Abstract
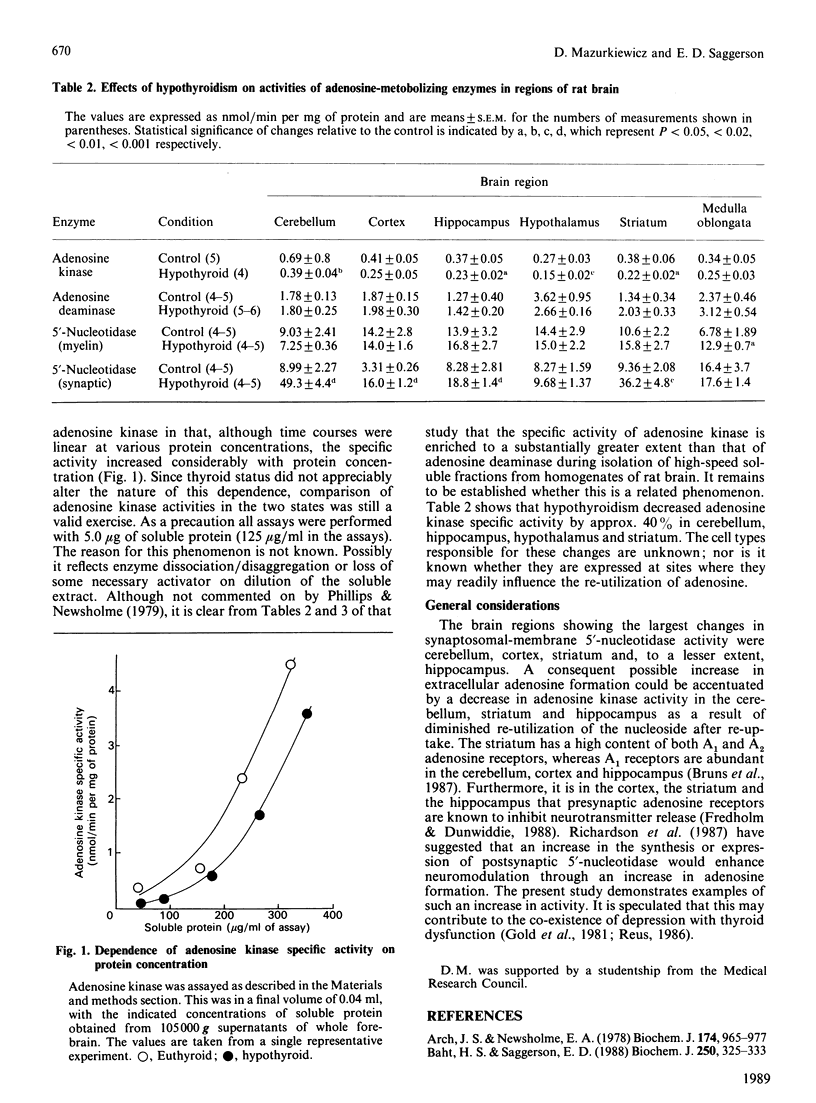
1. Rats (4 weeks old) were made hypothyroid by treatment with propylthiouracil and a low-iodine diet for a further period of 4 weeks. Synaptosomal membranes, myelin and 105,000 g soluble fractions were obtained from six regions of the brain. 2. Hypothyroidism resulted in 2-5-fold increases in membrane-bound 5'-nucleotidase activity in synaptosomal fractions obtained from cerebellum, cortex, striatum and hippocampus. By contrast, myelin 5'-nucleotidase activity was slightly increased only in the medulla oblongata. 3. Hypothyroidism did not change adenosine deaminase activity, but decreased adenosine kinase activity by approx. 40% in soluble fractions obtained from cerebellum, hippocampus, striatum and hypothalamus. 4. It is suggested that these changes in hypothyroidism, in particular the increases in 5'-nucleotidase activity, could enhance the neuromodulatory effect of adenosine to decrease neurotransmitter release.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Newsholme E. A. Activities and some properties of 5'-nucleotidase, adenosine kinase and adenosine deaminase in tissues from vertebrates and invertebrates in relation to the control of the concentration and the physiological role of adenosine. Biochem J. 1978 Sep 15;174(3):965–977. doi: 10.1042/bj1740965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baht H. S., Saggerson E. D. Comparison of triacylglycerol synthesis in rat brown and white adipocytes. Effects of hypothyroidism and streptozotocin-diabetes on enzyme activities and metabolic fluxes. Biochem J. 1988 Mar 1;250(2):325–333. doi: 10.1042/bj2500325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balázs R., Brooksbank B. W., Davison A. N., Eayrs J. T., Wilson D. A. The effect of neonatal thyroidectomy on myelination in the rat brain. Brain Res. 1969 Sep;15(1):219–232. doi: 10.1016/0006-8993(69)90321-7. [DOI] [PubMed] [Google Scholar]
- Bender A. S., Wu P. H., Phillis J. W. The characterization of [3H] adenosine uptake into rat cerebral cortical synaptosomes. J Neurochem. 1980 Sep;35(3):629–640. doi: 10.1111/j.1471-4159.1980.tb03702.x. [DOI] [PubMed] [Google Scholar]
- Bender A. S., Wu P. H., Phillis J. W. The rapid uptake and release of [3H]adenosine by rat cerebral cortical synaptosomes. J Neurochem. 1981 Feb;36(2):651–660. doi: 10.1111/j.1471-4159.1981.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Bisserbe J. C., Patel J., Marangos P. J. Autoradiographic localization of adenosine uptake sites in rat brain using [3H]nitrobenzylthioinosine. J Neurosci. 1985 Feb;5(2):544–550. doi: 10.1523/JNEUROSCI.05-02-00544.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R. F., Clark J. B. A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochem J. 1978 Nov 15;176(2):365–370. doi: 10.1042/bj1760365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammer W., Sirota S. R., Zimmerman T. R., Jr, Norton W. T. 5'-nucleotidase in rat brain myelin. J Neurochem. 1980 Aug;35(2):367–373. doi: 10.1111/j.1471-4159.1980.tb06273.x. [DOI] [PubMed] [Google Scholar]
- Casadó V., Mallol J., Bozal J. Localization of 5'-nucleotidase in bovine brain myelin fraction and myelin subfractions. Neurochem Res. 1988 Apr;13(4):359–368. doi: 10.1007/BF00972486. [DOI] [PubMed] [Google Scholar]
- Chohan P., Carpenter C., Saggerson E. D. Changes in the anti-lipolytic action and binding to plasma membranes of N6-L-phenylisopropyladenosine in adipocytes from starved and hypothyroid rats. Biochem J. 1984 Oct 1;223(1):53–59. doi: 10.1042/bj2230053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Prada M., Pletscher A. Isolated 5-hydroxytryptamine organelles of rabbit blood platelets: physiological properties and drug-induced changes. Br J Pharmacol. 1968 Nov;34(3):591–597. doi: 10.1111/j.1476-5381.1968.tb08487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:29–46. [PubMed] [Google Scholar]
- Dolphin A. C., Forda S. R., Scott R. H. Calcium-dependent currents in cultured rat dorsal root ganglion neurones are inhibited by an adenosine analogue. J Physiol. 1986 Apr;373:47–61. doi: 10.1113/jphysiol.1986.sp016034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Dunwiddie T. V. How does adenosine inhibit transmitter release? Trends Pharmacol Sci. 1988 Apr;9(4):130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Hedqvist P. Modulation of neurotransmission by purine nucleotides and nucleosides. Biochem Pharmacol. 1980 Jun 15;29(12):1635–1643. doi: 10.1016/0006-2952(80)90117-3. [DOI] [PubMed] [Google Scholar]
- Geffen L. B., Livett B. G. Synaptic vesicles in sympathetic neurons. Physiol Rev. 1971 Jan;51(1):98–157. doi: 10.1152/physrev.1971.51.1.98. [DOI] [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966 Aug;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Gold M. S., Pottash A. L., Extein I. Hypothyroidism and depression. Evidence from complete thyroid function evaluation. JAMA. 1981 May 15;245(19):1919–1922. doi: 10.1001/jama.245.19.1919. [DOI] [PubMed] [Google Scholar]
- Goodman R. R., Synder S. H. Autoradiographic localization of adenosine receptors in rat brain using [3H]cyclohexyladenosine. J Neurosci. 1982 Sep;2(9):1230–1241. doi: 10.1523/JNEUROSCI.02-09-01230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann D., Reddington M., Kreutzberg G. W. Subcellular localization of 5'-nucleotidase in rat brain. J Neurochem. 1984 Oct;43(4):971–978. doi: 10.1111/j.1471-4159.1984.tb12832.x. [DOI] [PubMed] [Google Scholar]
- Itoh R., Oka J., Ozasa H. Regulation of rat heart cytosol 5'-nucleotidase by adenylate energy charge. Biochem J. 1986 May 1;235(3):847–851. doi: 10.1042/bj2350847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal Z., Saggerson E. D. Enzymes involved in adenosine metabolism in rat white and brown adipocytes. Effects of streptozotocin-diabetes, hypothyroidism, age and sex differences. Biochem J. 1987 Aug 1;245(3):881–886. doi: 10.1042/bj2450881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonzon B., Fredholm B. B. Release of purines, noradrenaline, and GABA from rat hippocampal slices by field stimulation. J Neurochem. 1985 Jan;44(1):217–224. doi: 10.1111/j.1471-4159.1985.tb07133.x. [DOI] [PubMed] [Google Scholar]
- Karnieli E., Armoni M., Cohen P., Kanter Y., Rafaeloff R. Reversal of insulin resistance in diabetic rat adipocytes by insulin therapy. Restoration of pool of glucose transporters and enhancement of glucose-transport activity. Diabetes. 1987 Aug;36(8):925–931. doi: 10.2337/diab.36.8.925. [DOI] [PubMed] [Google Scholar]
- Katims J. J., Annau Z., Snyder S. H. Interactions in the behavioral effects of methylxanthines and adenosine derivatives. J Pharmacol Exp Ther. 1983 Oct;227(1):167–173. [PubMed] [Google Scholar]
- King R. A., Smith R. M., Dreosti I. E. Regional effects of hypothyroidism on 5'-nucleotidase and cyclic nucleotide phosphohydrolase activities in developing rat brain. Brain Res. 1983 Apr;283(2-3):287–294. doi: 10.1016/0165-3806(83)90185-2. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Douen A. G., Burdett E., Young D., Cartee G. D., Holloszy J. O. Insulin-induced decrease in 5'-nucleotidase activity in skeletal muscle membranes. FEBS Lett. 1988 Oct 10;238(2):419–423. doi: 10.1016/0014-5793(88)80524-6. [DOI] [PubMed] [Google Scholar]
- Kreutzberg G. W., Barron K. D. 5'-Nucleotidase of microglial cells in the facial nucleus during axonal reaction. J Neurocytol. 1978 Oct;7(5):601–610. doi: 10.1007/BF01260892. [DOI] [PubMed] [Google Scholar]
- Kreutzberg G. W., Barron K. D., Schubert P. Cytochemical localization of 5'-nucleotidase in glial plasma membranes. Brain Res. 1978 Dec 15;158(2):247–257. doi: 10.1016/0006-8993(78)90672-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lagercrantz H. Isolation and characterization of sympathetic nerve trunk vesicles. Acta Physiol Scand Suppl. 1971;366:1–44. [PubMed] [Google Scholar]
- Lewis M. E., Patel J., Edley S. M., Marangos P. J. Autoradiographic visualization of rat brain adenosine receptors using N6-cyclohexyl [3H]adenosine. Eur J Pharmacol. 1981 Jul 17;73(1):109–110. doi: 10.1016/0014-2999(81)90155-2. [DOI] [PubMed] [Google Scholar]
- Mallol J., Bozal J. Modification of 5'-nucleotidase activity by divalent cations and nucleotides. J Neurochem. 1983 May;40(5):1205–1211. doi: 10.1111/j.1471-4159.1983.tb13558.x. [DOI] [PubMed] [Google Scholar]
- McIlwain H., Poll J. D. Adenosine in cerebral homeostatic role: appraisal through actions of homocysteine, colchicine, and dipyridamole. J Neurobiol. 1986 Jan;17(1):39–49. doi: 10.1002/neu.480170105. [DOI] [PubMed] [Google Scholar]
- Montero J. M., Fes J. B. Purification and characterization of bovine brain 5'-nucleotidase. J Neurochem. 1982 Oct;39(4):982–989. doi: 10.1111/j.1471-4159.1982.tb11486.x. [DOI] [PubMed] [Google Scholar]
- Muller M. J., Paton D. M. Presynaptic inhibitory actions of 2-substituted adenosine derivatives on neurotransmission in rat vas deferens: effects of inhibitors of adenosine uptake and deamination. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jan;306(1):23–28. doi: 10.1007/BF00515589. [DOI] [PubMed] [Google Scholar]
- Nagata H., Mimori Y., Nakamura S., Kameyama M. Regional and subcellular distribution in mammalian brain of the enzymes producing adenosine. J Neurochem. 1984 Apr;42(4):1001–1007. doi: 10.1111/j.1471-4159.1984.tb12703.x. [DOI] [PubMed] [Google Scholar]
- Nagy A., Baker R. R., Morris S. J., Whittaker V. P. The preparation and characterization of synaptic vesicles of high purity. Brain Res. 1976 Jun 11;109(2):285–309. doi: 10.1016/0006-8993(76)90531-x. [DOI] [PubMed] [Google Scholar]
- Newby A. C., Luzio J. P., Hales C. N. The properties and extracellular location of 5'-nucleotidase of the rat fat-cell plasma membrane. Biochem J. 1975 Mar;146(3):625–633. doi: 10.1042/bj1460625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A. C. The pigeon heart 5'-nucleotidase responsible for ischaemia-induced adenosine formation. Biochem J. 1988 Jul 1;253(1):123–130. doi: 10.1042/bj2530123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips E., Newsholme E. A. Maximum activities, properties and distribution of 5' nucleotidase, adenosine kinase and adenosine deaminase in rat and human brain. J Neurochem. 1979 Aug;33(2):553–558. doi: 10.1111/j.1471-4159.1979.tb05187.x. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Edstrom J. P., Kostopoulos G. K., Kirkpatrick J. R. Effects of adenosine and adenine nucleotides on synaptic transmission in the cerebral cortex. Can J Physiol Pharmacol. 1979 Nov;57(11):1289–1312. doi: 10.1139/y79-194. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H. The role of adenosine and its nucleotides in central synaptic transmission. Prog Neurobiol. 1981;16(3-4):187–239. doi: 10.1016/0301-0082(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Proctor W. R., Dunwiddie T. V. Adenosine inhibits calcium spikes in hippocampal pyramidal neurons in vitro. Neurosci Lett. 1983 Feb 21;35(2):197–201. doi: 10.1016/0304-3940(83)90550-5. [DOI] [PubMed] [Google Scholar]
- Reddington M., Pusch R. Adenosine metabolism in a rat hippocampal slice preparation: incorporation into S-adenosylhomocysteine. J Neurochem. 1983 Jan;40(1):285–290. doi: 10.1111/j.1471-4159.1983.tb12684.x. [DOI] [PubMed] [Google Scholar]
- Reus V. I. Behavioral disturbances associated with endocrine disorders. Annu Rev Med. 1986;37:205–214. doi: 10.1146/annurev.me.37.020186.001225. [DOI] [PubMed] [Google Scholar]
- Richardson P. J., Brown S. J., Bailyes E. M., Luzio J. P. Ectoenzymes control adenosine modulation of immunoisolated cholinergic synapses. Nature. 1987 May 21;327(6119):232–234. doi: 10.1038/327232a0. [DOI] [PubMed] [Google Scholar]
- Richardson P. J. Presynaptic distribution of the cholinergic-specific antigen Chol-1 and 5'-nucleotidase in rat brain, as determined by complement-mediated release of neurotransmitters. J Neurochem. 1983 Sep;41(3):640–648. doi: 10.1111/j.1471-4159.1983.tb04789.x. [DOI] [PubMed] [Google Scholar]
- Rosman N. P., Malone M. J., Helfenstein M., Kraft E. The effect of thyroid deficiency on myelination of brain. A morphological and biochemical study. Neurology. 1972 Jan;22(1):99–106. doi: 10.1212/wnl.22.1.99. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Carnitine palmitoyltransferase in liver and five extrahepatic tissues in the rat. Inhibition by DL-2-bromopalmitoyl-CoA and effect of hypothyroidism. Biochem J. 1986 May 15;236(1):137–141. doi: 10.1042/bj2360137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. Lipogenesis in rat and guinea-pig isolated epididymal fat-cells. Biochem J. 1974 May;140(2):211–224. doi: 10.1042/bj1400211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker G., Rao G. S., Pieringer R. A. Investigations on myelinogenesis in vitro: regulation of 5'-nucleotidase activity by thyroid hormone in cultures of dissociated cells from embryonic mouse brain. J Neurosci Res. 1984;11(3):263–270. doi: 10.1002/jnr.490110306. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Patel A. J., Kingsbury A. E., Hunt A., Balázs R. Effects of thyroid state on brain development: beta-adrenergic receptors and 5'-nucleotidase activity. Brain Res. 1980 Oct 6;198(2):375–387. doi: 10.1016/0006-8993(80)90751-9. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Adenosine as a neuromodulator. Annu Rev Neurosci. 1985;8:103–124. doi: 10.1146/annurev.ne.08.030185.000535. [DOI] [PubMed] [Google Scholar]
- Sogin D. C. 2',3'-Cyclic NADP as a substrate for 2',3'-cyclic nucleotide 3'-phosphohydrolase. J Neurochem. 1976 Dec;27(6):1333–1337. doi: 10.1111/j.1471-4159.1976.tb02612.x. [DOI] [PubMed] [Google Scholar]
- Sun M. C., McIlwain H., Pull I. The metabolism of adenine derivatives in different parts of the brain of the rat, and their release from hypothalamic preparations on excitation. J Neurobiol. 1976 Mar;7(2):109–122. doi: 10.1002/neu.480070204. [DOI] [PubMed] [Google Scholar]
- Truong V. L., Collinson A. R., Lowenstein J. M. 5'-Nucleotidases in rat heart. Evidence for the occurrence of two soluble enzymes with different substrate specificities. Biochem J. 1988 Jul 1;253(1):117–121. doi: 10.1042/bj2530117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Adenosine-activated potassium conductance in cultured striatal neurons. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4857–4861. doi: 10.1073/pnas.82.14.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987 Oct;7(10):3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wylen D. G., Park T. S., Rubio R., Berne R. M. Increases in cerebral interstitial fluid adenosine concentration during hypoxia, local potassium infusion, and ischemia. J Cereb Blood Flow Metab. 1986 Oct;6(5):522–528. doi: 10.1038/jcbfm.1986.97. [DOI] [PubMed] [Google Scholar]
- Walters S. N., Morell P. Effects of altered thyroid states on myelinogenesis. J Neurochem. 1981 May;36(5):1792–1801. doi: 10.1111/j.1471-4159.1981.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Weber R. G., Jones C. R., Palacios J. M., Lohse M. J. Autoradiographic visualization of A1-adenosine receptors in brain and peripheral tissues of rat and guinea pig using 125I-HPIA. Neurosci Lett. 1988 May 3;87(3):215–220. doi: 10.1016/0304-3940(88)90451-x. [DOI] [PubMed] [Google Scholar]
- Winn H. R., Rubio G. R., Berne R. M. The role of adenosine in the regulation of cerebral blood flow. J Cereb Blood Flow Metab. 1981;1(3):239–244. doi: 10.1038/jcbfm.1981.29. [DOI] [PubMed] [Google Scholar]
- Winn H. R., Rubio R., Berne R. M. Brain adenosine production in the rat during 60 seconds of ischemia. Circ Res. 1979 Oct;45(4):486–492. doi: 10.1161/01.res.45.4.486. [DOI] [PubMed] [Google Scholar]
- Wojcik W. J., Neff N. H. Adenosine measurement by a rapid HPLC-fluorometric method: induced changes of adenosine content in regions of rat brain. J Neurochem. 1982 Jul;39(1):280–282. doi: 10.1111/j.1471-4159.1982.tb04736.x. [DOI] [PubMed] [Google Scholar]
- Zetterström T., Vernet L., Ungerstedt U., Tossman U., Jonzon B., Fredholm B. B. Purine levels in the intact rat brain. Studies with an implanted perfused hollow fibre. Neurosci Lett. 1982 Apr 16;29(2):111–115. doi: 10.1016/0304-3940(82)90338-x. [DOI] [PubMed] [Google Scholar]
- Zimmermann H., Dowdall M. J., Lane D. A. Purine salvage at the cholinergic nerve endings of the Torpedo electric organ: the central role of adenosine. Neuroscience. 1979;4(7):979–993. doi: 10.1016/0306-4522(79)90181-7. [DOI] [PubMed] [Google Scholar]


