Abstract
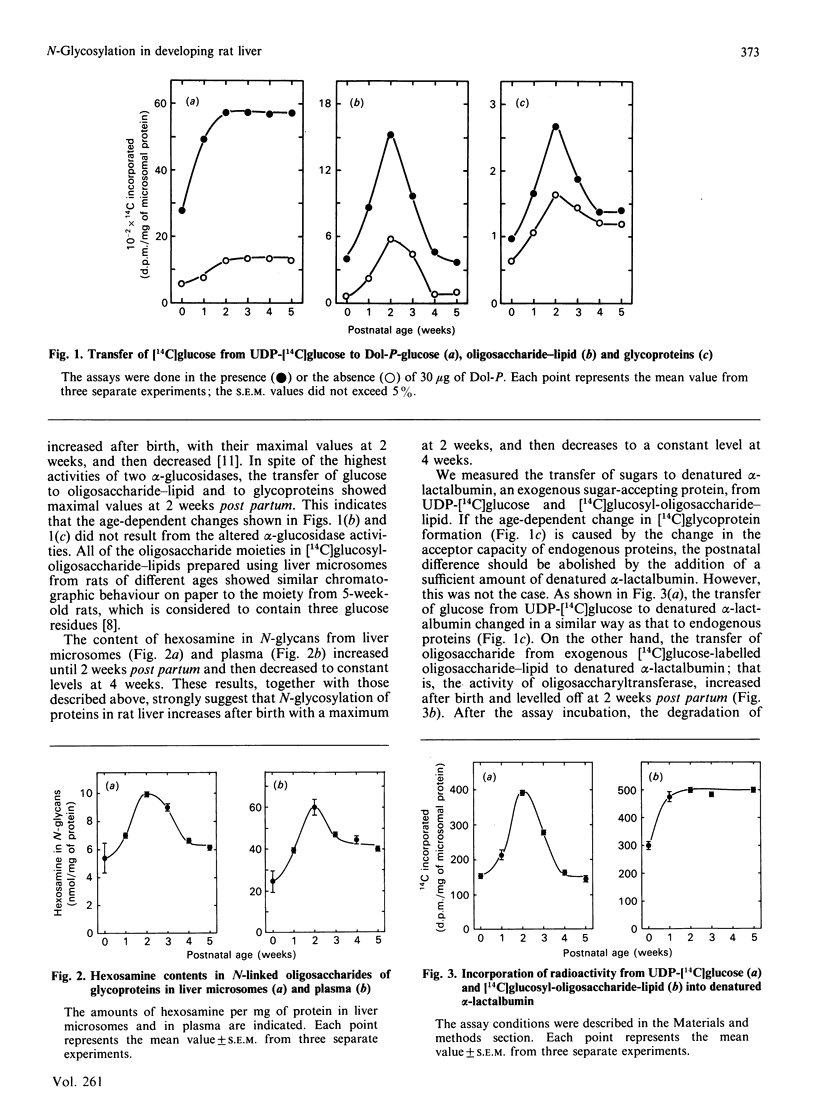
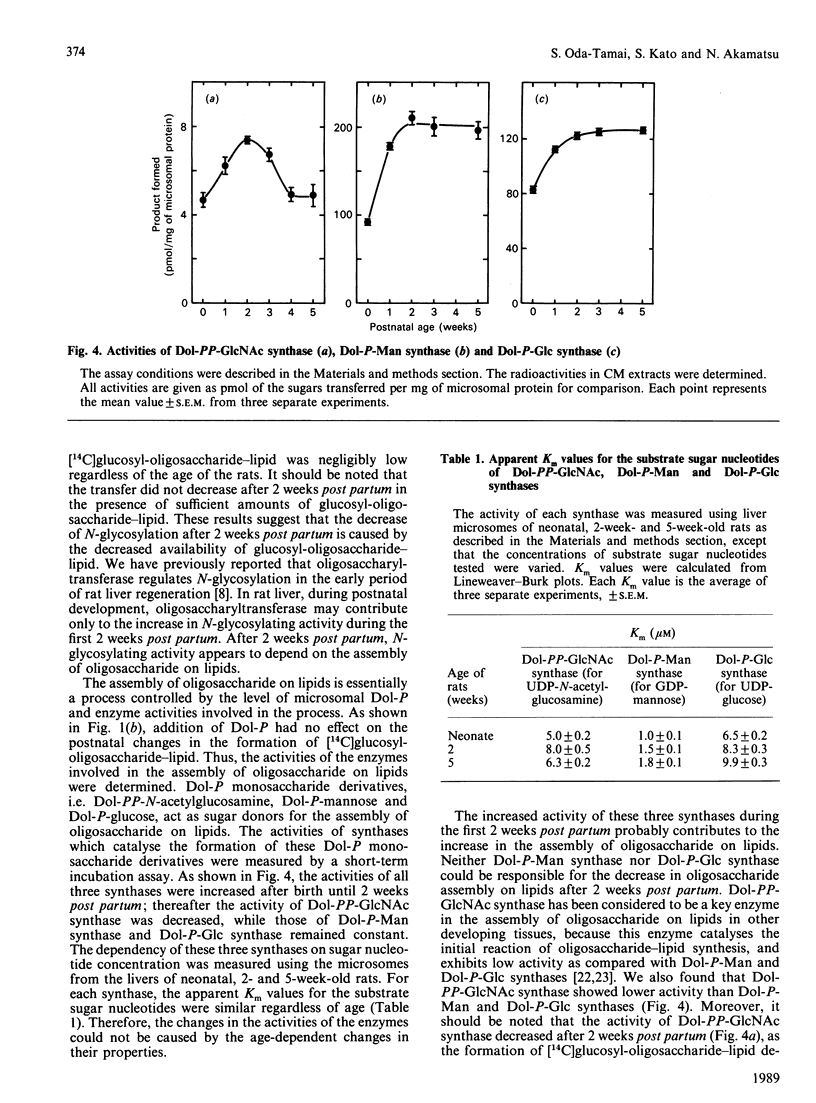
The activity of hepatic protein N-glycosylation was compared in rats of different ages by incubating UDP-[14C]glucose with liver microsomes. Dolichyl-phosphate [14C]glucose, [14C]glucosyl-oligosaccharide-lipid and [14C]glycoproteins formed were increased after birth to maximal levels at 2 weeks; thereafter dolichylphosphate [14C]glucose remained constant, while [14C]glucosyl-oligosaccharide-lipid and [14C]glycoproteins were decreased to constant levels at 4 weeks. The postnatal change in the formation of [14C]glycoproteins was similar to the change in the hexosamine content of N-glycans in liver microsomes and plasma, suggesting that the N-glycosylation of proteins in rat liver increases after birth to a maximum at 2 weeks, and thereafter decreases to a constant level at 4 weeks. The possibility of a regulatory role for dolichyl phosphate in glycoprotein synthesis in rat liver during postnatal development was eliminated by demonstrating the inefficiency of exogenous dolichyl phosphate on the postnatal changes in [14C]glycoprotein formation. The transfer of [14C]glucose from UDP-[14C]glucose to denatured alpha-lactalbumin in liver microsomes increased to a maximum at 2 weeks and then decreased to a constant level, as with transfer to endogenous proteins (i.e. the formation of [14C]glycoproteins). On the other hand, the transfer of oligosaccharide from exogenous [14C]glucosyl-oligosaccharide-lipid to denatured alpha-lactalbumin reached a maximum at 2 weeks and then remained constant. These results strongly suggest that oligosaccharide-lipid available for N-glycosylation is limiting in rat liver after 2 weeks post partum. The activities of dolichyl-phosphate glucose, dolichyl-phosphate mannose and dolichyl-pyrophosphate N-acetylglucosamine synthases increased until 2 weeks post partum. Thereafter, the activity of dolichyl-pyrophosphate N-acetylglucosamine synthase decreased to a constant level at 4 weeks, while the activities of dolichyl-phosphate glucose and dolichyl-phosphate mannose synthases remained constant. These results suggest that N-glycosylation of proteins in rat liver increases until 2 weeks post partum, and that this depends on the activities of dolichol-pathway enzymes as a whole rather than on the activity of specific enzymes. N-Glycosylation then decreases to a constant level at 4 weeks due to decreases in the activities of enzymes responsible for oligosaccharide assembly on lipids, including dolichyl-pyrophosphate N-acetylglucosamine synthase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carson D. D., Earles B. J., Lennarz W. J. Enhancement of protein glycosylation in tissue slices by dolichylphosphate. J Biol Chem. 1981 Nov 25;256(22):11552–11557. [PubMed] [Google Scholar]
- Clark G. F., Miller K. R., Smith P. B. Formation of dolichol-linked sugar intermediates during the postnatal development of skeletal muscle. J Biol Chem. 1983 Dec 10;258(23):14263–14270. [PubMed] [Google Scholar]
- Harford J. B., Waechter C. J. A developmental change in dolichyl phosphate mannose synthase activity in pig brain. Biochem J. 1980 May 15;188(2):481–490. doi: 10.1042/bj1880481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idoyaga-Vargas V., Carminatti H. Postnatal changes in dolichol-pathway enzyme activities in cerebral cortex neurons. Biochem J. 1982 Jan 15;202(1):87–95. doi: 10.1042/bj2020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Akamatsu N. Alterations in N-linked oligosaccharides of glycoproteins during rat liver regeneration. Biochim Biophys Acta. 1984 Mar 22;798(1):68–77. doi: 10.1016/0304-4165(84)90011-4. [DOI] [PubMed] [Google Scholar]
- Kato S., Akamatsu N. Alterations in fucosyl oligosaccharides of glycoproteins during rat liver regeneration. Biochem J. 1985 Jul 15;229(2):521–528. doi: 10.1042/bj2290521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Oda-Tamai S., Akamatsu N. Postnatal changes in N-linked oligosaccharides of glycoproteins in rat liver. Biochem J. 1988 Jul 1;253(1):59–66. doi: 10.1042/bj2530059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lucas J. J., Levin E. Increase in the lipid intermediate pathway of protein glycosylation during hen oviduct differentiation. J Biol Chem. 1977 Jun 25;252(12):4330–4336. [PubMed] [Google Scholar]
- Mookerjea S., Coolbear T., Sarkar M. L. Key role of dolichol phosphate in glycoprotein biosynthesis. Can J Biochem Cell Biol. 1983 Sep;61(9):1032–1040. doi: 10.1139/o83-132. [DOI] [PubMed] [Google Scholar]
- Oda-Tamai S., Kato S., Hara S., Akamatsu N. Decreased transfer of oligosaccharide from oligosaccharide-lipid to protein acceptors in regenerating rat liver. J Biol Chem. 1985 Jan 10;260(1):57–63. [PubMed] [Google Scholar]
- Okamoto Y., Akamatsu N. Further studies on lipid intermediates in glycoprotein synthesis during rat liver regeneration. Int J Biochem. 1981;13(7):791–798. doi: 10.1016/0020-711x(81)90097-5. [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Akamatsu N. Synthesis in vitro of glycoprotein in regenerating rat liver. Biochim Biophys Acta. 1977 Jul 21;498(1):272–281. doi: 10.1016/0304-4165(77)90265-3. [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Ito E., Akamatsu N. UDP-N-acetylglucosamine-glycoprotein N-acetylglucosaminyltransferase in regenerating rat liver. Biochim Biophys Acta. 1978 Aug 3;542(1):21–27. doi: 10.1016/0304-4165(78)90228-3. [DOI] [PubMed] [Google Scholar]
- Olden K., Parent J. B., White S. L. Carbohydrate moieties of glycoproteins. A re-evaluation of their function. Biochim Biophys Acta. 1982 May 12;650(4):209–232. doi: 10.1016/0304-4157(82)90017-x. [DOI] [PubMed] [Google Scholar]
- Parodi A. J., Behrens N. H., Leloir L. F., Dankert M. Glucose transfer from dolichol monophosphat glucose. The lipid moiety of the endogenous microsomal acceptor. Biochim Biophys Acta. 1972 Aug 11;270(4):529–536. doi: 10.1016/0005-2760(72)90118-x. [DOI] [PubMed] [Google Scholar]
- Sly W. S., Fischer H. D. The phosphomannosyl recognition system for intracellular and intercellular transport of lysosomal enzymes. J Cell Biochem. 1982;18(1):67–85. doi: 10.1002/jcb.1982.240180107. [DOI] [PubMed] [Google Scholar]
- Vijay I. K., Oka T. Developmental regulation of glycosyltransferases involved in biosynthesis of asparagine-linked glycoproteins in mouse mammary gland. Eur J Biochem. 1986 Jan 2;154(1):57–62. doi: 10.1111/j.1432-1033.1986.tb09358.x. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lucas J. J., Lennarz W. J. Membrane glycoproteins. I. Enzymatic synthesis of mannosyl phosphoryl polyisoprenol and its role as a mannosyl donor in glycoprotein synthesis. J Biol Chem. 1973 Nov 10;248(21):7570–7579. [PubMed] [Google Scholar]


