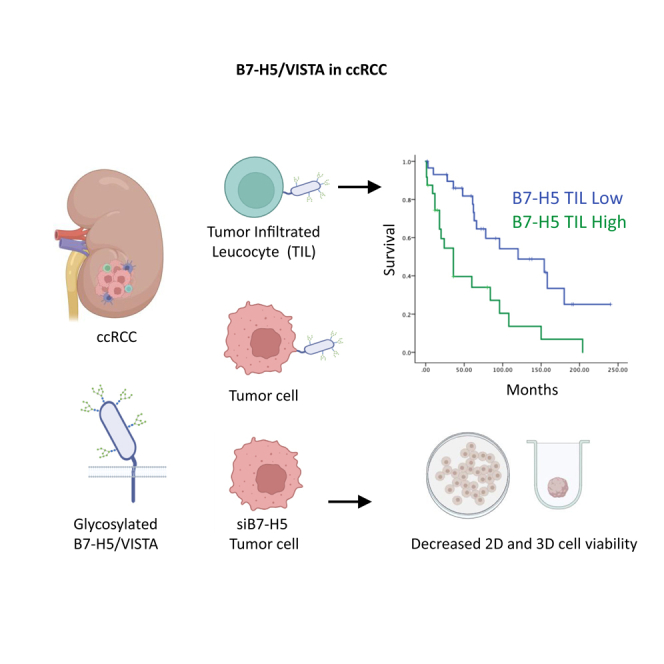
Summary
Increased expression of the B7 family of immune checkpoint proteins hinders tumor elimination by the immune system. Expression levels of the B7-H5 protein were found to be upregulated in clear cell renal cell carcinomas (ccRCC). We here report the molecular, functional, and clinical characterization of B7-H5 from renal cancer cells and metastatic ccRCC tumors. B7-H5 was highly glycosylated and mainly expressed in the cell membrane. Mutagenic studies on B7-H5 identified the residues targeted by N-glycosylation and revealed an impact of B7-H5 glycosylation on protein expression levels and localization. B7-H5 knockdown decreased the cell proliferation and viability of renal cancer cells. We analyzed B7-H5 expression on tumor cells and tumor-infiltrated leukocytes (TILs) in samples from metastatic ccRCC patients and found that B7-H5 expression on TILs correlated with syncronous metastases and poor outcomes. These results provide insights into the molecular properties and clinical impact of B7-H5 and support B7-H5 as a new immunotherapeutic target in metastatic ccRCC.
Subject areas: Biological sciences, Immunology, Immune response
Graphical abstract
Highlights
-
•
Mutagenic studies on B7-H5 identified the residues targeted by glycosylation
-
•
B7-H5 N-glycosylation revealed an impact on protein expression levels and localization
-
•
Knockdown of B7-H5 expression decreased 2D and 3D viability of renal cancer cells
-
•
B7-H5 expression on TILs correlated with poor outcomes in ccRCC
Biological sciences; Immunology; Immune response
Introduction
Cancer progression occurs via different processes that include cell death resistance, metastasis, and evasion of immune-mediated destruction. During cancer development, there is a dynamic period named immunoediting, in which immune cells may eliminate tumor cells. However, cancer cells are able to evade immune elimination via different mechanisms. This paradigm can be divided into three phases: elimination, equilibrium, and evasion. Firstly, cells that have undergone mutations that are not repaired by DNA repair mechanisms are identified and eliminated by the immune system (cancer immuno-surveillance); secondly, tumor cells that have not been eliminated by lymphocytes display restrained growth by the immune system; and thirdly, the lack of control by the immune system enables cancer cells to avoid recognition and elimination leading to tumor growth and metastasis.1,2 This capacity of the tumor cells to avoid immune attack is one of the main characteristics of cancer pathogenesis and can be carried out by different mechanisms, including the ability of cancer cells to express immune checkpoint proteins on the cell surface. Therefore, understanding the interaction between cancer development and the immune system is essential to improve current therapies and implement new ones.
The V-domain immunoglobulin suppressor of T cell activation (VISTA), or B7-H5 (official gene name, VSIR; GenBank: NM_022153), is an inhibitory B7 family member that under physiological conditions maintains T cell and myeloid quiescence. Under inflammatory conditions, however, B7-H5 induces myeloid cells to reduce the expression of pro-inflammatory cytokines by reducing Toll-like receptor (TLR) signaling and it also increases the release of anti-inflammatory mediators.3,4,5 B7-H5 can bind to V-set and Ig domain-containing 3 (VSIG-3) and to P-selectin glycoprotein ligand 1 (PSGL-1) ligands, probably with bidirectional signaling. Therefore, it can act both as a ligand and a receptor. In both interactions, pH is an important regulatory factor, being the interaction with PSGL-1 favored by the acidic pH in the tumor microenvironment (TME).6,7 Additional B7-H5 binding partners include VSIG-8 and the metalloproteinase MMP-13, among others (reviewed in8;9).
B7-H5 is a glycosylated type I transmembrane protein that shares structural features with B7 and CD28 protein families and shares sequence homology with both PD-1 and PD-L1. Different from other B7 family members, B7-H5 only contains one single large N-terminal IgV-like domain. The cytoplasmic domain has three C-terminal Src homology domain 3 (SH3) binding motifs and one Src homology domain 2 (SH2) binding motif, several casein kinase 2 and protein kinase C phosphorylation sites, and a potential ubiquitylation site.10,11 As other members from the B7-homologous family, B7-H5 does not contain immunoreceptor tyrosine-based signaling motifs (ITAM/ITIM motifs) in its cytoplasmic domain.12
B7-H5 is mostly expressed in hematopoietic tissues and on the myeloid and lymphoid compartments. In tumors, B7-H5 is expressed on myeloid-derived suppressive cells, tumor-infiltrating leukocytes (TILs), and antigen-presenting cells,10,11,13 and it has been reported increased B7-H5 expression in tumor-infiltrated hematopoietic cells after anti-CTLA-4/anti-PD-1 treatment, possibly due to adaptive resistance to immune checkpoint blockade.14,15 In addition, soluble forms of B7-H5 have been detected which are increased in the serum of patients with distinct cancers, and associated with high-stage and metastases.16,17 Less commonly, B7-H5 can also be expressed on tumor cells varying across cancer types. High expression of B7-H5 has been associated with poor prognosis in several human cancers, including ccRCC, making B7-H5 a suitable target for immunotherapy.18,19,20,21 In this regard, antibody blockade of B7-H5 by antagonist antibodies inhibits regulatory T cell immunosuppressive functions, and several of these antibodies are currently under preclinical development in cancer models, including combinatory therapies with antibodies against PD-1, CTLA-4, and PD-L1.5,9,22,23 In addition, global glycoproteomic analysis of ccRCC unveiled the potential of protein glycosylation in ccRCC development,24 however little is known about the glycosylation pattern of B7-H5 in renal cells.
The evolving therapeutic landscape of ccRCC includes immune checkpoint inhibitors in combination with targeted therapies, and despite its constant improvement, there is a need for alternative therapeutic modalities to improve efficacy, toxicities, response rates, and quality of life.4,25,26 B7-H5 is upregulated at both mRNA and protein levels in ccRCC tumors compared to normal adjacent tissues. Furthermore, B7-H5 expression in ccRCC tumors correlated with poor T cell responses, and blockade of B7-H5 with anti-B7-H5 antibody in in vivo mouse experiments decreased RCC tumor growth.20 In ccRCC with venous tumor thrombus, B7-H5 high expression in tumor-infiltrated leukocytes correlated with metastasis and with decreased overall survival.27 Here, we present the molecular, functional, and clinical characterization of B7-H5 in renal cancer cells. A positive role for highly glycosylated B7-H5 in renal cancer cell proliferation is disclosed, and we report the correlation of B7-H5 high expression in TILs with poor prognosis in metastatic ccRCC. Our findings suggest a role for B7-H5 in metastatic ccRCC and support the notion of B7-H5 as a suitable new target for ccRCC immunotherapy.
Results
Characterization and localization of glycosylated B7-H5
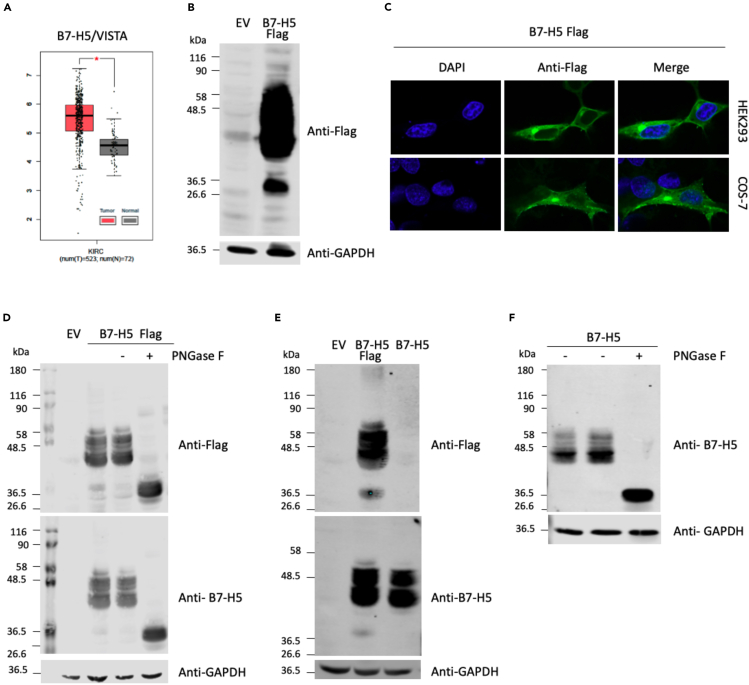
The comparison of the mRNA expression levels of B7-H5 in ccRCC tumor and normal renal tissues shows the upregulated expression of B7-H5 in ccRCC, suggesting a positive role for B7-H5 in ccRCC progression (Figure 1A). To characterize the B7-H5 protein, Western blot analysis was performed with total lysates from renal HEK293 cells transfected with pcDNA3.1+/C-DYK containing B7-H5 Flag (C-terminal Flag epitope tagging), and ectopic expression was visualized using anti-Flag antibody (Figure 1B). A cluster of protein bands was identified as B7-H5. We hypothesized that the larger-molecular weight range of bands (40–58 kDa) could correspond to different forms of glycosylated B7-H5 protein, whereas the smaller-molecular weight band (36.5 kDa) could correspond to the non-glycosylated form of the protein. Next, to study the subcellular localization of B7-H5, immunofluorescence assays were performed with HEK293 and COS-7 cells transfected to ectopically express B7-H5 Flag and localization of the proteins was visualized by confocal microscopy using an anti-Flag antibody (Figure 1C). In both HEK293 and COS-7 cells, the B7-H5 Flag protein was distributed mainly in the plasma membrane, with some cytoplasmic staining (Figure 1C).
Figure 1.
Expression and biochemical characterization of B7-H5 in renal cells
(A) Boxplot of B7-H5/VISTA mRNA expression in ccRCC tumor tissue (shown in red) in comparison to normal tissue (in gray). Data are represented in a logarithmic scale (Log2) and obtained from 523 tumor samples and 72 normal tissue samples (data from TCGA). The asterisk indicates a statistically significant difference (p < 0.01).
(B) Western blot of total lysates from HEK293 cells ectopically expressing B7-H5 Flag. Cells were transfected with pcDNA3.1+/C-DYK empty vector (EV) or containing B7-H5 Flag, and a Western blot was performed using anti-Flag or anti-GAPDH (loading control) antibodies. Migration of molecular weight markers is shown.
(C) Immunofluorescence confocal microscopy images at 63X showing subcellular localization of ectopically expressed B7-H5 Flag in HEK293 and COS-7 cells. Anti-Flag primary antibody and Alexa Fluor 488-conjugated secondary antibody were used for visualization of B7-H5 Flag (in green). Nuclei were stained with DAPI (in blue).
(D) Western blot of total lysates from HEK293 cells ectopically expressing B7-H5 Flag. Cells were transfected with pcDNA3.1+/C-DYK empty vector (EV) or containing B7-H5 Flag. Lysates were kept untreated or processed for PNGase F protocol in the absence (−) or in the presence of PNGase F enzyme (+). Western blot was performed using anti-Flag, anti-B7-H5, or anti-GAPDH antibodies. Migration of molecular weight markers is shown.
(E) Western blot of total lysates from HEK293 cells ectopically expressing B7-H5 Flag or B7-H5. Cells were transfected with pcDNA3.1+/C-DYK empty vector (EV), or containing B7-H5 Flag or B7-H5. Western blot was performed using anti-Flag, anti-B7-H5, or anti-GAPDH antibodies. Migration of molecular weight markers is shown.
(F) Western blot of total lysates from HEK293 cells ectopically expressing B7-H5.Lysates were kept untreated or treated with PNGase F, as indicated. Anti-B7-H5 was used for identification of B7-H5 protein and anti-GAPDH was used as a loading control.
To study the glycosylation of B7-H5, total lysates from HEK293 cells ectopically expressing B7-H5 Flag were treated with PNGase F endoglycosidase, which hydrolyses N-glycan chains from proteins. Western blot was performed and the migration of proteins was analyzed using anti-Flag and anti-B7-H5 antibodies (Figure 1D). Treatment with PNGase F shifted the migration of B7-H5 Flag proteins from the high molecular weight range (40–58 kDa) to the low molecular weight range (36.5 kDa) confirming that B7-H5 protein is heavily N-glycosylated. A similar migration pattern was observed for untagged B7-H5, as detected with anti-B7-H5 antibody (Figures 1E and 1F). Accordingly, further experiments were made ectopically expressing untagged B7-H5 in renal cells.
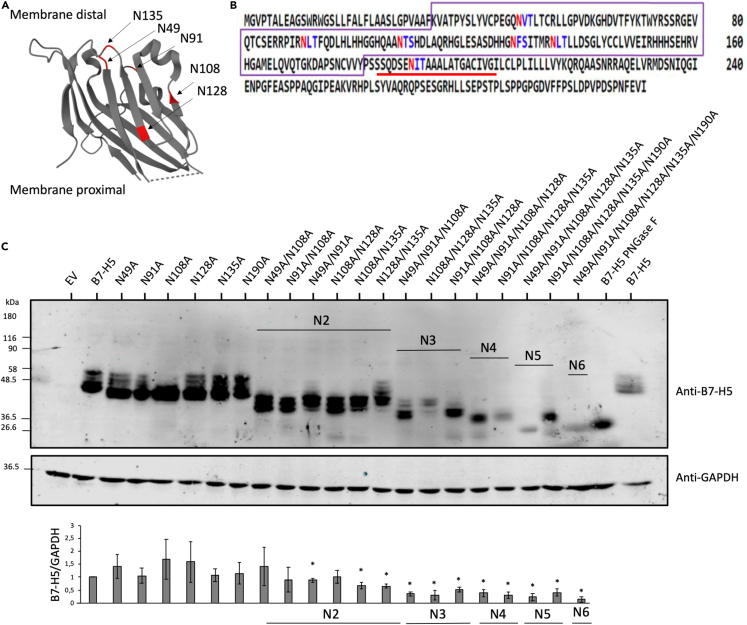
To identify potential N-glycosylated residues in B7-H5 protein sequence, we performed an in silico analysis using the online web server NetNGlyc, and six potential N-glycosylation motifs (Asn-Xxx-Ser/Thr; N-X-S/T) were found at residues N49, N91, N108, N128, N135, and N190 (Figures 2A and 2B). Next, site-directed mutagenesis was performed to create B7-H5 variants mutating to Ala (A) these Asn residues: N49A, N91A, N108A, N128A, N135A, and N190A, alone or in combinations. Western blot analysis was performed with total lysates from HEK293 cells ectopically expressing B7-H5 wild type (WT) or N/A variants. Protein bands corresponding to B7-H5 N49A, N91A, N108A, N128A, and N135A migrated at lower molecular weight than B7-H5 WT, indicating that N49, N91, N108, N128, and N135 are B7-H5 N-glycosylation sites. B7-H5 N190A migrated similarly to B7-H5 WT, suggesting that N190 is not targeted by N-glycosylation. Analysis of multiple N/A compound mutations revealed an additive effect on the migration of B7-H5 protein. In addition, B7-H5 protein expression levels were gradually diminished in the N/A compound mutations, in a manner dependent on multiple N-glycosylated residues (Figure 2C). This suggests that the lack of N-glycosylation affects the stability of B7-H5 proteins.
Figure 2.
N-glycosylation sites of B7-H5
(A) Depiction of B7-H5 3D structure and localization of the amino acids targeted by glycosylation in the extracellular region (N49, N91, N108, N128, N135). Extracellular domain of B7-H5 is shown according to accession 6OIL.28 Crystal structure visualization by Protein DataBank. N-glycosylated sites are highlighted in red.
(B) Potential N-glycosylation sites in B7-H5 protein sequences (NP_071436.1). Asn/N that have a high probability of being N-glycosylated are shown in red, whereas Asn/N that have a lower probability of being N-glycosylated are shown in blue. IgV domain is highlighted in a violet box, and transmembrane region is underlined in red.
(C) Western blot of total lysates from HEK293 cells ectopically expressing B7-H5, B7-H5 N49A, N91A, N108A, N128A, N135A, N190A single and multiple mutations (N2-N6), as indicated. Cells were transfected with pcDNA3.1+/C-DYK empty vector (EV), or with B7-H5 variants. B7-H5 WT lysate was kept untreated or treated with PNGase F, as indicated. Anti-B7-H5 was used for identification of B7-H5 protein and anti-GAPDH was used as a loading control. In the lower panel, quantification of B7-H5/GAPDH is shown from three independent blots, ± SD. Statistically significant results (p < 0.05) are marked with an asterisk.
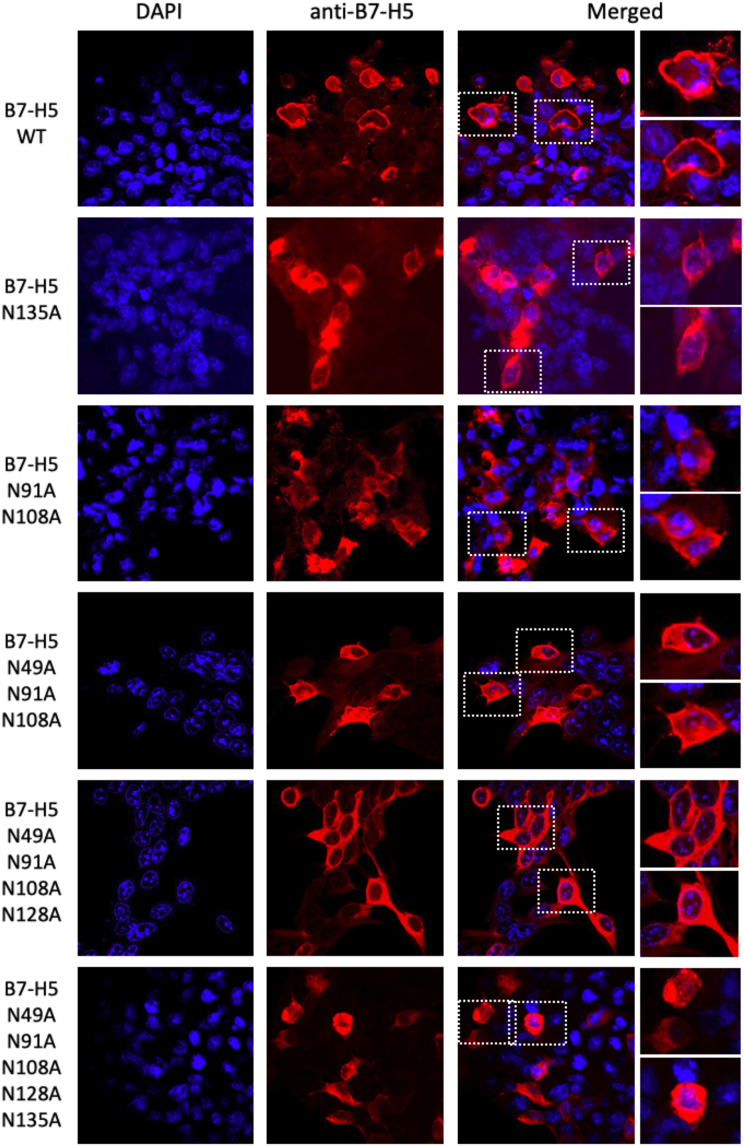
Next, immunofluorescence assays were performed in HEK293 cells ectopically expressing B7-H5 N/A mutations to study whether N-glycosylation affected its subcellular localization. All single B7-H5 N/A mutations, and 2-to-4 residues compound N/A mutations (N2-N4), were mainly distributed in the cell membrane with some cytoplasmic distribution, similar to B7-H5 WT. However, when all 5 N-glycosylated residues (N5) were mutated, the localization was not seen in the membrane and was mainly cytoplasmic (Figure 3).
Figure 3.
Protein localization of B7-H5 WT and N-glycosylation mutations
Immunofluorescence confocal microscope images at 63X showing subcellular localization of ectopically expressed B7-H5 WT and selected B7-H5 N-glycosylation mutations in HEK293 cells. Nuclei were stained with DAPI (in blue). Anti-B7-H5 primary antibody and Alexa Fluor 546 conjugated secondary antibody were used for visualization of the B7-H5 proteins (in red).
Functional role of B7-H5 in renal cancer cells
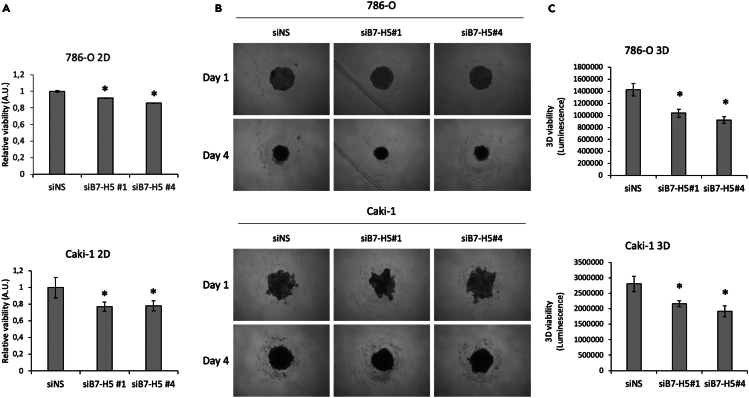
The proliferation and viability of renal cancer cells were studied after silencing of B7-H5 expression with different siRNAs, using non-specific siRNA (siNS) as a negative control. Firstly, 786-O and Caki-1 renal cancer cells were transfected with four different B7-H5 siRNAs, RNA was isolated and retro transcribed, and real-time quantitative polymerase chain reaction (RT-qPCR) was performed to test the efficacy of the silencing (Figure S1). In both cell lines siB7-H5 #1 and siB7-H5 #4 showed more efficiency for the silencing of B7-H5, and were selected for further functional assays. Next, the proliferation and viability of 786-O and Caki-1 cells were monitored by proliferation and viability assays after silencing of B7-H5 expression with siB7-H5 #1 and siB7-H5 #4. Silencing of B7-H5 expression in both renal cancer cell lines resulted in a modest, but significant, decrease of 2D cell proliferation (Figure 4A). B7-H5 expression was also silenced in 786-O and Caki-1 cell spheroids using siB7-H5#1 and siB7-H5#4. The evolution of the 3D spheroids was observed and photographed daily. The spheroid area decreased after silencing with both siRNAs (Figure 4B). To further verify the functional effect of B7-H5 silencing in 3D models, cell viability was measured and compared to the negative control (siNS). In spheroids of both cell lines, silencing of B7-H5 significantly reduced 3D cell viability and showed a more prone effect than differences in proliferation measured by 2D (Figure 4C).
Figure 4.
2D and 3D viability of renal cancers cells upon silencing of B7-H5
(A) Relative viability of 786-O and Caki-1 cells following transfection with siNS (negative control), siB7-H5 #1 or siB7-H5 #4. Data are presented relative to the negative control.
(B) 786-O and Caki-1 cell spheroid evolution the day of transfection (Day 1) and 3 days after transfection (Day 4).
(C) 3D viability of 786-O and Caki-1 cell spheroids after transfection with siNS, siB7-H5#1 or siB7-H5#4. Data are represented from one representative experiment out of three biological replicates, with 3–6 technical replicates, ±SD. Statistically significant results (p < 0.05) are marked with an asterisk.
Clinical impact of B7-H5 expression in metastatic clear cell renal cancer carcinomas
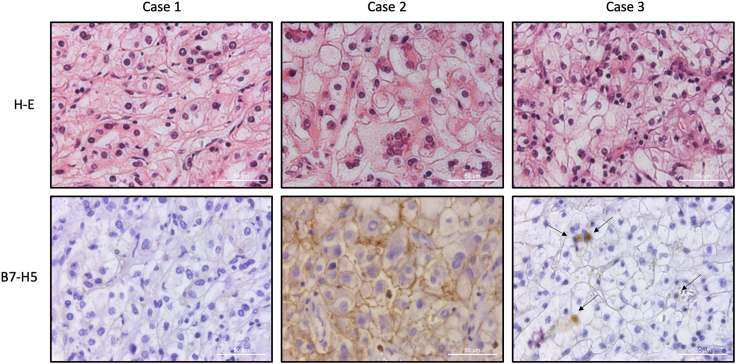
To gain further insights into the clinical impact of B7-H5 protein expression in ccRCC, we evaluated by IHC the expression of B7-H5 in tumor samples from a retrospective cohort of 54 metastatic ccRCC patients (Table 1). Expression was scored as negative (when low or no staining) or positive (when moderate or high staining) (see details in STAR methods section). Representative immunostaining images are shown in Figure 5. We observed expression of B7-H5 protein on tumor cells in 13/54 (24%) cases and on TILs in 24/54 (44%). B7-H5 expression displayed a major cytoplasmic/membranous localization. We analyzed the clinicopathological correlations of B7-H5 expression on tumor cells and TILs. We found a significant negative correlation of high B7-H5 expression in the tumor cells with lower grades (Table 1). Significant correlations were also found of B7-H5 expression on TILs with the stage (p = 0.033), necrosis (p = 0.004), sarcomatoid transformation (p = 0.020), disease-free survival (p = 0.015), synchronous metastasis (p 0.000) (Table 1), and correlation to metastasis to non-epithelial organs (p = 0.037) (Table 2). Importantly, we found a significant positive correlation of B7-H5 TIL immunostaining with lower overall survival (p = 0.027).
Table 1.
Correlation between clinical and pathological variables and B7-H5 protein expression in primary tumor specimens in metastatic ccRCC
| Metastatic ccRCC |
N = 54 | PRIMARY TUMOR |
|||
|---|---|---|---|---|---|
| Characteristic |
TUMOR |
TILs |
|||
|
B7-H5 low/no |
B7-H5 high |
B7-H5 low/no |
B7-H5 high |
||
| Patients | (N = 41) | (N = 13) | (N = 30) | (N = 24) | |
| Median follow-up time | 53.5 | ρ = 0.036/p = 0.795 | ρ = −0.406/p = 0.002 | ||
| Months | 39 | 61 | 69 | 22 | |
| Median age at surgery | 59 | ρ = −0.045/p = 0.749 | ρ = 0.133/p = 0.338 | ||
| Years | 59 | 59 | 59 | 59 | |
| Sex | ρ = 0.059/p = 0.671 | ρ = 0.222/p = 0.107 | |||
| Female | 15 | 12 | 3 | 11 | 4 |
| Male | 39 | 29 | 10 | 19 | 20 |
| Age at surgery | ρ = 0.131/p = 0.345 | ρ = 0.256/p = 0.061 | |||
| ≤70 years | 46 | 36 | 10 | 28 | 18 |
| >70 years | 8 | 5 | 3 | 2 | 6 |
| Gradea | ρ = −0.326/p = 0.016 | ρ = 0.059/p = 0.672 | |||
| Low | 22 | 13 | 9 | 13 | 9 |
| High | 32 | 28 | 4 | 17 | 15 |
| Stageb | ρ = −0.088/p = 0.525 | ρ = 0.291/p = 0.033 | |||
| Low | 28 | 20 | 8 | 19 | 9 |
| High | 26 | 21 | 5 | 11 | 15 |
| Diameterc | ρ = 0.024/p = 0.863 | ρ = 0.109/p = 0.439 | |||
| ≤4 cm | 9 | 7 | 2 | 6 | 3 |
| >4 cm | 44 | 33 | 11 | 23 | 24 |
| Tumor necrosis | ρ = −0.125/p = 0.369 | ρ = 0.382/p = 0.004 | |||
| No | 26 | 18 | 8 | 18 | 8 |
| Micro | 8 | 7 | 1 | 7 | 1 |
| Yes | 20 | 16 | 4 | 5 | 15 |
| Sarcomatoid transformation | ρ = 0.172/p = 0.215 | ρ = 0.316/p = 0.020 | |||
| No | 50 | 39 | 11 | 30 | 20 |
| Yes | 4 | 2 | 2 | 0 | 4 |
| Disease free survival | ρ = -0.088/p = 0.526 | ρ = 0.329/p = 0.015 | |||
| Yes | 13 | 9 | 4 | 11 | 2 |
| No | 41 | 32 | 9 | 19 | 22 |
| Survival | ρ = −0.106/p = 0.444 | ρ = 0.300/p = 0.027 | |||
| Alive | 20 | 14 | 6 | 15 | 5 |
| Dead | 34 | 27 | 7 | 15 | 19 |
| Metastasis | ρ = 0.014/p = 0.920 | ρ = 0.562/p = 0.000 | |||
| Metachronous | 38 | 29 | 9 | 28 | 10 |
| Synchronous | 16 | 12 | 4 | 2 | 14 |
Fuhrman’s grade, low (G1/2) vs. high (G3/4).
AJCC 2010 staging low (pT1/2) vs. high (≥pT3).
Tumor diameter, small (≤4 cm) vs. large (>4 cm) Spearman correlation ρ/p value.
Figure 5.
B7-H5 immunohistochemical expression patterns in ccRCC
Hematoxylin-eosin (H-E) staining and B7-H5 immunostaining is shown in three different ccRCC cases. Case 1 shows negative immunostaining. Case 2 shows positive immunostaining in tumor cells with a membranous pattern. Case 3 shows discrete positive immunostaining in TILs (arrows).
Table 2.
Correlation between clinical and pathological variables and B7-H5 protein expression in metastatic lesions in metastatic ccRCC
| Metastatic ccRCC |
N=54 |
METASTATIC SITE |
|||||
|---|---|---|---|---|---|---|---|
| Characteristic |
TUMOR |
TILs |
|||||
|
B7-H5 low/no |
B7-H5 high |
B7-H5 low/no |
B7-H5 high |
||||
| Patients | N=54 | (N=41) | (N=13) | (N=30) | (N=24) | ||
| Metastatic site | |||||||
| Lymph node | ρ = −0.070/p = 0.616 | ρ = 0.195/p = 0.157 | |||||
| No | 43 | 32 | 11 | 26 | 17 | ||
| Yes | 11 | 9 | 2 | 4 | 7 | ||
| Epithelial organs | ρ = 0.135/p = 0.332 | ρ = −0.285/p = 0.037 | |||||
| No | 23 | 19 | 4 | 9 | 14 | ||
| Yes | 31 | 22 | 9 | 21 | 10 | ||
| Soft tissues | ρ = −0.135/p = 0.329 | ρ = 0.066/p = 0.635 | |||||
| No | 40 | 29 | 11 | 23 | 17 | ||
| Yes | 14 | 12 | 2 | 7 | 7 | ||
Spearman correlation ρ/p value.
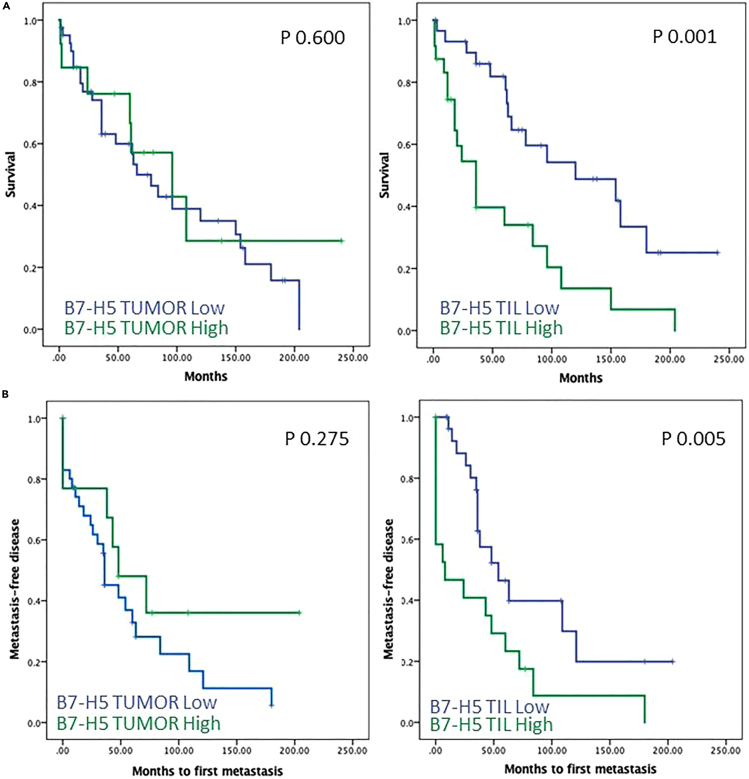
Kaplan–Meier curves for survival time and expression of B7-H5 on TILs showed significant correlations with overall survival (log rank test, p = 0.001) (Figure 6, upper panels). In addition, Kaplan–Meier curves for time to first metastasis and expression of B7-H5 on TILs showed differential significant correlations (log rank test, p = 0.005) (Figure 6, lower panels). Cox regression multivariate analysis, including statistically significant variables and variables previously shown to be clinically relevant, revealed that the necrosis and B7-H5 on TILs were independent prognostic parameters for the overall survival of metastatic ccRCC patients in this cohort (p = 0.019 and p = 0.002, respectively) (Table 3). Sarcomatoid transformation, metastasis synchronicity, and B7-H5 expression on tumor cells were excluded from the predictive model because they did not significantly correlate with patient survival (Table 3).
Figure 6.
Kaplan-Meier survival curves of ccRCC patients according to B7-H5 expression on tumor cells and TILs
Kaplan-Meier survival curves of ccRCC patients according to overall survival (A) or metastasis-free disease (B), and B7-H5 expression on tumor cells (left panels) and TILs (right panels).
Table 3.
Univariate and multivariate analysis to predict overall survival of metastatic ccRCC patients
| Variable | Description | Point Estimate | 95% Wald Confidence Limits | p-value Log rank | |
|---|---|---|---|---|---|
| Univariate analysis | |||||
| Grade | Low vs. high | 1.153 | 0.575 | 2.310 | 0.689 |
| Stage | Low vs. high | 0.959 | 0.478 | 1.923 | 0.905 |
| Diameter | <4 cm >4 cm |
1.132 | 0.461 | 2.779 | 0.787 |
| Tumor necrosis | No vs. yes | 2.282 | 1.097 | 4.747 | 0.027 |
| Sarcomatoid transformation | No vs. yes | 2.828 | 0.635 | 12.595 | 0.173 |
| Metastasis synchronicity |
Metachronous vs. Synchronous | 2.344 | 1.093 | 5.025 | 0.029 |
| Metastatic site Lymph node |
No vs. yes | 1.826 | 0.730 | 4.569 | 0.198 |
| Metastatic site Endothelial tissue |
No vs. yes | 0.798 | 0.399 | 1.596 | 0.523 |
| Metastatic site Soft tissue, sarcoma |
No vs. yes | 0.883 | 0.408 | 1.907 | 0.751 |
| B7-H5 tumor | No vs. yes | 0.801 | 0.346 | 1.853 | 0.604 |
| B7-H5 TILs | No vs. yes | 2.948 | 1.475 | 5.891 | 0.002 |
| Variable | Description | Point Estimate | 95% Wald Confidence Limits | p-value Cox | |
|---|---|---|---|---|---|
| Multivariate analysis | |||||
| Tumor necrosis | No vs. yes | 2.561 | 1.148 | 5.710 | 0.022 |
| Sarcomatoid transformation | No vs. yes | 0.782 | 0.155 | 3.959 | 0.767 |
| Metastasis synchronicity | Metachronous vs. Synchronous | 1.675 | 0.674 | 4.164 | 0.267 |
| B7-H5 tumor | No vs. yes | 1.078 | 0.453 | 2.564 | 0.865 |
| B7-H5 TILs | No vs. yes | 2.780 | 1.294 | 5.973 | 0.009 |
| Multivariate analysis (excluding variables with p-value > 0.2) | |||||
| Tumor necrosis | No vs. yes | 2.466 | 1.158 | 5.249 | 0.019 |
| B7-H5 TILs | No vs. yes | 3.132 | 1.542 | 6.364 | 0.002 |
Discussion
Increased expression of B7 immune checkpoint proteins on tumors evade elimination by the immune system. Some members of the B7 family are highly expressed in different types of cancer and immune cells, playing a role in cancer progression and immune evasion, in correlation with poor prognosis. This outlines B7 family members as new potential biomarkers and therapeutic targets in cancer, although knowledge on these proteins is limited.12 Currently, immune checkpoint inhibitors are in clinical use to target some of these proteins, which promotes T cell-mediated tumor elimination. Although ccRCC is a particularly chemo- and radio-resistant cancer, targeted therapies and PD-1/PD-L1-based immunotherapies have shown promising results.22,29 Nevertheless, many patients do not respond or develop resistance over time, possibly due to the co-expression of other checkpoint proteins in the tumor microenvironment.30 As a consequence, there is a need to identify better biomarkers as well as new therapeutic targets for ccRCC. The aim of this work was to characterize B7-H5 immune checkpoint proteins in ccRCC as a potential biomarker for high-risk ccRCC.
In silico analysis showed that B7-H5 mRNAs are upregulated in ccRCC bulk tumor tissue. In RCC, it has been reported that the activity of HIF is increased via the mTOR pathway and the inactivation of VHL,31 and HIF induced hypoxia in the TME upregulates B7-H5 expression in colorectal cancer.32 B7-H5 protein was recently found upregulated in tumor cells from patients with ccRCC, and its expression correlated with poor T cell responses.33 High expression of B7-H5 in ccRCC progression could be linked to VHL and HIF signaling and requires further studies.
In our study, we did not detect endogenous B7-H5 expression in ccRCC, HEK293 or COS-7 cell lines, suggesting low levels of protein expression. Since ccRCC cell lines were not efficiently transfected with plasmid-based cDNA, B7-H5 biochemical characterization was performed on transiently transfected HEK293 cells. Our analysis led to the identification of two different clusters of B7-H5 protein bands that corresponded to N-glycosylated forms of B7-H5 (40–58 kDa) and non-glycosylated B7-H5 (36.5 kDa), as indicated by treatment with PNGase F endoglycosidase. Similar protein patterns have been described for PD-L1, where a cluster of bands at 50 kDa corresponds to the PD-L1 N-glycosylated form of the protein, and the PD-L1 non-glycosylated form is detected at 33 kDa.34,35 IHC and immunofluorescence assays on cells overexpressing B7-H5 revealed that B7-H5 is distributed mainly at the cell membrane but also in the cytoplasm, consistent with previous reports.36 Site-directed mutagenesis was performed to create B7-H5 variants targeting potential N-glycosylation sites (B7-H5 N49A, N91A, N108A, N128A, N135A, N190A). Our analysis indicates that N49, N91, N108, N128, and N135, but not N190, are B7-H5 N-glycosylation sites and that single mutations alone do not alter significantly B7-H5 protein expression nor subcellular localization. However, multiple mutations showed a gradual impact of N-glycosylation on both protein expression levels and protein localization. In this regard, it has been described that non-glycosylated PD-L1 undergoes faster protein degradation,34 and the N-glycans in PD-L1 have been found to hinder recognition by PD-L1 antibodies and to reduce therapeutic efficacy, highlighting the N-glycosylation of B7 proteins as a potential therapeutic intervention mechanism in cancer.37 The potential impact of N-glycosylation on B7-H5 in their interaction with ligands and subsequent role in immunosuppressive function, as well as in their subcellular trafficking toward the cell membrane and in their protein stability, requires further studies.
Viability analysis of renal cancer cells upon silencing B7-H5 expression resulted in a modest, but significant, decrease in their proliferation and viability in 2D and 3D growth conditions. IHC analysis revealed the significance of high B7-H5 expression on tumor cells with lower stage. This could indicate that B7-H5 plays a role in the early progression of ccRCC. In this regard, low levels of B7-H5 in ccRCC tumor cells have been reported by others.20 Furthermore, we observed significant correlations of B7-H5 high expression on TILs in primary tumors with several clinical parameters, including higher disease stage, lower disease-free survival, and lower overall survival. This suggests a pro-oncogenic activity for B7-H5 in ccRCC, as already described in other tumor tissues.23 B7-H5 has previously been reported to be expressed on TILs.38 The correlation between TILs and ccRCC clinical parameters has been studied in several investigations. High levels of TILs may not always correspond to higher antitumor activity by immune cells, as TILs may be exhausted or inactive by immune checkpoint inhibitory activity. In line with our results, B7-H5 expression in TILs in venous tumor thrombi is associated with shorter overall survival in ccRCC.27 Immune checkpoint proteins play a critical role in tumor immune evasion, and it has been reported that tumors with T cell infiltrations have a higher success rate of immune checkpoint blockade therapy.39 This is consistent with the results of ccRCC studies that show that lower levels of TILs were observed in metastatic tumors compared with primary tumors, which could be explained by the immunoediting and immune evasion processes these tumors undergo.40 RCC tumors infiltrated by PD-1-expressing immune cells were reported to exhibit unfavorable pathological characteristics, such as the increased risk of cancer-specific death and overall mortality.41 Studies of immune checkpoint inhibitors in ccRCC have mainly been focused on PD-L1 expression.
In summary, our results provide an insight into the molecular features of B7-H5 protein and suggest the potential role of B7-H5 as a new immunotherapeutic target in advanced ccRCC for patients not responding to current therapies. Recently, an ex vivo cultured ccRCC patient-derived tumor tissue model has been used to mimic patient responses to an anti-B7-H5 monoclonal antibody, showing promising results.42 Furthermore, anti-B7-H5 monoclonal antibodies are under therapeutic development.43 In this regard, clinical trials (Phase 1/2) using anti-B7-H5 monoclonal antibodies are currently ongoing for solid tumors including renal cancer (ClinicalTrials.gov Identifier: NCT04475523, NCT05864144, and NCT05708950), whose results and conclusions will be important for the future implementation of B7-H5 based therapies in ccRCC. Additional studies are required to unravel the potential oncogenicity of B7-H5 and its suitability as a therapeutic target in ccRCC.
Limitations of the study
We have identified the B7-H5 residues targeted by glycosylation using in vitro cultures of renal cells. It would be interesting to explore the B7-H5 glycosylation patterns in ccRCC tumors, and their association to malignancy and therapy efficacy. Our study shows a correlation between B7-H5 expression on TILs and poor outcomes in ccRCC patients. These findings require further validation with additional independent cohorts.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-FLAG | Millipore | Cat#MAB3118; RRID: AB_11213007 |
| Anti-B7-H5/VISTA | Cell Signaling | Cat#64953; RRID: AB_2799671 |
| Anti-GAPDH | Santa Cruz Biotechnology | Cat#sc-32233; RRID: AB_627679 |
| Goat anti-mouse 800 | Li-Cor | Cat#926-32210; RRID: AB_621842 |
| Goat anti-rabbit 680 | Li-Cor | Cat#926-68071; RRID: AB_10956166 |
| Anti-mouse Alexa Fluor 488 | Thermo Scientific | Cat#A-11001; RRID: AB_2534069 |
| Anti-rabbit Alexa Fluor 546 | Thermo Scientific | Cat#A-11060; RRID: AB_2534107 |
| Bacterial and virus strains | ||
| E. coli DH5alpha | Thermo Scientific | 18265017 |
| Biological samples | ||
| Tissue microarray containing ccRCC and metastasis specimens | Cruces Hospital | NA |
| Critical commercial assays | ||
| CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) | Promega | G3581 |
| CellTiter-Glo® 3D Cell Viability Assay | Promega | G9683 |
| Experimental models: Cell lines | ||
| HEK293 | ATCC | CRL-1573 |
| COS-7 | ATCC | CRL-1651 |
| 786-O | ATCC | CRL-1932 |
| Caki-1 | ATCC | HTB-46 |
| Oligonucleotides | ||
| Primer B7-H5 N49A Forward GTCCCGAGGGGCAGGCCGTCACCCTCACCTG |
Life Technologies | A1561-2 |
| Primer B7-H5 N49A Reverse CAGGTGAGGGTGACGGCCTGCCCCTCGGGAC |
Life Technologies | A1561-2 |
| Primer B7-H5 N91A Forward CCGGCCCATCCGCGCCCTCACGTTCCAGG |
Life Technologies | A1561-2 |
| Primer B7-H5 N91A Reverse CCTGGAACGTGAGGGCGCGGATGGGCCGG |
Life Technologies | A1561-2 |
| Primer B7-H5 N108A Forward GCCACCAGGCTGCCGCCACCAGCCACGACCT |
Life Technologies | A1561-2 |
| Primer B7-H5 N108A Reverse AGGTCGTGGCTGGTGGCGGCAGCCTGGTGGC |
Life Technologies | A1561-2 |
| Primer B7-H5 N128A Forward CCGACCACCATGGCGCCTTCTCCATCACCAT |
Life Technologies | A1561-2 |
| Primer B7-H5 N128A Reverse ATGGTGATGGAGAAGGCGCCATGGTGGTCGG |
Life Technologies | A1561-2 |
| Primer B7-H5 N135A Forward CATCACCATGCGCGCCCTGACCCTGCTGG |
Life Technologies | A1561-2 |
| Primer B7-H5 N135A Reverse CCAGCAGGGTCAGGGCGCGCATGGTGATG |
Life Technologies | A1561-2 |
| Primer B7-H5 N190A Forward CCAGGATAGTGAAGCCATCACGGCTGCAG |
Life Technologies | A1561-2 |
| Primer B7-H5 N190A Reverse CTGCAGCCGTGATGGCTTCACTATCCTGG |
Life Technologies | A1561-2 |
| Primer removing DYK/Flag Forward CCAAACTTTGAGGTCATCTGATAAACCCGCTGATCA |
Life Technologies | A1561-2 |
| Primer removing DYK/Flag Reverse TGATCAGCGGGTTTATCAGATGACCTCAAAGTTTGG |
Life Technologies | A1561-2 |
| Recombinant DNA | ||
| pcDNA3.1/C-DYK/Flag B7-H5 | GenScript | NM_022153.1 |
| Software and algorithms | ||
| GEPIA http://gepia.cancer-pku.cn |
Peking University | RRID:SCR_018294 |
| NetNGlyc http://www.cbs.dtu.dk/services/NetNGlyc/ |
CBS Prediction Servers | RRID:SCR_001570 |
| Odessey Image Studio http://www.licor.com/bio/products/software/image_studio_lite/ |
Li-Cor | RRID:SCR_013715 v.4.0.21 software |
| ZEN Microscopy software https://www.zeiss.com/microscopy/en/products/software/zeiss-zen.html |
ZEISS | RRID:SCR_013672 |
| GraphPad Prism http://www.graphpad.com/ |
GraphPad | RRID:SCR_002798 |
| SPSS Statistics http://www-01.ibm.com/software/uk/analytics/spss/ |
IBM | RRID:SCR_002865 v.29 software |
| Other | ||
| DMEM | Corning | Cat#10-017-CV |
| RPMI 1640 medium | Corning | Cat#10-040-CV |
| McCoy’s 5A medium | Corning | Cat#10-050-CV |
| FBS | Gibco | Cat#A5256701 |
| L-Glutamine | Lonza | Cat#BE17-605E |
| Penicilin/Streptomycin | Lonza | Cat#DE17-602E |
| GenJet | SignaGen | Cat#SL100489 |
| PBS | Corning | Cat#21-040-CV |
| M-PER lysis buffer | Thermo Scientific | Cat#78501 |
| PhosStop | Roche | Cat#04 906 837 001 |
| cOmplete protease inhibitor | Roche | Cat#04 693 132 001 |
| PNGase F | New England Biolabs | Cat#P0704S |
| NuPAGE sample buffer | Thermo Scientific | Cat#NP0008 |
| Prestained molecular marker | Sigma Aldrich | Cat#SDS7B2 |
| Immobilon ®-FL PVDF Membrane | Merck | Cat# IPFL00010 |
| Odyssey Blocking Buffer | Li-Cor | Cat# 927-70001 |
| Mounting fluid with DAPI | Abcam | Cat# ab104139 |
| PepMute | SignaGen | Cat#SL100566 |
| siRNAs (siB7-H5#1–4) | Qiagen | Cat#1027416 |
| dNTPs | Thermo Scientific | R0193 |
| DpnI | Thermo Scientific | ER1701 |
| Pwo | Roche | PWOPOL-RO |
| OWL HEP-1 transfer system | Thermo Scientific | Cat#HEP-1 |
| Odyssey CLx Imager | Li-Cor | Cat#9140-09 |
| ZEISS LSM880 AIRYSCAN | Zeiss | RRID:SCR_020925 |
| AriaMx Real-Time PCR System | Agilent Technologies | Cat#G8830A |
| Mark™ Microplate Absorbance Reader | Bio-Rad | Cat#168-1130 |
| Infinite® M plex | Tecan | Cat# 30190085 |
| PTLink | Agilent Technologies | Cat#PT100 |
| Dako Autostainer Link 48 | Agilent Technologies | Cat#AS480 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Caroline E. Nunes-Xavier (carolinenunesxavier@gmail.com).
Materials availability
Plasmids generated in this study are available from the lead contact upon request.
Data and code availability
-
•
This study analyzed publicly available data from the TCGA. The gene expression data are available on the GEPIA http://gepia.cancer-pku.cn.
-
•
Uncropped original Western blot images are available as Data S1.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
Experimental model and study participant details
Clinical samples
Renal cancer cohorts with primary and metastatic ccRCC, consisted of 54 metastatic ccRCC tumors surgically removed at Cruces University Hospital in Spain between 1997 and 2001. Clinical follow-up has been recorded until October 1, 2016. FFPE blocks were preserved, and fresh tissue sections were prepared for the study. The series included primary and metastatic tissue samples from each patient. 29% of the patients had synchronous metastasis at the time of surgery, while the rest developed metachronous metastasis in a time oscillating between 6 and 204 months. The median diameter of the tumors was 8.5 cm. An experienced pathologist (JIL) selected formalin-fixed paraffin-embedded tumor areas of well-preserved tissue representative of the whole tumor from these patients, and TMA blocks were made from these areas. 4 μm sections were made from the TMA blocks, one of which was stained with hematoxylin and eosin to verify the presence and quality of tumor content. This renal cancer cohorts have been previously described in Errarte et al.44 Ethical approval has been obtained for the clinical material (CEIm-E number PI2022085).
Method details
In silico analysis of B7 expression and potential N-glycosylation sites in renal cancer
The mRNA expression levels of B7-H5 in ccRCC tumor tissue was analyzed in comparison to normal tissues using samples from TCGA database via online web server GEPIA (http://gepia.cancer-pku.cn).45 Online web server NetNGlyc (https://services.healthtech.dtu.dk/service.php?NetNGlyc-1.0) was used to identify the Asn-Xxx-Ser/Thr (N-X.S/T) motifs in B7-H5 protein sequence (NP_071436.1) which could be potential N-glycosylated sites.
Cell culture and transfections
The mammalian renal cell lines HEK293 (human embryonic kidney cell line), COS-7 (fibroblast-like cell line derived from monkey kidney tissue), and human ccRCC cell lines 786-O and Caki-1 cells were used. HEK293 and COS-7 cells were cultured in DMEM (Dulbecco’s Modified Eagle’s Medium, Corning), 786-O cells in RPMI 1640 medium (Corning), and Caki-1 cells in McCoy’s 5A medium (Lonza). HEK293 and COS-7 cells were supplemented with 5% FBS (Fetal Bovine Serum, Gibco), and 786-O and Caki-1 cells were supplemented with 10% FBS. All medias were supplemented with 1% L-Glutamine and 1% penicillin/streptomycin (Lonza). All cells were incubated at 37°C and 5% CO2. The vector used to ectopically express B7-H5 in mammalian cells was pcDNA3.1/C-DYK B7-H5 cDNA (NM_022153.1) (GenScript). Site-directed mutagenesis of pCDNA3.1 B7-H5 Flag was performed as described in Mingo et al.46 and it was carried out to remove the B7-H5 C-terminal FLAG epitope and to create B7-H5 N/A mutations. GenJet DNA In Vitro Transfection Reagent (SignaGen) protocol was used to transfect HEK293 and COS-7 cells with pCDNA3.1 B7-H5 Flag, or B7-H5 WT and mutations, for immunofluorescence assays and Western blot analysis.
Cell lysate and Western blot
Cells were washed twice with cold Phosphate Buffered Saline (PBS) and were lysed in lysis buffer M-PER (Mammalian Protein Extraction Reagent) (Thermo Scientific) supplemented with PhosSTOP Phosphatase Inhibitor Cocktail Tablets (Roche) and cOmplete protease inhibitor cocktail tablet (Roche). PNGase F protocol (New England Biolabs) was used to hydrolyze N-glycan chains from B7-H5 Flag, or B7-H5 WT from total cell lysates according to manufacturer’s protocol. Lysates were mixed with loading buffer 4X (NuPAGE sample buffer, Life Technologies) containing 5% β-mercaptoethanol. Prestained Molecular Weight Marker (Sigma-Aldrich) was used as a molecular weight marker. Protein content in the gels was transferred to Immobilon-FL PVDF Membrane using semi-dry OWL HEP-1 transfer system (Thermo Scientific). Membranes were blocked with blocking buffer (1:1, Odyssey Blocking Buffer (OBB, Li-Cor ): PBS). Primary antibodies used were mouse monoclonal anti-Flag (1:500, Flag #MAB3118, Millipore), rabbit monoclonal anti-B7-H5 (1:1000, VISTA (D1L2G) #64953, Cell Signaling), or mouse monoclonal anti-GAPDH (1:500, GAPDH (6C5) #sc-32233, SantaCruz Biotechnology). Membranes were washed with washing buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 0.05% Triton X-100, and 0.25% gelatin) as described in Emaldi et al.47 Secondary antibodies used were goat anti-mouse green (1:5000) or goat anti-rabbit red (1:5000). To visualize fluorescence signals on the membranes, Odyssey CLx Imager and Odyssey Image Studio v4.0.21 software (Li-Cor) were used. Data S1 contains all uncropped Western blots.
Immunofluorescence assay
HEK293 and COS-7 cells were plated in a 12-well plate and transiently transfected with plasmids to ectopically express B7-H5 Flag or B7-H5 following the GenJetˆ transient transfection protocol. Cells were fixed with Metanol, and subsequently blocked in blocking solution (PBS 3% BSA). Cells were incubated with mouse anti-Flag(1:100 in blocking solution, Flag #MAB3118, Millipore), or rabbit anti-B7-H5 primary antibodies (1:100 in blocking solution, VISTA (D1L2G) #64953, Cell Signaling). Secondary antibodies used were anti-mouse Alexa Fluor 488 (1:100) or anti-rabbit Alexa Fluor 546 (1:100). Mounting fluid with DAPI (Abcam) was added for the staining of the nuclei. Visualization was seen in a confocal microscope (ZEISS LSM880 AIRYSCAN - objective 63×, 1024×1024 pixels).
Silencing of B7-H5, RNA isolation, reverse transcription and RT-qPCR
PepMute siRNA Transfection Reagent (SignaGen) protocol was used for B7-H5 silencing according to manufacturer’s protocol. 786-O and Caki-1 cell lines were transfected with non-specific siRNA (siNS), glyceraldehyde-3-phosphate dehydrogenase siRNA (siGAPDH) and four different siRNAs for human B7-H5 gene (Qiagen). 786-O and Caki-1 cells were plated in 6-well plates and B7-H5 expression was silenced following PepMute silencing protocol. Total mRNA was extracted using Illustra RNAspin Mini RNA Isolation Kit following the manufacturer’s protocol. Thermo Scientific RevertAid Reverse Transcriptase protocol was followed for cDNA synthesis, and Real time quantitative PCR (RT-qPCR) was performed to monitor B7-H5 mRNA expression using RealQ Plus 2× Master Mix Green with low ROX (Ampliqon), QuantiTect Primers (Qiagen) for HPRT, GAPDH and B7-H5, using Agilent AriaMx Real-Time PCR System (Agilent).
2D cell proliferation assay
3000 Caki-1 cells and 1500 786-O cells were plated per well and incubated for 24 h in 96-well plates. The next day B7-H5 expression was silenced following the Pepmute silencing protocol, and cells were incubated for 72 h. Cell proliferation was measured using CellTiter 96AQueous One Solution Cell Proliferation Assay Kit (Promega). Absorbance was measured at 490 nm using Mark Microplate Absorbance Reader (Bio-Rad). Relative proliferation was calculated by normalizing data to cells treated with siNS.
Spheroid culture and 3D cell viability assay
8000 Caki-1 and 786-O cells were plated per well in low attachment 96 well plates (Thermo Scientific) to create three-dimensional (3D) spheroids of ccRCC cells to better resemble the in vivo characteristics of a solid tumor. B7-H5 was silenced immediately after seeding for spheroid generation following PepMute silencing protocol. The evolution of the spheroids was photographed daily for 5 days using the ZEN Microscopy software (ZEISS). 96 h after transfection, viability of cells in 3D cell cultures was measured following the CellTiter-Glo 3D Cell Viability Assay (Promega) protocol. Luminescent signal was measured using the Infinite M plex (TECAN) plate reader.
Immunohistochemical staining and scoring
Anti-B7-H5 rabbit monoclonal antibody VISTA (D1L2G) #64953 (Cell Signaling, dilution 1:100) was used for immunohistochemistry (IHC). Immunostaining was performed in fully automated immunostainers following routine methods as explained in Nunes-Xavier et al.48 Antigen retrieval was performed at pH 9 using PT link system (Agilent Technologies). B7-H5 immunostaining was performed with EnVision FLEX and Dako Autostainer Link 48 (Agilent). B7-H5 antibody was incubated for 30 min, followed by secondary antibody incubation for 15 min using secondary polyclonal anti-rabbit Ig/HRP (Dako), FLEX/HPR for 20 min, FLEX DAB/Sub Chromo for 10 min, and finally counterstaining with hematoxylin. Slides were dehydrated through incubations with sequentially increasing alcohol concentrations, before xylene incubated and cover-slipped. TMAs and tissue slides were evaluated manually by an experienced uropathologist (JIL). B7-H5 immunoscoring was made following the immunoreactivity scoring system (IRS). Immunostaining for the tumor cells: low/no: <10% positive cells and 0–1 intensity; high: >10% positive cells and 2–4 intensity. Immunostaining for the TILs: low/no: <1% positive cells; high: >1% positive cells.
Quantification and statistical analysis
Statistical analysis of protein expression levels was performed by using a two-tailed Student's t-test and p values were calculated using GraphPad Prism for the differences in B7-H5/GAPDH. Standard deviation (SD) is represented in results by error bars. All experiments were performed at least twice, and results are shown from one representative experiment. p values smaller than 0.05 were considered significant and are indicated in results with an asterisk. The Spearman correlation was used to correlate B7-H5 expression to clinicopathologic parameters. The estimated survival curves were compared using the log rank test. Univariate and multivariate Cox correlation analysis was used to test the independent effects of variables of interest on survival. A stepwise variable selection for a Cox proportional hazards predictive model was used with an entry criterion of p = 0.05 and a stay value of p = 0.2. Correlations were calculated using SPSS Statistics V.29 (IBM). A two-sided p value of less than 0.05 was considered significant.
Acknowledgments
This work was funded by Instituto de Salud Carlos III grant numbers CP20/00008 and PI22/00386, (Spain and co-financed by European Union) to C.E.N-X; and Stiftelsen til fremme av forskning innen nyresykdommer/Foundation for promoting research in kidney diseases (Unifor, Norway) to C.E.N-X. M.E. is the recipient of a Biobizkaia Fellowship 2023/2024 (Biobizkaia Health Research Institute, Spain), and fellowships from Fundacin Jesús Gangoiti Barrera (FJGB21/006, FJGB22/006). E.R-I. is the recipient of a predoctoral fellowship from Asociación Española Contra el Cáncer (AECC, Junta Provincial de Bizkaia, Spain, PRDVZ222375REY). We would like to thank Javier Díez García (Microscope Core facility) and personnel at Genetic and Genomic Core facility for their expert assistance with microscopy and DNA sequencing, respectively, at the Biobizkaia Health Research Institute (Spain); and to Arantza Perez Dobaran (University of the Basque Country UPV/EHU, Spain) for expert technical IHC support.
Author contributions
M.E., P.A-M.: methodology, investigation, visualization, analysis, validation, and writing; E.R-I., L.M., D.L: investigation, visualization, analysis, and validation; R.P., J.I.L.: conceptualization, investigation, visualization, analysis and validation, writing - review and editing; C.E.N-X.: investigation, conceptualization, visualization, analysis, validation, supervision, funding acquisition, project administration, and writing – review, and editing.
Declaration of interests
The authors declare no competing interests.
Published: July 25, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.110587.
Supplemental information
References
- 1.Abbott M., Ustoyev Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019;35 doi: 10.1016/j.soncn.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J. Clin. Invest. 2015;125:3335–3337. doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan L., Tatineni J., Mahoney K.M., Freeman G.J. VISTA: A Mediator of Quiescence and a Promising Target in Cancer Immunotherapy. Trends Immunol. 2021;42:209–227. doi: 10.1016/j.it.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J.J., Hsieh J.J. The Therapeutic Landscape of Renal Cell Carcinoma: From the Dark Age to the Golden Age. Semin. Nephrol. 2020;40:28–41. doi: 10.1016/j.semnephrol.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu W., Hiếu T., Malarkannan S., Wang L. The structure, expression, and multifaceted role of immune-checkpoint protein VISTA as a critical regulator of anti-tumor immunity, autoimmunity, and inflammation. Cell. Mol. Immunol. 2018;15:438–446. doi: 10.1038/cmi.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston R.J., Su L.J., Pinckney J., Critton D., Boyer E., Krishnakumar A., Corbett M., Rankin A.L., Dibella R., Campbell L., et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature. 2019;574:565–570. doi: 10.1038/s41586-019-1674-5. [DOI] [PubMed] [Google Scholar]
- 7.Wang J., Wu G., Manick B., Hernandez V., Renelt M., Erickson C., Guan J., Singh R., Rollins S., Solorz A., et al. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology. 2019;156:74–85. doi: 10.1111/imm.13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Im E., Sim D.Y., Lee H.J., Park J.E., Park W.Y., Ko S., Kim B., Shim B.S., Kim S.H. Immune functions as a ligand or a receptor, cancer prognosis potential, clinical implication of VISTA in cancer immunotherapy. Semin. Cancer Biol. 2022;86:1066–1075. doi: 10.1016/j.semcancer.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Shekari N., Shanehbandi D., Kazemi T., Zarredar H., Baradaran B., Jalali S.A. VISTA and its ligands: the next generation of promising therapeutic targets in immunotherapy. Cancer Cell Int. 2023;23:265. doi: 10.1186/s12935-023-03116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L., Rubinstein R., Lines J.L., Wasiuk A., Ahonen C., Guo Y., Lu L.F., Gondek D., Wang Y., Fava R.A., et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flies D.B., Wang S., Xu H., Chen L. Cutting edge: A monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J. Immunol. 2011;187:1537–1541. doi: 10.4049/jimmunol.1100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flem-Karlsen K., Fodstad Ø., Nunes-Xavier C.E. B7-H3 Immune Checkpoint Protein in Human Cancer. Curr. Med. Chem. 2020;27:4062–4086. doi: 10.2174/0929867326666190517115515. [DOI] [PubMed] [Google Scholar]
- 13.Lines J.L., Pantazi E., Mak J., Sempere L.F., Wang L., O'Connell S., Ceeraz S., Suriawinata A.A., Yan S., Ernstoff M.S., Noelle R. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014;74:1924–1932. doi: 10.1158/0008-5472.CAN-13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao J., Ward J.F., Pettaway C.A., Shi L.Z., Subudhi S.K., Vence L.M., Zhao H., Chen J., Chen H., Efstathiou E., et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017;23:551–555. doi: 10.1038/nm.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kakavand H., Jackett L.A., Menzies A.M., Gide T.N., Carlino M.S., Saw R.P.M., Thompson J.F., Wilmott J.S., Long G.V., Scolyer R.A. Negative immune checkpoint regulation by VISTA: a mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod. Pathol. 2017;30:1666–1676. doi: 10.1038/modpathol.2017.89. [DOI] [PubMed] [Google Scholar]
- 16.Shi T., Zhou S., Zhang T., Han S., Zhang L., Fu F., Yan R., Zhang X. Establishment of a Monoclonal Antibody-Based Enzyme-Linked Immunosorbent Assay to Measure Soluble B7-H5 in Patients with Cancer. J. Immunol. Res. 2022;2022 doi: 10.1155/2022/3013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu W., Xia X., Cheng C., Niu L., Wu J., Qian Y. Serum Soluble PD-L1, PD-L2, and B7-H5 as Potential Diagnostic Biomarkers of Human Pancreatic Cancer. Clin. Lab. 2021;67:1512. doi: 10.7754/Clin.Lab.2021.210103. [DOI] [PubMed] [Google Scholar]
- 18.Ghouzlani A., Lakhdar A., Rafii S., Karkouri M., Badou A. The immune checkpoint VISTA exhibits high expression levels in human gliomas and associates with a poor prognosis. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-00835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie X., Zhang J., Shi Z., Liu W., Hu X., Qie C., Chen W., Wang Y., Wang L., Jiang J., Liu J. The Expression Pattern and Clinical Significance of the Immune Checkpoint Regulator VISTA in Human Breast Cancer. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.563044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong S., Yuan Q., Xia H., Zhu G., Feng Y., Wang Q., Zhang Z., He W., Lu J., Dong C., Ni L. Analysis of VISTA expression and function in renal cell carcinoma highlights VISTA as a potential target for immunotherapy. Protein Cell. 2019;10:840–845. doi: 10.1007/s13238-019-0642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong C., Lang Q., Yu J., Wu S., Xu F., Tian Y. Phenotypical and potential functional characteristics of different immune cells expressing CD28H/B7-H5 and their relationship with cancer prognosis. Clin. Exp. Immunol. 2020;200:12–21. doi: 10.1111/cei.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deleuze A., Saout J., Dugay F., Peyronnet B., Mathieu R., Verhoest G., Bensalah K., Crouzet L., Laguerre B., Belaud-Rotureau M.A., et al. Immunotherapy in Renal Cell Carcinoma: The Future Is Now. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21072532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noelle R.J., Lines J.L., Lewis L.D., Martell R.E., Guillaudeux T., Lee S.W., Mahoney K.M., Vesely M.D., Boyd-Kirkup J., Nambiar D.K., Scott A.M. Clinical and research updates on the VISTA immune checkpoint: immuno-oncology themes and highlights. Front. Oncol. 2023;13 doi: 10.3389/fonc.2023.1225081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lih T.M., Cho K.C., Schnaubelt M., Hu Y., Zhang H. Integrated glycoproteomic characterization of clear cell renal cell carcinoma. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzo A., Mollica V., Dall'Olio F.G., Ricci A.D., Maggio I., Marchetti A., Rosellini M., Santoni M., Ardizzoni A., Massari F. Quality of life assessment in renal cell carcinoma Phase II and III clinical trials published between 2010 and 2020: a systematic review. Future Oncol. 2021;17:2671–2681. doi: 10.2217/fon-2021-0069. [DOI] [PubMed] [Google Scholar]
- 26.Dall'Olio F.G., Rizzo A., Mollica V., Massucci M., Maggio I., Massari F. Immortal time bias in the association between toxicity and response for immune checkpoint inhibitors: a meta-analysis. Immunotherapy. 2021;13:257–270. doi: 10.2217/imt-2020-0179. [DOI] [PubMed] [Google Scholar]
- 27.Zapała Ł., Kunc M., Sharma S., Peksa R., Popeda M., Biernat W., Radziszewski P. Immune checkpoint receptor VISTA on immune cells is associated with expression of T-cell exhaustion marker TOX and worse prognosis in renal cell carcinoma with venous tumor thrombus. J. Cancer Res. Clin. Oncol. 2023;149:4131–4139. doi: 10.1007/s00432-022-04329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta N., Maddineni S., Mathews I.I., Andres Parra Sperberg R., Huang P.S., Cochran J.R. Structure and Functional Binding Epitope of V-domain Ig Suppressor of T Cell Activation. Cell Rep. 2019;28:2509–2516.e5. doi: 10.1016/j.celrep.2019.07.073. [DOI] [PubMed] [Google Scholar]
- 29.Barata P.C., Rini B.I. Treatment of renal cell carcinoma: Current status and future directions. CA. Cancer J. Clin. 2017;67:507–524. doi: 10.3322/caac.21411. [DOI] [PubMed] [Google Scholar]
- 30.Nunes-Xavier C.E., Angulo J.C., Pulido R., López J.I. A Critical Insight into the Clinical Translation of PD-1/PD-L1 Blockade Therapy in Clear Cell Renal Cell Carcinoma. Curr. Urol. Rep. 2019;20:1. doi: 10.1007/s11934-019-0866-8. [DOI] [PubMed] [Google Scholar]
- 31.Baldewijns M.M., van Vlodrop I.J.H., Vermeulen P.B., Soetekouw P.M.M.B., van Engeland M., de Bruïne A.P. VHL and HIF signalling in renal cell carcinogenesis. J. Pathol. 2010;221:125–138. doi: 10.1002/path.2689. [DOI] [PubMed] [Google Scholar]
- 32.Deng J., Li J., Sarde A., Lines J.L., Lee Y.C., Qian D.C., Pechenick D.A., Manivanh R., Le Mercier I., Lowrey C.H., et al. Hypoxia-Induced VISTA Promotes the Suppressive Function of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Cancer Immunol. Res. 2019;7:1079–1090. doi: 10.1158/2326-6066.CIR-18-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulati K., Hamanishi J., Matsumura N., Chamoto K., Mise N., Abiko K., Baba T., Yamaguchi K., Horikawa N., Murakami R., et al. VISTA expressed in tumour cells regulates T cell function. Br. J. Cancer. 2019;120:115–127. doi: 10.1038/s41416-018-0313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C.W., Lim S.O., Xia W., Lee H.H., Chan L.C., Kuo C.W., Khoo K.H., Chang S.S., Cha J.H., Kim T., et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016;7 doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y.N., Lee H.H., Hsu J.L., Yu D., Hung M.C. The impact of PD-L1 N-linked glycosylation on cancer therapy and clinical diagnosis. J. Biomed. Sci. 2020;27:77. doi: 10.1186/s12929-020-00670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ElTanbouly M.A., Croteau W., Noelle R.J., Lines J.L. VISTA: a novel immunotherapy target for normalizing innate and adaptive immunity. Semin. Immunol. 2019;42 doi: 10.1016/j.smim.2019.101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H.H., Wang Y.N., Xia W., Chen C.H., Rau K.M., Ye L., Wei Y., Chou C.K., Wang S.C., Yan M., et al. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell. 2019;36:168–178.e4. doi: 10.1016/j.ccell.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lines J.L., Sempere L.F., Broughton T., Wang L., Noelle R. VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol. Res. 2014;2:510–517. doi: 10.1158/2326-6066.CIR-14-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendry S., Salgado R., Gevaert T., Russell P.A., John T., Thapa B., Christie M., van de Vijver K., Estrada M.V., Gonzalez-Ericsson P.I., et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017;24:235–251. doi: 10.1097/PAP.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mansfield A.S., Aubry M.C., Moser J.C., Harrington S.M., Dronca R.S., Park S.S., Dong H. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann. Oncol. 2016;27:1953–1958. doi: 10.1093/annonc/mdw289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson R.H., Dong H., Lohse C.M., Leibovich B.C., Blute M.L., Cheville J.C., Kwon E.D. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin. Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 42.Hong S., Yuan Q., Xia H., Dou Y., Sun T., Xie T., Zhang Z., He W., Dong C., Lu J., et al. Establishment of an Ex Vivo Tissue Culture Model for Evaluation of Antitumor Efficacy in Clear Cell Renal Cell Carcinoma. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.851191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ElTanbouly M.A., Schaafsma E., Noelle R.J., Lines J.L. VISTA: Coming of age as a multi-lineage immune checkpoint. Clin. Exp. Immunol. 2020;200:120–130. doi: 10.1111/cei.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Errarte P., Guarch R., Pulido R., Blanco L., Nunes-Xavier C.E., Beitia M., Gil J., Angulo J.C., López J.I., Larrinaga G. The Expression of Fibroblast Activation Protein in Clear Cell Renal Cell Carcinomas Is Associated with Synchronous Lymph Node Metastases. PLoS One. 2016;11 doi: 10.1371/journal.pone.0169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang Z., Li C., Kang B., Gao G., Li C., Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mingo J., Erramuzpe A., Luna S., Aurtenetxe O., Amo L., Diez I., Schepens J.T.G., Hendriks W.J.A.J., Cortés J.M., Pulido R. One-Tube-Only Standardized Site-Directed Mutagenesis: An Alternative Approach to Generate Amino Acid Substitution Collections. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emaldi M., Nunes-Xavier C.E. B7-H4 Immune Checkpoint Protein Affects Viability and Targeted Therapy of Renal Cancer Cells. Cells. 2022;11 doi: 10.3390/cells11091448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nunes-Xavier C.E., Kildal W., Kleppe A., Danielsen H.E., Waehre H., Llarena R., Maelandsmo G.M., Fodstad Ø., Pulido R., López J.I. Immune checkpoint B7-H3 protein expression is associated with poor outcome and androgen receptor status in prostate cancer. Prostate. 2021;81:838–848. doi: 10.1002/pros.24180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
This study analyzed publicly available data from the TCGA. The gene expression data are available on the GEPIA http://gepia.cancer-pku.cn.
-
•
Uncropped original Western blot images are available as Data S1.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.