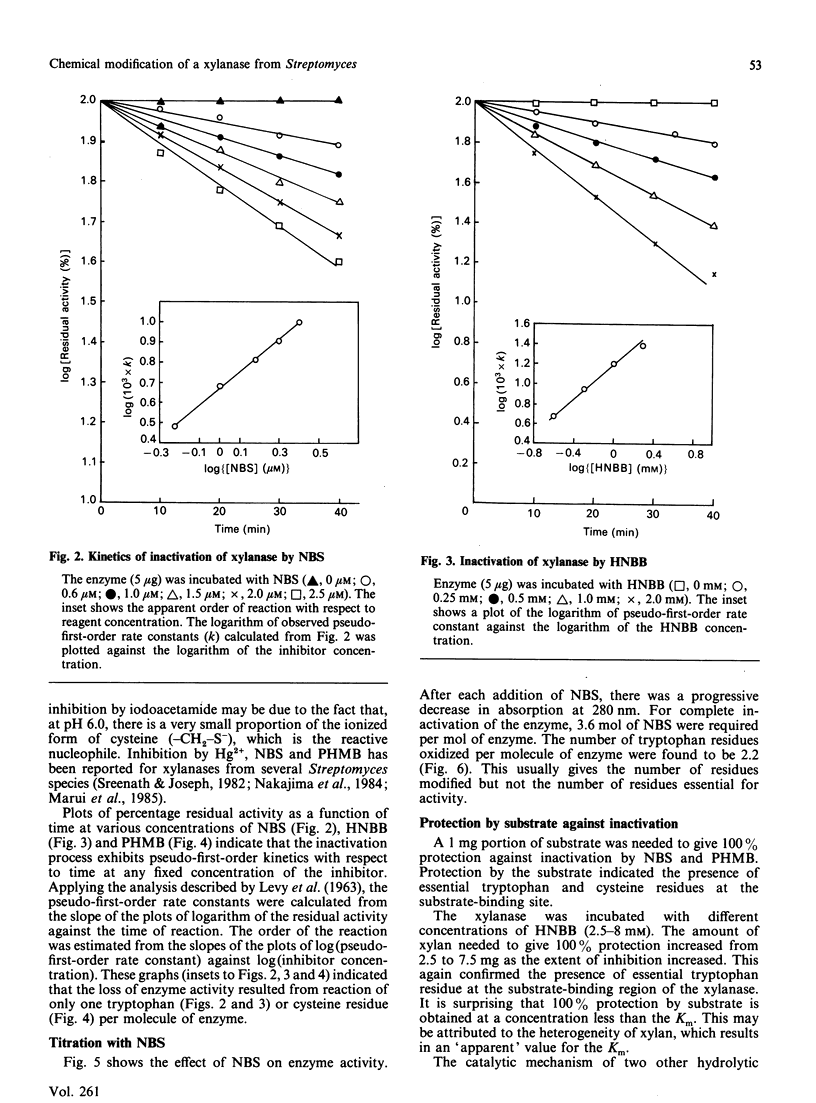
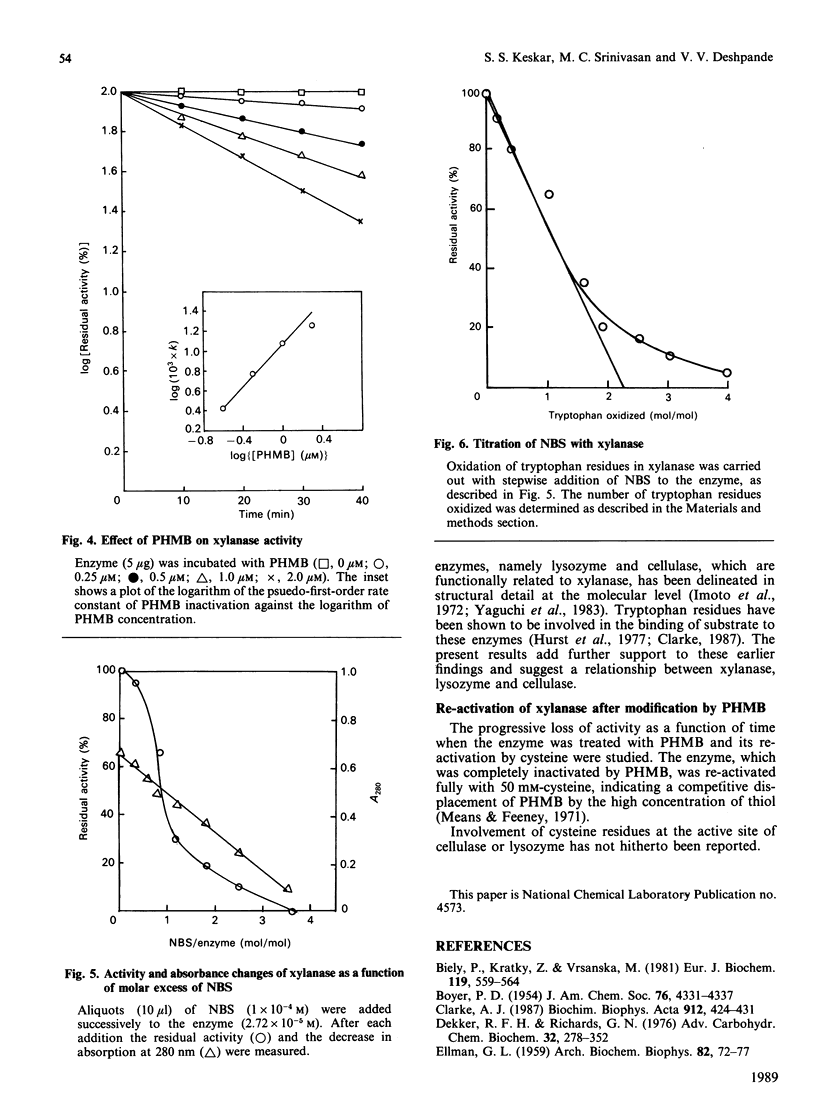
Extracellular xylanase produced in submerged culture by a thermotolerant Streptomyces T7 growing at 37-50 degrees C was purified to homogeneity by chromatography on DEAE-cellulose and gel filtration on Sephadex G-50. The purified enzyme has an Mr of 20,463 and a pI of 7.8. The pH and temperature optima for the activity were 4.5-5.5 and 60 degrees C respectively. The enzyme retained 100% of its original activity on incubation at pH 5.0 for 6 days at 50 degrees C and for 11 days at 37 degrees C. The Km and Vmax. values, as determined with soluble larch-wood xylan, were 10 mg/ml and 7.6 x 10(3) mumol/min per mg of enzyme respectively. The xylanase was devoid of cellulase activity. It was completely inhibited by Hg2+ (2 x 10(-6) M). The enzyme degraded xylan, producing xylobiose, xylo-oligosaccharides and a small amount of xylose as end products, indicating that it is an endoxylanase. Chemical modification of xylanase with N-bromosuccinimide, 2-hydroxy-5-nitrobenzyl bromide and p-hydroxymercuribenzoate (PHMB) revealed that 1 mol each of tryptophan and cysteine per mol of enzyme were essential for the activity. Xylan completely protected the enzyme from inactivation by the above reagents, suggesting the presence of tryptophan and cysteine at the substrate-binding site. Inactivation of xylanase by PHMB could be restored by cysteine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biely P., Krátký Z., Vrsanská M. Substrate-binding site of endo-1,4-beta-xylanase of the yeast Cryptococcus albidus. Eur J Biochem. 1981 Oct;119(3):559–564. doi: 10.1111/j.1432-1033.1981.tb05644.x. [DOI] [PubMed] [Google Scholar]
- Clarke A. J. Essential tryptophan residues in the function of cellulase from Schizophyllum commune. Biochim Biophys Acta. 1987 Apr 30;912(3):424–431. doi: 10.1016/0167-4838(87)90048-3. [DOI] [PubMed] [Google Scholar]
- Dekker R. F., Richards G. N. Hemicellulases: their occurrence, purification, properties, and mode of action. Adv Carbohydr Chem Biochem. 1976;32:277–352. doi: 10.1016/s0065-2318(08)60339-x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Hurst P. L., Sullivan P. A., Shepherd M. G. Chemical modification of cellulase from Aspergillus niger. Biochem J. 1977 Dec 1;167(3):549–556. doi: 10.1042/bj1670549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY H. M., LEBER P. D., RYAN E. M. INACTIVATION OF MYOSIN BY 2,4-DINITROPHENOL AND PROTECTION BY ADENOSINE TRIPHOSPHATE AND OTHER PHOSPHATE COMPOUNDS. J Biol Chem. 1963 Nov;238:3654–3659. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meagher M. M., Tao B. Y., Chow J. M., Reilly P. J. Kinetics and subsite mapping of a D-xylobiose- and D-xylose-producing Aspergillus niger endo-(1----4)-beta-D-xylanase. Carbohydr Res. 1988 Mar 1;173(2):273–283. doi: 10.1016/s0008-6215(00)90823-1. [DOI] [PubMed] [Google Scholar]
- Morosoli R., Bertrand J. L., Mondou F., Shareck F., Kluepfel D. Purification and properties of a xylanase from Streptomyces lividans. Biochem J. 1986 Nov 1;239(3):587–592. doi: 10.1042/bj2390587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenath H. K., Joseph R., Murthy V. S. Studies on xylan hydrolases from different strains of Streptomyces and their mutual influences in the breakdown of xylan. Folia Microbiol (Praha) 1978;23(4):299–303. doi: 10.1007/BF02876684. [DOI] [PubMed] [Google Scholar]
- Sreenath H. K., Joseph R. Purification and properties of extracellular xylan hydrolases of Streptomyces exfoliatus. Folia Microbiol (Praha) 1982;27(2):107–115. doi: 10.1007/BF02879768. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Vrsanská M., Gorbacheva I. V., Krátký Z., Biely P. Reaction pathways of substrate degradation by an acidic endo-1,4-beta-xylanase of Aspergillus niger. Biochim Biophys Acta. 1982 May 21;704(1):114–122. doi: 10.1016/0167-4838(82)90138-8. [DOI] [PubMed] [Google Scholar]
- WITKOP B. Nonenzymatic methods for the preferential and selective cleavage and modification of proteins. Adv Protein Chem. 1961;16:221–321. doi: 10.1016/s0065-3233(08)60031-5. [DOI] [PubMed] [Google Scholar]
- Yaguchi M., Roy C., Rollin C. F., Paice M. G., Jurasek L. A fungal cellulase shows sequence homology with the active site of hen egg-white lysozyme. Biochem Biophys Res Commun. 1983 Oct 31;116(2):408–411. doi: 10.1016/0006-291x(83)90537-5. [DOI] [PubMed] [Google Scholar]


