Abstract
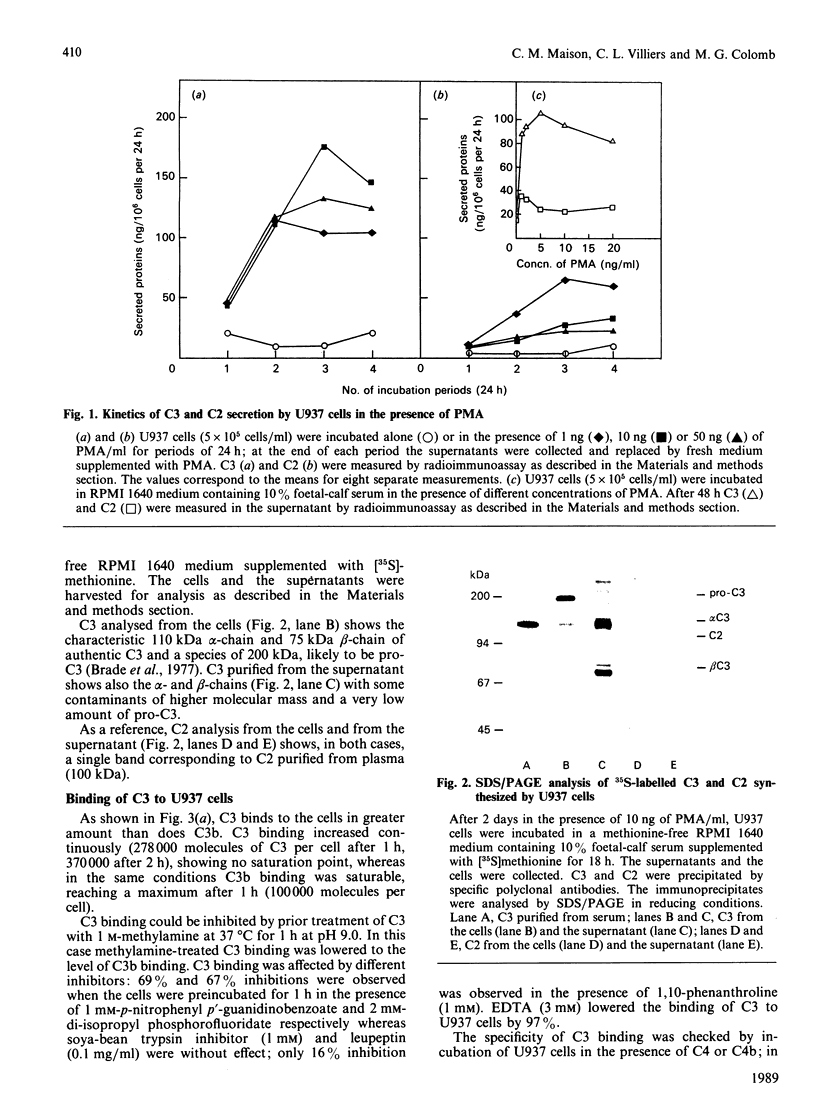
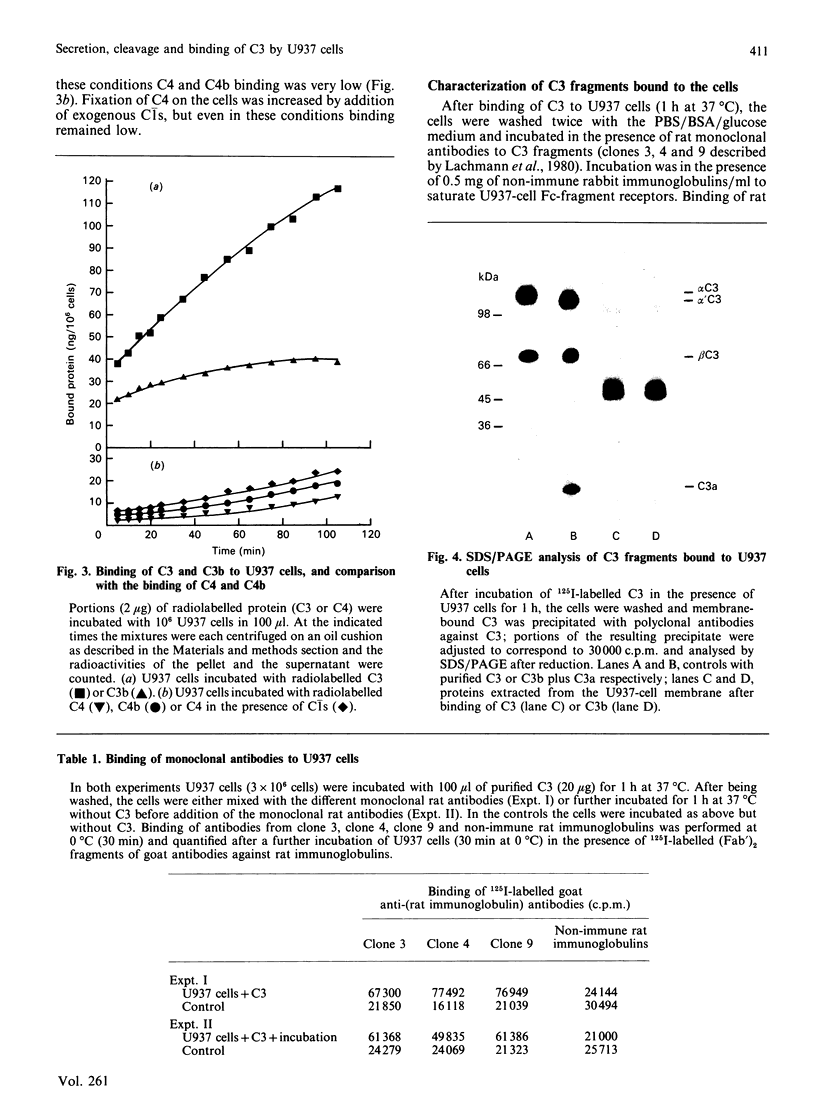
Secretion of complement component C3 by U937 cells was studied. Preliminary evidence for a cell-associated proteolytic activity specific for C3 is given, as well as for a covalent-like binding of C3 fragments to the cell membranes. Secretion of C3, in the presence of 10 ng of phorbol 12-myristate 13-acetate/ml, is 120-140 ng/10(6) cells per 24 h on the third day after addition of the activator. As shown by SDS/polyacrylamide-gel electrophoresis, the intracellular pro-C3 (200 kDa) and the extracellular secreted C3 (alpha-chain 110 kDa and beta-chain 75 kDa) are identical with the forms of C3 previously characterized from human serum. Incubation of U937 cells in the presence of exogenous radiolabelled C3 shows that membrane-bound proteinase(s), not related to the classical-pathway or the alternative-pathway C3 convertases, is (are) able to cleave C3; this cleavage leads to the binding of the resulting C3 fragments to the cell membrane through reaction of membrane acceptors with the carbonyl group of C3 revealed after disruption of the intramolecular thioester bond. The proteolysis appears to be fairly specific to C3, as C4, which also possesses an intramolecular thioester bond, is not cleaved and does not bind to the cells. p-Nitrophenyl p'-guanidinobenzoate (1 mM) and di-isopropyl phosphorofluoridate (2 mM) are potent inhibitors of the proteolysis, whereas soya-bean trypsin inhibitor (1 mM), leupeptin (0.1 mg/ml) and 1,10-phenanthroline (1 mM) were ineffective. Immunological characterization of the cell-bound C3 fragments with monoclonal antibodies shows an evolution of the proteolysis of the fragments from iC3b to C3dg epitopes. Extraction of membrane-bound fragments by detergent, followed by SDS/polyacrylamide-gel electrophoresis, shows two fragments, of 43 kDa and 46 kDa, with C3dg-like characteristics.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. L., Abraham G. N. Characterization of the Fc receptor for IgG on a human macrophage cell line, U937. J Immunol. 1980 Dec;125(6):2735–2741. [PubMed] [Google Scholar]
- Arlaud G. J., Sim R. B., Duplaa A. M., Colomb M. G. Differential elution of Clq, Clr and Cls from human Cl bound to immune aggregates. Use in the rapid purification of Cl subcomponents. Mol Immunol. 1979 Jul;16(7):445–450. doi: 10.1016/0161-5890(79)90069-5. [DOI] [PubMed] [Google Scholar]
- Barnum S. R., Volanakis J. E. In vitro biosynthesis of complement protein D by U937 cells. J Immunol. 1985 Mar;134(3):1799–1803. [PubMed] [Google Scholar]
- Bengio S., Gilbert D., Peulve P., Daveau M., Fontaine M. Biosynthesis of the third component of complement (C3) by the human monocytic-cell line U-937. Induction by phorbol myristate acetate. Biochem J. 1986 Nov 1;239(3):711–716. doi: 10.1042/bj2390711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensa J. C., Reboul A., Colomb M. G. Biosynthesis in vitro of complement subcomponents C1q, C1s and C1 inhibitor by resting and stimulated human monocytes. Biochem J. 1983 Nov 15;216(2):385–392. doi: 10.1042/bj2160385. [DOI] [PMC free article] [PubMed] [Google Scholar]
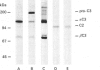
- Brade V., Hall R. E., Colten H. R. Biosynthesis of pro-C3, a precursor of the third component of complement. J Exp Med. 1977 Sep 1;146(3):759–765. doi: 10.1084/jem.146.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttger E. C., Metzger S., Bitter-Suermann D., Stevenson G., Kleindienst S., Burger R. Impaired humoral immune response in complement C3-deficient guinea pigs: absence of secondary antibody response. Eur J Immunol. 1986 Oct;16(10):1231–1235. doi: 10.1002/eji.1830161008. [DOI] [PubMed] [Google Scholar]
- Erdei A., Bajtay Z., Fábry Z., Sim R. B., Gergely J. Appearance of acceptor-bound C3b on HLA-DR positive macrophages and on stimulated U937 cells; inhibition of Fc gamma-receptors by the covalently fixed C3 fragments. Mol Immunol. 1988 Mar;25(3):295–303. doi: 10.1016/0161-5890(88)90021-1. [DOI] [PubMed] [Google Scholar]
- Erdei A., Melchers F., Schulz T., Dierich M. The action of human C3 in soluble or cross-linked form with resting and activated murine B lymphocytes. Eur J Immunol. 1985 Feb;15(2):184–188. doi: 10.1002/eji.1830150214. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., Hill M., Gordon S. Local opsonization by secreted macrophage complement components. Role of receptors for complement in uptake of zymosan. J Exp Med. 1984 Jan 1;159(1):244–260. doi: 10.1084/jem.159.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Fábry Z., Erdei A., Gergely J. A possible self-regulating mechanism mediated by C3b-acceptor-bound C3b generated by stimulated macrophages. Scand J Immunol. 1985 Nov;22(5):549–555. doi: 10.1111/j.1365-3083.1985.tb01914.x. [DOI] [PubMed] [Google Scholar]
- Gilbert D., Peulve P., Daveau M., Ripoche J., Fontaine M. Modulation of complement receptors of a human monocyte cell line, U-937, during incubation with phorbol myristate acetate: expression of an iC3b-specific receptor (CR3). Eur J Immunol. 1985 Oct;15(10):986–991. doi: 10.1002/eji.1830151005. [DOI] [PubMed] [Google Scholar]
- Hamilton A. O., Jones L., Morrison L., Whaley K. Modulation of monocyte complement synthesis by interferons. Biochem J. 1987 Mar 15;242(3):809–815. doi: 10.1042/bj2420809. [DOI] [PMC free article] [PubMed] [Google Scholar]
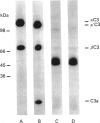
- Lachmann P. J., Oldroyd R. G., Milstein C., Wright B. W. Three rat monoclonal antibodies to human C3. Immunology. 1980 Nov;41(3):503–515. [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Pangburn M. K., Oldroyd R. G. Breakdown of C3 after complement activation. Identification of a new fragment C3g, using monoclonal antibodies. J Exp Med. 1982 Jul 1;156(1):205–216. doi: 10.1084/jem.156.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Littman B. H., Hall R. E., Muchmore A. V. Lymphokine and phorbol (PMA) regulation of complement (C2) synthesis using U937. Cell Immunol. 1983 Feb 15;76(1):189–195. doi: 10.1016/0008-8749(83)90360-x. [DOI] [PubMed] [Google Scholar]
- Malhotra V., Sim R. B. Expression of complement factor H on the cell surface of the human monocytic cell line U937. Eur J Immunol. 1985 Sep;15(9):935–941. doi: 10.1002/eji.1830150913. [DOI] [PubMed] [Google Scholar]
- Melchers F., Erdei A., Corbel C., Leptin M., Schulz T., Dierich M. P. Cell cycle control of activated, synchronized murine B lymphocytes--roles of macrophages and complement C3. Mol Immunol. 1986 Nov;23(11):1173–1176. doi: 10.1016/0161-5890(86)90148-3. [DOI] [PubMed] [Google Scholar]
- Minta J. O., Isenman D. E. Biosynthesis of the third component of complement by the human monocyte-like cell line, U-937. Mol Immunol. 1987 Oct;24(10):1105–1111. doi: 10.1016/0161-5890(87)90079-4. [DOI] [PubMed] [Google Scholar]
- Minta J. O., Pambrun L. In vitro induction of cytologic and functional differentiation of the immature human monocytelike cell line U-937 with phorbol myristate acetate. Am J Pathol. 1985 Apr;119(1):111–126. [PMC free article] [PubMed] [Google Scholar]
- Morgan E. L. Modulation of the immune response by anaphylatoxins. Complement. 1986;3(3):128–136. doi: 10.1159/000467890. [DOI] [PubMed] [Google Scholar]
- Pepys M. B. Role of complement in induction of antibody production in vivo. Effect of cobra factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J Exp Med. 1974 Jul 1;140(1):126–145. doi: 10.1084/jem.140.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahl J. W., Porter R. R. Allotype-related sequence variation of the heavy chain of rabbit immunoglobulin G. Biochem J. 1968 May;107(6):753–763. doi: 10.1042/bj1070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo B. P., Dattwyler R. J., Kaplan A. P., Ghebrehiwet B. Synthesis of C1 inhibitor (C1-INA) by a human monocyte-like cell line, U937. J Immunol. 1985 Aug;135(2):1313–1319. [PubMed] [Google Scholar]
- Reboul A., Thielens N., Villiers M. B., Colomb M. G. Purification of human complement subcomponent C4. C4 cleavage by C1s. FEBS Lett. 1979 Jul 1;103(1):156–161. doi: 10.1016/0014-5793(79)81271-5. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Medof M. E. Membrane complement receptors specific for bound fragments of C3. Adv Immunol. 1985;37:217–267. doi: 10.1016/s0065-2776(08)60341-7. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Twose T. M., Paterson D. S., Sim E. The covalent-binding reaction of complement component C3. Biochem J. 1981 Jan 1;193(1):115–127. doi: 10.1042/bj1930115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Thielens N. M., Villiers M. B., Reboul A., Villiers C. L., Colomb M. G. Human complement subcomponent C2: purification and proteolytic cleavage in fluid phase by C1s, C1r2-C1s2 and C1. FEBS Lett. 1982 May 3;141(1):19–24. doi: 10.1016/0014-5793(82)80006-9. [DOI] [PubMed] [Google Scholar]
- Venkatesh Y. P., Levine R. P. The esterase-like activity of covalently bound human third complement protein. Mol Immunol. 1988 Sep;25(9):821–828. doi: 10.1016/0161-5890(88)90118-6. [DOI] [PubMed] [Google Scholar]
- Venkatesh Y. P., Minich T. M., Law S. K., Levine R. P. Natural release of covalently bound C3b from cell surfaces and the study of this phenomenon in the fluid-phase system. J Immunol. 1984 Mar;132(3):1435–1439. [PubMed] [Google Scholar]
- Villiers M. B., Ward R. H., Lachmann P. J. Antibody-dependent cytotoxicity on chicken red blood cell targets mediated by the U937 cell line. Immunology. 1987 Jul;61(3):277–282. [PMC free article] [PubMed] [Google Scholar]
- Zimmer B., Hartung H. P., Scharfenberger G., Bitter-Suermann D., Hadding U. Quantitative studies of the secretion of complement component C3 by resident, elicited and activated macrophages. Comparison with C2, C4 and lysosomal enzyme release. Eur J Immunol. 1982 May;12(5):426–430. doi: 10.1002/eji.1830120513. [DOI] [PubMed] [Google Scholar]