Abstract
In Parkinson's disease (PD), exogenous ghrelin protects dopaminergic neurons through its receptor, growth hormone secretagogue receptor (GHSR). However, in contrast to the strikingly low levels of ghrelin, GHSR is highly expressed in the substantia nigra (SN). What role does GHSR play in dopaminergic neurons is unknown. In this study, using GHSR knockout mice (Ghsr−/− mice) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD model, we found that GHSR deletion aggravated dopaminergic neurons degeneration, and the expression and activity of GHSR were significantly reduced in PD. Furthermore, we explored the potential mechanism that GHSR deficiency aggregated PD-related neurodegeneration. We showed that DEPTOR, a subunit of mTORC1, was overexpressed in Ghsr−/− mice, positively regulating autophagy and enhancing autophagy initiation. The expression of lysosomal markers was abnormal, implying lysosomal dysfunction. As a result, the damaged mitochondria could not be effectively eliminated, which ultimately exacerbated the injury of nigral dopaminergic neurons. In particular, we demonstrated that DEPTOR could be transcriptionally regulated by KLF4. Specific knockdown of KLF4 in dopaminergic neurons effectively alleviated neurodegeneration in Ghsr−/− mice. In summary, our results suggested that endogenous GHSR deletion-compromised autophagy by impairing lysosomal function, is a key contributor to PD, which provided ideas for therapeutic approaches involving the manipulation of GHSR.
Keywords: Parkinson's disease, GHSR, Autophagy, KLF4, DEPTOR
Graphical abstract
GHSR knockout upregulated KLF4, subsequently transcriptionally regulating the mTORC1 subunit DEPTOR and resulting in autophagy activation and an increase in the formation of autophagosomes. Dysfunction of lysosomes could block the elimination of damaged mitochondria, thus induce the occurrence of apoptosis, finally exacerbate the loss of dopaminergic neurons (A). The above process was reversed when knocking down KLF4 in the nigral dopaminergic neurons of Ghsr−/− mice. This reduced the formation of autophagosomes and restored the lysosomal degradation capacity. Finally, damaged mitochondria could be degraded by lysosomes and eliminated from the cell, thereby alleviating the death of dopaminergic neurons (B).

Highlights
-
•
GHSR knockout aggravated the damage of dopaminergic neurons in PD, which was closely related to the dysregulation of autophagy.
-
•
GHSR knockout regulated DEPTOR by up-regulating KLF4 transcription, resulting in continuous autophagy activation.
-
•
Knocking-down KLF4 in nigral dopaminergic neurons of Ghsr−/− mice alleviated the death of dopaminergic neurons.
1. Introduction
Parkinson's disease (PD) is an age-related neurodegenerative disorder, characterized by the loss of dopaminergic neurons in the substantia nigra (SN), leading to decreased dopamine (DA) content in the striatum [1]. To date, the pathogenesis of PD has not been elucidated, and genetic factors, oxidative stress, environmental changes, inflammation, aging and mitochondrial dysfunction are involved in the occurrence of PD [2,3]. Our previous studies showed that plasma ghrelin levels were significantly reduced in patients with early-stage PD [4]. The administration of exogenous ghrelin antagonized 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity and protected MES23.5 cells from rotenone-induced apoptosis [[5], [6], [7]]. As a brain-gut peptide composed of 28 amino acids, ghrelin acts primarily through the activation of growth hormone secretagogue receptor (GHSR).
GHSR is a G protein-coupled receptor (GPCR) that is highly expressed not only in the hypothalamus and pituitary gland, but also in the SN, cortex, hippocampus and ventral tegmental area (VTA), which is essential for ghrelin to exert its neuroprotective effects [8,9]. Notably, the probability of circulating ghrelin entering the brain is very low, in this case, ghrelin cannot target most of the GHSR in the brain [10,11]. Therefore, what roles do the highly expressed receptor GHSR play in the brain? Studies have demonstrated that GHSR knockout mice (Ghsr−/− mice) exhibit depression-like behavior [12], and Ghsr−/− rats exhibit a decrease in dendritic density and the number of new neurons in the hippocampus, which ultimately impairs the learning and memory ability [13]. In male mice, GHSR signaling in dopaminergic neurons of the VTA facilitate social approach, and GHSR signaling deficits lead to reduced social motivation [14]. Several studies have also proven that decrease the expression of GHSR could result in PD-like motor dysfunction, and the activation of GHSR in dopaminergic neurons in the SN greatly improved motor dysfunction in mice, but its role and specific mechanism in dopaminergic neurons of SN remains unclear [15,16].
Activation of GHSR has been shown to regulate autophagy. For example, GHSR activated by ghrelin could efficiently stimulate SIRT1/AMPK axis to upregulate autophagy in lymphoblastic cell lines [17]. Besides, ghrelin/GHSR can also upregulate autophagy via AMPK/mTOR to reduce lipotoxicity in high-fat diet mice [18]. However, whether GHSR in the SN could regulate autophagy and thus affect PD process has not been reported. Autophagy is a conserved, lysosomal dependent degradation pathway, in which the damaged cellular organelles could be degraded to maintain normal physiological activities [19]. It has been confirmed that the dysfunction of autophagy caused by the deletion of autophagy related genes (such as Atg5, Atg7) can lead to PD-like symptoms in mice [20,21]. In addition, the deficiency or dysfunction of lysosomal protease (such as Cathepsin D (CTSD), Cathepsin B) can seriously impair the function of autophagy-lysosomes and thus affect PD progression, which suggesting that PD may be associated with autophagy and lysosomal dysfunction [[22], [23], [24], [25]]. mTORC1 is the major inhibitor of autophagy and is a conserved regulator of autophagy, which is composed of five subunits: DEPTOR, RAPTOR, PRAS40, mLST8 and mTOR. As an important subunit of mTORC1, DEPTOR is involved in the activation of the autophagy pathway [26]. DEPTOR knockout can lead to decreased expression of autophagy-related proteins (such as ATG5, LC3), indicating that DEPTOR is a positive regulator of autophagy initiation [27,28].
In this study, we used MPTP to induce PD model in Ghsr−/− mice to clarify the importance of GHSR in dopaminergic neurons. We found that GHSR knockout accelerated the degeneration of dopaminergic neurons in PD mice model, besides, the expression and activity of GHSR were significantly reduced in PD model. Then, we testified that GHSR knockout-induced autophagy dysfunction via DEPTOR might be a potential mechanism for promoting dopaminergic neurons degeneration. The present study expands the current knowledge about the involvement of GHSR in PD-related neurodegeneration and provides a new theoretical basis for the treatment of PD.
2. Results
2.1. GHSR knockout aggravated neurodegeneration in PD mice
To explore the role of endogenous GHSR in nigral dopaminergic neurons in MPTP-induced PD mice model, we injected Ghsr−/− and WT mice with MPTP and normal saline (NS). First, we measured the number of TH-positive neurons and the protein expression of tyrosine hydroxylase (TH). Compared with that in WT-NS and Ghsr−/−-NS groups, the number of TH-positive neurons in the WT-MPTP and Ghsr−/−-MPTP groups was decreased dramatically, i.e., by 47.8% and 58.1%, respectively. In addition, compared with that in the WT-MPTP group, the number of nigral TH-positive neurons in the Ghsr−/−-MPTP group was decreased by 29.5% (Fig. 1A and B). Similar results were observed for nigral TH protein levels, which were still significantly reduced in the WT-MPTP and Ghsr−/−-MPTP groups compared with the WT-NS and Ghsr−/−-NS groups (44.8% and 65.5% respectively). Moreover, compared with those in WT-MPTP group, the nigral TH protein levels in the Ghsr−/−-MPTP group were 45.2% lower (Fig. 1K and L). In the pole test, we tested the total time and the time to turn (Fig. 1F and G). Our results showed that, compared with WT-MPTP mice, the Ghsr−/−-MPTP group exhibited a 105.3% and 97.0% longer in total time and time to turn, respectively (Fig. 1F and G). Moreover, the rotarod test showed that the latency to fall in the Ghsr−/−-MPTP groups was 46.8% greater than that in the WT-MPTP group (Fig. 1H).
Fig. 1.
GHSR knockout aggravated dopaminergic neuron degeneration in PD mice A: Representative images showing TH-positive neurons in the SN in the different groups following immunofluorescence staining. Scale bar = 100 μm. B: Group data showing the number of TH-positive neurons in the different groups. C–E: Group data showing the levels of striatal DA, DOPAC and HVA in the different groups. F–H: Behavioral changes in the different groups were detected by the pole test and rotarod test. I: Representative images showing microglia in the SN in the different groups following immunofluorescent staining. Scale bar = 100 μm. J: Group data showing the number of microglia in the different groups. K–M: Western blotting was used to measure TH and SOD1 protein levels in the SN. Each bar represents the mean ± S.E.M. (one-way ANOVA; *P<0.05, **P<0.01, ***P<0.001).
Next, we observed the levels of DA and its metabolites DOPAC and HVA in the striatum by HPLC. Compared with that in the WT-NS and Ghsr−/−-NS groups, the DA content in the WT-MPTP and Ghsr−/−-MPTP groups was reduced by 51.8% and 67.3%, respectively. Moreover, compared with that in the WT-MPTP group, the DA levels in the striatum in the Ghsr−/−-MPTP group was 42.6% lower (Fig. 1C). As indicated in Fig. 1E, compared with those in the WT-NS and Ghsr−/−-NS group, the striatal HVA levels in the WT-MPTP and Ghsr−/−-MPTP groups were reduced by 64.4% and 71.4%, respectively. These results indicated that GHSR knockout could aggravate the loss of nigral dopaminergic neurons and decrease DA levels in striatum.
Notably, inflammation is a causative factor of PD, so we detected changes in the number of microglia [29]. In the present study, we observed that microglia were activated after MPTP treatment, exhibiting enlarged cell bodies and shortened protuberances. Moreover, in Ghsr−/−-MPTP mice, the number of microglia was 2.11-fold greater than that in Ghsr−/−-NS mice. However, in WT mice, the number of microglia increased only 1.73-fold after MPTP treatment (Fig. 1I and J).
We measured the protein levels of SOD1, which strongly protects against oxidative stress, in the SN [[30], [31], [32]]. After MPTP treatment, the Ghsr−/−-MPTP group exhibited a 60.1% reduction in SOD1 protein levels compared to the Ghsr−/−-NS group. The protein expression of SOD1 in the SN was reduced by only 30.5% in the WT-MPTP group compared with the WT-NS group. Moreover, a 34.4% reduction in SOD1 protein expression was observed in the Ghsr−/−-MPTP group compared with the WT-MPTP group, and the difference was very notable (Fig. 1K and M). The above results demonstrated that GHSR knockout could stimulate microglial activation and exacerbate apoptosis and oxidative stress, thereby aggravating MPTP-induced the degeneration of dopaminergic neurons.
2.2. The expression and activity of GHSR were decreased in PD
We clarified the expression of GHSR in PD models (Fig. 2A and B). The results showed that the protein levels of GHSR in N2a cells was reduced by 31.01% after 1-methyl-4-phenylpyridinium ion (MPP+) treatment, and the difference was statistically significant compared with the control group. Besides, the protein levels of GHSR were decreased by 39.53% after MPTP treatment compared with that in the control group. The above results showed that the expression of GHSR was reduced in PD. To determine the effect of MPP+ on the constitutive activity of GHSR, we used HTRF technique to detect the intracellular IP content. The experimental results showed that the IP content after MPP+ treatment was reduced by 38.95%, which had significant difference compared with that in the control group (Fig. 2C–E). This result indicated that the activity of GHSR in N2a cells was decreased after MPP+ treatment.
Fig. 2.
The expression and activity of GHSR were decreased in PD A: The effect of MPP+ on GHSR protein expression in N2a cells. B: The effect of MPTP on GHSR protein expression in the SN of mice. C: The standard curve of IP. D: Schematic diagram of HTRF technology detecting the intracellular IP content. E: The effect of MPP+ on the intracellular IP content. Each bar represents the mean ± S.E.M. (one-way ANOVA, Student's t-test; *P<0.05, **P<0.01).
2.3. GHSR knockout promoted autophagy initiation and suppressed autophagic flux in PD mice
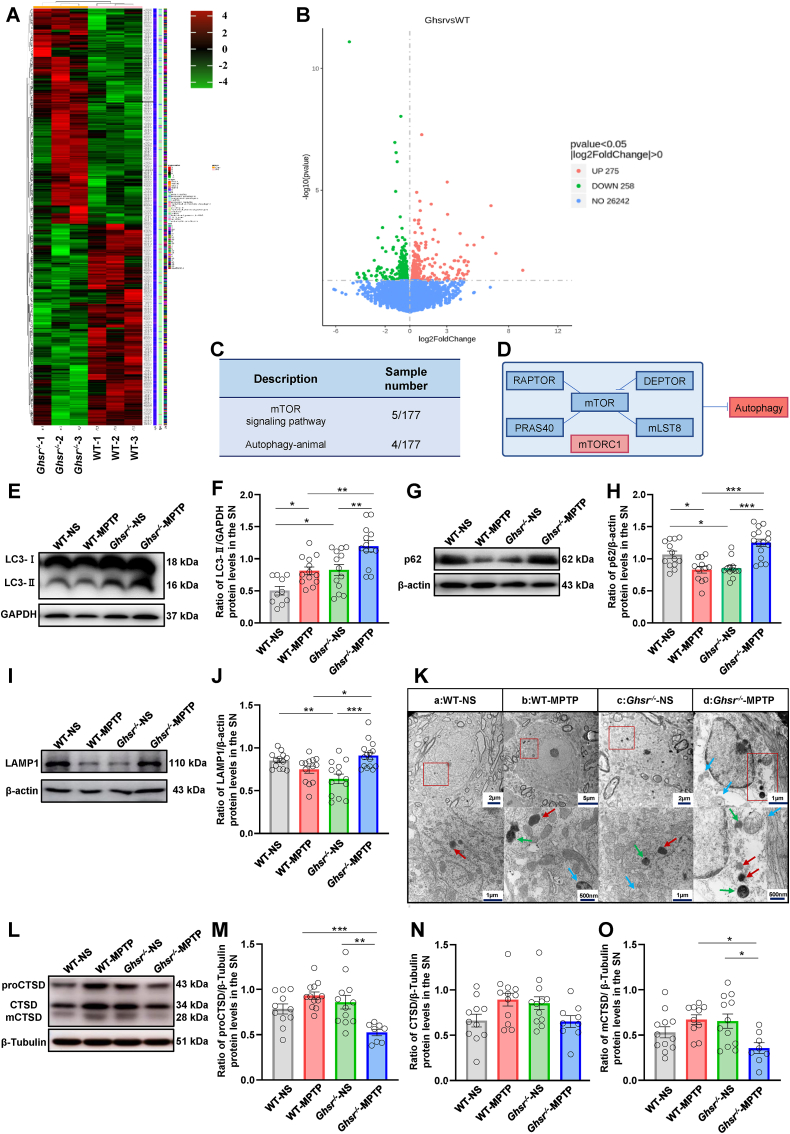
We next investigated the mechanism by which GHSR knockout exacerbated the degeneration of dopaminergic neurons. We performed transcriptome sequencing of the SN of WT and Ghsr−/− mice. After GHSR was knocked out, a total of 533 differentially expressed genes (DEGs) were detected, including 275 upregulated genes and 258 downregulated genes (Fig. 3A–B). The relevant quality inspection results are shown in Supplement Fig. 2. DEPTOR, Slc7a5, Fzd7, Fzd9 and other DEGs were enriched in the mTOR and autophagy signaling pathways. Transcriptome sequencing suggested that autophagy dysregulation might be the cause of the death of dopaminergic neurons after GHSR knockout (Fig. 3C–D). Therefore, we first detected changes in LC3-II and p62 during autophagy [33]. The results showed that, compared with that in the WT-NS group, the protein expression of LC3-II in the WT-MPTP group was increased by 61.45%, but the protein expression of p62 was decreased by 22.10%, which indicated that intraperitoneal injection of MPTP could induce autophagy initiation in the SN of dopaminergic neurons and that autophagic flow was unobstructed during this process (Fig. 3E–H). In addition, compared with that in the WT-MPTP group, the protein content of LC3-II in the Ghsr−/−-MPTP group was increased by 47.25%, while the expression of p62 was also significantly increased by 50.41%, indicating that GHSR knockout further promoted MPTP-induced autophagy initiation, but autophagic flow was blocked (Fig. 3E–H).
Fig. 3.
GHSR knockout enhanced autophagy initiation and blocked autophagic flux in PD mice A–B: Analysis of the transcriptomic results. C–D: Signaling pathways identified according to the transcriptomic results. E–J: Western blotting was used to measure the protein levels of LC3, p62, and LAMP1 in the SN of WT and Ghsr−/− mice. K: The morphological characteristics of the autophagosomes, lysosomes and autophagosomes in dopaminergic neurons in the SN were observed via transmission electron microscopy. The blue arrows represent autophagosomes, the red arrows represent lysosomes, and the green arrows represent autolysosomes. L–O: Western blotting was used to measure the protein level of CTSD in the SN of WT and Ghsr−/− mice. Each bar represents the mean ± S.E.M. (one-way ANOVA; *P<0.05, **P<0.01, ***P<0.001). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Lysosomes play an important role in the whole process of autophagy. This is because the lysosomes can form autolysosomes with autophagosomes, and cellular substances are degraded and recycled in the acidic environment of lysosomes [34]. To investigate whether the accumulation of the p62 protein in the Ghsr−/−-MPTP group was related to the accumulation of lysosomes, we further measured the protein levels of lysosome-associated membrane protein (LAMP1). The expression of LAMP1 exhibited the same change trend as did the expression of p62; that is, compared with that in the WT-MPTP group, the LAMP1 protein content in the Ghsr−/−-MPTP group increased by 21.80%. The above experimental results indicated that GHSR knockout aggravated the accumulation of the lysosome-associated protein LAMP1 in the MPTP-induced PD mice model and eventually led to an increase in p62 levels (Fig. 3I–J).
Next, we observed the changes of autophagosomes, lysosomes and autolysosomes in dopaminergic neurons in the SN more intuitively via transmission electron microscopy (Fig. 3K). In Fig. 3K, the blue arrows represent autophagosomes, the red arrows represent lysosomes, and the green arrows represent autolysosomes. Electron microscopy revealed that, compared with those in the WT-NS group and the Ghsr−/−-NS group, the numbers of autophagosomes, lysosomes and autolysosomes in the Ghsr−/−-MPTP group were greater. These findings indicated that autophagy in the Ghsr−/−-MPTP group was increased, resulting in increased autophagosome formation; however, the lysosomal degradation was impaired, causing massive accumulation of autolysosomes (Fig. 3K).
However, recently, many LAMP1-labeled organelles were shown not to be functional lysosomes in neurons but rather to be widespread endocytosed organelles; thus, LAMP1-labeled organelles do not contain lysosomal hydrolases and have degradative capacity [35,36]. mCTSD, an important member of the cathepsin family, is expressed mainly in lysosomes and is an important marker of lysosomal function. These results showed that, compared with that in the WT-MPTP group, the protein content of mCTSD in the Ghsr−/−-MPTP group was significantly lower (by 46.88%) (Fig. 3L–O). Therefore, intraperitoneal injection of MPTP after GHSR knockout could lead to impaired lysosomal function, which was the reason for the blockade of autophagic flow.
2.4. GHSR knockout-induced autophagy dysregulation was attributed to the mTORC1 subunit DEPTOR
mTORC1 is composed of five subunits: DEPTOR, RAPTOR, PRAS40, mLST8 and mTOR (Fig. 4A). The transcriptomic analysis revealed that among the five subunits, only the mRNA expression levels of DEPTOR was significantly increased in Ghsr−/− mice, while the expression of the other subunits was not significantly changed. We further validated the expression of these subunits. The results showed that the mRNA levels of the DEPTOR subunit in the SN was significantly increased by 63.25% in Ghsr−/− mice than in WT mice, which was consistent with the transcriptomic results (Fig. 4B). The mRNA and protein levels of RAPTOR, mLST8, PRAS40 and mTOR did not significantly change after GHSR knockout (Fig. 4B, 4E-4L). In addition, we further verified the protein levels of DEPTOR in the SN of mice. Compared with that in the WT-NS group, the expression levels of the DEPTOR protein in the WT-MPTP group was significantly greater (by 56.54%). Compared with that in the WT-MPTP group, the expression levels of DEPTOR in the Ghsr−/−-MPTP group was increased by 19.41% (Fig. 4C–D). These findings suggested that GHSR knockout could enhance MPTP-induced DEPTOR expression in PD mice, which was consistent with the trend observed for LC3-II expression (Fig. 3E–F). Besides, the protein level of p-mTOR was decreased in Ghsr−/− mice (Supplement Fig. 2E and 2F). Considering that DEPTOR is a negative regulator of mTORC1, which in turn negatively regulates autophagy, we concluded that enhanced autophagy initiation after GHSR knockout was associated with the changes in DEPTOR expression.
Fig. 4.
GHSR knockout-induced autophagy dysregulation was associated with the mTORC1 subunit DEPTOR A: Constituent subunits of mTORC1. B: The mRNA expression levels of the five subunits of mTORC1 in WT mice and Ghsr−/− mice were verified via RT-PCR. C–D: Western blotting was used to measure the protein levels of DEPTOR in the SN. E–L: The protein expression levels of RAPTOR, mLST8, PRAS40, and mTOR in WT mice and Ghsr−/− mice were verified by western blotting. Each bar represents the mean ± S.E.M. (one-way ANOVA, Student's t-test; *P<0.05, **P<0.01, ***P<0.001).
2.5. The transcription factor KLF4 could bind to the promoter of DEPTOR
To further explore the reasons for the change in DEPTOR expression, we made predictions via bioinformatics analysis. The prediction results combined with the transcriptome data, indicated that KLF4 and SOX10 might be upstream transcription factors of DEPTOR (Fig. 5A, Supplement Fig. 3A). Next, we carried out experimental verification through a dual-luciferase reporter gene experiment. The results showed that the relative fluorescence intensity was significantly greater in cells cotransfected with the DEPTOR luciferase plasmid and KLF4/SOX10 plasmid than those transfected with the DEPTOR luciferase plasmid along (2.65-fold and 1.56-fold, respectively) (Fig. 5B–C, Supplement Fig. 3). These findings indicated that the transcription factors KLF4 and SOX10 could bind to the DEPTOR promoter to regulate the expression of the DEPTOR gene. According to the statistical analysis, we draw the following conclusions: the ability of KLF4 to transcriptionally regulate DEPTOR was stronger than that of SOX10. Considering the findings in the literature that KLF4 has protective effects on the central nervous system [37,38], KLF4 was selected as our target molecule for the following experiments. Nucleic acid-protein interaction analysis can be used to verify the binding of transcription factors to promoters. The experiment confirmed that when DEPTOR was immobilized on the SA chip as a nucleic acid ligand and KLF4 was used as a protein analyte, DEPTOR and KLF4 could bind to each other, which was consistent with the findings of the dual-luciferase reporter gene experiment (Fig. 5E–I). When they DEPTOR and KLF4 bound each other, the combination rate Ka (1/Ms) was 1.728E+5, the dissociation rate Kd (1/s) was 0.008781, and the affinity Kd (M) was 5.083E-8. The specific sequence of the binding site was 5′-CCTTGGTCTA-3’ (Fig. 5D–H). To further verify the specific sequence involved in the binding of KLF4 to DEPTOR, we conducted further experimental verification through dual-luciferase reporter gene experiments. A dual-luciferase reporter was generated after mutating the sequence 5′-CCTTGGTCTA-3′ in the promoter. We observed that the ability of the transcription factor KLF4 to bind to the promoter of DEPTOR was significantly reduced, by 55.7%. This finding once again confirmed that the binding sequence identified by nucleic acid-protein interaction analysis was reliable (Fig. 5I).
Fig. 5.
The transcription factor KLF4 could bind to the promoter of DEPTOR A: The transcription factor upstream of DEPTOR was predicted to be KLF4 by bioinformatics methods. B–C: Dual-luciferase reporter gene experiments confirmed that KLF4 and DEPTOR could bind to each other. D–H: The Biacore T200 instrument was used to analyze the interaction between DEPTOR and KLF4. KD was 5.083*10^ (−8), and the binding site sequence was 5′-CCTTGGTCTA-3'. I: The dual-luciferase reporter gene experiment confirmed that KLF4-DEPTOR binding ability was decreased after the promoter of DEPTOR was mutated. Each bar represents the mean ± S.E.M. (one-way ANOVA; ***P<0.001).
2.6. Dopaminergic neuron-specific knockdown of KLF4 prevented MPTP-induced neurodegeneration in Ghsr−/− mice
We subsequently validated the effect of GHSR on KLF4 expression. GHSR knockout resulted in a significant increase in KLF4 expression (Fig. 6A). Next, we knockdown KLF4 specifically in SN dopaminergic neurons to observe the effects on mice behavior and the levels of various biochemical indicators of autophagy (Fig. 6B). The mice were divided into four groups: the Ghsr−/−-Vector-NS, Ghsr−/−-Vector-MPTP, Ghsr−/−-AAV shKLF4-NS and Ghsr−/−-AAV shKLF4-MPTP groups. A schematic of the shKLF4-expressing virus is shown in Supplement Fig. 4. Viruses expressing shKLF4 under the control of a dopaminergic neuron-specific promoter were used to selectively knockdown KLF4 in dopaminergic neurons in the SN in Ghsr−/− mice. Supplemental Fig. 4 shows image taken after stereotactic injection of Pontamine Sky Blue and AAV-shKLF4. By comparing the location of Pontamine Sky Blue, fluorescence images and brain maps, we confirmed that the location of virus injection was correct and that the infection efficiency was good. Additionally, the protein expression of KLF4 in dopaminergic neurons in the SN decreased (Fig. 6C). On the basis of these results, the following experiment was carried out.
Fig. 6.
Knocking down KLF4 in dopaminergic neurons prevented MPTP-induced neurodegeneration in Ghsr−/− mice A: The protein expression of KLF4 increased after GHSR gene knockout. B: Experimental design for stereotactic injection of AAV-shKLF4 into the SN of Ghsr−/− mice. C: Western blotting was used to measure the protein level of KLF4 after AAV-shKLF4 injection. D–F: The movement of Ghsr−/− mice after stereotactic injection of AAV-shKLF4 was assessed via the pole test and rotarod test. G–J: Western blotting was used to measure the expressions of TH and LC3 in the SN of Ghsr−/− mice after stereotactic injection of AAVshKLF4. K–N: Western blotting was used to measure the expression of p62 and DEPTOR in the SN of Ghsr−/− mice after stereotactic injection of AAV-shKLF4. O–R: Western blotting was used to measure the expression of CTSD in the SN of Ghsr−/− mice after stereotactic injection of AAV-shKLF4. Each bar represents the mean ± S.E.M. (one-way ANOVA; *P<0.05, **P<0.01, ***P<0.001).
In accordance with the above findings, the motor behaviors of the mice were tested by the pole test (Fig. 6D–E) and rotarod test (Fig. 6F). Compared with those in the Ghsr−/−-AAV shKLF4-NS group, the mice in the Ghsr−/−-AAV shKLF4-MPTP group tended to exhibit motor impairment. Compared with those in the Ghsr−/−-Vector-MPTP group, the head turning and landing times of the mice in the Ghsr−/−-AAV shKLF4-MPTP group were significantly lower (by 44.71% and 31.83%, respectively), and the latency to fall from the rotarod was also significantly greater (by 70.72%), which indicated that knocking down of KLF4 in the SN of Ghsr−/− mice could improve the behavior of the mice (Fig. 6D–F).
Next, we collected SN from the mice to measure the expression of the TH protein (Fig. 6G–H). As shown in Fig. 6H, compared with that in the Ghsr−/−-Vector-MPTP group, the expression of TH in the Ghsr−/−-AAV shKLF4-MPTP group was 42.47% greater, which indicated that knocking down of KLF4 in the SN of Ghsr−/− mice could protect dopaminergic neurons. As shown in Fig. 4, enhanced autophagy initiation was related to DEPTOR. What is the effect of administrating AAV-shKLF4 followed by injecting MPTP on DEPTOR expression in Ghsr−/− mice? As shown in Fig. 6N, compared with that in the Ghsr−/−-Vector-MPTP group, DEPTOR protein expression in the Ghsr−/−-AAV shKLF4-MPTP group was significantly decreased by 22.87% (Fig. 6M–N). Are processes related to autophagy changed under these conditions? The first parameter we measured was the change in LC3-II expression. We observed that the expression of LC3-II protein in the Ghsr−/−-AAV shKLF4-MPTP group was 21.27% lower than that in the Ghsr−/−-Vector-MPTP group, which indicated that stereotaxic preinjection of AAV-shKLF4 followed by intraperitoneal injection of MPTP could attenuate the initiation of autophagy (Fig. 6I–J). In addition, the expression of p62 showed a trend consistent with that of LC3- II. Compared with that in the Ghsr−/−-Vector-MPTP group, the protein expression of p62 in the Ghsr−/−-AAV shKLF4-MPTP group tended to decrease, which indicated that preinjection of AAV-shKLF4 followed by intraperitoneal injection of MPTP could attenuate the disruption of autophagic flux (Fig. 6K–L). We observed this phenomenon more directly by electron microscopy (Supplement Fig. 4E). As previously described, CTSD deficiency or dysfunction severely impaired autophagy lysosomal function, leading to aberrant α-syn aggregation [39]. Next, we measured the protein expression of CTSD, and we observed that the expression of mCTSD in the Ghsr−/−-AAV shKLF4-MPTP group was 53.57% greater than that in the Ghsr−/−-Vector-MPTP group, indicating that shKLF4 preinjection could also improve lysosomal function (Fig. 6O and P).
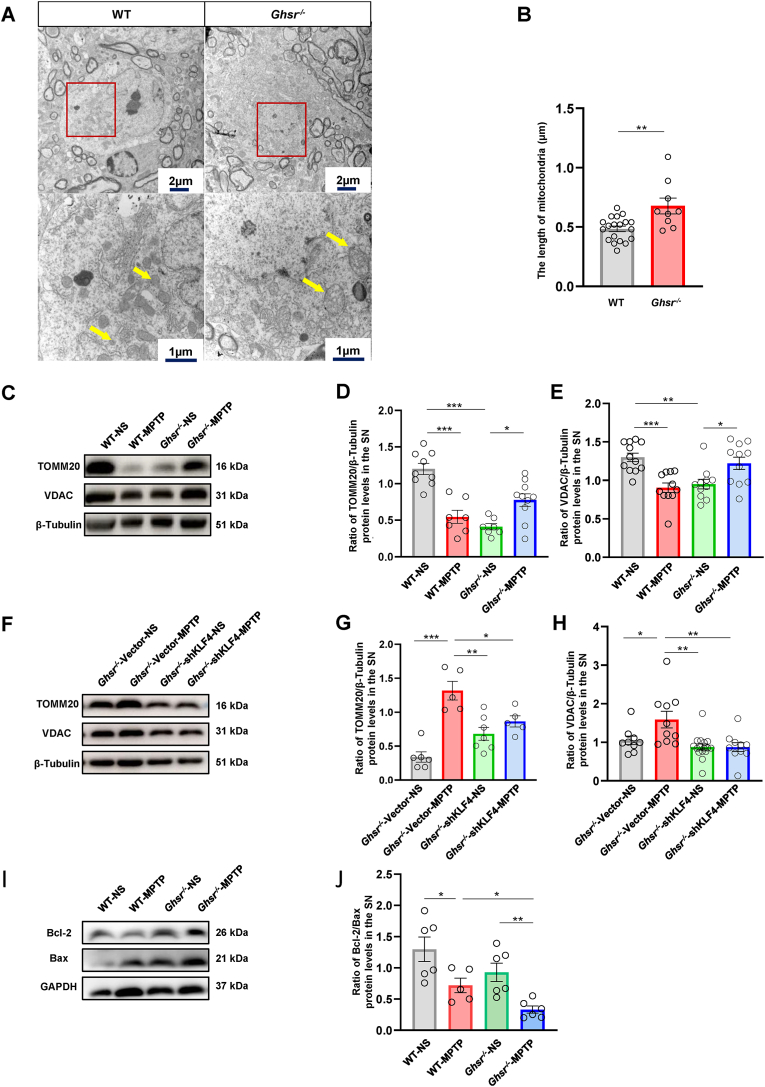
2.7. GHSR knockout-induced autophagy dysregulation suppressed mitochondrial clearance
Compared with those in WT mice, mitochondria in the SN dopaminergic neurons of Ghsr−/− mice exhibited obvious swelling and enlargement, suggesting that mitochondrial homeostasis could also be affected by GHSR knockout (Fig. 7A–B). Next, we measured the protein expression of TOMM20 and VDAC, which are important components of mitochondria that perform normal biological functions. Compared with those in the WT-NS group, the expression levels of TOMM20 and VDAC in the WT-MPTP and Ghsr−/−-NS groups were lower (Fig. 7C–E), which indicated that MPTP or GHSR knockout could affect the expression of proteins involved in mitochondrial functions. Combined with the changes in autophagy in Fig. 3, these findings indicated that the mitochondria damaged by the above-mentioned process could be engulfed by autophagosomes and cleared through the autophagy-lysosomal pathway. However, the damaged mitochondria could not be removed due to abnormal autophagy-lysosomal processes in the Ghsr−/−-MPTP group; thus, the damaged mitochondria accumulated in neurons and aggravated the death of dopaminergic neurons. The expression of the TOMM20 and VDAC proteins in the Ghsr−/−-shKLF4-MPTP group was decreased compared with that in the Ghsr−/−-Vector-MPTP group (Fig. 7F–H). Mitochondria play a central role in apoptosis. The antiapoptotic protein Bcl-2 and the proapoptotic protein Bax play important roles in MPTP-induced apoptosis to contribute to the occurrence and progression of PD [7]. We next asked whether the abnormal accumulation of damaged mitochondria in the Ghsr−/−-MPTP group exacerbated apoptosis and thus neuronal death. We examined the changes in the Bcl-2/Bax ratio in the SN. As indicated in Fig. 7I and J, after MPTP treatment, the Bcl-2/Bax ratio in the Ghsr−/−-MPTP group was decreased by 64.4% compared with that in the Ghsr−/−-NS group. However, there was only a 53.8% reduction in the Bcl-2/Bax ratio in WT mice after MPTP treatment (Fig. 7I and J).
Fig. 7.
GHSR knockout-induced autophagy dysregulation inhibited mitochondrial clearance in PD mice A–B: Mitochondria in dopaminergic neurons in the SN of WT and Ghsr−/− mice were observed via transmission electron microscopy. C–H: Western blotting was used to measure the expression of TOMM20 and VDAC in the SN of WT and Ghsr−/− mice. I–J: Western blotting was used to measure the Bcl-2/Bax ratio in the SN. Each bar represents the mean ± S.E.M. (one-way ANOVA; *P<0.05, **P<0.01, ***P<0.001).
3. Discussion
In the present study, we observed that GHSR deletion significantly aggravated dopaminergic neurons degeneration in PD mice model, besides, the expression and activity of GHSR is decreased in PD models, which all demonstrated that GHSR play an important role in the normal physiological function of dopaminergic neurons. GHSR knockout leads to continuous activation of autophagy and lysosomal dysfunction, which suppresses the elimination of damaged mitochondria and ultimately causes neurodegeneration in PD mice model. Mechanistically, GHSR deletion enhanced the expression of KLF4, subsequently transcriptionally regulating the mTORC1 subunit DEPTOR. In this case, KLF4 knockdown effectively prevented the loss of dopaminergic neurons and PD-like phenotypes, highlighting the importance of the KLF4-DEPTOR axis in autophagy dysregulation in Ghsr−/− mice.
GHSR is a member of the GPCR family, it has been shown that GHSR is dominantly expressed in dopaminergic neurons in the SN, which is essential for ghrelin to exert its neuroprotective effect [8,15]. The expression of GHSR was dramatically decreased in iPSCs, which differentiated from skin fibroblasts of patients with PARK2 familial PD patients [16]. In our experiments, GHSR was also decreased in MPP+/MPTP induced models. It has been confirmed that Ghsr−/− mice exhibit improved spatial memory, deficits in contextual memory and social motivation [40]. Here, we observed that endogenous GHSR knockout significantly aggravated MPTP-induced damage to dopaminergic neurons in the SN. For example, the motor coordination of mice was greatly reduced, and TH protein levels were also significantly decreased when GHSR knockout in PD mice model. Moreover, GHSR knockout could aggravate oxidative damage, apoptosis, and the inflammatory reaction in PD. To explore the mechanism underlying this phenomenon, transcriptome sequencing was performed. And it was found that among the 533 identified DEGs (e.g., DEPTOR, AKT, and JNK1), many were enriched in the autophagy signaling pathway, and several (e.g., Slc7a5, Fzd7, and Fzd9) were enriched in the mTOR signaling pathway. The mTOR signaling pathway plays a crucial role in regulating autophagy [41,42], indicating the involvement of autophagy in neurodegeneration in Ghsr−/− mice.
We confirmed that the expression of DEPTOR in the SN of Ghsr−/− mice was significantly increased, but the expression of other subunits of mTORC1 was not changed. DEPTOR, an important subunit of mTORC1, interacts with mTOR to inhibit its kinase activity, which involves in activation of the autophagy pathway [26]. Continuous accumulation of DEPTOR may induce the expression of the autophagy marker LC3-II by inhibiting the activity of mTOR in a colitis-associated colorectal cancer mice model [43]. In human multiple myeloma cells, DEPTOR knockout inhibits the activation of the PI3K/AKT signaling pathway, decreases the expression of autophagy-related proteins (such as ATG5, LC3) and thus inhibits autophagy in vitro, which confirms that DEPTOR is a positive regulator of autophagy initiation [27,28,44]. In pituitary tumor cell lines, overexpression of DEPTOR promote autophagy by inhibiting the mTOR signaling pathway [45], which demonstrates that DEPTOR can affect cell autophagy and survival by regulating mTOR [27,28]. Based on the above evidence, the increase in autophagy initiation in Ghsr−/− mice was related to the change in DEPTOR expression.
Several studies have shown that PD is closely related to autophagy, and a variety of PD-related genes (such as LRRK2, PINK1, and DJ-1) participate in the regulation of the autophagy pathway [25,[46], [47], [48], [49], [50], [51], [52], [53]]. Autopsy reports of PD patients also showed that lysosome and autophagosome aggregation occur in dopaminergic neurons [54,55]. In our study, the expression of LC3-II in the SN of Ghsr−/−-MPTP mice was significantly increased, indicating that GHSR knockout significantly enhanced autophagy initiation in PD mice model. We further examined the expression of p62, which is a specific substrate for autophagic degradation. We demonstrated that the p62 and LAMP1 protein levels in the Ghsr−/−-MPTP group were significantly increased, indicating that autophagic flux was blocked after GHSR knockout in PD mice model, which may be related to lysosomal dysfunction.
Autophagy is a conserved, lysosomal dependent degradation pathway [56]. The substrate is degraded after autophagosome and lysosome fuse to form autolysosome. In order to maintain the stability of the intracellular lysosome, the autolysosome will unite into tube and break to form new lysosomes, a process that depends on mTOR activation, known as autophagic lysosome reformation (ALR) [57]. In Ghsr−/−-MPTP group, the increased expression of DEPTOR can continuously inhibit mTOR, initiate autophagy and generate a large number of autophagosomes. Due to the decreased expression of lysosomal enzyme mCTSD, a large number of accumulated substrates cannot be degraded. In this process, lysosomes are not consumed and the ALR process is impaired, which resulting in the dysfunction of lysosomal and imbalance of autophagy.
Based on the analysis of bioinformatics results, the transcription factors KLF4 and SOX10 may be involved in the upregulation of DEPTOR in Ghsr−/− mice. SOX10, a member of the SOXE family of transcription factors (SOX8, SOX9, and SOX10), which can serve as a tumor marker [58,59]. KLF4, a member of the zinc finger protein transcription family, which participates in the regulation of important biological processes, such as cell proliferation and differentiation, the cell cycle and embryonic development [60]. Previous studies have shown that KLF4 inhibits axonal regeneration by inhibiting JAK-STAT3 signaling pathway [[61], [62], [63]]. And KLF4 knockout can alleviate traumatic brain injury-induced neuronal damage through p53 and JAK-STAT3 signaling pathway [61]. KLF4 also has the potential in the treatment of neurodegenerative diseases such as PD and Alzheimer's disease (AD) [64]. In the present study, the ability of KLF4 binding to the DEPTOR promoter was greater than that of SOX10, so we determined KLF4 as our target molecule for the following experiments. Knockdown KLF4 in dopaminergic neurons in Ghsr−/− mice followed by intraperitoneal injection of MPTP ameliorated behavioral impairment and dopaminergic neuron injury in the SN. In this process, the dysregulation of autophagy is also alleviated, which suggesting that KLF4 knockdown not only reduced the formation of autophagosomes but also promoted lysosomal degradation, which partially restored autophagic flow. However, how GHSR regulates KLF4 expression and whether it is related to a certain signaling pathway remains to be further studied.
Mitochondrial abnormalities have adverse effects in neurodegenerative diseases such as PD and AD. In our experiments, mitochondria exhibited abnormal morphology after GHSR knockout, mainly manifested as mitochondrial swelling. In the WT-MPTP and Ghsr−/−-NS groups, the protein expression of mitochondrial marker proteins, such as TOMM20 and VDAC, significantly decreased, which indicated that the number of mitochondria was reduced. However, in the Ghsr−/−-MPTP group, the expression of TOMM20 significantly increased, which indicated the abnormal accumulation of mitochondria. Combined with the changes in autophagy mentioned above, these findings suggested that damaged mitochondria could not be cleared by autophagy and deposited in cells, resulting in abnormal expression of TOMM20 and VDAC. Mitochondria play a central role in apoptosis. Mitochondrial permeability transition pore allows apoptotic factors, such as cytochrome C and apoptosis-inducing factors, release into the cytoplasm during apoptosis, triggering downstream apoptotic executors and ultimately leading to cell death [65]. In our study, the damaged mitochondria cannot be cleared through the autophagy pathway, inducing the decrease of Bcl-2/Bax and the occurrence of apoptosis and ultimately leading to neuronal death. The process was exacerbated by MPTP in Ghsr−/− mice.
In summary, GHSR knockout exacerbated dopaminergic neuron damage, which was associated with dysregulation of autophagy. In PD models, GHSR deletion upregulated KLF4 and subsequently transcriptionally regulated the expression of DEPTOR, which led to the continuous activation of autophagy. However, lysosomal dysfunction prevented the clearance of damaged mitochondria and exacerbated the death of dopaminergic neurons. Dopaminergic neuron-specific knockdown of KLF4 in Ghsr−/− mice reduced autophagy activation and increased lysosomal degradation, which allowed the elimination of damaged mitochondria and ultimately alleviated the death of dopaminergic neurons. Therefore, endogenous GHSR plays an important role in maintaining the function of dopaminergic neurons. This study provides a new theoretical and experimental basis for the treatment of PD.
4. Materials and methods
4.1. Cell culture
Fetal bovine serum was added to DMEM high glucose medium, and the mixture of cyan/streptomycin was prepared in the complete medium. The cells were placed in 37°C, 5% CO2 constant temperature incubator for culture. Cells were passaged in 96-well plates, and transfected with plasmids when the cells had grown to a suitable density. Experiments were carried out according to the following groups, and the plasmids were transfected with basal medium and Lip2000 in a certain proportion. The basic medium was replaced with complete medium 5–6 h after transfection, and the double luciferase reporter gene assay/HTRF assay were performed 24 h after transfection.
4.2. Animals and treatment
All operations were performed in accordance with the “Guidelines for the Care and Use of Laboratory Animals” by the National Institutes of Health, and were approved by the Animal Ethics Committee of Qingdao University. 8-10-weeks old mice (22 ± 2) g were kept in cages with tap water and food freely, with a 12 h light-dark cycle at room temperature (22 ± 2)°C.
4.3. HTRF assay to detect the cell IP content
The cell suspension was inoculated into the 12-well plate, and the cells were transfected with GHSR high expression plasmid when the cells grew to an appropriate density; Cells were collected after the appropriate time of drug treatment, 50 μL protein lysate was added to each well and collected, centrifuged at 4°C and 12000 g for 10 min, and supernatant was taken for subsequent determination; Preparation and testing of standard products (IP1 Gq Kit): Each well was filled with 14 μL IP1 standard, followed by 3 μL IP1 d2 reagent, and finally with 3 μL IP1 Tb Cryptate Antibody after incubation at room temperature for 1 h, the value was read in the HTRF program of the ELISA. Sample detection: 7 μL cell lysate was added to 386 microplates, followed by 7 μL Stim B solution, and incubated at 37°C. After incubation, 3 μL of d2 Reagent and 3 μL of IP1Tb Cryptate Antibody were added, the well plate was incubated at room temperature for 1 h. The values were read in the HTRF program of the enzyme label.
4.4. Animal identification
WT and Ghsr−/− mice on a C57BL/6 background were used for this study. Genotypes were confirmed by PCR of tail DNA. The PCR reaction system, the following primers and the reaction conditions were as follows. Finally, after electrophoresis, the DNA bands were analyzed. The size of the products was 1550bp in Ghsr−/−, the products were 1550bp∼473bp in Ghsr+/− mice, and the products were 473bp in WT mice. Reaction component 1: ddH2O (9 μL), 2 × Taq Plus Master Mix (13 μL), P1 (0.5 μL), P2 (0.5 μL), Genomic DNA (2 μL), Total (25 μL); Reaction component 2: ddH2O (9 μL), 2 × Taq Plus Master Mix (13 μL), P3 (0.5 μL), P4 (0.5 μL), Genomic DNA (2 μL), Total (25 μL). The reaction process is as follows: Step1 (94°C, 3 min); Step2 (94°C, 30 s); Step3 (61°C, 30 s); Step4 (72°C, 2 min; 35 Times to 2); Step5 (72°C, 5 min); Step6 (4°C, hold).
The primer sequence is as follows (5′-3′):
P1 (Mut): GTGCGCACTGTCTCCTCTGATTTG;
P2 (Mut): GTGCTTTGGGGTGCGTGTGATGGA;
P3 (WT): CACGCCCACCAGCACGAAGA;
P4 (WT): ACGACTCACTCTCTGACGAA.
4.5. Dual-luciferase assay
The complete medium was aspirated from the 96-well plate, 30 μL 1 × PLB solution was added, and cell lysates were obtained by shaking vigorously on a shaker. 10 μL cell lysate was pipetted into a white opaque 96-well plate, and 30 μL LAR II was added into the target well. The fluorescence value of firefly was detected at a wavelength of 560 nm. 30 μL Stop & Glo® Reagent was added to the above wells for fluorescence detection at 460 nm. The relative fluorescence value was measured by Firefly fluorescence value/Renilla luciferase value. Experimental grouping of DEPTOR-SOX10 is as follows: group1 (pGL4.10+pcDNA3.1-FLAG+PRL-CMV); group2 (pGL4.10-DEPTOR promoter+pcDNA3.1-FLAG+PRL-CMV); group3 (pGL4.10+pcDNA3.1(+)-SOX10-FLAG+PRL-CMV); group4 (pGL4.10-DEPTOR promoter+pcDNA3.1(+)+SOX10-FLAG+PRL-CMV). Experimental grouping of DEPTOR-KLF4 is as follows: group1 (pGL4.10+pcDNA3.1-Myc+PRL-CMV); group2 (pGL4.10-DEPTOR promoter+pcDNA3.1-Myc+PRL-CMV); group3 (pGL4.10+pcDNA3.1(+)-KLF4-Myc+PRL-CMV); group4 (pGL4.10-DEPTOR promoter+pcDNA3.1(+)+KLF4-Myc+PRL-CMV). Experimental grouping of DEPTOR (Mut)-KLF4 is as follows: group1 (pGL4.10+pcDNA3.1-Myc+PRL-CMV); group2 (pGL4.10-DEPTORpromoter+pcDNA3.1-Myc+PRL-CMV); group3 (pGL4.10+pcDNA3.1(+)-KLF4-Myc+PRL-CMV); group4 (pGL4.10-DEPTOR promoter+pcDNA3.1(+)+KLF4-Myc+PRL-CMV); group5 (pGL4.10+pcDNA3.1-Myc+PRL-CMV); group6 (pGL4.10-DEPTOR promoter (Mut)+pcDNA3.1-Myc+PRL-CMV); group7 (pGL4.10+pcDNA3.1(+)-KLF4-Myc+PRL-CMV); group8 (pGL4.10-DEPTOR promoter (Mut)+pcDNA3.1(+)+KLF4-Myc+PRL-CMV).
4.6. Time and dose of drug injection
MPTP was purchased from Sigma company, and a 6 g/L solution of MPTP was prepared using medical NS, and the PD mice model were prepared by chronic modeling method. That is, MPTP was injected intraperitoneally on Monday and Thursday every week (5 weeks in total), and the dosage of each mouse was 30 mg/kg.
5. Behavioral test
5.1. Pole test
The equipment used in the experiment is a rough wooden pole with a diameter of 1 cm and a length of 50 cm, and a small wooden ball with a diameter of 2 cm is fixed on the top of the pole. During the experiment, the mice were placed at the bottom of the wooden ball. The time required for the mice to climb over the wooden ball was time to turn, and the time required for the mice to reach the bottom of the wooden pole was the total time. Five experiments were performed for each mouse, and the average value was calculated.
5.2. Rotarod test
The rotating rod experiment system is composed of rotating rod instrument and display screen, and the rotating rod instrument is divided into 4 compartments. Before the start of the experiment, four mice were placed in each of the four compartments with their heads facing inward and their tails placed at 45°. The experiment was carried out after the mice were acclimated to the rotating rod apparatus for an appropriate time. The time of the mice staying on the rotary rod was recorded respectively. Two experiments were performed for each mouse, and the average value was calculated.
5.3. RNA-seq of SN in mice
In order to facilitate gene function and structure analysis in the later stage, RNA-seq technology was used to study the expression of all mRNA in the SN. After the mice were anesthetized, obtaining the mice brain tissue using surgical scissors, surgical forceps and other instruments, the blood on the surface of the brain tissue was washed with 0.9% NS. The mice brain tissues were immediately frozen in dry ice. A microtome was used to cut the mice brain tissue into 100 μm brain slices, which were observed under a desktop microscope and compared with the brain atlas. The SN on both sides were extracted by fine ophthalmic tweezers. And then placed them in an EP tube immediately and preserved in liquid nitrogen. The sequencing samples were transported to Beijing Novo Biotechnology Co., Ltd. for testing at low temperature. The results of transcriptome sequencing of Ghsr−/− mice and WT mice were analyzed.
5.4. Western blotting
The SN of mice was sampled and the protein concentration was detected by BCA method. Gel plates with different concentrations were prepared according to molecular weight. The extracted protein was loaded and electrophoresed at a stable voltage of 80 V, moreover, the voltage was adjusted to 120 V after protein marker separation. After electrophoresis, the membrane was transferred under a constant current of 300 mA for 120 min/60 min. Blocking was performed with 10% nonfat dry milk to remove nonspecific binding sites. Bands were incubated with antibody overnight, and the band rinsed with 1 × TBST solution three times for 10 min each time. The secondary antibody was prepared with TBST, and after incubation for 1 h, the target band was rinsed three times with 1 × TBST solution for 10 min each time. Finally, the bands were photographed with a developing machine. Specific information about antibodies is as follows: GHSR (Abclonal; A1840); TH (Millipore; Ab152); LC3 (Protein; 14600-1-AP); p62 (Cell Signaling Technology; #5114); LAMP1 (Abcam; Ab208943/Ab24170); DEPTOR (NOVUS; #NBPI-49674); RAPTOR (Cell Signaling Technology; 2280S); mLST8 (Cell Signaling Technology; 3274T); PRAS40 (Cell Signaling Technology; 2691T); mTOR (Cell Signaling Technology; 2983S); p-mTOR (Cell Signaling Technology; 5536T); CTSD (Cell Signaling Technology; #69854); KLF4 (STNTA CRUZ; sc-393462); β-Actin (Bioss; bs0061R); GAPDH (Cell Signaling Technology; 5174S); β-Tubulin (Abcam; Ab21058); Bax (Abclonal; A19684); Bcl-2 (Absin; Abs131701); SOD1 (Absin; Abs135597); TOMM20 (Abclonal; A19403); VDAC (Abclonal; A19707).
5.5. High performance liquid chromatography
(1) Solution preparation in HPLC: Solution A: 4.6 mL HClO4 diluted to 200 mL with ddH2O; Solution B: 20 mmol/L potassium citrate (1.6 g), 300 mmol/L K2HPO4 (13.06 g), 2 mmol/L EDTA·2Na (0.185 g) dissolved in 250 mL ddH2O. Mobile phase preparation: 20 mmol/L citric acid (H2O) (4.2028 g); 50 mmol/L sodium acetate (6.804 g); 0.134 mmol/L EDTA·2Na (2H2O) (49.87 mg); 1 mmol/L dibutylamine (0.17 mL); 50 mL methanol diluted to 1 L with ddH2O. (2) Standard solution configuration: weighing 0.5 mg DA, DOPAC and HVA respectively, dissolving them in 0.5 mL ddH2O to obtain 1 μg/μL store liquid, then taking 10 μL of the above liquid and adding 990 μL of the mobile phase dilute into 10 ng/μL. Seven concentration gradients were obtained by mobile phase dilution: 16 ng/20 μL, 8 ng/20 μL, 4 ng/20 μL, 2 ng/20 μL, 1 ng/20 μL, 0.5 ng/20 μL, 0.25 ng/20 μL. (3) Tissue samples were obtained by the following methods: The brain tissues were taken after the mice were anesthetized, and the bilateral striatum of the mice was extracted using tweezers under stereo microscope after the brain tissue was frozen in dry ice. The striatum was accurately weighed and pre-treated with 80 μL solution A. Transferring 40 μL of supernatant to 1.5 mL EP tube, adding 80 μL solution B, then mixing and leaving it on ice for 1 h. Centrifuging at 4°C and 12 000 rpm for 20 min, then removing the supernatant and store it at −80°C. (4) The HPLC instrument parameters were set as follows: the maximum column pressure was 3500 psi, the flow rate was 1.0 mL/min, the ECD set voltage was 0.65 V, and each sample was tested for 40 min. Each standard product is injected separately, and its retention time is tested as a qualitative index. Drawing of standard curve: Mixing each standard product and then dilute it into 7 concentration gradients, that is, inject 0.25, 0.5, 1, 2, 4, 8, 16 ng, drawing the standard curve, finding out the linear regression equation of each standard product, and obtain the sample content.
5.6. Observing the morphological changes of cells were under transmission electron microscope
After anesthetizing the mice, 2.5% paraformaldehyde was used to perfuse the mice and the brains were taken out, then 0.9% NS was used to wash the blood on the surface of the brain tissue. The SN was cut into 1 cm3 tissue blocks using a pre-cooled blade. The brain tissue blocks were transferred to EP tubes and fixed for 3 days. Afterwards, the samples were sent to the Electron Microscopy Laboratory of Qingdao University for follow-up processing.
5.7. Quantitative real-time PCR
The SN were inserted into an EP tube and fully ground with 500 μL TRIzo1. The tube was placed on ice for 5 min, and 200 μL of CHCl₃ was added to mix well. The supernatant was discarded after centrifugation (Temperature: 4°C; rotate speed: 12000 rpm/min; Time: 15 min), and then 1 mL 75% ethanol was added into the tube to clean the RNA. After the supernatant was discarded, it was dried on ice. After drying, an appropriate amount of RNase-free H2O was added to dissolve the RNA. The mRNA extracted in the above step was used as template to synthesize complementary cDNA. RT-PCR was conducted according to the PCR reaction system. The reaction conditions are as follows: Stage1 (1cycle, 95°C); Stage2 (40 cycles, 95°C, 60°C); Stage3 (1 cycle, 95°C, 60°C, 95°C). The primer sequences as follows:
mLST8 (Sense primer: CAACTCCAATAACCCCAACCC; Antisense primer: GGAAGATACGCTGACACTGCAAG);
RAPTOR (Sense primer: GCCCGAGTCTGTGAATGTAATG; Antisense primer: TTCCGTTGTAGATGGCTGTGA);
PRAS40 (Sense primer: TTCAAGGAGAAGAGGACAGA; Antisense primer: GAAGATCCCCGAAGACC);
mTOR (Sense primer: ACAGGCACCCATCCAATC; Antisense primer: CCAGACCCGTAACCTCCATA);
DEPTOR (Sense primer: ACCGAGAGACGGCGATAAAA; Antisense primer: GGTCCCATCATCCTTCCTAAA);
GAPDH (Sense primer: GCACCGTCAAGGCTGAGAAC; Antisense primer: TGGTGAAGACGCCAGTGGA).
5.8. Protein-nucleic acid interaction analysis experiment
The kinetic data of Ka, Kd and affinity data KD of nucleic acid-protein binding were detected by Biacore SA chip. The nucleic acid used in this experiment is DEPTOR and the protein is KLF4 (ab169841). KLF4 protein powder was diluted to 100 μg/mL with ddH2O. The concentration of mother solution of KLF4 protein analyte was at least 10 μM, the sample volume was above 50 μL, and the purity was more than 90%. The KLF4 protein solution was diluted to 0.125 μg/mL with running buffer HBS-EP. The diluted ligand solution was denatured in a 95°C H2O bath for 10 min, and then cooled naturally at room temperature. The KLF4 protein concentration was serially diluted to: 20 nM, 10 nM, 5 nM, 2.5 nM, 1.25 nM, 0.625 nM with running buffer. And then, the multi-cycle detection method was used to carry out the experiment. Finally, open the data analysis software Biacore T200 Evaluation Software for data analysis.
5.9. Preparation of AAV virus
AAV-shKLF4 virus were synthesized in Shanghai GeneChem Co., Ltd. The serotype of the virus was AAV9 and the titer of the virus was 7.26E +13v.g/ml. The specific details of the virus are shown in Supplement Fig. 4A.
Stereotactic brain injection.
The mice were anesthetized by small animal gas anesthesia machine for 2 min, and the anesthetized mice were placed on the brain stereotaxic positioning instrument. The mice incisors were clamped on the adapter incisors clamp, and then the ear rod was fixed on the ear canal of the mice. After fixation, the mouse brain was checked whether it was in the same plane and whether the mouse brain was fixed well. The skin at the top of the head is disinfected with iodophor and 75% alcohol, followed by ophthalmic tweezers cut along the midline to remove the connective tissue on the skull surface and wipe with hydrogen peroxide, exposing the anterior and posterior fontanelle. Adjust the anterior fontanelle and the posterior fontanelle on the same plane, and place the microsyringe needle at the anterior fontanelle point, and the coordinates return to zero. The SN was observed according to the injection position of the brain region, and the position was marked. The skull drill was used to gently drill holes, and the skull was carefully opened with a syringe needle. After cleaning the microsyringe with PBS, 1 μL of virus was absorbed, and the microsyringe was fixed on brain stereotaxic device and connected with the microinjection pump. The microsyringe was slowly inserted into the target area of the brain, and the micro-injection pump was started. The injection speed was 0.2 μL/min. After the injection, the needle was left for 5–8 min, then the microsyringe was slowly drawn out. The skin was sutured with medical suture, and iodine was used to disinfect the wound and the nearby skin. Finally, the mice were removed from the stereoscopic brain locator and placed in a cage, with an electric heater placed next to the cage if necessary.
5.10. Immunofluorescence
The mice after modeling were perfused with PFA, and the brains were removed and placed in PFA for 24 h for fixation, and 20% and 30% sugar were fixed to the mice brain on the second and third days. Frozen sectioning can be performed after completing the above steps. The brain regions of the SN required for the experiment were sectioned into 20 μm thick brain slices. The mouse brain slices of experimental group and control group were placed in 24-well plates, blocked with 10% goat serum, and incubated with TH antibody (Millipore, Ab318, 1: 2000) and IBA1 antibody (Wako, 019–19741, 1: 500) after blocking for 1.5 h. After 24 h, the brain slices were washed three times with 1 × PBST (Tween) for 15 min each time. After that, the secondary antibody was incubated for 1.5 h. Then, the brain slices were washed three times with 1 × PBST (Tween) for 15 min each time. DAPI staining was then performed as required. Finally, the slices were spread, sealed and observed under a fluorescence microscope.
Ethics approval and consent to participate
In the project, the rights and interests of the subjects are fully protected and meet the ethical requirements.
The study protocol has been approved.
Consent for publication
All authors have read the final version of the manuscript and approved its submission.
Availability of data and material
The data used in this article is available.
CRediT authorship contribution statement
Xue Xiao: Writing – original draft, Software, Formal analysis, Data curation. Tingting Tang: Methodology, Formal analysis, Data curation. Mingxia Bi: Writing – review & editing, Supervision, Software, Funding acquisition. Jing Liu: Methodology, Formal analysis, Data curation. Mengru Liu: Writing – review & editing, Methodology. Qian Jiao: Supervision, Methodology, Funding acquisition. Xi Chen: Supervision, Methodology. Chunling Yan: Supervision, Methodology. Xixun Du: Writing – review & editing, Supervision, Software, Methodology, Funding acquisition. Hong Jiang: Writing – review & editing, Project administration, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgements
National Natural Science Foundation of China, Grant/Award Numbers: 32371181, 32171131, 32371013, 32270113 and 82071429; Natural Science Foundation of Shandong Province, Grant/Award Numbers: 2021ZDSYS11, ZR2019ZD31 and ZR2022MC098; Taishan Scholars Construction Project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103322.
Contributor Information
Xixun Du, Email: xunxundu@qdu.edu.cn.
Hong Jiang, Email: hongjiang@qdu.edu.cn.
Abbreviations
- AD
Alzheimer's disease
- ALR
autophagic lysosome reformation
- DA
dopamine
- DEGs
differentially expressed genes
- GHSR
growth hormone secretagogue receptor
- Ghsr−/− mice
GHSR knockout mice
- GPCR
G protein-coupled receptor
- LAMP1
lysosome-associated membrane protein 1
- MPP+
1-methyl-4-phenylpyridinium ion
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- CTSD
Cathepsin D
- NS
normal saline
- PD
Parkinson's disease
- SN
substantia nigra
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
Appendix A. Supplementary data
The following is the supplementary data to this article.
Data availability
No data was used for the research described in the article.
References
- 1.Goldman S.M. Environmental toxins and Parkinson's disease. Annu. Rev. Pharmacol. Toxicol. 2014;54:141–164. doi: 10.1146/annurev-pharmtox-011613-135937. [DOI] [PubMed] [Google Scholar]
- 2.Samii A., Nutt J.G., Ransom B.R. Parkinson's disease. Lancet. 2004;363(9423):1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 3.Ascherio A., Schwarzschild M.A. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 4.Song N., et al. Assessments of plasma ghrelin levels in the early stages of Parkinson's disease. Mov. Disord. 2017;32(10):1487–1491. doi: 10.1002/mds.27095. [DOI] [PubMed] [Google Scholar]
- 5.Yu J., et al. Ghrelin protects MES23.5 cells against rotenone via inhibiting mitochondrial dysfunction and apoptosis. Neuropeptides. 2016;56:69–74. doi: 10.1016/j.npep.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Dong J., et al. Ghrelin antagonized 1-methyl-4-phenylpyridinium (MPP(+))-induced apoptosis in MES23.5 cells. J. Mol. Neurosci. 2009;37(2):182–189. doi: 10.1007/s12031-008-9162-7. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H., et al. Ghrelin antagonizes MPTP-induced neurotoxicity to the dopaminergic neurons in mouse substantia nigra. Exp. Neurol. 2008;212(2):532–537. doi: 10.1016/j.expneurol.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Xiao X., et al. A new understanding of GHSR1a--independent of ghrelin activation. Ageing Res. Rev. 2020;64 doi: 10.1016/j.arr.2020.101187. [DOI] [PubMed] [Google Scholar]
- 9.Shi L., et al. Ghrelin and neurodegenerative disorders-a review. Mol. Neurobiol. 2017;54(2):1144–1155. doi: 10.1007/s12035-016-9729-1. [DOI] [PubMed] [Google Scholar]
- 10.Perello M., et al. Brain accessibility delineates the central effects of circulating ghrelin. J. Neuroendocrinol. 2019;31(7) doi: 10.1111/jne.12677. [DOI] [PubMed] [Google Scholar]
- 11.Cabral A., De Francesco P.N., Perello M. Brain circuits mediating the orexigenic action of peripheral ghrelin: narrow gates for a vast kingdom. Front. Endocrinol. 2015;6:44. doi: 10.3389/fendo.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang H.J., et al. The protective effects of Ghrelin/GHSR on hippocampal neurogenesis in CUMS mice. Neuropharmacology. 2019;155:31–43. doi: 10.1016/j.neuropharm.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Cahill S.P., et al. An examination of early neural and cognitive alterations in hippocampal-spatial function of ghrelin receptor-deficient rats. Behav. Brain Res. 2014;264:105–115. doi: 10.1016/j.bbr.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Park S.B., et al. Contribution of growth hormone secretagogue receptor (GHSR) signaling in the ventral tegmental area (VTA) to the regulation of social motivation in male mice. Transl. Psychiatry. 2021;11(1):230. doi: 10.1038/s41398-021-01350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suda Y., et al. Effect of ghrelin on the motor deficit caused by the ablation of nigrostriatal dopaminergic cells or the inhibition of striatal dopamine receptors. Biochem. Biophys. Res. Commun. 2018;496(4):1102–1108. doi: 10.1016/j.bbrc.2018.01.145. [DOI] [PubMed] [Google Scholar]
- 16.Suda Y., et al. Down-regulation of ghrelin receptors on dopaminergic neurons in the substantia nigra contributes to Parkinson's disease-like motor dysfunction. Mol. Brain. 2018;11(1):6. doi: 10.1186/s13041-018-0349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heshmati M., et al. Ghrelin induces autophagy and CXCR4 expression via the SIRT1/AMPK axis in lymphoblastic leukemia cell lines. Cell. Signal. 2020;66 doi: 10.1016/j.cellsig.2019.109492. [DOI] [PubMed] [Google Scholar]
- 18.Mao Y., et al. Ghrelin attenuated lipotoxicity via autophagy induction and nuclear factor-κb inhibition. Cell. Physiol. Biochem. 2015;37(2):563–576. doi: 10.1159/000430377. [DOI] [PubMed] [Google Scholar]
- 19.Klionsky D.J., et al. Autophagy in major human diseases. EMBO J. 2021;40(19) doi: 10.15252/embj.2021108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng J., et al. Microglial autophagy defect causes Parkinson disease-like symptoms by accelerating inflammasome activation in mice. Autophagy. 2020;16(12):2193–2205. doi: 10.1080/15548627.2020.1719723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu X.Y., et al. Deletion of autophagy-related gene 7 in dopaminergic neurons prevents their loss induced by MPTP. Neuroscience. 2016;339:22–31. doi: 10.1016/j.neuroscience.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 22.Prieto Huarcaya S., et al. Recombinant pro-CTSD (cathepsin D) enhances SNCA/α-Synuclein degradation in α-Synucleinopathy models. Autophagy. 2022;18(5):1127–1151. doi: 10.1080/15548627.2022.2045534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou X., et al. Autophagy in Parkinson's disease. J. Mol. Biol. 2020;432(8):2651–2672. doi: 10.1016/j.jmb.2020.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arotcarena M.L., Teil M., Dehay B. Autophagy in synucleinopathy: the overwhelmed and defective machinery. Cells. 2019;8(6) doi: 10.3390/cells8060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerri S., Blandini F. Role of autophagy in Parkinson's disease. Curr. Med. Chem. 2019;26(20):3702–3718. doi: 10.2174/0929867325666180226094351. [DOI] [PubMed] [Google Scholar]
- 26.Catena V., Fanciulli M. Deptor: not only a mTOR inhibitor. J. Exp. Clin. Cancer Res. 2017;36(1):12. doi: 10.1186/s13046-016-0484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y., Xiong X., Sun Y. DEPTOR, an mTOR inhibitor, is a physiological substrate of SCF(βTrCP) E3 ubiquitin ligase and regulates survival and autophagy. Mol. Cell. 2011;44(2):304–316. doi: 10.1016/j.molcel.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao D., et al. mTOR drives its own activation via SCF(βTrCP)-dependent degradation of the mTOR inhibitor DEPTOR. Mol. Cell. 2011;44(2):290–303. doi: 10.1016/j.molcel.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun S.P., et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nat. Med. 2018;24(7):931–938. doi: 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenner P. Oxidative stress and Parkinson's disease. Handb. Clin. Neurol. 2007;83:507–520. doi: 10.1016/S0072-9752(07)83024-7. [DOI] [PubMed] [Google Scholar]
- 31.Jiang M., et al. Puerarin prevents inflammation and apoptosis in the neurocytes of a murine Parkinson's disease model. Genet. Mol. Res. 2016;15(4) doi: 10.4238/gmr.15047501. [DOI] [PubMed] [Google Scholar]
- 32.Trist B.G., et al. Amyotrophic lateral sclerosis-like superoxide dismutase 1 proteinopathy is associated with neuronal loss in Parkinson's disease brain. Acta Neuropathol. 2017;134(1):113–127. doi: 10.1007/s00401-017-1726-6. [DOI] [PubMed] [Google Scholar]
- 33.Janda E., et al. Parkinsonian toxin-induced oxidative stress inhibits basal autophagy in astrocytes via NQO2/quinone oxidoreductase 2: implications for neuroprotection. Autophagy. 2015;11(7):1063–1080. doi: 10.1080/15548627.2015.1058683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahapatra K.K., et al. The lysosome as an imperative regulator of autophagy and cell death. Cell. Mol. Life Sci. 2021;78(23):7435–7449. doi: 10.1007/s00018-021-03988-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng X.T., et al. Revisiting LAMP1 as a marker for degradative autophagy-lysosomal organelles in the nervous system. Autophagy. 2018;14(8):1472–1474. doi: 10.1080/15548627.2018.1482147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng X.T., et al. Characterization of LAMP1-labeled nondegradative lysosomal and endocytic compartments in neurons. J. Cell Biol. 2018;217(9):3127–3139. doi: 10.1083/jcb.201711083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marote A., et al. Generation of an induced pluripotent stem cell line (CSC-44) from a Parkinson's disease patient carrying a compound heterozygous mutation (c.823C>T and EX6 del) in the PARK2 gene. Stem Cell Res. 2018;27:90–94. doi: 10.1016/j.scr.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Song Y., Liu Y., Chen X. MiR-212 attenuates MPP⁺-Induced neuronal damage by targeting KLF4 in SH-SY5Y cells. Yonsei Med. J. 2018;59(3):416–424. doi: 10.3349/ymj.2018.59.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hossain M.I., et al. Restoration of CTSD (cathepsin D) and lysosomal function in stroke is neuroprotective. Autophagy. 2021;17(6):1330–1348. doi: 10.1080/15548627.2020.1761219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albarran-Zeckler R.G., Brantley A.F., Smith R.G. Growth hormone secretagogue receptor (GHS-R1a) knockout mice exhibit improved spatial memory and deficits in contextual memory. Behav. Brain Res. 2012;232(1):13–19. doi: 10.1016/j.bbr.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y.C., Guan K.L. mTOR: a pharmacologic target for autophagy regulation. J. Clin. Invest. 2015;125(1):25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Z., et al. Balancing mTOR signaling and autophagy in the treatment of Parkinson's disease. Int. J. Mol. Sci. 2019;20(3) doi: 10.3390/ijms20030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian Y., et al. Chemopreventive effect of dietary glutamineon colitis-associated colorectal cancer is associated with modulation of the DEPTOR/mTOR signaling pathway. Nutrients. 2016;8(5) doi: 10.3390/nu8050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H., et al. Knockdown of DEPTOR induces apoptosis, increases chemosensitivity to doxorubicin and suppresses autophagy in RPMI-8226 human multiple myeloma cells in vitro. Int. J. Mol. Med. 2013;31(5):1127–1134. doi: 10.3892/ijmm.2013.1299. [DOI] [PubMed] [Google Scholar]
- 45.Yao H., et al. DEPTOR inhibits cell proliferation and confers sensitivity to dopamine agonist in pituitary adenoma. Cancer Lett. 2019;459:135–144. doi: 10.1016/j.canlet.2019.05.043. [DOI] [PubMed] [Google Scholar]
- 46.Dunn W.A., Jr. Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol. 1994;4(4):139–143. doi: 10.1016/0962-8924(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 47.Plowey E.D., et al. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J. Neurochem. 2008;105(3):1048–1056. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu C.T. A pivotal role for PINK1 and autophagy in mitochondrial quality control: implications for Parkinson disease. Hum. Mol. Genet. 2010;19(R1):R28–R37. doi: 10.1093/hmg/ddq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michiorri S., et al. The Parkinson-associated protein PINK1 interacts with Beclin1 and promotes autophagy. Cell Death Differ. 2010;17(6):962–974. doi: 10.1038/cdd.2009.200. [DOI] [PubMed] [Google Scholar]
- 50.Repici M., Giorgini F. DJ-1 in Parkinson's disease: clinical insights and therapeutic perspectives. J. Clin. Med. 2019;8(9) doi: 10.3390/jcm8091377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanik S.A., et al. Lewy body-like α-synuclein aggregates resist degradation and impair macroautophagy. J. Biol. Chem. 2013;288(21):15194–15210. doi: 10.1074/jbc.M113.457408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin Y., et al. ER stress and impaired autophagy flux in neuronal degeneration and brain injury. Ageing Res. Rev. 2017;34:3–14. doi: 10.1016/j.arr.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao H., et al. DJ-1 protects dopaminergic neurons against rotenone-induced apoptosis by enhancing ERK-dependent mitophagy. J. Mol. Biol. 2012;423(2):232–248. doi: 10.1016/j.jmb.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 54.Nixon R.A., Yang D.S. Autophagy and neuronal cell death in neurological disorders. Cold Spring Harbor Perspect. Biol. 2012;4(10) doi: 10.1101/cshperspect.a008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dehay B., et al. Pathogenic lysosomal depletion in Parkinson's disease. J. Neurosci. 2010;30(37):12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levine B., Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176(1–2):11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dai A., Yu L., Wang H.W. WHAMM initiates autolysosome tubulation by promoting actin polymerization on autolysosomes. Nat. Commun. 2019;10(1):3699. doi: 10.1038/s41467-019-11694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J.A., et al. Fbxo9 functions downstream of Sox10 to determine neuron-glial fate choice in the dorsal root ganglia through Neurog2 destabilization. Proc. Natl. Acad. Sci. U. S. A. 2020;117(8):4199–4210. doi: 10.1073/pnas.1916164117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miettinen M., et al. Sox10--a marker for not only schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue: a systematic analysis of 5134 tumors. Am. J. Surg. Pathol. 2015;39(6):826–835. doi: 10.1097/PAS.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng Z., et al. The role of KLF(4) in alzheimer's disease. Front. Cell. Neurosci. 2018;12:325. doi: 10.3389/fncel.2018.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui D.M., et al. KLF4 knockdown attenuates TBI-induced neuronal damage through p53 and JAK-STAT3 signaling. CNS Neurosci. Ther. 2017;23(2):106–118. doi: 10.1111/cns.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore D.L., et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326(5950):298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qin S., Zou Y., Zhang C.L. Cross-talk between KLF4 and STAT3 regulates axon regeneration. Nat. Commun. 2013;4:2633. doi: 10.1038/ncomms3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ebben J.D., et al. Introduction to induced pluripotent stem cells: advancing the potential for personalized medicine. World Neurosurg. 2011;76(3–4):270–275. doi: 10.1016/j.wneu.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bock F.J., Tait S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020;21(2):85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this article is available.
No data was used for the research described in the article.