Abstract
The first two steps of urea synthesis in liver of marine elasmobranchs involve formation of glutamine from ammonia and of carbamoyl phosphate from glutamine, catalysed by glutamine synthetase and carbamoyl-phosphate synthetase, respectively [Anderson & Casey (1984) J. Biol. Chem. 259, 456-462]; both of these enzymes are localized exclusively in the mitochondrial matrix. The objective of this study was to establish the enzymology of carbamoyl phosphate formation and utilization for pyrimidine nucleotide biosynthesis in Squalus acanthias (spiny dogfish), a representative elasmobranch. Aspartate carbamoyltransferase could not be detected in liver of dogfish. Spleen extracts, however, had glutamine-dependent carbamoyl-phosphate synthetase, aspartate carbamoyltransferase, dihydro-orotase, and glutamine synthetase activities, all localized in the cytosol; dihydro-orotate dehydrogenase, orotate phosphoribosyltransferase, and orotidine-5'-decarboxylase activities were also present. Except for glutamine synthetase, the levels of all activities were very low. The carbamoyl-phosphate synthetase activity is inhibited by UTP and is activated by 5-phosphoribosyl 1-pyrophosphate. The first three enzyme activities of the pyrimidine pathway were eluted in distinctly different positions during gel filtration chromatography under a number of different conditions; although complete proteolysis of inter-domain regions of a multifunctional complex during extraction cannot be excluded, the evidence suggests that in dogfish, in contrast to mammalian species, these three enzymes of the pyrimidine pathway exist as individual polypeptide chains. These results: (1) establish that dogfish express two different glutamine-dependent carbamoyl-phosphate synthetase activities, (2) confirm the report [Smith, Ritter & Campbell (1987) J. Biol. Chem. 262, 198-202] that dogfish express two different glutamine synthetases, and (3) provide indirect evidence that glutamine may not be available in liver for biosynthetic reactions other than urea formation.
Full text
PDF


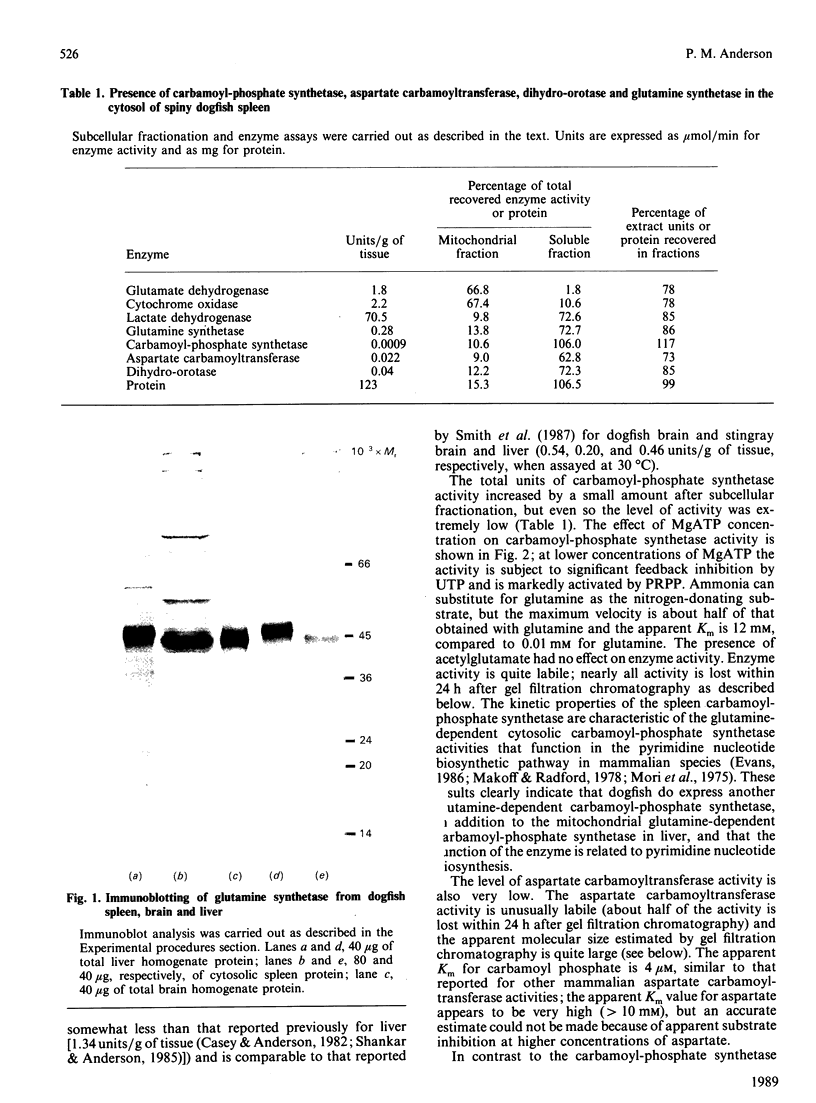
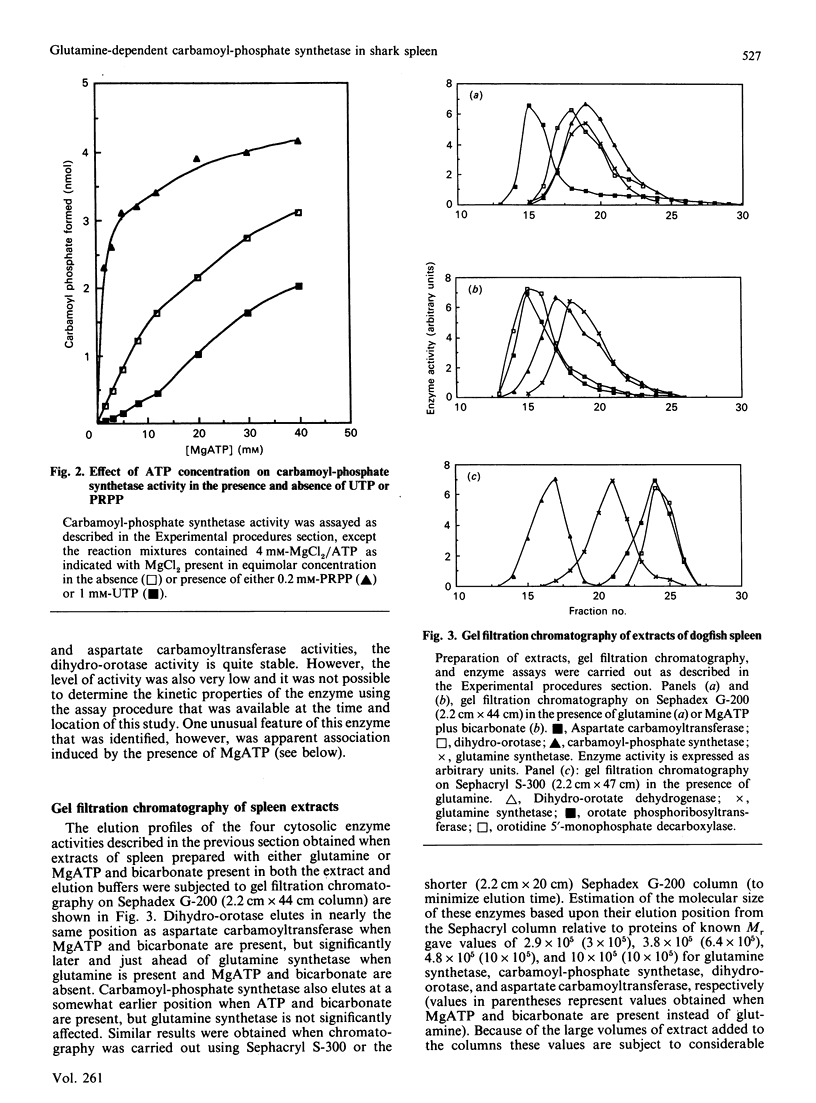


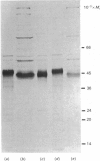
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M. A glutamine- and N-acetyl-L-glutamate-dependent carbamyl phosphate synthetase activity in the teleost Micropterus salmoides. Comp Biochem Physiol B. 1976;54(2):261–263. doi: 10.1016/0305-0491(76)90154-1. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Casey C. A. Glutamine-dependent synthesis of citrulline by isolated hepatic mitochondria from Squalus acanthias. J Biol Chem. 1984 Jan 10;259(1):456–462. [PubMed] [Google Scholar]
- Anderson P. M. Glutamine- and N-acetylglutamate-dependent carbamoyl phosphate synthetase in elasmobranchs. Science. 1980 Apr 18;208(4441):291–293. doi: 10.1126/science.6245445. [DOI] [PubMed] [Google Scholar]
- Anderson P. M. Purification and properties of the glutamine- and N-acetyl-L-glutamate-dependent carbamoyl phosphate synthetase from liver of Squalus acanthias. J Biol Chem. 1981 Dec 10;256(23):12228–12238. [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Oya H. Regulatory properties of carbamoyl-phosphate synthetase II from the parasitic protozoan Crithidia fasciculata. Comp Biochem Physiol B. 1987;87(4):655–658. doi: 10.1016/0305-0491(87)90369-5. [DOI] [PubMed] [Google Scholar]
- Bodine A. B., Luer C. A., Gangjee S. A comparative study of monooxygenase activity in elasmobranchs and mammals: activation of the model pro-carcinogen aflatoxin B1 by liver preparations of calf, nurse shark and clearnose skate. Comp Biochem Physiol C. 1985;82(2):255–257. doi: 10.1016/0742-8413(85)90159-8. [DOI] [PubMed] [Google Scholar]
- Campbell J. W., Vorhaben J. E., Smith D. D., Jr Uricoteley:its nature and origin during the evolution of tetrapod vertebrates. J Exp Zool. 1987 Sep;243(3):349–363. doi: 10.1002/jez.1402430302. [DOI] [PubMed] [Google Scholar]
- Carrey E. A. Nucleotide ligands protect the inter-domain regions of the multifunctional polypeptide CAD against limited proteolysis, and also stabilize the thermolabile part-reactions of the carbamoyl-phosphate synthase II domains within the CAD polypeptide. Biochem J. 1986 Jun 1;236(2):327–335. doi: 10.1042/bj2360327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey C. A., Anderson P. M. Glutamine- and N-acetyl-L-glutamate-dependent carbamoyl phosphate synthetase from Micropterus salmoides. Purification, properties, and inhibition by glutamine analogs. J Biol Chem. 1983 Jul 25;258(14):8723–8732. [PubMed] [Google Scholar]
- Casey C. A., Anderson P. M. Subcellular location of glutamine synthetase and urea cycle enzymes in liver of spiny dogfish (Squalus acanthias). J Biol Chem. 1982 Jul 25;257(14):8449–8453. [PubMed] [Google Scholar]
- Casey C. A., Anderson P. M. Submitochondrial localization of arginase and other enzymes associated with urea synthesis and nitrogen metabolism, in liver of Squalus acanthias. Comp Biochem Physiol B. 1985;82(2):307–315. doi: 10.1016/0305-0491(85)90246-9. [DOI] [PubMed] [Google Scholar]
- Christopherson R. I., Jones M. E. Interconversion of carbamayl-L-aspartate and L-dihydroorotate by dihydroorotase from mouse Ehrlich ascites carcinoma. J Biol Chem. 1979 Dec 25;254(24):12506–12512. [PubMed] [Google Scholar]
- Davidson J. N., Rumsby P. C., Tamaren J. Organization of a multifunctional protein in pyrimidine biosynthesis. Analyses of active, tryptic fragments. J Biol Chem. 1981 May 25;256(10):5220–5225. [PubMed] [Google Scholar]
- Grayson D. R., Evans D. R. The isolation and characterization of the aspartate transcarbamylase domain of the multifunctional protein, CAD. J Biol Chem. 1983 Apr 10;258(7):4123–4129. [PubMed] [Google Scholar]
- Grayson D. R., Lee L., Evans D. R. Immunochemical analysis of the domain structure of CAD, the multifunctional protein that initiates pyrimidine biosynthesis in mammalian cells. J Biol Chem. 1985 Dec 15;260(29):15840–15849. [PubMed] [Google Scholar]
- Hoogenraad N. J., Levine R. L., Kretchmer N. Copurification of carbamoyl phosphate synthetase and aspartate transcarbamoylase from mouse spleen. Biochem Biophys Res Commun. 1971 Aug 20;44(4):981–988. doi: 10.1016/0006-291x(71)90808-4. [DOI] [PubMed] [Google Scholar]
- Jones M. E. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu Rev Biochem. 1980;49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]
- Kelly R. E., Mally M. I., Evans D. R. The dihydroorotase domain of the multifunctional protein CAD. Subunit structure, zinc content, and kinetics. J Biol Chem. 1986 May 5;261(13):6073–6083. [PubMed] [Google Scholar]
- Lee A., Langer R. Shark cartilage contains inhibitors of tumor angiogenesis. Science. 1983 Sep 16;221(4616):1185–1187. doi: 10.1126/science.6193581. [DOI] [PubMed] [Google Scholar]
- Makoff A. J., Radford A. Genetics and biochemistry of carbamoyl phosphate biosynthesis and its utilization in the pyrimidine biosynthetic pathway. Microbiol Rev. 1978 Jun;42(2):307–328. doi: 10.1128/mr.42.2.307-328.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreadith R. W., Lehninger A. L. Purification, kinetic behavior, and regulation of NAD(P)+ malic enzyme of tumor mitochondria. J Biol Chem. 1984 May 25;259(10):6222–6227. [PubMed] [Google Scholar]
- Mori M., Ishida H., Tatibana M. Aggregation states and catalytic properties of the multienzyme complex catalyzing the initial steps of pyrimidine biosynthesis in rat liver. Biochemistry. 1975 Jun 17;14(12):2622–2630. doi: 10.1021/bi00683a010. [DOI] [PubMed] [Google Scholar]
- Mori M., Tatibana M. Dissociation by elastase digestion of enzyme complex catalyzing the initial steps of pyrimidine biosynthesis in rat liver. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1525–1531. doi: 10.1016/0006-291x(73)91159-5. [DOI] [PubMed] [Google Scholar]
- Mukherjee T., Ray M., Bhaduri A. Aspartate transcarbamylase from Leishmania donovani. A discrete, nonregulatory enzyme as a potential chemotherapeutic site. J Biol Chem. 1988 Jan 15;263(2):708–713. [PubMed] [Google Scholar]
- Powers C. N., Pierson D. L. Stabilization and purification of ornithine transcarbamylase from Neisseria gonorrhoeae. J Bacteriol. 1980 Feb;141(2):544–549. doi: 10.1128/jb.141.2.544-549.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A. R., Medina M. A., Márquez J., Sánchez-Jiménez F. M., Núez de Castro I. Contribution by host tissues to circulating glutamine in mice inoculated with Ehrlich ascites tumor cells. Cancer Res. 1988 Mar 15;48(6):1551–1553. [PubMed] [Google Scholar]
- Ratner S. Enzymes of arginine and urea synthesis. Adv Enzymol Relat Areas Mol Biol. 1973;39:1–90. doi: 10.1002/9780470122846.ch1. [DOI] [PubMed] [Google Scholar]
- Shankar R. A., Anderson P. M. Purification and properties of glutamine synthetase from liver of Squalus acanthias. Arch Biochem Biophys. 1985 May 15;239(1):248–259. doi: 10.1016/0003-9861(85)90833-1. [DOI] [PubMed] [Google Scholar]
- Smith D. D., Jr, Ritter N. M., Campbell J. W. Glutamine synthetase isozymes in elasmobranch brain and liver tissues. J Biol Chem. 1987 Jan 5;262(1):198–202. [PubMed] [Google Scholar]
- Smith J. M., Kelln R. A., O'Donovan G. A. Repression and derepression of the enzymes of the pyrimidine biosynthetic pathway in Salmonella typhimurium. J Gen Microbiol. 1980 Nov;121(1):27–38. doi: 10.1099/00221287-121-1-27. [DOI] [PubMed] [Google Scholar]
- Tampitag S., O'Sullivan W. J. Enzymes of pyrimidine biosynthesis in Crithidia luciliae. Mol Biochem Parasitol. 1986 May;19(2):125–134. doi: 10.1016/0166-6851(86)90117-9. [DOI] [PubMed] [Google Scholar]