Abstract
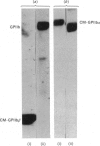
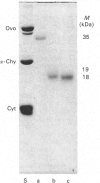
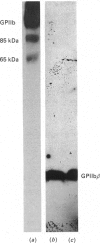
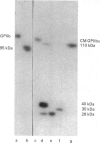
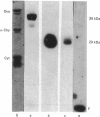
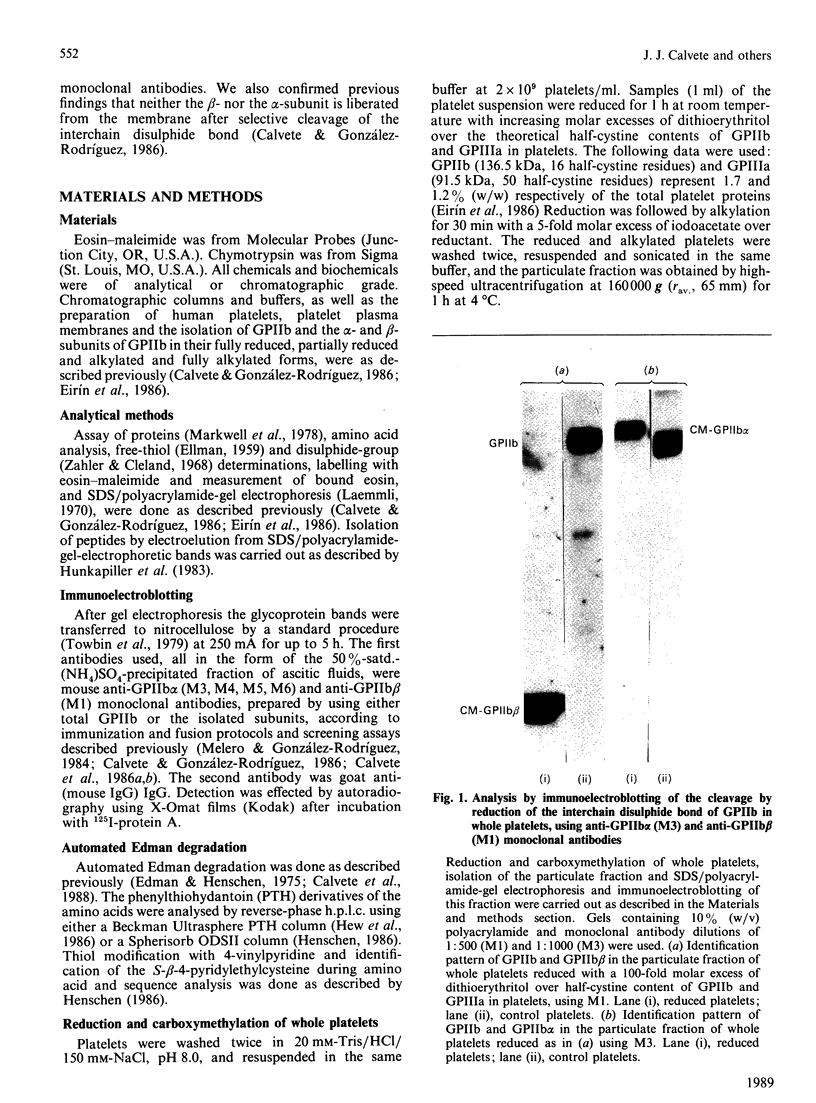
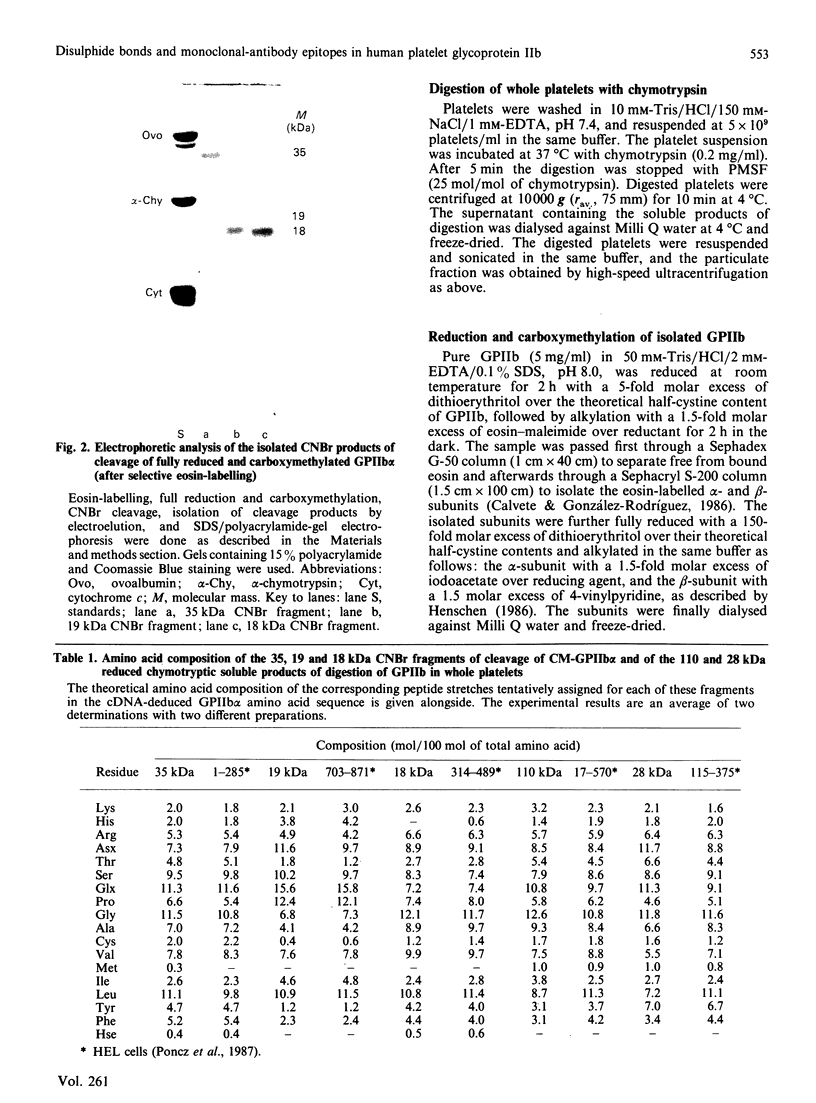
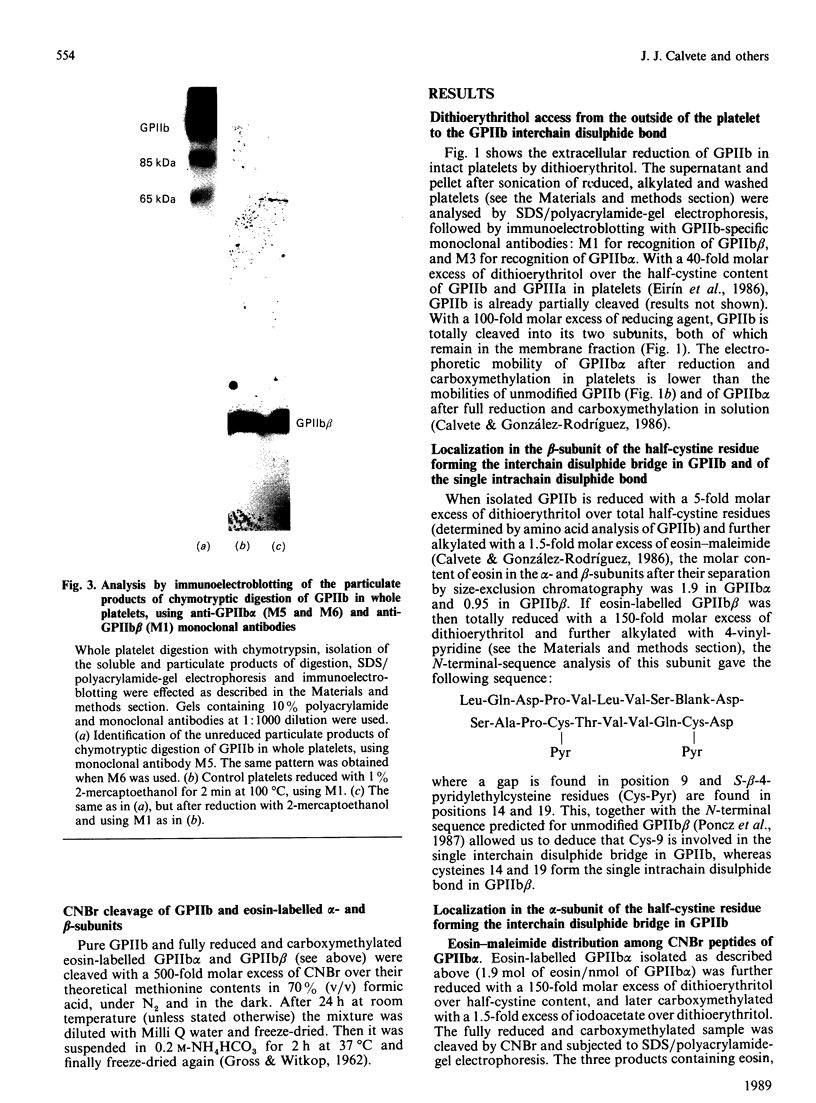
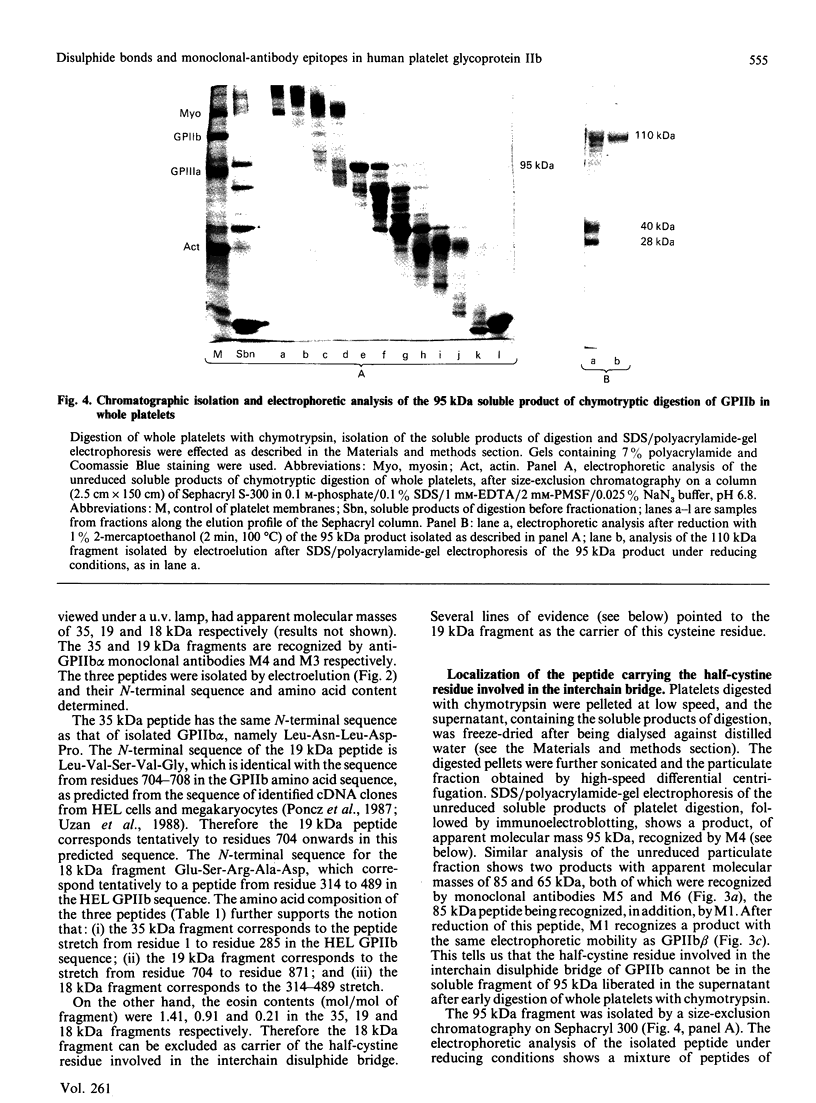
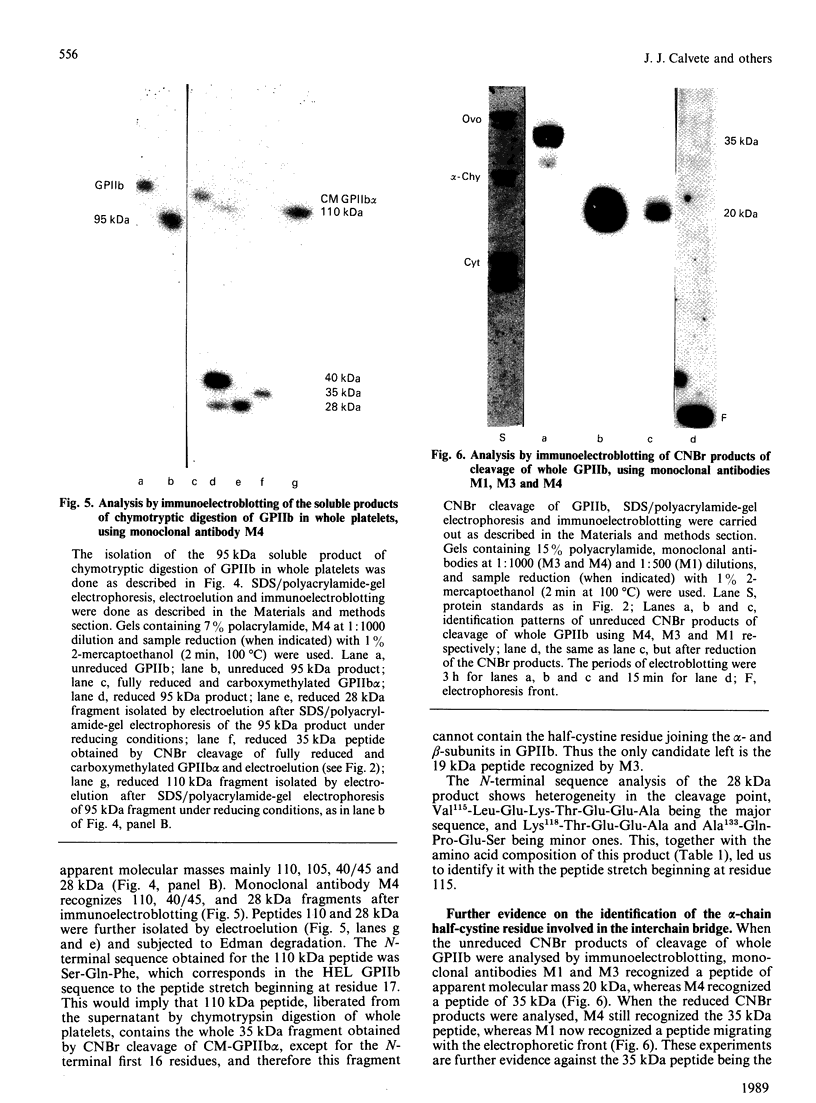
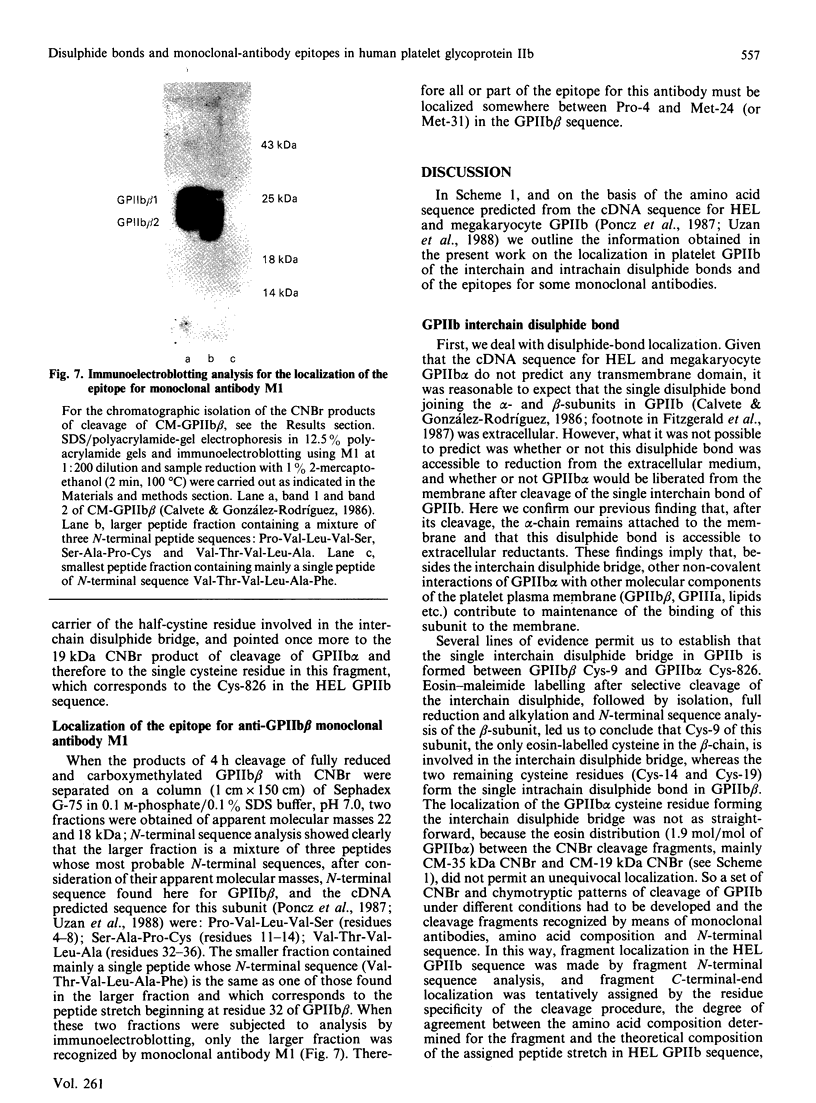
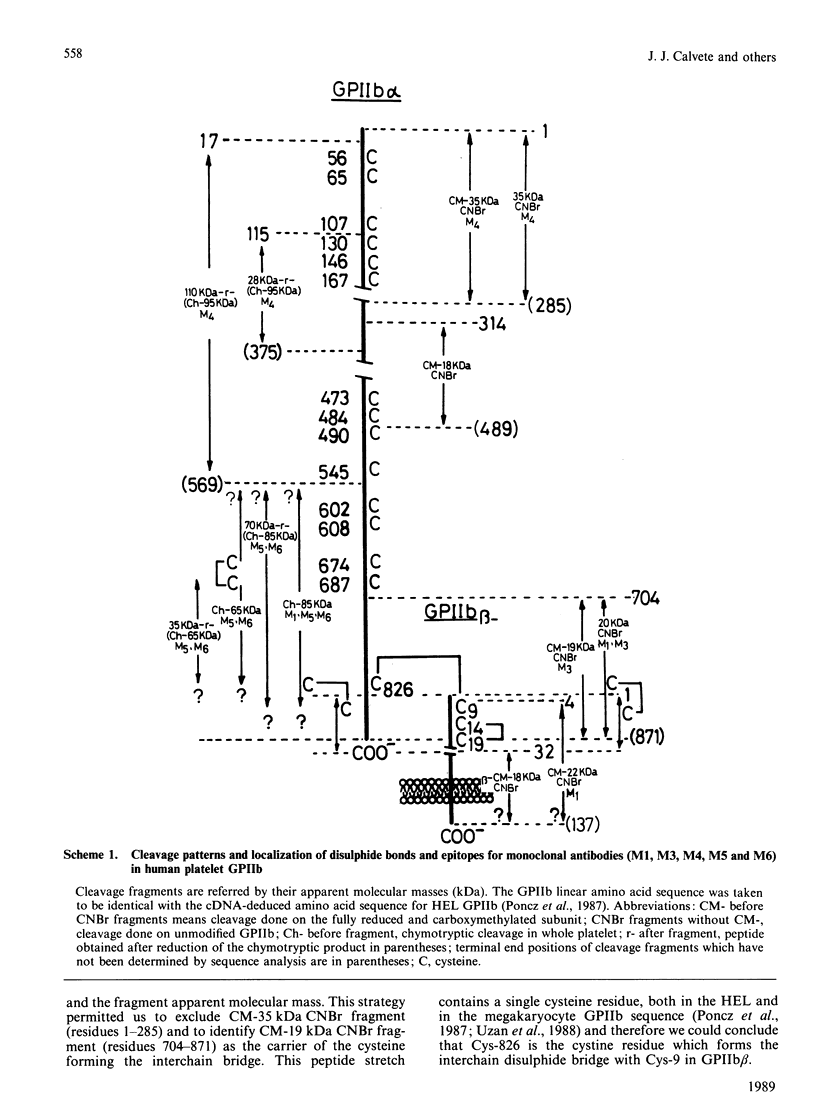
The single interchain disulphide bond in platelet glycoprotein IIb (GPIIb) is accessible to extracellular reductants, and selective cleavage does not liberate GPIIb alpha from platelet plasma membrane, confirming that non-covalent interactions contribute to maintaining attachment of this subunit to the membrane. Eosin-maleimide labelling of isolated GPIIb after selective cleavage of this interchain disulphide bond, followed by full reduction and alkylation, CNBr cleavage, and analysis of the cleavage products allowed us to establish that this interchain disulphide bridge is formed between GPIIb beta (GPIIb beta-subunit) Cys-9 and GPIIb alpha Cys-826, and this conclusion was confirmed by independent routes. The other two cysteines of GPIIb beta (Cys-14 and Cys-19) form the single intrachain disulphide bond in this subunit. Last, the intrachain disulphides in GPIIb alpha (GPIIb alpha-subunit) are distributed in four main peptide domains which are not disulphide-bonded among themselves. The linear epitope for monoclonal antibody M1 is localized between Pro-4 and Met-24 (or Met-31) of GPIIb beta. The linear epitope for M3 is situated between Cys-826 and the C-terminus of GPIIb alpha. The M4 epitope is also linear and localized somewhere between residues 115 and 285 of GPIIb alpha. Finally, the epitopes for M5 and M6 are somewhere between Cys-608 and Met-704, within a 35 kDa membrane-bound chymotryptic product of digestion of GPIIb in whole platelets. The N-terminal amino acid sequences determined for eight different cleavage products of GPIIb alpha and GPIIb beta agree with the corresponding amino acid sequences predicted by cDNA sequence for human-erythroleukaemic-cell GPIIb [Poncz, Eisman, Heindenreich, Silver, Vilaire, Surrey, Schwartz & Bennett (1987) J. Biol. Chem. 262, 8476-8482].
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray P. F., Rosa J. P., Lingappa V. R., Kan Y. W., McEver R. P., Shuman M. A. Biogenesis of the platelet receptor for fibrinogen: evidence for separate precursors for glycoproteins IIb and IIIa. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1480–1484. doi: 10.1073/pnas.83.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete J. J., González-Rodríguez J. Isolation and biochemical characterization of the alpha- and beta-subunits of glycoprotein IIb of human platelet plasma membrane. Biochem J. 1986 Nov 15;240(1):155–161. doi: 10.1042/bj2400155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete J. J., Henschen A., González-Rodríguez J. Complete localization of the intrachain disulphide bonds and the N-glycosylation points in the alpha-subunit of human platelet glycoprotein IIb. Biochem J. 1989 Jul 15;261(2):561–568. doi: 10.1042/bj2610561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete J. J., Rivas G., Maruri M., Alvarez M. V., McGregor J. L., Hew C. L., Gonzalez-Rodriguez J. Tryptic digestion of human GPIIIa. Isolation and biochemical characterization of the 23 kDa N-terminal glycopeptide carrying the antigenic determinant for a monoclonal antibody (P37) which inhibits platelet aggregation. Biochem J. 1988 Mar 15;250(3):697–704. doi: 10.1042/bj2500697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo I. F., Fitzgerald L. A., Steiner B., Rall S. C., Jr, Bekeart L. S., Phillips D. R. Platelet glycoproteins IIb and IIIa: evidence for a family of immunologically and structurally related glycoproteins in mammalian cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8351–8355. doi: 10.1073/pnas.83.21.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duperray A., Berthier R., Chagnon E., Ryckewaert J. J., Ginsberg M., Plow E., Marguerie G. Biosynthesis and processing of platelet GPIIb-IIIa in human megakaryocytes. J Cell Biol. 1987 Jun;104(6):1665–1673. doi: 10.1083/jcb.104.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Eirín M. T., Calvete J. J., González-Rodríguez J. New isolation procedure and further biochemical characterization of glycoproteins IIb and IIIa from human platelet plasma membrane. Biochem J. 1986 Nov 15;240(1):147–153. doi: 10.1042/bj2400147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald L. A., Poncz M., Steiner B., Rall S. C., Jr, Bennett J. S., Phillips D. R. Comparison of cDNA-derived protein sequences of the human fibronectin and vitronectin receptor alpha-subunits and platelet glycoprotein IIb. Biochemistry. 1987 Dec 15;26(25):8158–8165. doi: 10.1021/bi00399a021. [DOI] [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Hew C. L., Wang N. C., Yan S., Cai H., Sclater A., Fletcher G. L. Biosynthesis of antifreeze polypeptides in the winter flounder. Characterization and seasonal occurrence of precursor polypeptides. Eur J Biochem. 1986 Oct 15;160(2):267–272. doi: 10.1111/j.1432-1033.1986.tb09966.x. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Jennings L. K., Phillips D. R. Purification of glycoproteins IIb and III from human platelet plasma membranes and characterization of a calcium-dependent glycoprotein IIb-III complex. J Biol Chem. 1982 Sep 10;257(17):10458–10466. [PubMed] [Google Scholar]
- Kieffer N., Boizard B., Didry D., Wautier J. L., Nurden A. T. Immunochemical characterization of the platelet-specific alloantigen Leka: a comparative study with the PlA1 alloantigen. Blood. 1984 Dec;64(6):1212–1219. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung L. L., Kinoshita T., Nachman R. L. Isolation, purification, and partial characterization of platelet membrane glycoproteins IIb and IIIa. J Biol Chem. 1981 Feb 25;256(4):1994–1997. [PubMed] [Google Scholar]
- Loftus J. C., Plow E. F., Frelinger A. L., 3rd, D'Souza S. E., Dixon D., Lacy J., Sorge J., Ginsberg M. H. Molecular cloning and chemical synthesis of a region of platelet glycoprotein IIb involved in adhesive function. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7114–7118. doi: 10.1073/pnas.84.20.7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus J. C., Plow E. F., Jennings L. K., Ginsberg M. H. Alternative proteolytic processing of platelet membrane glycoprotein IIb. J Biol Chem. 1988 Aug 15;263(23):11025–11028. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Baenziger J. U., Majerus P. W. Isolation and structural characterization of the polypeptide subunits of membrane glycoprotein IIb-IIIa from human platelets. Blood. 1982 Jan;59(1):80–85. [PubMed] [Google Scholar]
- Melero J. A., Gonzalez-Rodriguez J. Preparation of monoclonal antibodies against glycoprotein IIIa of human platelets. Their effect on platelet aggregation. Eur J Biochem. 1984 Jun 1;141(2):421–427. doi: 10.1111/j.1432-1033.1984.tb08208.x. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. An abnormal platelet glycoprotein pattern in three cases of Glanzmann's thrombasthenia. Br J Haematol. 1974 Oct;28(2):253–260. doi: 10.1111/j.1365-2141.1974.tb06660.x. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Jenkins C. S., Lüscher E. F., Larrieu M. Molecular differences of exposed surface proteins on thrombasthenic platelet plasma membranes. Nature. 1975 Oct 16;257(5527):599–600. doi: 10.1038/257599a0. [DOI] [PubMed] [Google Scholar]
- Poncz M., Eisman R., Heidenreich R., Silver S. M., Vilaire G., Surrey S., Schwartz E., Bennett J. S. Structure of the platelet membrane glycoprotein IIb. Homology to the alpha subunits of the vitronectin and fibronectin membrane receptors. J Biol Chem. 1987 Jun 25;262(18):8476–8482. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usobiaga P., Calvete J. J., Saíz J. L., Eirín M. T., González-Rodríguez J. Molecular characterization of human platelet glycoproteins IIIa and IIb and the subunits of the latter. Eur Biophys J. 1987;14(4):211–218. doi: 10.1007/BF00256354. [DOI] [PubMed] [Google Scholar]
- Uzan G., Frachet P., Lajmanovich A., Prandini M. H., Denarier E., Duperray A., Loftus J., Ginsberg M., Plow E., Marguerie G. cDNA clones for human platelet GPIIb corresponding to mRNA from megakaryocytes and HEL cells. Evidence for an extensive homology to other Arg-Gly-Asp adhesion receptors. Eur J Biochem. 1988 Jan 15;171(1-2):87–93. doi: 10.1111/j.1432-1033.1988.tb13762.x. [DOI] [PubMed] [Google Scholar]
- Zahler W. L., Cleland W. W. A specific and sensitive assay for disulfides. J Biol Chem. 1968 Feb 25;243(4):716–719. [PubMed] [Google Scholar]