Abstract
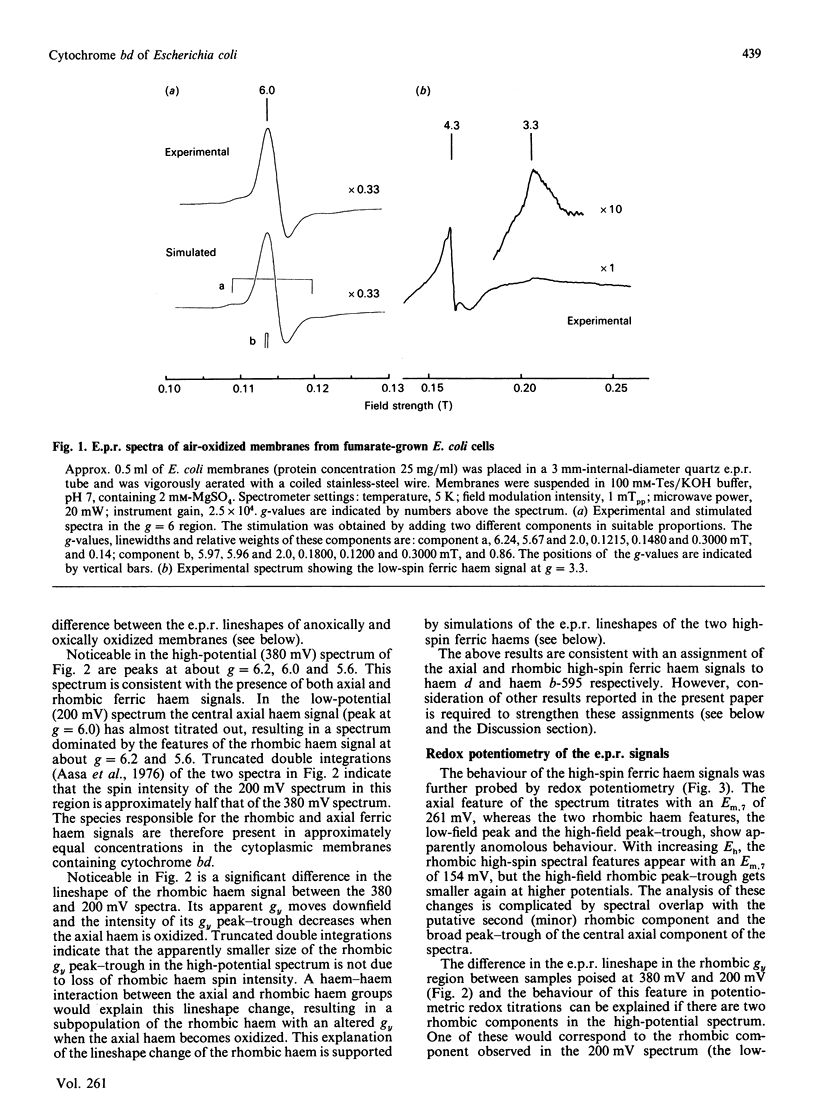
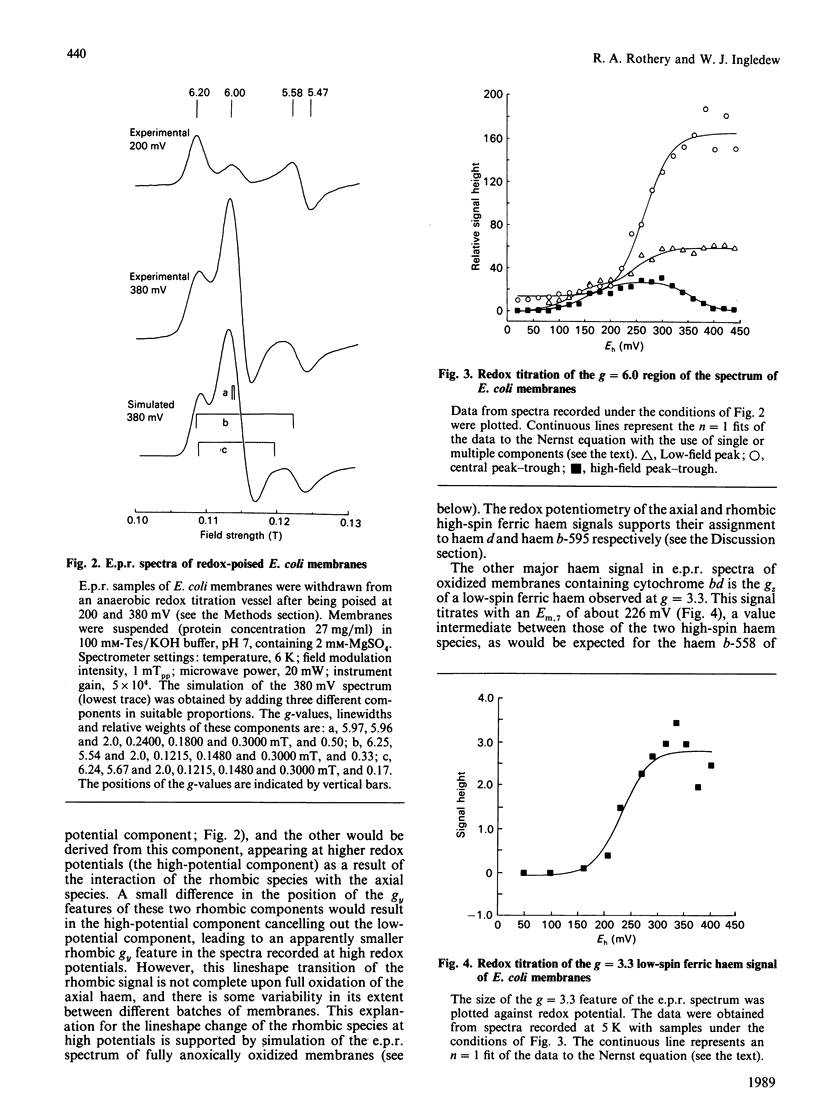
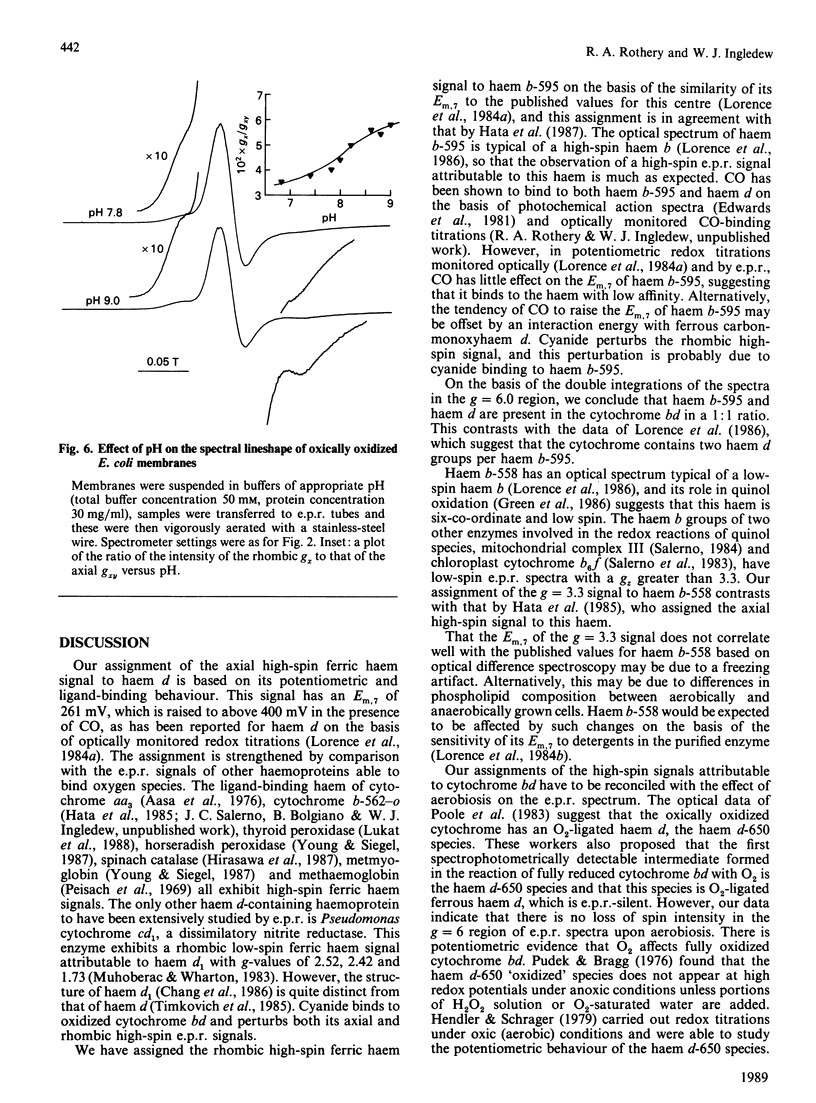
The e.p.r. signals attributable to a cytochrome bd-type ubiquinol:O2 oxidoreductase (cytochrome b-558-b-595-d) were studied in a cytoplasmic membrane preparation of Escherichia coli that had been grown on glycerol with fumarate as respiratory-chain oxidant. Two major high-spin ferric haem signals were resolved on the basis of their potentiometric behaviour: a rhombic high-spin species (gx = 6.25, gy = 5.54) was assigned to haem b-595, and an axial high-spin (gx = 5.97, gy = 5.96) species was assigned to the haem d. These signals titrated with Em.7 values of 154 and 261 mV respectively, corresponding closely to optically determined values for haem b-595 and haem d. At high potentials (greater than 300 mV) the rhombic species attributable to haem b-595 underwent a partial transition to a second rhombic species with g-values of 6.24 (gx) and 5.67 (gy). The high-spin ferric haem spectra were affected by O2, CO, cyanide and pH. A low-spin ferric haem signal was observed at g = 3.3 (gz), which titrated with an Em.7 of 226 mV, and this was assigned to haem b-558. The data support a model for cytochrome bd with two ligand-binding sites, a single haem d and a single haem b-595.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasa R., Albracht P. J., Falk K. E., Lanne B., Vänngard T. EPR signals from cytochrome c oxidase. Biochim Biophys Acta. 1976 Feb 13;422(2):260–272. doi: 10.1016/0005-2744(76)90137-6. [DOI] [PubMed] [Google Scholar]
- Chang C. K., Timkovich R., Wu W. Evidence that heme d1 is a 1,3-porphyrindione. Biochemistry. 1986 Dec 30;25(26):8447–8453. doi: 10.1021/bi00374a019. [DOI] [PubMed] [Google Scholar]
- Dutton P. L. Redox potentiometry: determination of midpoint potentials of oxidation-reduction components of biological electron-transfer systems. Methods Enzymol. 1978;54:411–435. doi: 10.1016/s0076-6879(78)54026-3. [DOI] [PubMed] [Google Scholar]
- Edwards C., Beer S., Siviram A., Chance B. Photochemical action spectra of bacterial a- and o-type oxidases using a dye laser. FEBS Lett. 1981 Jun 15;128(2):205–207. doi: 10.1016/0014-5793(81)80081-6. [DOI] [PubMed] [Google Scholar]
- Green G. N., Lorence R. M., Gennis R. B. Specific overproduction and purification of the cytochrome b558 component of the cytochrome d complex from Escherichia coli. Biochemistry. 1986 May 6;25(9):2309–2314. doi: 10.1021/bi00357a002. [DOI] [PubMed] [Google Scholar]
- Hata-Tanaka A., Matsuura K., Itoh S., Anraku Y. Electron flow and heme-heme interaction between cytochromes b-558, b-595 and d in a terminal oxidase of Escherichia coli. Biochim Biophys Acta. 1987 Sep 10;893(2):289–295. doi: 10.1016/0005-2728(87)90050-8. [DOI] [PubMed] [Google Scholar]
- Hata A., Kirino Y., Matsuura K., Itoh S., Hiyama T., Konishi K., Kita K., Anraku Y. Assignment of ESR signals of Escherichia coli terminal oxidase complexes. Biochim Biophys Acta. 1985 Oct 29;810(1):62–72. doi: 10.1016/0005-2728(85)90206-3. [DOI] [PubMed] [Google Scholar]
- Hendler R. W., Shrager R. I. Potentiometric analysis of Escherichia coli cytochromes in the optical absorbance range of 500 nm to 700 nm. J Biol Chem. 1979 Nov 25;254(22):11288–11299. [PubMed] [Google Scholar]
- Ingledew W. J. The electron transport chain of Escherichia coli grown anaerobically with fumarate as terminal electron acceptor: an electron paramagnetic resonance study. J Gen Microbiol. 1983 Jun;129(6):1651–1659. doi: 10.1099/00221287-129-6-1651. [DOI] [PubMed] [Google Scholar]
- Kita K., Konishi K., Anraku Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J Biol Chem. 1984 Mar 10;259(5):3375–3381. [PubMed] [Google Scholar]
- Kumar C., Poole R. K., Salmon I., Chance B. The oxygen reaction of the cytochrome d-terminated respiratory chain of Escherichia coli at sub-zero temperatures. Kinetic resolution by EPR spectroscopy of two high-spin cytochromes. FEBS Lett. 1985 Oct 14;190(2):227–231. doi: 10.1016/0014-5793(85)81289-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lorence R. M., Green G. N., Gennis R. B. Potentiometric analysis of the cytochromes of an Escherichia coli mutant strain lacking the cytochrome d terminal oxidase complex. J Bacteriol. 1984 Jan;157(1):115–121. doi: 10.1128/jb.157.1.115-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorence R. M., Koland J. G., Gennis R. B. Coulometric and spectroscopic analysis of the purified cytochrome d complex of Escherichia coli: evidence for the identification of "cytochrome a1" as cytochrome b595. Biochemistry. 1986 May 6;25(9):2314–2321. doi: 10.1021/bi00357a003. [DOI] [PubMed] [Google Scholar]
- Lorence R. M., Miller M. J., Borochov A., Faiman-Weinberg R., Gennis R. B. Effects of pH and detergent on the kinetic and electrochemical properties of the purified cytochrome d terminal oxidase complex of Escherichia coli. Biochim Biophys Acta. 1984 Oct 23;790(2):148–153. doi: 10.1016/0167-4838(84)90218-8. [DOI] [PubMed] [Google Scholar]
- Lukat G. S., Jabro M. N., Rodgers K. R., Goff H. M. Electron paramagnetic resonance spectroscopy of thyroid peroxidase. Biochim Biophys Acta. 1988 Jun 13;954(3):265–270. doi: 10.1016/0167-4838(88)90081-7. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Gennis R. B. The purification and characterization of the cytochrome d terminal oxidase complex of the Escherichia coli aerobic respiratory chain. J Biol Chem. 1983 Aug 10;258(15):9159–9165. [PubMed] [Google Scholar]
- Muhoberac B. B., Wharton D. C. Electron paramagnetic resonance study of the interaction of some anionic ligands with oxidized Pseudomonas cytochrome oxidase. J Biol Chem. 1983 Mar 10;258(5):3019–3027. [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Wittenberg B. A., Wittenberg J. B., Kampa L. Hemoglobin A: an electron paramagnetic resonance study of the effects of interchain contacts on the heme symmetry of high-spin and low-spin derivatives of ferric alpha chains. Proc Natl Acad Sci U S A. 1969 Jul;63(3):934–939. doi: 10.1073/pnas.63.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K., Kumar C., Salmon I., Chance B. The 650 and chromophore in Escherichia coli is an 'oxy-' or oxygenated compound, not the oxidized form of cytochrome oxidase d: an hypothesis. J Gen Microbiol. 1983 May;129(5):1335–1344. doi: 10.1099/00221287-129-5-1335. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Scott R. I., Chance B. The light-reversible binding of carbon monoxide to cytochrome a1 in Escherichia coli K12. J Gen Microbiol. 1981 Aug;125(2):431–438. doi: 10.1099/00221287-125-2-431. [DOI] [PubMed] [Google Scholar]
- Pudek M. R., Bragg P. D. Redox potentials of the cytochromes in the respiratory chain of aerobically grown Escherichia coli. Arch Biochem Biophys. 1976 Jun;174(2):546–552. doi: 10.1016/0003-9861(76)90382-9. [DOI] [PubMed] [Google Scholar]
- Reid G. A., Ingledew W. J. Characterization and phenotypic control of the cytochrome content of Escherichia coli. Biochem J. 1979 Aug 15;182(2):465–472. doi: 10.1042/bj1820465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothery R. A., Houston A. M., Ingledew W. J. The respiratory chain of anaerobically grown Escherichia coli: reactions with nitrite and oxygen. J Gen Microbiol. 1987 Nov;133(11):3247–3255. doi: 10.1099/00221287-133-11-3247. [DOI] [PubMed] [Google Scholar]
- Salerno J. C. Cytochrome electron spin resonance line shapes, ligand fields, and components stoichiometry in ubiquinol-cytochrome c oxidoreductase. J Biol Chem. 1984 Feb 25;259(4):2331–2336. [PubMed] [Google Scholar]
- Young L. J., Siegel L. M. On the reaction of ferric heme proteins with nitrite and sulfite. Biochemistry. 1988 Apr 19;27(8):2790–2800. doi: 10.1021/bi00408a020. [DOI] [PubMed] [Google Scholar]


