Abstract
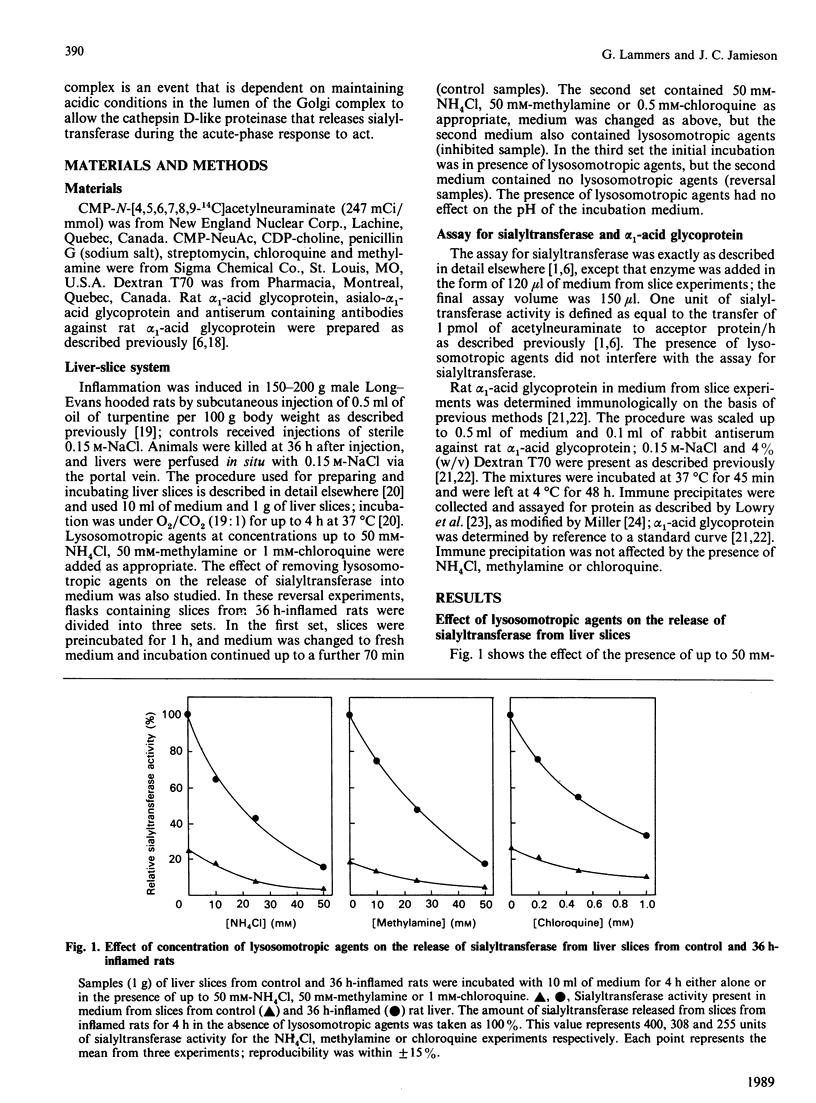
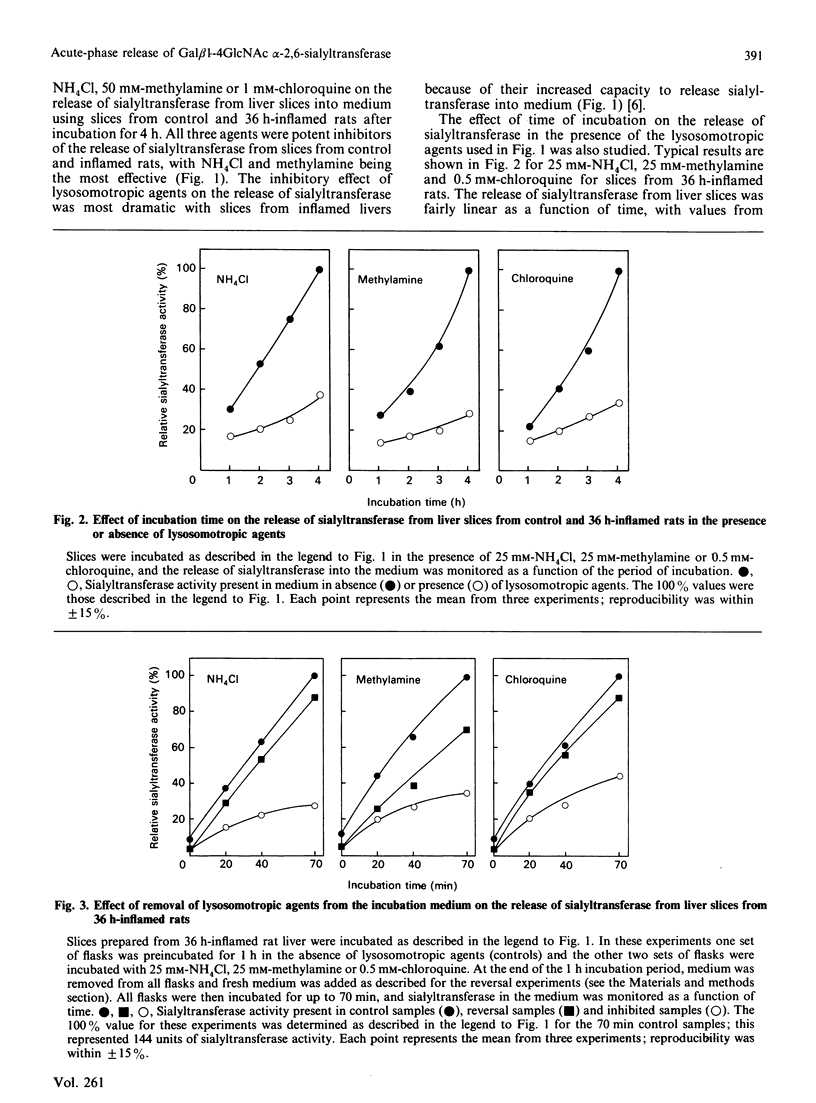
The mechanism of release of Gal beta 1-4GlcNAc alpha-2,6-sialyltransferase (CMP-N-acetylneuraminate: beta-galactoside alpha-2,6-sialytransferase, EC 2.4.99.1) from rat liver during the acute-phase response is due to the action of a cathepsin D-like proteinase that cleaves the trans-Golgi membrane-bound enzyme from a membrane anchor; this allows a major portion of the enzyme containing the catalytic site to escape into the extracellular space [Lammers & Jamieson (1988) Biochem. J. 256, 623-631]. The release of sialytransferase was most effective at pH 5.6, suggesting that release of sialyltransferase from the Golgi in whole cells is dependent on maintaining an acidic environment in the trans-Golgi compartment of the hepatocyte. Golgi membranes contain a proton pump that maintains the acidic pH in these compartments [Glickman, Croen, Kelly & Al-Awquati (1983) J. Cell Biol. 97, 1303-1308; Yamashiro, Tycko & Maxfield (1984) Cell (Cambridge, Mass.) 37, 789-800; Zhang & Schneider (1983) Biochem. Biophys. Res. Commun. 114, 620-625; Anderson & Pathak (1985) Cell (Cambridge, Mass.) 40, 635-643]. Lysosomotropic agents, such as NH4Cl, chloroquine and methylamine can penetrate acidic compartments of the cell, such as the Golgi complex, raise the pH, and thus affect proteolytic cleavage events. The present paper describes the effect of lysosomotropic agents on the release of sialyltransferase from the hepatocyte using liver slices as a whole-cell system. Slices were prepared from control rats and rats suffering from the acute-phase response, where release of sialyltransferase is increased substantially [Lammers & Jamieson (1988) Biochem. J. 256, 623-631; Kaplan, Woloski, Hellman & Jamieson (1983) J. Biol. Chem. 258, 11505-11509]. Release of sialyltransferase was almost abolished in presence of 50 mM-NH4Cl, 50 mM-methylamine or 1 mM-chloroquine. Inhibition of release of sialyltransferase was reversed when the lysosomotropic agents were removed from the medium, showing that these agents are not cytotoxic to the cells under the conditions used. The secretion of rat alpha 1-acid glycoprotein, which is not subject to proteolytic processing in the Golgi complex, was not found to be substantially affected by the presence of lysosomotropic agents. The results suggest that proteolytic cleavage of the catalytic site of sialyltransferase is a process that is significantly affected by the intra-Golgi pH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Pathak R. K. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985 Mar;40(3):635–643. doi: 10.1016/0092-8674(85)90212-0. [DOI] [PubMed] [Google Scholar]
- Ashton F. E., Jamieson J. C., Friesen A. D. Studies on the effect of inflammation on rat serum proteins. Can J Biochem. 1970 Aug;48(8):841–850. doi: 10.1139/o70-133. [DOI] [PubMed] [Google Scholar]
- Beisel W. R. Effects of infection on nutritional status and immunity. Fed Proc. 1980 Nov;39(13):3105–3108. [PubMed] [Google Scholar]
- Caplan M. J., Stow J. L., Newman A. P., Madri J., Anderson H. C., Farquhar M. G., Palade G. E., Jamieson J. D. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987 Oct 15;329(6140):632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Jessup W., Roberts C. R. Effects of exogenous amines on mammalian cells, with particular reference to membrane flow. Biochem J. 1984 Jan 1;217(1):27–40. doi: 10.1042/bj2170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson A. H., Conner G. E., Blobel G. Biosynthesis of a lysosomal enzyme. Partial structure of two transient and functionally distinct NH2-terminal sequences in cathepsin D. J Biol Chem. 1981 Nov 10;256(21):11224–11231. [PubMed] [Google Scholar]
- Glickman J., Croen K., Kelly S., Al-Awqati Q. Golgi membranes contain an electrogenic H+ pump in parallel to a chloride conductance. J Cell Biol. 1983 Oct;97(4):1303–1308. doi: 10.1083/jcb.97.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., von Figura K., Conzelmann E., Nehrkorn H., Sandhoff K. Lysosomal enzyme precursors in human fibroblasts. Activation of cathepsin D precursor in vitro and activity of beta-hexosaminidase A precursor towards ganglioside GM2. Eur J Biochem. 1982 Jul;125(2):317–321. doi: 10.1111/j.1432-1033.1982.tb06685.x. [DOI] [PubMed] [Google Scholar]
- Jamieson J. C., Ashton F. E., Friesen A. D., Chou B. Studies on acute phase proteins of rat serum. II. Determination of the contents of 1 -acid glycoprotein, 2-macroglobulin, and albumin in serum from rats suffering from induced inflammation. Can J Biochem. 1972 Aug;50(8):871–880. doi: 10.1139/o72-122. [DOI] [PubMed] [Google Scholar]
- Jamieson J. C., Ashton F. E. Studies on acute phase proteins of rat serum. 3. Site of synthesis of albumin and alpha 1-acid glycoprotein and the contents of these proteins in liver microsome fractions from rats suffering from induced inflammation. Can J Biochem. 1973 Jul;51(7):1034–1045. doi: 10.1139/o73-135. [DOI] [PubMed] [Google Scholar]
- Jamieson J. C., Ashton F. E. Studies on acute phase proteins of rat serum. IV. Pathway of secretion of albumin and alpha1-acid glycoprotein from liver. Can J Biochem. 1973 Sep;51(9):1281–1291. doi: 10.1139/o73-168. [DOI] [PubMed] [Google Scholar]
- Jamieson J. C., Friesen A. D., Ashton F. E., Chou B. Studies on acute phase proteins of rat serum. 1. Isolation and partial characterization of an 1 -acid glycoprotein and an 2 -macroglobulin. Can J Biochem. 1972 Aug;50(8):856–870. doi: 10.1139/o72-121. [DOI] [PubMed] [Google Scholar]
- Jamieson J. C., Kaplan H. A., Woloski B. M., Hellman M., Ham K. Glycoprotein biosynthesis during the acute-phase response to inflammation. Can J Biochem Cell Biol. 1983 Sep;61(9):1041–1048. doi: 10.1139/o83-133. [DOI] [PubMed] [Google Scholar]
- Jamieson J. C., Kaplan H. A., Woloski B. M., Hellman M., Ham K. Glycoprotein biosynthesis during the acute-phase response to inflammation. Can J Biochem Cell Biol. 1983 Sep;61(9):1041–1048. doi: 10.1139/o83-133. [DOI] [PubMed] [Google Scholar]
- Jamieson J. C., Lammers G., Janzen R., Woloski B. M. The acute phase response to inflammation: the role of monokines in changes in liver glycoproteins and enzymes of glycoprotein metabolism. Comp Biochem Physiol B. 1987;87(1):11–15. doi: 10.1016/0305-0491(87)90463-9. [DOI] [PubMed] [Google Scholar]
- Jamieson J. C., Morrison K. E., Molasky D., Turchen B. Studies on acute phase proteins of rat serum. V Effect on induces inflammation on the synthesis of albumin and alpha-1-acid glycoprotein by liver slices. Can J Biochem. 1975 Apr;53(4):401–414. doi: 10.1139/o75-056. [DOI] [PubMed] [Google Scholar]
- Kaplan H. A., Woloski B. M., Hellman M., Jamieson J. C. Studies on the effect of inflammation on rat liver and serum sialyltransferase. Evidence that inflammation causes release of Gal beta 1 leads to 4GlcNAc alpha 2 leads to 6 sialyltransferase from liver. J Biol Chem. 1983 Oct 10;258(19):11505–11509. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lammers G., Jamieson J. C. The role of a cathepsin D-like activity in the release of Gal beta 1-4GlcNAc alpha 2-6-sialyltransferase from rat liver Golgi membranes during the acute-phase response. Biochem J. 1988 Dec 1;256(2):623–631. doi: 10.1042/bj2560623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K., Ikehara Y. Weakly basic amines inhibit the proteolytic conversion of proalbumin to serum albumin in cultured rat hepatocytes. Eur J Biochem. 1985 Nov 4;152(3):605–609. doi: 10.1111/j.1432-1033.1985.tb09238.x. [DOI] [PubMed] [Google Scholar]
- Oda K., Koriyama Y., Yamada E., Ikehara Y. Effects of weakly basic amines on proteolytic processing and terminal glycosylation of secretory proteins in cultured rat hepatocytes. Biochem J. 1986 Dec 15;240(3):739–745. doi: 10.1042/bj2400739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke R., Feigelson P. Rat alpha 1-acid glycoprotein. Gene sequence and regulation by glucocorticoids in transfected L-cells. J Biol Chem. 1985 Apr 10;260(7):4397–4403. [PubMed] [Google Scholar]
- Ricca G. A., Taylor J. M. Nucleotide sequence of rat alpha 1-acid glycoprotein messenger RNA. J Biol Chem. 1981 Nov 10;256(21):11199–11202. [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Lucocq J. M., Weinstein J., Paulson J. C. Demonstration of an extensive trans-tubular network continuous with the Golgi apparatus stack that may function in glycosylation. Cell. 1985 Nov;43(1):287–295. doi: 10.1016/0092-8674(85)90034-0. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Strous G. J., Slot J. W., Geuze H. J. Immunoelectron microscopic localization of acidic intracellular compartments in hepatoma cells. EMBO J. 1985 Apr;4(4):899–904. doi: 10.1002/j.1460-2075.1985.tb03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin J. L., Jamieson J. C. Studies on the site of biosynthesis of acidic glycoproteins of guinea-pig serum. Biochem J. 1967 Apr;103(1):153–164. doi: 10.1042/bj1030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J., Du Maine A., Zijderhand-Bleekemolen J. E., Slot J. W., Schwartz A. L. Effect of lysosomotropic amines on the secretory pathway and on the recycling of the asialoglycoprotein receptor in human hepatoma cells. J Cell Biol. 1985 Aug;101(2):531–539. doi: 10.1083/jcb.101.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro D. J., Tycko B., Fluss S. R., Maxfield F. R. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984 Jul;37(3):789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- Yonezawa S., Takahashi T., Wang X. J., Wong R. N., Hartsuck J. A., Tang J. Structures at the proteolytic processing region of cathepsin D. J Biol Chem. 1988 Nov 5;263(31):16504–16511. [PubMed] [Google Scholar]
- Zhang F., Schneider D. L. The bioenergetics of Golgi apparatus function: evidence for an ATP-dependent proton pump. Biochem Biophys Res Commun. 1983 Jul 29;114(2):620–625. doi: 10.1016/0006-291x(83)90825-2. [DOI] [PubMed] [Google Scholar]
- de Duve C. Lysosomes revisited. Eur J Biochem. 1983 Dec 15;137(3):391–397. doi: 10.1111/j.1432-1033.1983.tb07841.x. [DOI] [PubMed] [Google Scholar]


