ABSTRACT
Pseudomonas aeruginosa is an opportunistic pathogen that thrives in environments associated with human activity, including soil and water altered by agriculture or pollution. Because L-lactate is a significant product of plant and animal metabolism, it can serve as a carbon source for P. aeruginosa in the diverse settings that it inhabits. In this study, we evaluate the production and use of two redundant P. aeruginosa L-lactate dehydrogenases, termed LldD and LldA. We confirm that the protein LldR represses lldD and identify a new transcription factor, called LldS, that activates lldA; these distinct regulators and the genomic contexts of lldD and lldA contribute to their differential expression. We demonstrate that the lldD and lldA genes are conditionally controlled in response to lactate isomers as well as to glycolate and ɑ-hydroxybutyrate, which, like lactate, are ɑ-hydroxycarboxylates. We also show that lldA is induced when iron availability is low. Our examination of lldD and lldA expression across depth in biofilms indicates a complex pattern that is consistent with the effects of glycolate production, iron availability, and cross-regulation on enzyme preference. Finally, macrophage infection assays reveal that both lldD and lldA contribute to persistence within host cells, underscoring the potential role of L-lactate as a carbon source during P. aeruginosa–eukaryote interactions. Together, these findings help us understand the metabolism of a key resource that may promote P. aeruginosa’s success as a resident of contaminated environments and animal hosts.
IMPORTANCE
Pseudomonas aeruginosa is a major cause of lung infections in people with cystic fibrosis, of hospital-acquired infections, and of wound infections. It consumes L-lactate, which is found at substantial levels in human blood and tissues. In this study, we investigated the spatial regulation of two redundant enzymes, called LldD and LldA, which enable L-lactate metabolism in P. aeruginosa biofilms. We uncovered mechanisms and identified compounds that control the preference of P. aeruginosa for LldD versus LldA. We also showed that both enzymes contribute to its ability to survive within macrophages, a behavior that is thought to augment the chronicity and recalcitrance of infections. Our findings shed light on a key metabolic strategy used by P. aeruginosa and have the potential to inform the development of therapies targeting bacterial metabolism during infection.
KEYWORDS: lactate, biofilm, Pseudomonas aeruginosa, glycolate, macrophages
INTRODUCTION
Lactate is a small organic compound and a metabolite that is present in diverse environments. In the rhizosphere or within animal hosts, lactate can constitute a major carbon source for commensal and pathogenic microbes (1–4). The bacterial “NAD-independent” lactate dehydrogenases, referred as “iLDHs,” enable growth on lactate by oxidizing it to pyruvate (5). iLDHs are specific for either the D- or L-isomer of lactate, and bacteria generally show variation in their complements of these enzymes (6). Some bacteria also show iLDH redundancy, i.e., the presence of more than one enzyme that can act on a given isomer, which is particularly prevalent among species that produce iLDHs that act on L-lactate (“L-iLDHs”) (File S1) (6). This type of redundancy can make biological sense if redundant genes differ in their spatial or temporal regulation and are thus specialized for distinct environmental contexts (7–10).
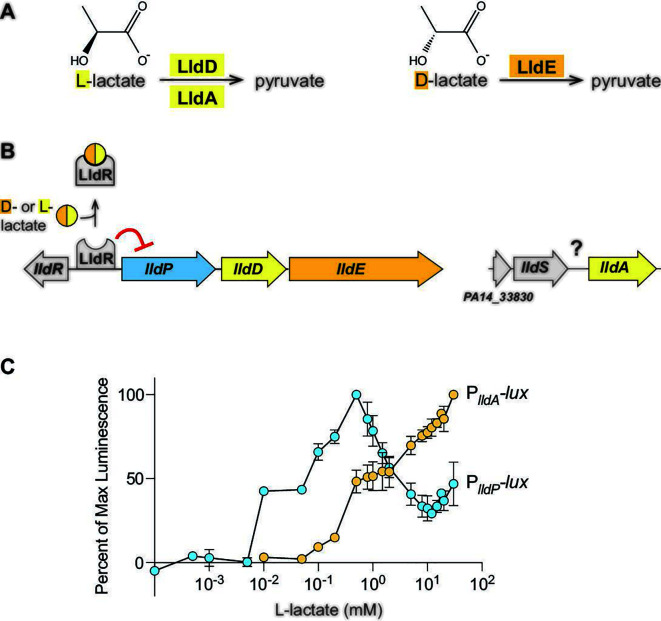
Bacteria of the genus Pseudomonas are found in aquatic and terrestrial settings and are common colonizers of eukaryotic hosts (11). One outstanding feature of pseudomonads is their ability to use a wide range of carbon sources and their preferential use of organic acids, including lactate, over sugars (12). The opportunistic pathogen P. aeruginosa is a common cause of hospital-acquired infections and infections in immunocompromised individuals (13). P. aeruginosa is able to grow using both D- and L-lactate, and our group has previously shown that it produces functionally redundant L-iLDHs (Fig. 1A) (14). Both LldD and LldA are predicted to bind a flavin mononucleotide (FMN) cofactor and to couple L-lactate oxidation to reduction of the quinone pool (15). The maintenance of redundant L-iLDHs in P. aeruginosa, which is unusual among the pseudomonads in that it can thrive in natural environments and in human infection sites (16), raises the question of whether context-dependent production of L-iLDHs contributes to its adaptability.
Fig 1.
Genes for P. aeruginosa L-lactate dehydrogenases are sensitive to different lactate isoforms and concentrations. (A) Reactions carried out by the L-lactate dehydrogenases (LldD; LldA) and the D-lactate dehydrogenase (LldE). (B) Chromosomal arrangement of genes associated with lactate utilization in P. aeruginosa. The lldR gene encodes a transcriptional repressor for the lldPDE operon, while details regarding the regulation of lldA are unknown. (C) Activities of the lldA and lldPDE promoters at L-lactate concentrations ranging from 0.1 µM to 30 mM. Cultures of luminescent reporter strains (PlldA-lux and PlldP-lux) were grown with shaking in a 96-well plate at 37°C for 24 hours in a base medium buffered with 3-(N-morpholino) propanesulfonic acid (MOPS) and containing 20 mM succinate. Each value shown represents the maximum luminescence produced during growth in the indicated L-lactate concentration, normalized to the maximum luminescence value produced in the most stimulatory L-lactate concentration. Values shown for each concentration are averages of two biological replicates, and error bars represent standard deviation.
In addition to its metabolic versatility and ability to colonize humans, P. aeruginosa is notorious for its formation of biofilms: aggregates of microbial cells encased in a self-produced and protective matrix (17). These multicellular structures are well-suited to the study of condition-dependent gene expression because they contain steep chemical gradients that lead to the formation of microniches and metabolically differentiated subpopulations (18). Prior observations by our group have suggested that P. aeruginosa lldD is heterogeneously expressed in laboratory-grown biofilms (14, 19), while a study that applied a machine learning approach to transcriptomic data identified lldA as a locus specifically associated with human infection (20). In this work, we set out to identify conditions and mechanisms that control L-lactate dehydrogenase gene expression in P. aeruginosa. Our findings illustrate the complexity of redundant gene utilization, which has consequences for in vitro growth and facilitates bacterial survival during infection. Moreover, our study results show that differential regulation of redundant genes translates into physiological heterogeneity during multicellular P. aeruginosa growth. This work provides insight into context-dependent differentiation that may contribute to P. aeruginosa’s success as a prominent cause of biofilm-based and chronic infections.
RESULTS
lldPDE and lldA are differentially expressed in response to isomer identity and L-lactate concentration
The genes for the L-lactate dehydrogenases LldD and LldA are situated at distinct sites on the P. aeruginosa chromosome. lldD is located within an operon that also codes for a lactate permease (LldP) and a D-lactate dehydrogenase (LldE), while lldA is monocistronic (Fig. 1A and B). Previously, we showed that both lactate isomers induce lldPDE expression, while only L-lactate induces lldA expression, and that lldPDE and lldA exhibit different expression dynamics in liquid cultures (14). These results are consistent with the idea that differential expression might allow the redundant L-iLDHs to contribute to fitness under distinct environmental conditions. Accordingly, we sought to examine the parameters, and investigate the roles of potential regulators, that might differentially affect lldD and lldA expression.
First, we asked if lldD and lldA are induced by different L-lactate concentrations. To address this question, we engineered the reporter strains PlldP-lux and PlldA-lux, which express the luxCDABE operon under control of the indicated promoters (21). lux-based reporter constructs produce the luciferase enzyme (LuxAB) as well as its substrate (via LuxCDE) and have increased sensitivity to promoter activity compared to fluorescent protein-based reporters (22). In the PlldP-lux and PlldA-lux strains, the constructs have been cloned into a neutral site on the chromosome; each luciferase signal serves as a readout for promoter activity and therefore reports lldPDE or lldA expression. We grew planktonic cultures of the PlldP-lux and PlldA-lux reporter strains with 20 mM succinate and various concentrations of L-lactate ranging from 0.1 µM to 30 mM. We detected luminescence from PlldP-lux expression already at 10 µM L-lactate, while that of PlldA-lux required 10 x higher concentrations. In contrast to PlldA-lux, whose expression positively correlated with the L-lactate concentration, PlldP-lux expression peaked at 500 µM L-lactate (Fig. 1C). These findings indicate that the expression of lldPDE and lldA is fine-tuned to different L-lactate concentrations and suggest distinct regulatory mechanisms.
LldS is required for lldA expression
Although previous studies have identified the transcriptional repressor LldR, which controls the expression of lldPDE (23), regulators of P. aeruginosa lldA expression have not yet been described. To test whether LldR impacts lldA expression, we deleted lldR in a PlldA-gfp reporter strain (which contains a PlldA-driven GFP expression construct cloned at a neutral site on the chromosome) and grew this strain in a liquid medium containing L-lactate. Deletion of the lldR gene had only a modest effect on PlldA activity (Fig. S1), indicating that LldR is not a major regulator of lldA expression and supporting our finding that lldA and lldD are differentially regulated (Fig. 1C).
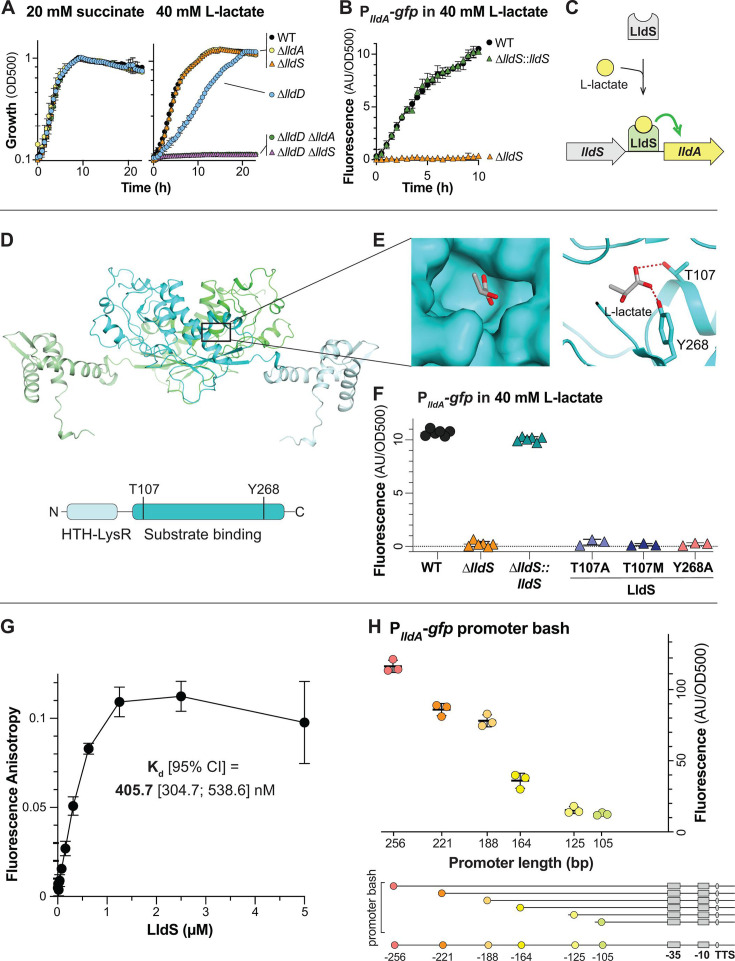
In bacterial genomes, including that of P. aeruginosa, it is common for the gene encoding a transcriptional regulator to lie adjacent to a target of the regulator (24). The gene just upstream of lldA, which we have named lldS (Fig. 1B), encodes a regulatory protein predicted to contain a LysR-type substrate-binding domain and a DNA-binding domain (25). LysR-type transcription factors have been implicated in the regulation of lactate utilization in other bacteria (6, 26). To test whether LldS affects lldA expression, we deleted lldS (PA14_33840) in a ∆lldD mutant. This strain was unable to grow when L-lactate was provided as the sole carbon source, recapitulating the phenotype of a ∆lldD ∆lldA mutant (14), but did not show a growth defect when succinate was provided as the sole carbon source (Fig. 2A). Moreover, we deleted lldS in the PlldA-gfp reporter strain background and found that this abolished PlldA activity in the presence of L-lactate (Fig. 2B). Chromosomal complementation (insertion of the wild-type lldS allele at the native site in ∆lldS) restored lldA expression to wild-type levels. Together, these observations suggest that lldS encodes a transcriptional regulator that directly activates PlldA expression in response to L-lactate (Fig. 2C).
Fig 2.
LldS (PA14_33840) is necessary for expression of lldA and likely senses L-lactate via its ligand-binding domain. (A) Growth of wild-type (WT) and mutants, lacking various genes associated with lactate metabolism, as liquid cultures in MOPS medium containing 20 mM succinate (left) or 40 mM L-lactate (right) as the sole carbon source. (B) lldA promoter activity in liquid cultures of WT, ∆lldS, and the ∆lldS complementation strain grown in MOPS medium containing 40 mM L-lactate. We observe that growth of ∆lldS under this condition is supported by LldD, as indicated by the results in panel A. (C) Schematic of the proposed mechanism regulating lldA expression. (D) (Top) AlphaFold-predicted structure of the LldS dimer with the individual monomers colored green and cyan and with lighter shades representing the DNA-binding domains. (Bottom) Domain architecture of the LldS protein. (E) Left: molecular surface of the predicted LldS binding pocket, containing L-lactate. Right: ribbon model of the predicted LldS binding pocket with two residues, T107 and Y268, shown interacting with L-lactate. (F) lldA promoter activity in liquid cultures of ∆lldS strains complemented with wild-type lldS or with the LldS point mutants T107A, T107M, and Y268A. Strains were grown in MOPS medium containing 40 mM L-lactate. Fluorescence values were taken 5–6 hours after the onset of the stationary phase. Data points represent biological replicates, and error bars represent standard deviation. (G) Binding curve of LldS to a 5’FAM-labeled DNA probe containing 256 bp upstream of the start codon of lldA. Protein concentrations ranged from 9.3 nM to 5 µM, and the probe concentration was 5 nM. The calculated Kd value is shown, and error bars represent standard deviation of two to three replicates per concentration. (H) Top: fluorescence of PlldA-gfp reporter strains with promoter regions of the indicated length. Each value was normalized by subtracting the average background fluorescence value of ∆lldS containing the full-length promoter construct. Cultures were incubated for 15 hours in MOPS medium containing 40 mM L-lactate. Data points represent biological replicates, and error bars represent standard deviation. Bottom: diagram depicting the truncations made for “promoter bash” constructs. The predicted −10 and −35 boxes and transcription start site (TSS) are indicated. These motifs were identified using the SAPPHIRE tool (27). For plots shown in panels A and B, error bars represent the standard deviation of biological triplicates and are obscured by the point marker in some cases.
To further evaluate the potential of LldS as an L-lactate-dependent transcriptional activator, we used AlphaFold2 (28) to predict its structure and identified a potential L-lactate binding pocket based on the structure of an LldS homolog in complex with a peptide ligand that has a D-alanine at the C-terminus (PDB 4WKM) (29) (Fig. 2D). This pocket contains two residues that might coordinate the carboxyl group of L-lactate, T107 and Y268 (Fig. 2E), which are equivalent to the interactions for the carboxyl group of D-alanine. To test whether these residues contribute to L-lactate-dependent expression of lldA, we generated the LldS point mutants T107A, T107M, and Y268A. We found that all three mutations prevented the activation of PlldA in response to L-lactate (Fig. 2F). While it is possible that these mutations abolished lldA expression by yielding unstable variants, the fact that the residue changes are expected to have diverse chemical and structural effects supports the interpretation that their impact on gene expression arises from defects in substrate binding. Specifically, when paired with AlphaFold2 modeling results, our observations suggest that conversion of the polar threonine and tyrosine residues to alanine interferes with hydrogen bonding to L-lactate and that the conversion of threonine to methionine sterically hinders L-lactate in the binding pocket.
LldS binds the lldA promoter sequence
To examine the interaction between LldS and the sequence upstream of lldA, we purified 6×-histidine-tagged P. aeruginosa PA14 LldS protein from Escherichia coli and assayed its binding to a 5′-fluorophore-labeled DNA composed of the 256-bp sequence upstream of lldA (i.e., the putative lldA promoter region) using a fluorescence polarization experiment. We generated binding curves by incubating 5 nM of the DNA with LldS at concentrations ranging from 9.37 nM to 5 µM and determined Kd values for the DNA–protein interaction (Fig. 2G). We calculated an average Kd (95% CI) value of 405.7 (304.7; 538.6) nM for LldS probe binding.
Having found that LldS binds to the 256-bp sequence upstream of lldA, we sought to further narrow down the sequence required for lldA expression. We therefore characterized the region upstream of this gene using a “promoter bash” approach (30). PlldA-gfp reporter strains with promoters of different lengths (256, 221, 188, 164, 125, and 105 bp) were grown as liquid cultures in MOPS medium containing L-lactate. We observed a gradual decrease in PlldA-gfp activity with decreasing promoter length. However, we observed the most pronounced difference in PlldA-gfp activity between the constructs that contained 164 versus 188 bp of the sequence upstream of the start codon, which may indicate that a transcription factor binds in this region (Fig. 2H).
lldPDE and lldA respond differentially to ɑ-hydroxycarboxylates
To test whether metabolites other than D- or L-lactate affect PlldP or PlldA activity, we screened 95 compounds using the phenotype microarray plate PM1 (Biolog, Inc.) (File S2). A dual-fluorescent transcriptional reporter strain, containing the lldA promoter driving mScarlet expression and the lldPDE promoter driving gfp expression, was used for these screens (Fig. S2). In this reporter strain, the constructs have been cloned into a neutral site on the chromosome; each fluorescence signal serves as a readout for promoter activity and therefore reports lldPDE or lldA expression. To screen for activating compounds, we tested for increased expression in a base medium containing succinate as the sole carbon source. For lldPDE expression, we did not identify any activating compounds other than L-lactate (plate PM1 does not contain D-lactate). For lldA expression, we found that, in addition to L-lactate, α-hydroxybutyrate (α-HB) had a significant stimulatory effect (Fig. S2). To screen for inhibitory compounds, we tested for decreased expression in a base medium containing succinate and L-lactate. For lldA expression, we did not identify any inhibitory compounds, but for lldPDE expression, we found that glycolate, which differs from lactate by the removal of one methyl group, had a strong inhibitory effect.
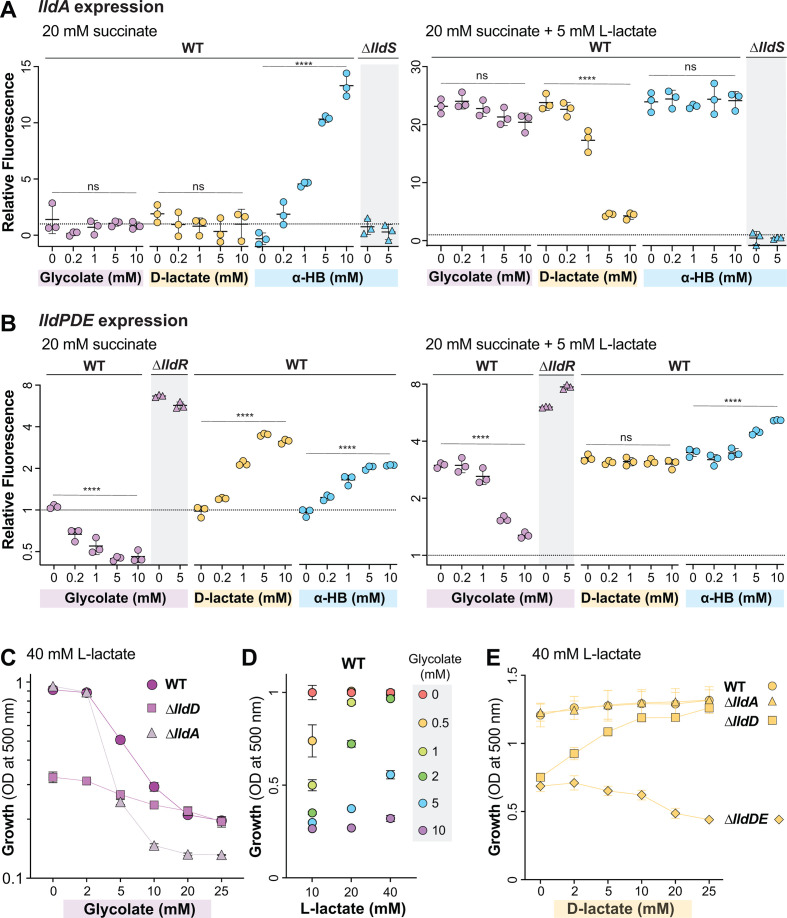
To verify the screen results, we surveyed the effects of α-HB, glycolate, or D-lactate on lldA (Fig. 3A) and lldPDE expression (Fig. 3B) by adding the compounds at concentrations ranging from 0.2 to 10 mM to a base medium containing either succinate alone or succinate in combination with L-lactate. In cultures grown in a base medium containing succinate, we confirmed that α-HB was the only compound of the three that affected lldA expression and found that this effect was abolished in the ∆lldS background. This suggested that LldS activity might be affected by both L-lactate and α-HB; we therefore examined the activity of the more-sensitive PlldA-lux reporter in media containing a range of concentrations of each compound and found that the promoter is more sensitive to L-lactate (Fig. S3). lldPDE expression, meanwhile, was affected by all three surveyed compounds: D-lactate showed the expected stimulatory effect, α-HB showed a moderate stimulatory effect, and glycolate slightly inhibited even background expression. This background expression is the residual expression detected in the absence of an exogenous stimulant and may arise from endogenous production of D-lactate. (P. aeruginosa can produce D-lactate via the enzyme LdhA [31]; it does not produce L-lactate.) The glycolate effect is likely to be LldR-mediated, as its inhibitory function is lost in a ∆lldR strain (Fig. 3B), which has high lactate-independent lldPDE expression.
Fig 3.
Identification of additional effectors of lactate gene regulation. (A, B) Fluorescence values 5–6 hours after the onset of the stationary phase produced by the lldA and lldPDE reporter strains. The dashed line represents fluorescence of a wild-type culture grown with 20 mM succinate, and fluorescence values are expressed in relation to this value. Cultures were grown in liquid MOPS medium containing 20 mM succinate (left graphs) or 20 mM succinate +5 mM L-lactate (right graphs) and amended with the indicated compounds. Fluorescence values of mutant strains (∆lldS for the lldA graphs and ∆lldR for the lldPDE graphs) are also shown for select compounds, as indicated. Each dot is representative of a biological replicate, and error bars represent standard deviation. **** indicates a P-value < 0.0001; ns = not significant. (C) Growth at 10 hours of WT, ∆lldD, and ∆lldA in MOPS medium containing 40 mM L-lactate with glycolate concentrations ranging from 0 to 25 mM. (D) Growth of WT in MOPS medium containing L-lactate concentrations of 10, 20, or 40 mM with added glycolate concentrations ranging from 0 to 10 mM. Measurements were taken at the time point when the 0 mM glycolate condition for each L-lactate concentration reached the stationary phase. Values shown are relative to growth yield at 0 mM glycolate for each respective L-lactate concentration. (E) Growth at 15 hours of WT, ∆lldD, ∆lldA, and ∆lldDE strains grown in MOPS medium containing 40 mM L-lactate, with D-lactate concentrations ranging from 0 to 25 mM.
In the “stimulatory” base medium containing both succinate (20 mM) and L-lactate (5 mM), the addition of glycolate had no significant effect on lldA expression. In contrast, increasing amounts of D-lactate had an inhibitory effect on lldA expression, beginning at 1 mM. We also observed that in the stimulatory base medium, α-HB had no effect on lldA expression. This finding is consistent with our study results, suggesting that PlldA is more sensitive to L-lactate than α-HB (Fig. S3) and indicates that α-HB is not a strong competitor for LldS binding in the presence of L-lactate. With respect to lldPDE expression, we found that glycolate showed an inhibitory effect even in the presence of L-lactate, particularly when it was added at an equimolar concentration. D-lactate did not stimulate lldPDE expression beyond the level arising from L-lactate-dependent induction. Finally, α-HB showed a slight stimulatory effect comparable to that observed in the medium without lactate.
Overall, the results of these experiments show that the activities of PlldP and PlldA are differentially affected by various α-hydroxy acid compounds. While lldPDE expression is stimulated by D-lactate and inhibited by glycolate, lldA expression is inhibited by D-lactate and relatively unaffected by glycolate. The effect of glycolate on PlldP is mediated by the repressor LldR (Fig. 3B). The effect of D-lactate on PlldA could indicate that LldS, which is activated by L-lactate, is deactivated by the D-isomer of lactate. An alternative explanation, however, is that D-lactate appears to inhibit L-lactate-dependent lldA expression because less L-lactate enters the cell when both isomers are provided simultaneously due to competition for the lactate permease (32). Finally, both promoters are activated by α-HB, but in the case of PlldA, this requires the regulator LldS (Fig. 3A), while for PlldP, we do not yet have an indication as to how this activation is mediated.
Consistent with the observations that LldD is the primary contributor to growth on L-lactate under standard conditions (Fig. 2A) (14) and that lldPDE expression is inhibited by glycolate (Fig. 3B), previous study results have indicated that P. aeruginosa growth on lactate is inhibited by the addition of glycolate (23, 33). This raised the question of whether growth of P. aeruginosa on L-lactate plus glycolate increases its reliance on LldA, whose expression is unaffected by glycolate (Fig. 3A). To test this, we grew PA14 WT alongside ∆lldD and ∆lldA mutants in 40 mM L-lactate with added glycolate concentrations ranging from 0 to 25 mM (Fig. 3C). As expected, we found that glycolate inhibited wild-type growth, causing a sharp decrease in growth yield at 5 mM glycolate. In contrast, although ∆lldD showed decreased growth consistent with the LldD’s primary role in L-lactate utilization, the effect of increasing glycolate concentrations on this yield was less pronounced and was indistinguishable from the effect of WT at concentrations of 20 mM and higher. The residual growth of WT and ∆lldD at high glycolate concentrations indicates LldD-independent L-lactate utilization that is less sensitive to glycolate. Accordingly, in contrast to ∆lldD, the ∆lldA mutant showed a pronounced decrease in growth yield at glycolate concentrations of 5 mM and higher when compared to the WT. These results show that LldA makes an important contribution to growth of P. aeruginosa on L-lactate in the presence of glycolate. Finally, to test whether sensitivity to glycolate is affected by the concentration of L-lactate provided, we performed similar experiments with a base L-lactate concentration of 10 or 20 mM for a direct comparison with the effects of 40 mM L-lactate on growth (Fig. 3D). We found that cultures grown on lower concentrations of L-lactate were sensitive to lower concentrations of glycolate, with glycolate negatively affecting growth starting at only 0.5 mM glycolate in the 10 mM-L-lactate culture and at 2 mM glycolate in the 20 mM-L-lactate culture. This observation suggests that glycolate might competitively bind to LldR and enhance its repression of lldPDE expression.
In addition to the negative effect of glycolate on PlldP activity, our expression analysis revealed an inhibition of PlldA activity by D-lactate (Fig. 3A). We hypothesized that this would lead to an increased reliance on LldD under conditions where both lactate isomers were present. We therefore predicted that addition of D-lactate would be detrimental to the growth of the ∆lldD mutant on L-lactate. We grew WT P. aeruginosa alongside ∆lldD and ∆lldA mutants in 40 mM L-lactate with added D-lactate concentrations ranging from 0 to 25 mM. We found that added D-lactate had no effect on WT and ∆lldA growth. Unexpectedly, however, it had a stimulatory effect on ∆lldD growth (Fig. 3E). We reasoned that this stimulation arose from the utilization of D-lactate via LldE activity (Fig. 1A) and therefore repeated the experiment, this time including a ∆lldDE mutant. As expected, this mutant showed decreased growth in D-lactate concentrations of 10 mM or higher.
The genomic context of lldA suggests a link to iron availability
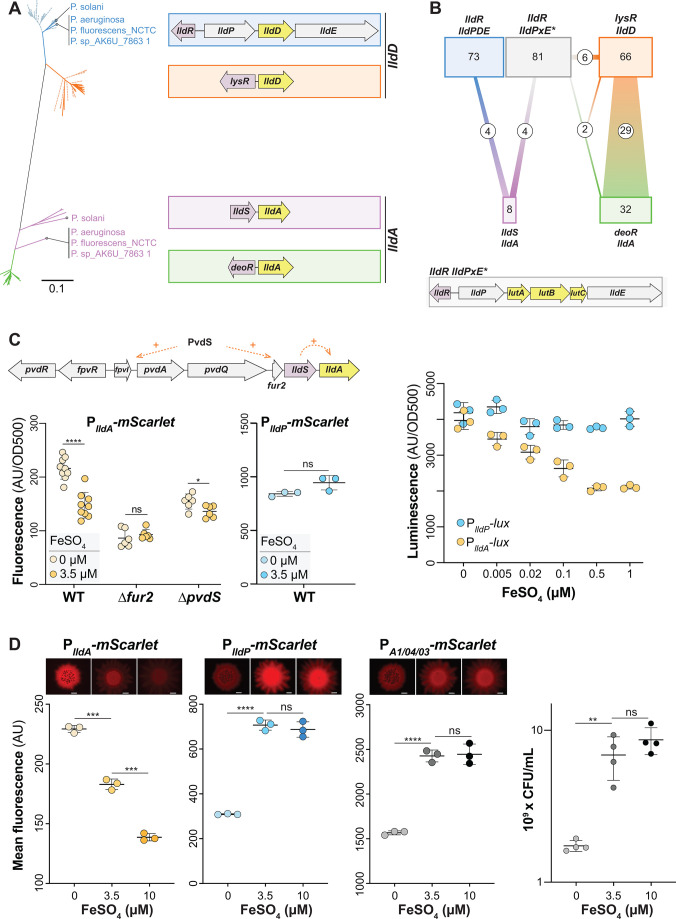
Having observed effects of organic metabolites and local regulators on the expression of P. aeruginosa’s redundant L-iLDH genes, we next examined the phylogenetic relationship of these proteins for clues regarding their respective physiological roles. Instead of or in addition to LldD and/or LldA, some organisms possess an L-iLDH encoded by a three-gene cluster referred to as lutABC (34). We searched for lldD, lldA, and lutABC homologs in Pseudomonas genomes from the Pseudomonas Genome DB (35), choosing one representative strain genome for each Pseudomonas species. Out of the 213 strains with L-iLDHs, we identified LldD and LldA homologs in 179 of these genomes. Fig. 4A shows a phylogenetic tree generated using the corresponding sequences and depiction of their gene arrangements. File S1 lists all 213 analyzed strains and indicates their L-iLDH profiles; those containing LldA or LldD are arranged according to the Lld phylogeny. Fig. 4B shows the total numbers of analyzed genomes that contain each of the indicated L-iLDH gene arrangements and the numbers of genomes that contain two or more L-iLDH homologs. In the tree shown in Fig. 4A, the lldD and lldA homologs formed independent clusters. Each of these clusters, however, also contained subgroupings that largely correlated with the genomic neighborhood/arrangement of lldD and lldA genes. lldD genes separated into two main subgroups: (i) those contained within an lldPDE operon, adjacent to a divergently transcribed lldR homolog (indicated in blue; as in P. aeruginosa PA14), and (ii) those that were monocistronic, located next to a divergently transcribed gene for a LysR-family transcriptional regulator (indicated in orange) (Fig. 4A).
Fig 4.
lldA, but not lldPDE, expression is sensitive to iron availability. (A) Phylogenetic tree of lldD and lldA genes obtained from 213 pseudomonad genomes. Only one representative strain per species was included, unless strains showed different lld arrangements. Blue and orange lines represent genes with homology to lldD, while green and purple lines represent genes with homology to lldA. Dotted lines indicate strains with one L-iLDH; solid lines indicate strains with more than one L-iLDH. The color of each cluster corresponds to the outlines surrounding the gene arrangement patterns shown on the right. Species/strain name is written if the strain has two L-iLDH genes. Only eight Pseudomonas species (including P. aeruginosa) possess the lldS–lldA gene arrangement outlined in purple. (B) Diagram depicting genome associations for the arrangement patterns shown (A) (indicated by a consistent color coding). An additional gene locus with the L-lactate utilization genes lutABC, which is not reflected in the tree, is outlined in gray and depicted at the bottom of this panel. The number of species possessing each gene arrangement is indicated in its corresponding box, and the connections represent the number of species with multiple L-iLDHs and their corresponding gene arrangements. (C) Top: in P. aeruginosa, lldS–lldA lies directly downstream of several genes involved in iron regulation and uptake. Left: lldA promoter activity in liquid cultures of WT, ∆fur2, and ∆pvdS strains grown in MOPS medium containing 40 mM L-lactate with ferrous sulfate added (3.5 µM) or no iron added (0 µM). Fluorescence values were taken 7 hours into the growth curve. Center: lldPDE promoter activity under the same conditions used for the PlldA reporter. Right: lldA and lldPDE promoter activity assayed using luciferase reporters. Cultures were grown in MOPS liquid medium with 40 mM L-lactate, and ferrous sulfate was added as indicated. The maximum luminescence value for each condition is shown for each reporter. (D) Representative top-down fluorescent biofilm images and quantification of the average fluorescence across the width of the center of the biofilm for the lldA reporter (left), lldPDE reporter (center left), and constitutive mScarlet (center right) strains. Biofilms were grown on MOPS medium with 20 mM succinate and 10 mM L-lactate, amended with ferrous sulfate as indicated. Scale bars = 2 mm. Right: quantification of colony-forming units (CFUs) of biofilms grown under each iron-amendment condition. Each dot is representative of a biological replicate, and error bars represent standard deviation. **P < 0.01, ***P < 0.001, and ****P < 0.0001; ns = not significant.
lldA homologs showed a more scattered phylogeny overall but also formed two subgroups with consistent genomic arrangements: (i) a monocistronic lldA gene located next to a divergently transcribed gene for the DeoR-family transcriptional regulator (indicated in green) and (ii) a much less common lldA gene located directly downstream of an lldS homolog (indicated in purple) (Fig. 4A and B). The P. aeruginosa lldA sequence fell within the latter subgroup. P. aeruginosa, however, stood out as the only species in which lldS and lldA are situated near a large chromosomal region involved in the uptake of iron (Fig. 4C). PA14_33830 (“fur2”), which lies upstream of lldS and bears homology to the global iron regulator Fur, is highly upregulated under low iron availability (36, 37) and has been implicated in the regulation of a wide range of genes, including those involved in iron uptake and siderophore production (38).
Expression of lldA is enhanced under low-iron conditions
Given the proximity of lldS and lldA to the cluster of iron-related genes, we tested the effect of iron availability on lldA expression. The defined medium that we typically use for P. aeruginosa growth contains iron added as freshly prepared ferrous sulfate at a concentration of 3.5 µM. We grew the PlldA-mScarlet strain in this liquid medium with L-lactate as the carbon source, with or without added iron. Because iron chelators were not added to the “low-iron” medium, residual amounts of contaminating iron enabled P. aeruginosa growth (albeit at levels that were significantly lower than that observed for the standard (i.e., iron-amended or -replete) medium) (Fig. S4A). We found that OD-corrected lldA expression was enhanced under the low-iron condition when compared to the expression in the standard medium (Fig. 4C, left). This effect was not observed for lldPDE expression (Fig. 4C, center). While here only a single time point is shown, we also provide the full-time course data in Fig. S4B. Increasing the concentration of added iron to 10 µM also had no effect on lldA expression under these (liquid culture) conditions (Fig. S5). To better visualize subtle effects on gene expression that might arise under various conditions of iron availability, we once again utilized the luciferase reporters (PlldA-lux and PlldP-lux) and tested concentrations of ferrous iron sulfate ranging from 5 nM to 1 µM. While lldA expression decreased in a stepwise fashion with increasing iron concentrations, we did not observe significant changes in lldPDE expression (Fig. 4C, right).
How is the effect of iron on lldA expression mediated? To address this question, we tested the contributions of two regulatory genes, fur2 and pvdS, to lldA expression under conditions of added or omitted ferrous iron sulfate. PvdS is a sigma factor that directly controls the expression of several loci in the region adjacent to lldS, including fpvI (Fig. 4C), and that has been implicated in the regulation of lldS (36, 39). We found that, in both the ∆fur2 and ∆pvdS strain backgrounds, the enhancement of lldA expression observed in the WT under low-iron conditions was abolished (Fig. 4C). These results suggest that both Fur2 and PvdS contribute to the iron-dependent effects on lldA expression.
Nutrient limitation is a key determinant of the physiological status in biofilms because multicellularity promotes chemical gradient formation (18). We therefore tested the effect of iron availability on lldA and lldPDE expression in biofilms. Our group studies the physiology of bacteria in biofilms using a macrocolony assay, in which a suspension of cells is pipetted onto an agar-solidified growth medium and incubated in a standard atmosphere at high humidity for several days (40, 41). To examine the effects of iron availability on lld expression in biofilms, we grew the PlldA- and PlldP-mScarlet reporter strains in the macrocolony assay on a defined medium containing L-lactate with different amounts of added ferrous iron sulfate. We also included a strain containing the PA1/04/03-mScarlet construct; this strain constitutively synthesizes fluorescent protein and can therefore serve as a control for the effects of iron availability on overall biomass production. Additionally, to test for effects of iron availability on growth, we homogenized biofilms and plated for CFUs. As expected, biofilms grown on the medium without added iron yielded a CFU count that was approximately one order of magnitude lower than that of biofilms grown on the medium with added iron concentrations of 3.5 or 10 µM, and fluorescence levels of the PA1/04/03-mScarlet biofilms (imaged top-down on a fluorescence microscope) correlated with these relative CFU counts (Fig. 4D). We observed similar changes in fluorescence for PlldP-mScarlet biofilms. Notably, in comparison to PA1/04/03-mScarlet and PlldP-mScarlet biofilms, PlldA-mScarlet biofilms showed an inverse trend. In spite of the decreased biofilm growth yield observed on the medium without added iron, the PlldA-mScarlet reporter biofilm showed a significant increase in fluorescence in this medium when compared to the 3.5-µM-added-iron condition. Additionally, although growth yields were similar with 3.5 µM- and 10 µM-added-iron, lldA expression was significantly lower in the high-iron condition. In summary, our experiments examining lld gene expression in liquid cultures and biofilms indicate that iron limitation enhances the expression of lldA, but not lldPDE, consistent with the lldA’s chromosomal location (near iron-related genes). In biofilm experiments specifically, we also observed that the addition of excess iron was inhibitory to lldA expression.
lldPDE and lldA are differentially expressed across biofilm depth
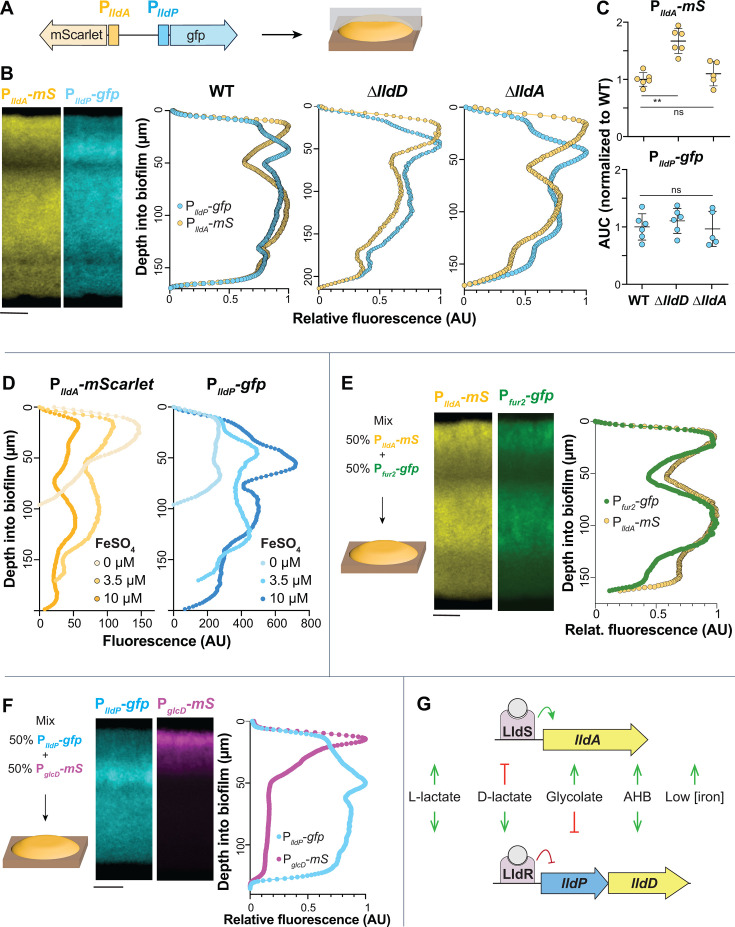
We predicted that the opposing gradients of oxygen (from the biofilm–air interface) and other resources (from the biofilm–medium interface) in macrocolony biofilms would differentially affect the expression of lldPDE and lldA. To examine this, we grew biofilms of the dual transcriptional reporter strain (PlldP-gfp PlldA-mScarlet) for 3 days before preparing thin sections (Fig. 5A) (42). For this experiment, the medium contained 20 mM succinate as the primary carbon source so that growth would not depend solely on LldD/A activity. Imaging via fluorescence microscopy revealed intriguing and robust patterns of lldA and lldPDE expression (Fig. 5B). Most notably, the activities of PlldA and PlldP showed an inverse relationship in the top ~60 µm of the biofilm. Specifically, we detected maximal expression of lldA and relatively low expression of lldPDE at the biofilm–air interface and maximal expression of lldPDE in a region where lldA expression shows a pronounced decline (30–40 µm from the biofilm–air interface). We observed a similar pattern for biofilms inoculated with an equal mixture of individual PlldP-gfp and PlldA-mScarlet or of individual PlldP-mScarlet and PlldA-gfp reporter strains (Fig. S6). The exclusionary patterning led us to hypothesize that LldD might negatively affect the expression of lldA and/or vice versa. To test this, we created mutants lacking the L-lactate dehydrogenase genes (∆lldA and ∆lldD) in the background of the dual (PlldP-gfp PlldA-mScarlet) transcriptional reporter strain and examined promoter activity in biofilm thin sections. We found that although overall lldA expression was enhanced in the ∆lldD mutant (Fig. 5C), the characteristic expression patterns of lldPDE and lldA were unaffected by the respective gene deletions (Fig. 5B), indicating that this tight regulatory patterning is determined by other factors.
Fig 5.
Biofilms display patterns of lldA and lldPDE expression across depth, which might arise from local differences in iron availability and glycolate production. (A) Schematic showing the genomic arrangement of the dual reporter construct (PlldA-mScarlet, PlldP-gfp) used in these experiments and the orientation of thin-sectioning for a macrocolony biofilm. (B) Left: fluorescence images of a thin-section from a biofilm formed by the dual PlldA-mScarlet (“mS”), PlldP-gfp reporter strain. mScarlet fluorescence is shown in yellow, and gfp fluorescence is shown in cyan. Right: fluorescence across biofilm depth for the PlldA and PlldP reporters in the indicated strain backgrounds. Images and quantification are representative of at least three biological replicates. (C) Quantification of total dual-reporter thin-section fluorescence expressed as the area under the curve (AUC) for lldA (top) and lldPDE (bottom) reporters in WT, ∆lldD, and ∆lldA, normalized to average wild-type fluorescence. Each dot is representative of a single biological replicate, and error bars represent standard deviation. **P < 0.01, ns = not significant. (D) Fluorescence across biofilm depth for each reporter in biofilms of the dual-reporter strain grown on medium amended with ferrous sulfate as indicated. Profiles are representative of three biological replicates for each iron availability condition. (E) Left: schematic of the experimental design for growth of biofilms containing two reporter strains: PlldA-mScarlet and Pfur2-gfp. Center: fluorescence images of thin-section from a mixed biofilm. mScarlet fluorescence is shown in yellow, and gfp fluorescence is shown in green. Right: fluorescence across biofilm depth for the PlldA-mScarlet and Pfur2-gfp reporters. Images and quantification are representative of four biological replicates. (F) Left: schematic of the experimental design for growth of biofilms containing two reporter strains: PglcD-mScarlet and PlldP-gfp. Center: fluorescence images of a thin-section from a mixed biofilm. mScarlet fluorescence is shown in magenta, and gfp fluorescence is shown in cyan. Right: fluorescence across biofilm depth for the PglcD-mScarlet and PlldP-gfp reporters. Images and quantification are representative of four biological replicates. (G) Visual summary of the cues that activate or inhibit the expression of P. aeruginosa lldA and lldPDE. Biofilms in panels B, D, E, and F were grown on MOPS medium containing 20 mM succinate and 10 mM L-lactate. Scale bars = 25 µm.
The fact that resource gradients form within biofilms raised the question of whether iron limitation contributes to induction of lldA at the biofilm–air interface. We, therefore, sought to test whether iron availability influences the spatial patterning of lldA and lldPDE expression across biofilm depth. We grew the dual transcriptional reporter strain for 3 days on agar containing succinate and L-lactate and various concentrations of added iron sulfate and prepared biofilm thin sections. While we found, once again, that the general patterning of lldA and lldPDE expression was retained, we also noted that lldA expression increased with decreasing iron availability, specifically in the region close to the air interface. Further, overall levels of expression varied. Iron availability affected the expression levels of both lldA and lldPDE, but in opposite ways (Fig. 5D). With increasing concentrations of added iron (0 µM, 3.5 µM, and 10 µM), lldA expression levels decreased, while those for lldPDE increased (the latter may be due to a general increase in biomass, as indicated in Fig. 4D). To further investigate iron availability across biofilm depth, we created a strain that reports the expression of fur2 (“Pfur2-gfp”), which is induced by low iron conditions (36). We then grew biofilms, starting from an equal mixture of Pfur2-gfp and PlldA-mScarlet, on medium containing succinate and L-lactate and found that lldA and fur2 expression were aligned across biofilm depth (Fig. 5E). Together, these results suggest that iron availability is a significant parameter determining the pattern of lldA expression in biofilms.
While iron availability could explain some of the lldA expression features in biofilms, it is not responsible for the distinct decrease in lldPDE expression close to the air interface (Fig. 5D). We also excluded that the high lldA expression in this region affects lldPDE expression (Fig. 5B), indicating that its spatial patterning is influenced by other cues. Since we had found in our compound screen that glycolate inhibits lldPDE expression (Fig. 3A and C), we followed up on this lead. P. aeruginosa contains the glcC gene and the adjacent glcDEFG operon, which are homologous to E. coli genes for (i) a glycolate-sensing transcription factor that induces glcDEFG expression and (ii) a glycolate oxidase complex, respectively (43). We constructed a PglcD-mScarlet reporter strain and confirmed using liquid-culture experiments that its activity is induced by glycolate (Fig. S7). To test the hypothesis that glycolate contributes to the pattern of lldPDE expression in biofilms, we used equal mixtures of PglcD-mScarlet and PlldP-gfp to inoculate agar plates containing succinate and L-lactate (Fig. 5F). We found that glcD expression was highly induced specifically at the biofilm–air interface. This finding suggests a build-up of glycolate at the top of the biofilm, which could explain the decreased lldPDE expression in this zone.
Having shown that both iron availability and exposure to specific α-hydroxycarboxylates influence the differential regulation of lldD and lldA (Fig. 5G), we next tested the contributions of LldD and LldA during macrophage infection.
lldD and lldA contribute to persistence of P. aeruginosa in macrophages
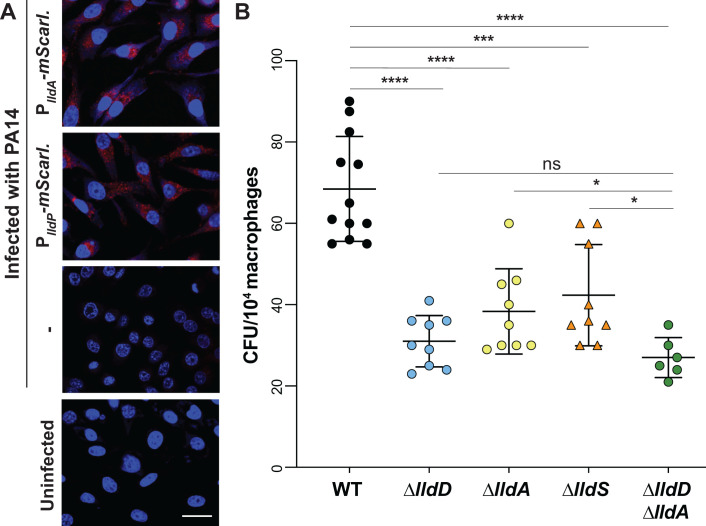
Exposure of macrophages to bacteria or bacterial products has been reported to induce a “Warburg-like effect” in which macrophages exhibit increased glycolysis and decreased oxidative phosphorylation (44). Accordingly, studies have suggested that levels of lactate, a byproduct of glycolysis (45), are increased in infected macrophages and that lactate promotes growth of intracellular bacterial pathogens within these cells (46, 47). We therefore used RAW264.7 cells to test whether L-lactate dehydrogenase genes are expressed in the presence of macrophages and whether they contribute to P. aeruginosa success during infection. Macrophages incubated with the PA14 PlldA-mScarlet or PlldP-mScarlet reporter strains showed red fluorescence, which was absent in reporterless and uninfected controls, indicating that lldA and lldD are expressed during infection (Fig. 6A). To evaluate whether the L-lactate dehydrogenases contribute to P. aeruginosa’s success during macrophage infection, we used a gentamicin protection assay (48). We found that macrophages infected with deletion mutants lacking the L-lactate dehydrogenase genes lldD or lldA or the mutant lacking the regulator LldS showed a comparable decrease in bacterial burden when compared to those infected with WT P. aeruginosa (Fig. 6B). Complementation removed or mitigated this defect (Fig. S8). The observation that the double-mutant ∆lldD∆lldA phenotype is similar to the individual L-iLDH mutants suggests that the contributions of LldD and LldA are not additive under this condition. Nevertheless, these results indicate that both LldD and LldA contribute to P. aeruginosa persistence within macrophages.
Fig 6.
Expression of lldA and contributions of lld genes during macrophage infection. (A) Fluorescence images of RAW264.7 macrophages infected with the PlldA-mScarlet strain (top), the PlldP-mScarlet strain, or WT PA14, and of an uninfected control (bottom). 4′,6-Diamidino-2-phenylindole (DAPI) fluorescence is shown in blue, and mScarlet fluorescence is shown in red. Scale bar is 10 µm. (B) Intracellular burden of P. aeruginosa WT and indicated mutant strains in RAW264.7 macrophages 3 hours post-infection and subjected to the gentamicin protection assay. Each dot represents one replicate, and error bars represent standard deviation. *P < 0.05, ***P < 0.001, and ****P < 0.0001; ns = not significant.
DISCUSSION
Enzymes that convert L-lactate to pyruvate allow bacteria to directly link a common carbon source to central metabolism. They contribute to growth alongside fermentative bacteria and eukaryotes and during colonization of eukaryotes. Within the human body, for example, L-lactate has been shown to accumulate to 1.5–3 mM in blood and tissue under normal physiological conditions and up to 40 mM under inflammatory or cancerous conditions (45), making it a significant carbon source for pathogens. Studies in various bacteria, including Neisseria gonorrhoeae, Staphylococcus aureus, and Mycobacterium tuberculosis, support the idea that L-lactate utilization contributes to success in the host and/or to virulence (46, 49–53). Coincidentally, all of these organisms have the capacity to produce multiple L-lactate dehydrogenases.
P. aeruginosa is a biofilm-forming, opportunistic pathogen that harbors traits similar to those of the nonpathogenic members of the Pseudomonas genus in addition to adaptations that allow it to cause disease in diverse hosts, including humans. These include the ability to grow at high temperatures and the production of a broad array of virulence factors (54–56). We were intrigued by P. aeruginosa’s possession of a redundant L-lactate dehydrogenase gene and the fact that this gene lies adjacent to the “pvd region” of the chromosome (36, 57), which contains multiple genes involved in iron acquisition and the response to iron limitation (Fig. 4C). This close proximity of the lldS–lldA locus and iron-related genes is unique to P. aeruginosa among the pseudomonad genomes surveyed by us. In our prior work, we showed that both of P. aeruginosa’s genes for L-iLDH enzymes—lldD and lldA—are expressed in liquid cultures in media containing L-lactate, including artificial sputum media, which contain this carbon source at millimolar concentrations. In liquid batch cultures, lldD is expressed before lldA and is sufficient to support wild-type growth dynamics (14). However, transcriptomic studies have shown that lldA is specifically induced in infection models and under infection conditions (20, 58, 59), which indicates an important role for LldA during association with hosts. We therefore investigated the regulation of P. aeruginosa’s lldD and lldA genes, the pattern of their expression in biofilms, and their contributions to host cell infection.
In this study, we confirmed that lldD expression is induced by both isomers of lactate (Fig. 3B), which alleviate repression by LldR (23). We discovered that lldA induction is controlled by the activator LldS (Fig. 2), which is encoded by an adjacent gene and responds specifically to the L- isomer. The chromosomal location of lldA led us to hypothesize that its expression could be affected by iron availability, and we indeed observed an inverse relationship between gene expression and iron availability that was unique to this L-lactate dehydrogenase gene (Fig. 4C and D). We also found that the expression of lldA correlates with the expression of fur2 (Fig. 5E), a putative regulatory gene located next to lldS that has previously been shown to respond to iron limitation (36, 37). Moreover, we observed reduced lldA expression for mutants lacking fur2 and pvdS (Fig. 4B), which codes for a sigma factor that controls a large regulon of genes induced by iron limitation, including fur2 (36). We hypothesize that in response to low iron availability, PvdS increases fur2 transcription, and Fur2 acts to increase lldA transcription in the presence of L-lactate, either by directly binding the lldA promoter or through increasing the expression of lldS. Iron limitation may act as a proxy signal for conditions in infection sites, where host metabolites act to sequester iron and decrease its availability for pathogens (60, 61), and where L-lactate may be available as a carbon source. Accordingly, in N. gonorrhoeae, it has also been suggested that the induction of an L-lactate dehydrogenase gene by iron limitation is an adaptation that promotes this pathogen’s utilization of L-lactate during infection (52). Another notable aspect of the iron-responsive regulation of lldA is that P. aeruginosa’s other L-lactate dehydrogenase gene, lldD, is cotranscribed with lldE. LldE codes for a multidomain D-lactate dehydrogenase that is predicted to contain Fe–S clusters (62). By inducing lldA, as opposed to lldPDE, under low-iron conditions, P. aeruginosa poises itself to utilize L-lactate but avoids production of a costly, iron-containing, and potentially superfluous enzyme.
In addition to the effect of iron availability, we evaluated the effects of a suite of metabolites on PlldP and PlldA reporter strains and found that two α-hydroxycarboxylates, α-HB and glycolate, affected the expression of one or both of the lldPDE and lldA loci. α-HB induced the expression of both lldPDE and lldA but was a more potent inducer of lldA. The α-HB-dependent induction of lldA was also dependent on LldS, suggesting that this regulator may recognize α-HB. The notion that L-lactate-binding sites can also accommodate α-HB is consistent with the structural similarity of these two compounds and the fact that studies of two L-lactate dehydrogenase enzymes from P. stutzeri strains have been shown to oxidize α-HB (63, 64). Little is known about the abundance of α-HB, so the physiological significance of its effects on L-lactate dehydrogenase expression and its oxidation by these enzymes is unclear.
Glycolate had an inhibitory effect on gene expression, and this effect was specific to lldPDE. This compound, which is also structurally similar to lactate, is found in diverse environments as it is present in large quantities in aquatic settings (65, 66) and has also been detected in chronic pressure ulcer wounds (67). In a study examining the transcriptomes of dual-species biofilms, the P. aeruginosa glc genes as well as lldA were induced during coculture with S. aureus when compared to monoculture conditions (68), suggesting that S. aureus produces both glycolate and L-lactate. In addition to these exogenous sources of glycolate, metabolic pathways within P. aeruginosa have the potential to produce this compound. A metabolome analysis of P. aeruginosa PAO1 detected enhanced glycolate levels under conditions that stimulated flux through the glyoxylate shunt, indicating that glycolate may be a byproduct of this pathway (69). Another potential source of endogenous glycolate is detoxification pathways for glyoxals, which are toxic side-products of metabolic processes and are often associated with oxidative stress (70). In organisms where glyoxal detoxification has been studied, it has been found to occur via conversion of glyoxal into glycolate by the enzymes glyoxalase I and II. P. aeruginosa contains homologs to these enzymes and therefore has the potential to carry out this pathway (71). Our results, which indicate that glycolate production is localized to the biofilm–air interface of macrocolonies, are consistent with both potential sources of glycolate because this region is subject to carbon limitation (a condition that promotes use of the glyoxylate shunt) and oxidative stress (a condition that would be expected to correlate with glyoxal production and detoxification) (72–75).
In previous studies examining the physiological heterogeneity and architecture of P. aeruginosa macrocolonies, we have used stimulated Raman scattering microscopy to detect metabolic activity (76) across biofilm depth (19). Our results show that metabolic activity levels are spatially heterogeneous in biofilms. They also show that in biofilms grown on complex or defined media and with a range of carbon source concentrations, maximum metabolic activity occurs at a distance from the biofilm–air interface (19, 75, 77). This suggests that the hypoxic biofilm subzone is more conducive to metabolism than the oxic region and raises the possibility that the redundant L-iLDH enzymes are optimized to function in, and may play a role in determining, the different levels of metabolic activity occurring in biofilm subzones. Interestingly, despite the marked differences we observed in the responses of lldD and lldA to metabolic cues, we found that both loci contribute to persistence within macrophages, indicating that while multicellular growth can promote differential use of LldD and LldA, an in vivo condition has the potential to promote their simultaneous use. Together, our observations provide a case study of the conditional use of redundant enzymes and provide insight into the metabolic strategies employed by a devastating bacterium that forms multicellular structures and survives intracellularly during infection (78–82).
MATERIALS AND METHODS
Bacterial strains and culture conditions
Pseudomonas aeruginosa strain UCBPP-PA14 (“PA14”) was used for all experiments. Overnight cultures were grown in lysogeny broth (LB) (83) shaking at 200 rpm at 37°C for 16–18 hours.
Construction of markerless deletions and reporter strains
To make deletion strains, approximately 1 kb of the flanking sequence from each side of the target locus was amplified using the primers listed in Table S3 and inserted into pMQ30 through gap repair cloning in Saccharomyces cerevisiae InvSc1. Plasmids used in this study are listed in Table S2. Each plasmid was transformed into Escherichia coli strain UQ950, verified by sequencing, and moved into P. aeruginosa PA14 using biparental conjugation via the E. coli donor strain BW29427. PA14 single recombinants were selected on LB agar plates containing 70 µg/mL gentamicin. Double recombinants (markerless mutants) were selected on a modified LB medium (containing 10%[wt/vol] sucrose and lacking NaCl) containing 1.5% agar, and genotypes were confirmed by PCR. Combinatorial mutants were constructed by using single mutants as hosts for biparental conjugation, as indicated in Table S1.
To construct reporter strains, promoter regions of varying lengths were amplified from the PA14 genome using primers listed in Table S3 and inserted upstream of the coding sequence of gfp, mScarlet, or luxCDABE on their respective plasmids via ligation. Plasmids were transformed into E. coli UQ950 cells and verified by sequencing. Verified plasmids were introduced into PA14 using biparental conjugation with E. coli S17-1. Single recombinants were selected on agar plates with M9 minimal medium (47.8 mM Na2HPO47H2O, 2 mM KH2PO4, 8.6 mM NaCl, 18.6 mM NH4Cl, 1 mM MgSO4, 0.1 mM CaCl2, 20 mM sodium citrate dihydrate, 1.5% agar) containing 70 µg/mL gentamicin or 150 µg/mL tetracycline for the luciferase reporters. The plasmid backbone was resolved out of PA14 using Flp-FRT recombination using the pFLP2 plasmid (84) and selection on M9 minimal medium 1.5% agar plates containing 300 µg/mL carbenicillin. Strains were cured of the pFLP2 plasmid by streaking on LB agar plates without NaCl and with 10% (wt/vo)l sucrose. The presence of gfp, mScarlet, or luxCDABE in final clones was confirmed by PCR.
Liquid culture growth assays
Biological triplicates of overnight pre-cultures were diluted 1:100 in 200 µL MOPS medium (50 mM MOPS, 43 mM sodium chloride, 93 mM ammonium chloride, 2.2 mM monobasic potassium phosphate, 1 mM magnesium sulfate) containing carbon sources as indicated, in a flat-bottomed, black polystyrene 96-well plate (Greiner Bio-One 655001). Ferrous sulfate was added, at a concentration of 1 µg/mL (3.5 µM), to the MOPS medium, unless otherwise indicated. Carbon sources (succinate, lactate, glycolate, etc.) were added as sodium salts at the concentration indicated. Plates were incubated at 37°C with continuous shaking on the medium setting in a Biotek Synergy H1 plate reader. Expression of mScarlet, GFP, or luciferase was assessed by taking fluorescence or luminescence readings every 30 minutes for up to 24 hours. mScarlet was detected at excitation and emission wavelengths of 569 nm and 599 nm, respectively. GFP was detected at excitation and emission wavelengths of 480 nm and 510 nm, respectively. Growth was assessed by taking OD readings at 500 nm simultaneously with the fluorescence/luminescence readings. Unless otherwise stated, the reported values were obtained by first subtracting the fluorescence values of “no-reporter” strains and then normalizing to growth (OD at 500 nm).
Biolog metabolite screens
Phenotypic microarray plate PM1 (Biolog, Cat. No. 12111) contains a unique carbon source in each well of a 96-well plate (File S2). For activator screens, 100 µL of MOPS medium containing 20 mM sodium succinate was added to each well of this phenotypic microarray plate, and for inhibitor screens, 100 µL of MOPS medium containing 20 mM sodium succinate and 5 mM sodium L-lactate was added to each well. Plates were incubated with shaking at 37°C for approximately 1 hour to fully dissolve each compound. The compound solutions were then transferred to a black 96-well plate pre-filled with 100 µL of the base medium per well so that the total volume per well was 200 µL. Overnight pre-cultures were diluted 1:100 into each well. Plates were placed in a Biotek Synergy H1 plate reader, and experimental parameters were as described above. A compound was considered a “hit” if the raw fluorescence value in its well (at 7.5 hours of incubation; representing the early stationary phase) was two standard deviations above (for activators) or below (for inhibitors) the median raw fluorescence value for all wells. Each screen was performed in two independent experiments, and only metabolites that were identified as hits in both experiments are reported.
Titration experiments with luciferase reporters
The same protocol was used as for liquid culture growth assays, but with different concentrations of L-lactate, α-HB, or ferrous sulfate used to supplement the MOPS base medium. The L-lactate and α-HB titrations were performed in MOPS medium containing 20 mM succinate with L-lactate concentrations ranging from 0.1 µM to 30 mM and α-HB concentrations from 10 µM to 30 mM. The ferrous sulfate titration was done in MOPS containing 40 mM L-lactate, with the concentration of added ferrous sulfate ranging from 5 nM to 1 µM. Plates were placed in a Biotek Synergy H1 plate reader, and experimental parameters were the same as described above. Cultures were grown for 15–24 hours, and the maximum luminescence value was identified for each concentration, then normalized to growth, and to the luminescence values recorded in a promoterless lux strain. For lactate and α-HB titrations, this value was also normalized by subtracting the maximum fluorescence value recorded during the full incubation period in the base medium (i.e., without added L-lactate/α-HB). Where indicated, the maximum luminescence value for each L-lactate/α-HB concentration is expressed as its proportion of the maximum value exhibited for that strain at any concentration. For PlldP-lux, the maximum luminescence value was detected during growth in 500 µM L-lactate, while for PlldA-lux, the maximum luminescence value was detected during growth in 30 mM L-lactate or 30 mM α-HB (the highest concentrations tested).
AlphaFold-based modeling
AlphaFold-Multimer (85) was used to predict models of an LldS dimer, which were manually examined with PyMOL and Coot (86). For the model of the L-lactate complex, a search for structural homologs of LldS in the Protein Data Bank (PDB) was carried out with the program Dali (87). The structure of the AmpR effector binding domain in complex with a peptide ligand (PDB entry 4WKM) (29) was found to be a close homolog, and the C-terminal Ala residue of the peptide ligand was used as the starting point for modeling L-lactate. The hydrogen-bonding interactions between the carboxylate group of Ala and the protein was maintained for L-lactate, while the position of the rest of the compound was adjusted manually. The Thr and Tyr residues in AmpR that are hydrogen-bonded to the C-terminal carboxylate of the peptide are conserved in LldS, and these residues have the same conformation in the two structures.
Protein expression and purification
PA14 lldS was cloned into the pET28a vector with an N-terminal 6×His-tag. The proteins was overexpressed in E. coli BL21 (DE3) cells at 16°C overnight. For purification, the cell pellet was resuspended and lysed by sonication in a buffer containing 20 mM Tris (pH 8.0), 500 mM NaCl, 2 mM bME, 5% (vol/vol) glycerol, and one tablet of the protease inhibitor mixture (Sigma). The cell lysate was then centrifuged at 13,000 rpm for 30 minutes at 4°C. The protein was purified from the supernatant via nickel affinity chromatography (Qiagen). The protein was further purified by a Hiload 16/60 Superdex 200 column (Cytiva) equilibrated with a buffer containing 20 mM Tris (pH 8.0), 500 mM NaCl, and 2 mM DTT. The purified protein was concentrated to 20 mg/mL, supplemented with 5% (vol/vol) glycerol, and stored at −80°C.
Fluorescence anisotropy
Fluorophore-labeled DNA was generated using PCR amplification with a 5′FAM-labeled reverse primer (IDT) and an unlabeled forward primer and purified using the E.Z.N.A Gel Extraction Kit (Omega Bio-tek). The amplified region contained the 256-bp region directly upstream of the lldA start codon. Purified LldS protein and 5 nM of FAM-labeled probe were added to the reaction buffer (50 mM HEPES [pH 7.5], 30 mM KCl, 3 mM magnesium acetate, 5% glycerol) with the protein concentration ranging from 9.37 nM to 5 µM. Parallel and perpendicular polarization values were measured in a black 384-well plate using a Biotek Synergy Neo2 plate reader with excitation and emission wavelengths of 485 nm and 528 nm, respectively, and fluorescence anisotropy was calculated using the formula (parallel − perpendicular)/parallel + (2 × perpendicular) (88). Before plotting, the minimum anisotropy value was subtracted from all data points. Kd was calculated in GraphPad Prism by curve-fitting using a nonlinear, least squares regression model and assuming one-site, specific binding.
Gene arrangement and association analysis
Genomic sequences (i.e., GenBank files) of 1,323 strains were downloaded from the Pseudomonas DB (https://pseudomonas.com/) (35). For P. aeruginosa, only one strain, PA14, was chosen, while strains from all other species were kept for analysis. This resulted in 689 strains for the search of lactate dehydrogenase-related genes. The gene arrangement search was implemented in a custom-built tool named “Locus Hunter” (https://github.com/linyc74/locus_hunter). For each gene arrangement, blastp was used to search for homologous genes (e-value = 10−60) (89). The interval of each homologous gene was extended by a flanking length of 5,000 bp, resulting in intervals that overlap with each other. Intervals that overlap with each other were then fused to form a gene arrangement containing genes of interest. For each species, only one representative strain was chosen; however, if two or more strains showed different gene arrangements, both were represented. These considerations reduced the number of strains to 213. For the association analysis, two gene arrangements were defined to be associated when they coexist in a given strain. The number of associations between a pair of gene arrangements is the number of strains harboring both arrangements.
Phylogenetic analysis
Out of the 213 strains identifed in the gene arrangement analysis (described above), 179 strains contained lldD and lldA homologs. In Geneious Prime (Version 2024.0.3), the corresponding LldD and LldA protein sequences were aligned using Clustal Omega, and a tree was constructed using Geneious Tree Builder (Jukes-Cantor; neighbor-joining; no outgroup).
Preparation of P. aeruginosa macrocolony biofilms
Overnight P. aeruginosa cultures were subcultured at a ratio of 1:100 in 3 mL LB and were grown at 37°C, 200 rpm to an OD500 of ~0.5–0.6. Then, 5 µL of liquid subcultures was spotted onto 1% agar plates (100 mm x 15 mm, Simport Scientific D210-16) containing 60 mL of MOPS medium with 20 mM sodium succinate and 10 mM sodium L-lactate. For biofilms used for thin-sectioning experiments, agar plates were prepared in two layers: a 45-mL base layer and a 15-mL top layer. The base MOPS medium was prepared the same in every case, except in experiments testing the effect of iron availability, in which ferrous sulfate was omitted for the “0 µM” condition and added in excess for the “10 µM” condition. For mixed biofilms containing two reporter strains, the OD500 of each subculture was corrected to exactly 0.5, and subcultures were mixed in a 1:1 ratio before being spotted. Macrocolony biofilms were grown in the dark at 25°C. Macrocolony biofilm experiments were conducted with at least three biological replicates of each strain and condition.
Top-down fluorescence imaging of P. aeruginosa macrocolony biofilms
After 3 days of growth as described above, bright-field and fluorescence images were obtained using a Zeiss Axio Zoom V16 fluorescence stereo zoom microscope (excitation, 545 nm; emission, 605 nm for imaging of mScarlet). Images were processed using the Zeiss Zen software and analyzed using Fiji/ImageJ.
Quantification of colony-forming units (CFUs) from macrocolony biofilms
After 3 days of growth as described above, biofilms were homogenized in 1 mL PBS using a bead mill homogenizer (Omni [Kennesaw, GA] Bead Ruptor 12; at high setting for 90 s) and 0.5 g ceramic beads (Thermo Fisher 15 340 159, diameter of 1.4 mm). The cell suspension was serially diluted in PBS, plated on 1% tryptone, 1.5% agar plates and incubated at 37°C for 16 hours before CFU counting.
Thin sectioning of P. aeruginosa macrocolony biofilms
After 3 days of growth on bilayer agar plates as described above, biofilms were overlaid with 15 mL 1% agar and sandwiched biofilms were lifted from the bottom agar layer and fixed in 4% paraformaldehyde in PBS at room temperature for 24 hours. Fixed biofilms were processed, infiltrated with wax, sectioned in 10-μm-thick sections, and collected onto slides as described in reference 77. Slides were air-dried overnight, heat-fixed on a hotplate for 30 minutes at 45°C, and rehydrated in the reverse order of processing. Rehydrated colonies were immediately mounted in Tris-buffered DAPI:fluorogel (Electron Microscopy Sciences) and overlaid with a coverslip. Thin sections were imaged using a Zeiss Axio Zoom V16 fluorescence stereo zoom microscope, at the same settings as described above. Images were processed using the Zeiss Zen software and analyzed using Fiji/ImageJ.
Assessment of intracellular bacterial load in macrophages
RAW264.7 macrophage cells were plated overnight on six-well plates. Bacterial strains (WT, ∆lldD, ∆lldA, ∆lldS, and ∆lldD∆lldA) were grown in LB to an OD600 of 2.0 and then washed with PBS. Macrophages were washed with antibiotic-free Dulbecco’s modified Eagle medium (DMEM) and infected with bacteria at 5 MOI for 30 minutes at 37°C in 5% CO2. Bacteria that were unbound to macrophages were removed by one wash with cold DMEM, and to remove any excess extracellular bacteria, 100 µg/mL of gentamicin was added for 30 minutes; cells were washed with DMEM and transferred to the medium without gentamicin and incubated at 37°C in 5% CO2 for 3 hours. Infected RAW cells were lysed in distilled water. The lysed cells were immediately diluted in PBS and plated on LB agar plates to assess the intracellular bacterial load in macrophages. Bacterial CFUs were counted after incubating the plates overnight at 37°C.
Fluorescence imaging of macrophages
RAW264.7 cells were seeded at 1 × 105 cells per well on three-well-chambered coverglass slides the day prior to the experiment. Cells were infected with WT PA14 or PA14 bacteria carrying the PlldA-mScarlet reporter at an MOI of 5, as described in the previous section. Cells were then fixed in 4% paraformaldehyde in PBS (pH 7.4) for 15 minutes at room temperature. Slides were washed in 1× PBS (pH 7.4). Cells were stained with DAPI (1:10,000) at room temperature and washed in 1× PBS (pH 7.4) three times for 5 minutes each. The cells were examined using a confocal microscope (Nikon ECLIPSE Ti2; Nikon Instruments Inc., Tokyo) at 400× optical magnification. The assay was conducted in triplicate and repeated three times.
ACKNOWLEDGMENTS
This work was supported by NIH/NIAID grant R01AI103369 to L.E.P.D., R35GM118093 to L.T., and NIH awards R01AI134857 and R01AI177555 and Shriner’s grant 83009 to L.R.
The authors thank Hannah Peha for assistance with strain engineering and Riley Gentry, Nicholas Ide, Ji Huang, and Columbia’s Precision Biomolecular Characterization Facility (PBCF) for assistance with the anisotropy experiments.
Contributor Information
Lars E. P. Dietrich, Email: LDietrich@columbia.edu.
Deborah A. Hogan, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.00852-24.
Phylogram.
List of all the metabolites present in Biolog's PM1 plate and their corresponding well number.
Figures S1-S8.
Tables S1-S3. Strains, plasmids, primers.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Macias-Benitez S, Garcia-Martinez AM, Caballero Jimenez P, Gonzalez JM, Tejada Moral M, Parrado Rubio J. 2020. Rhizospheric organic acids as biostimulants: monitoring feedbacks on soil microorganisms and biochemical properties. Front Plant Sci 11:633. doi: 10.3389/fpls.2020.00633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borer B, Kleyer H, Or D. 2022. Primary carbon sources and self-induced metabolic landscapes shape community structure in soil bacterial hotspots. Soil Biol Biochem 168:108620. doi: 10.1016/j.soilbio.2022.108620 [DOI] [Google Scholar]
- 3. Jensen PØ, Nielsen BU, Kolpen M, Pressler T, Faurholt‐Jepsen D, Mathiesen IHM. 2022. Increased sputum lactate during oral glucose tolerance test in cystic fibrosis. APMIS 130:535–539. doi: 10.1111/apm.13233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Llibre A, Grudzinska FS, O’Shea MK, Duffy D, Thickett DR, Mauro C, Scott A. 2021. Lactate cross-talk in host-pathogen interactions. Biochem J 478:3157–3178. doi: 10.1042/BCJ20210263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiang T, Gao C, Ma C, Xu P. 2014. Microbial lactate utilization: enzymes, pathogenesis, and regulation. Trends Microbiol 22:589–599. doi: 10.1016/j.tim.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 6. Pinchuk GE, Rodionov DA, Yang C, Li X, Osterman AL, Dervyn E, Geydebrekht OV, Reed SB, Romine MF, Collart FR, Scott JH, Fredrickson JK, Beliaev AS. 2009. Genomic reconstruction of Shewanella oneidensis MR-1 metabolism reveals a previously uncharacterized machinery for lactate utilization. Proc Natl Acad Sci U S A 106:2874–2879. doi: 10.1073/pnas.0806798106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahadevan R, Lovley DR. 2008. The degree of redundancy in metabolic genes is linked to mode of metabolism. Biophys J 94:1216–1220. doi: 10.1529/biophysj.107.118414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martínez-Núñez MA, Pérez-Rueda E, Gutiérrez-Ríos RM, Merino E. 2010. New insights into the regulatory networks of paralogous genes in bacteria. Microbiology (Reading) 156:14–22. doi: 10.1099/mic.0.033266-0 [DOI] [PubMed] [Google Scholar]
- 9. Keane OM, Toft C, Carretero-Paulet L, Jones GW, Fares MA. 2014. Preservation of genetic and regulatory robustness in ancient gene duplicates of Saccharomyces cerevisiae. Genome Res 24:1830–1841. doi: 10.1101/gr.176792.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schada von Borzyskowski L, Bernhardsgrütter I, Erb TJ. 2020. Biochemical unity revisited: microbial central carbon metabolism holds new discoveries, multi-tasking pathways, and redundancies with a reason. Biol Chem 401:1429–1441. doi: 10.1515/hsz-2020-0214 [DOI] [PubMed] [Google Scholar]
- 11. Crone S, Vives‐Flórez M, Kvich L, Saunders AM, Malone M, Nicolaisen MH, Martínez‐García E, Rojas‐Acosta C, Catalina Gomez‐Puerto M, Calum H, Whiteley M, Kolter R, Bjarnsholt T. 2020. The environmental occurrence of Pseudomonas aeruginosa. APMIS 128:220–231. doi: 10.1111/apm.13010 [DOI] [PubMed] [Google Scholar]
- 12. McGill SL, Yung Y, Hunt KA, Henson MA, Hanley L, Carlson RP. 2021. Pseudomonas aeruginosa reverse diauxie is a multidimensional, optimized, resource utilization strategy. Sci Rep 11:1457. doi: 10.1038/s41598-020-80522-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qin S, Xiao W, Zhou C, Pu Q, Deng X, Lan L, Liang H, Song X, Wu M. 2022. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Sig Transduct Target Ther 7:199. doi: 10.1038/s41392-022-01056-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin Y-C, Cornell WC, Jo J, Price-Whelan A, Dietrich LEP. 2018. The Pseudomonas aeruginosa complement of lactate dehydrogenases Enables use of d- and l-lactate and metabolic cross-feeding. mBio 9:e00961-18. doi: 10.1128/mBio.00961-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Xiao D, Liu Q, Zhang Y, Hu C, Sun J, Yang C, Xu P, Ma C, Gao C. 2018. Two NAD-independent l-lactate dehydrogenases drive l-lactate utilization in Pseudomonas aeruginosa PAO1. Environ Microbiol Rep 10:569–575. doi: 10.1111/1758-2229.12666 [DOI] [PubMed] [Google Scholar]
- 16. Girard L, Lood C, Höfte M, Vandamme P, Rokni-Zadeh H, van Noort V, Lavigne R, De Mot R. 2021. The ever-expanding Pseudomonas genus: description of 43 new species and partition of the Pseudomonas putida group. Microorganisms 9:1766. doi: 10.3390/microorganisms9081766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sauer K, Stoodley P, Goeres DM, Hall-Stoodley L, Burmølle M, Stewart PS, Bjarnsholt T. 2022. The biofilm life cycle: expanding the conceptual model of biofilm formation. Nat Rev Microbiol 20:608–620. doi: 10.1038/s41579-022-00767-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jo J, Price-Whelan A, Dietrich LEP. 2022. Gradients and consequences of heterogeneity in biofilms. Nat Rev Microbiol 20:593–607. doi: 10.1038/s41579-022-00692-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schiessl KT, Hu F, Jo J, Nazia SZ, Wang B, Price-Whelan A, Min W, Dietrich LEP. 2019. Phenazine production promotes antibiotic tolerance and metabolic heterogeneity in Pseudomonas aeruginosa biofilms. Nat Commun 10:762. doi: 10.1038/s41467-019-08733-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cornforth DM, Dees JL, Ibberson CB, Huse HK, Mathiesen IH, Kirketerp-Møller K, Wolcott RD, Rumbaugh KP, Bjarnsholt T, Whiteley M. 2018. Pseudomonas aeruginosa transcriptome during human infection. Proc Natl Acad Sci U S A 115:E5125–E5134. doi: 10.1073/pnas.1717525115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Craney A, Hohenauer T, Xu Y, Navani NK, Li Y, Nodwell J. 2007. A synthetic luxCDABE gene cluster optimized for expression in high-GC bacteria. Nucleic Acids Res 35:e46. doi: 10.1093/nar/gkm086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan F, Wood KV. 2007. Bioluminescent assays for high-throughput screening. Assay Drug Dev Technol 5:127–136. doi: 10.1089/adt.2006.053 [DOI] [PubMed] [Google Scholar]
- 23. Gao C, Hu C, Zheng Z, Ma C, Jiang T, Dou P, Zhang W, Che B, Wang Y, Lv M, Xu P. 2012. Lactate utilization is regulated by the FadR-type regulator LldR in Pseudomonas aeruginosa. J Bacteriol 194:2687–2692. doi: 10.1128/JB.06579-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Korbel JO, Jensen LJ, von Mering C, Bork P. 2004. Analysis of genomic context: prediction of functional associations from conserved bidirectionally transcribed gene pairs. Nat Biotechnol 22:911–917. doi: 10.1038/nbt988 [DOI] [PubMed] [Google Scholar]
- 25. Matilla MA, Velando F, Martín-Mora D, Monteagudo-Cascales E, Krell T. 2022. A catalogue of signal molecules that interact with sensor kinases, chemoreceptors and transcriptional regulators. FEMS Microbiol Rev 46:fuab043. doi: 10.1093/femsre/fuab043 [DOI] [PubMed] [Google Scholar]
- 26. Brutinel ED, Gralnick JA. 2012. Preferential utilization of D-lactate by Shewanella oneidensis. Appl Environ Microbiol 78:8474–8476. doi: 10.1128/AEM.02183-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coppens L, Lavigne R. 2020. SAPPHIRE: a neural network based classifier for σ70 promoter prediction in Pseudomonas. BMC Bioinformatics 21:415. doi: 10.1186/s12859-020-03730-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, Yuan D, Stroe O, Wood G, Laydon A, et al. 2022. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res 50:D439–D444. doi: 10.1093/nar/gkab1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vadlamani G, Thomas MD, Patel TR, Donald LJ, Reeve TM, Stetefeld J, Standing KG, Vocadlo DJ, Mark BL. 2015. The β-lactamase gene regulator AmpR is a tetramer that recognizes and binds the D-Ala-D-Ala motif of its repressor UDP-N-acetylmuramic acid (MurNAc)-pentapeptide. J Biol Chem 290:2630–2643. doi: 10.1074/jbc.M114.618199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chalfie M, Kain SR. 2005. Green fluorescent protein vol. 47: properties, applications and protocols. Wiley & Sons, Limited. [Google Scholar]
- 31. Eschbach M, Schreiber K, Trunk K, Buer J, Jahn D, Schobert M. 2004. Long-term anaerobic survival of the opportunistic pathogen Pseudomonas aeruginosa via pyruvate fermentation. J Bacteriol 186:4596–4604. doi: 10.1128/JB.186.14.4596-4604.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matin A, Konings WN. 1973. Transport of lactate and succinate by membrane vesicles of Escherichia coli, Bacillus subtilis and a Pseudomonas species. Eur J Biochem 34:58–67. doi: 10.1111/j.1432-1033.1973.tb02728.x [DOI] [PubMed] [Google Scholar]
- 33. Brown PR, Tata R. 1987. Glycollate inhibition of growth of Pseudomonas aeruginosa on lactate medium. J Gen Microbiol 133:1521–1526. doi: 10.1099/00221287-133-6-1521 [DOI] [PubMed] [Google Scholar]
- 34. Chai Y, Kolter R, Losick R. 2009. A widely conserved gene cluster required for lactate utilization in Bacillus subtilis and its involvement in biofilm formation. J Bacteriol 191:2423–2430. doi: 10.1128/JB.01464-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Winsor GL, Griffiths EJ, Lo R, Dhillon BK, Shay JA, Brinkman FSL. 2016. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res 44:D646–D653. doi: 10.1093/nar/gkv1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ochsner UA, Wilderman PJ, Vasil AI, Vasil ML. 2002. GeneChip expression analysis of the iron starvation response in Pseudomonas aeruginosa: identification of novel pyoverdine biosynthesis genes. Mol Microbiol 45:1277–1287. doi: 10.1046/j.1365-2958.2002.03084.x [DOI] [PubMed] [Google Scholar]
- 37. Cai Z, Yang F, Shao X, Yue Z, Li Z, Song Y, Pan X, Jin Y, Cheng Z, Ha U-H, Feng J, Yang L, Deng X, Wu W, Bai F. 2022. ECF sigma factor HxuI is critical forIn Vivo fitness of Pseudomonas aeruginosa during infection. Microbiol Spectr 10. doi: 10.1128/spectrum.01620-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zheng P, Sun J, Geffers R, Zeng A-P. 2007. Functional characterization of the gene PA2384 in large-scale gene regulation in response to iron starvation in Pseudomonas aeruginosa. J Biotechnol 132:342–352. doi: 10.1016/j.jbiotec.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 39. Schulz S, Eckweiler D, Bielecka A, Nicolai T, Franke R, Dötsch A, Hornischer K, Bruchmann S, Düvel J, Häussler S. 2015. Elucidation of sigma factor-associated networks in Pseudomonas aeruginosa reveals a modular architecture with limited and function-specific crosstalk. PLoS Pathog 11:e1004744. doi: 10.1371/journal.ppat.1004744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Friedman L, Kolter R. 2004. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol Microbiol 51:675–690. doi: 10.1046/j.1365-2958.2003.03877.x [DOI] [PubMed] [Google Scholar]
- 41. Jo J, Cortez KL, Cornell WC, Price-Whelan A, Dietrich LE. 2017. An orphan cbb3-type cytochrome oxidase subunit supports Pseudomonas aeruginosa biofilm growth and virulence. Elife 6:171538. doi: 10.7554/eLife.30205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cornell WC, Morgan CJ, Koyama L, Sakhtah H, Mansfield JH, Dietrich LEP. 2018. Paraffin embedding and thin sectioning of microbial colony biofilms for microscopic analysis. J Vis Exp:57196. doi: 10.3791/57196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pellicer MT, Badía J, Aguilar J, Baldomà L. 1996. Glc locus of Escherichia coli: characterization of genes encoding the subunits of glycolate oxidase and the glc regulator protein. J Bacteriol 178:2051–2059. doi: 10.1128/jb.178.7.2051-2059.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Escoll P, Buchrieser C. 2018. Metabolic reprogramming of host cells upon bacterial infection: why shift to a warburg-like metabolism? FEBS J 285:2146–2160. doi: 10.1111/febs.14446 [DOI] [PubMed] [Google Scholar]
- 45. Li X, Yang Y, Zhang B, Lin X, Fu X, An Y, Zou Y, Wang J-X, Wang Z, Yu T. 2022. Lactate metabolism in human health and disease. Sig Transduct Target Ther 7:305. doi: 10.1038/s41392-022-01151-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Billig S, Schneefeld M, Huber C, Grassl GA, Eisenreich W, Bange F-C. 2017. Lactate oxidation facilitates growth of Mycobacterium tuberculosis in human macrophages. Sci Rep 7:6484. doi: 10.1038/s41598-017-05916-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang X, Yang B, Ma S, Yan X, Ma S, Sun H, Sun Y, Jiang L. 2023. Lactate promotes Salmonella intracellular replication and systemic infection via driving macrophage M2 polarization. Microbiol Spectr 11:e0225323. doi: 10.1128/spectrum.02253-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chakraborty A, Kabashi A, Wilk S, Rahme LG. 2023. Quorum-sensing signaling molecule 2-aminoacetophenone mediates the persistence of Pseudomonas aeruginosa in macrophages by interference with autophagy through epigenetic regulation of lipid biosynthesis. mBio 14:e0015923. doi: 10.1128/mbio.00159-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Exley RM, Goodwin L, Mowe E, Shaw J, Smith H, Read RC, Tang CM. 2005. Neisseria meningitidis lactate permease is required for nasopharyngeal colonization. Infect Immun 73:5762–5766. doi: 10.1128/IAI.73.9.5762-5766.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smith H, Tang CM, Exley RM. 2007. Effect of host lactate on gonococci and meningococci: new concepts on the role of metabolites in pathogenicity. Infect Immun 75:4190–4198. doi: 10.1128/IAI.00117-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fuller JR, Vitko NP, Perkowski EF, Scott E, Khatri D, Spontak JS, Thurlow LR, Richardson AR. 2011. Identification of a lactate-quinone oxidoreductase in Staphylococcus aureus that is essential for virulence. Front Cell Infect Microbiol 1:19. doi: 10.3389/fcimb.2011.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen NH, Ong C-LY, O’sullivan J, Ibranovic I, Davey K, Edwards JL, McEwan AG. 2020. Two distinct L-lactate dehydrogenases play a role in the survival of Neisseria gonorrhoeae in cervical epithelial cells. J Infect Dis 221:449–453. doi: 10.1093/infdis/jiz468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Atack JM, Ibranovic I, Ong C-L, Djoko KY, Chen NH, Vanden Hoven R, Jennings MP, Edwards JL, McEwan AG. 2014. A role for lactate dehydrogenases in the survival of Neisseria gonorrhoeae in human polymorphonuclear leukocytes and cervical epithelial cells. J Infect Dis 210:1311–1318. doi: 10.1093/infdis/jiu230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wurtzel O, Yoder-Himes DR, Han K, Dandekar AA, Edelheit S, Greenberg EP, Sorek R, Lory S. 2012. The single-nucleotide resolution transcriptome of Pseudomonas aeruginosa grown in body temperature. PLoS Pathog 8:e1002945. doi: 10.1371/journal.ppat.1002945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nikolaidis M, Mossialos D, Oliver SG, Amoutzias GD. 2020. Comparative analysis of the core proteomes among the Pseudomonas major evolutionary groups reveals species-specific adaptations for Pseudomonas aeruginosa and Pseudomonas chlororaphis. Diversity 12:289. doi: 10.3390/d12080289 [DOI] [Google Scholar]
- 56. Yi B, Dalpke AH. 2022. Revisiting the intrageneric structure of the genus Pseudomonas with complete whole genome sequence information: insights into diversity and pathogen-related genetic determinants. Infect Genet Evol 97:105183. doi: 10.1016/j.meegid.2021.105183 [DOI] [PubMed] [Google Scholar]
- 57. Tsuda M, Miyazaki H, Nakazawa T. 1995. Genetic and physical mapping of genes involved in pyoverdin production in Pseudomonas aeruginosa PAO. J Bacteriol 177:423–431. doi: 10.1128/jb.177.2.423-431.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lewin GR, Kapur A, Cornforth DM, Duncan RP, Diggle FL, Moustafa DA, Harrison SA, Skaar EP, Chazin WJ, Goldberg JB, Bomberger JM, Whiteley M. 2023. Application of a quantitative framework to improve the accuracy of a bacterial infection model. Proc Natl Acad Sci U S A 120:e2221542120. doi: 10.1073/pnas.2221542120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rossi E, Falcone M, Molin S, Johansen HK. 2018. High-resolution in situ transcriptomics of Pseudomonas aeruginosa unveils genotype independent patho-phenotypes in cystic fibrosis lungs. Nat Commun 9:3459. doi: 10.1038/s41467-018-05944-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Singh PK, Parsek MR, Greenberg EP, Welsh MJ. 2002. A component of innate immunity prevents bacterial biofilm development. Nature 417:552–555. doi: 10.1038/417552a [DOI] [PubMed] [Google Scholar]
- 61. Weaver VB, Kolter R. 2004. Burkholderia spp. alter Pseudomonas aeruginosa physiology through iron sequestration. J Bacteriol 186:2376–2384. doi: 10.1128/JB.186.8.2376-2384.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jiang T, Guo X, Yan J, Zhang Y, Wang Y, Zhang M, Sheng B, Ma C, Xu P, Gao C. 2017. A bacterial multidomain NAD-independent d-lactate dehydrogenase utilizes flavin adenine dinucleotide and Fe-S clusters as cofactors and quinone as an electron acceptor for d-lactate oxidization. J Bacteriol 199:e00342-17. doi: 10.1128/JB.00342-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gao C, Jiang T, Dou P, Ma C, Li L, Kong J, Xu P. 2012. NAD-independent L-lactate dehydrogenase is required for L-lactate utilization in Pseudomonas stutzeri SDM. PLoS One 7:e36519. doi: 10.1371/journal.pone.0036519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gao C, Wang Y, Zhang Y, Lv M, Dou P, Xu P, Ma C. 2015. NAD-Independent L-lactate dehydrogenase required for L-lactate utilization in Pseudomonas stutzeri A1501. J Bacteriol 197:2239–2247. doi: 10.1128/JB.00017-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim Y-M, Nowack S, Olsen MT, Becraft ED, Wood JM, Thiel V, Klapper I, Kühl M, Fredrickson JK, Bryant DA, Ward DM, Metz TO. 2015. Diel metabolomics analysis of a hot spring chlorophototrophic microbial mat leads to new hypotheses of community member metabolisms. Front Microbiol 6:209. doi: 10.3389/fmicb.2015.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wright RT, Shah NM. 1977. The trophic role of glycolic acid in coastal seawater. II. Seasonal changes in concentration and heterotrophic use in Ipswich Bay, Massachusetts, USA. Mar Biol 43:257–263. doi: 10.1007/BF00402318 [DOI] [Google Scholar]
- 67. Ammons MCB, Morrissey K, Tripet BP, Van Leuven JT, Han A, Lazarus GS, Zenilman JM, Stewart PS, James GA, Copié V. 2015. Biochemical association of metabolic profile and microbiome in chronic pressure ulcer wounds. PLoS One 10:e0126735. doi: 10.1371/journal.pone.0126735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Magalhães AP, França A, Pereira MO, Cerca N. 2022. Unveiling co-Infection in cystic fibrosis airways: transcriptomic analysis of Pseudomonas aeruginosa and Staphylococcus aureus dual-species biofilms. Front Genet 13:883199. doi: 10.3389/fgene.2022.883199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Meylan S, Porter CBM, Yang JH, Belenky P, Gutierrez A, Lobritz MA, Park J, Kim SH, Moskowitz SM, Collins JJ. 2017. Carbon sources tune antibiotic susceptibility in Pseudomonas aeruginosa via tricarboxylic acid cycle control. Cell Chem Biol 24:195–206. doi: 10.1016/j.chembiol.2016.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thornalley PJ. 1998. Glutathione-dependent detoxification of alpha-oxoaldehydes by the glyoxalase system: involvement in disease mechanisms and antiproliferative activity of glyoxalase I inhibitors. Chem Biol Interact 111–112:137–151. doi: 10.1016/s0009-2797(97)00157-9 [DOI] [PubMed] [Google Scholar]
- 71. Sukdeo N, Honek JF. 2007. Pseudomonas aeruginosa contains multiple glyoxalase I-encoding genes from both metal activation classes. Biochim Biophys Acta 1774:756–763. doi: 10.1016/j.bbapap.2007.04.005 [DOI] [PubMed] [Google Scholar]
- 72. Dolan SK, Welch M. 2018. The glyoxylate shunt, 60 years on. Annu Rev Microbiol 72:309–330. doi: 10.1146/annurev-micro-090817-062257 [DOI] [PubMed] [Google Scholar]
- 73. Ahn S, Jung J, Jang I-A, Madsen EL, Park W. 2016. Role of glyoxylate shunt in oxidative stress response. J Biol Chem 291:11928–11938. doi: 10.1074/jbc.M115.708149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lee C, Park C. 2017. Bacterial responses to glyoxal and methylglyoxal: reactive electrophilic species. Int J Mol Sci 18:169. doi: 10.3390/ijms18010169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Evans CR, Smiley MK, Asahara Thio S, Wei M, Florek LC, Dayton H, Price-Whelan A, Min W, Dietrich LEP. 2023. Spatial heterogeneity in biofilm metabolism elicited by local control of phenazine methylation. Proc Natl Acad Sci U S A 120:e2313208120. doi: 10.1073/pnas.2313208120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shi L, Zheng C, Shen Y, Chen Z, Silveira ES, Zhang L, Wei M, Liu C, de Sena-Tomas C, Targoff K, Min W. 2018. Optical imaging of metabolic dynamics in animals. Nat Commun 9:2995. doi: 10.1038/s41467-018-05401-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dayton H, Kiss J, Wei M, Chauhan S, LaMarre E, Cornell WC, Morgan CJ, Janakiraman A, Min W, Tomer R, Price-Whelan A, Nirody JA, Dietrich LEP. 2024. Cellular arrangement impacts metabolic activity and antibiotic tolerance in Pseudomonas aeruginosa biofilms. PLoS Biol 22:e3002205. doi: 10.1371/journal.pbio.3002205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kolpen M, Kragh KN, Enciso JB, Faurholt-Jepsen D, Lindegaard B, Egelund GB, Jensen AV, Ravn P, Mathiesen IHM, Gheorge AG, Hertz FB, Qvist T, Whiteley M, Jensen PØ, Bjarnsholt T. 2022. Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax 77:1015–1022. doi: 10.1136/thoraxjnl-2021-217576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Heimer SR, Evans DJ, Stern ME, Barbieri JT, Yahr T, Fleiszig SMJ. 2013. Pseudomonas aeruginosa utilizes the type III secreted toxin ExoS to avoid acidified compartments within epithelial cells. PLoS ONE 8:e73111. doi: 10.1371/journal.pone.0073111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kroken AR, Chen CK, Evans DJ, Yahr TL, Fleiszig SMJ. 2018. The impact of ExoS on Pseudomonas aeruginosa internalization by epithelial cells is independent of fleQ and correlates with bistability of type three secretion system gene expression. mBio 9:e00668-18. doi: 10.1128/mBio.00668-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. DePas WH, Starwalt-Lee R, Van Sambeek L, Ravindra Kumar S, Gradinaru V, Newman DK. 2016. Exposing the three-dimensional biogeography and metabolic States of pathogens in cystic fibrosis sputum via hydrogel embedding, clearing, and rRNA labeling. mBio 7:e00796-16. doi: 10.1128/mBio.00796-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kroken AR, Klein KA, Mitchell PS, Nieto V, Jedel EJ, Evans DJ, Fleiszig SMJ. 2023. Intracellular replication of Pseudomonas aeruginosa in epithelial cells requires suppression of the caspase-4 inflammasome. mSphere 8:e0035123. doi: 10.1101/2023.02.13.528260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bertani G. 2004. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J Bacteriol 186:595–600. doi: 10.1128/JB.186.3.595-600.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. doi: 10.1016/s0378-1119(98)00130-9 [DOI] [PubMed] [Google Scholar]
- 85. Evans R, O’Neill M, Pritzel A, Antropova N, Senior A, Green T, Žídek A, Bates R, Blackwell S, Yim J, Ronneberger O, Bodenstein S, Zielinski M, Bridgland A, Potapenko A, Cowie A, Tunyasuvunakool K, Jain R, Clancy E, Kohli P, Jumper J, Hassabis D. 2022. Protein complex prediction with AlphaFold-multimer. bioRxiv. doi: 10.1101/2021.10.04.463034 [DOI]
- 86. Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132. doi: 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 87. Holm L, Kääriäinen S, Rosenström P, Schenkel A. 2008. Searching protein structure databases with DaliLite v.3. Bioinformatics 24:2780–2781. doi: 10.1093/bioinformatics/btn507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lundblad JR, Laurance M, Goodman RH. 1996. Fluorescence polarization analysis of protein-DNA and protein-protein interactions. Mol Endocrinol 10:607–612. doi: 10.1210/mend.10.6.8776720 [DOI] [PubMed] [Google Scholar]
- 89. Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10:421. doi: 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogram.
List of all the metabolites present in Biolog's PM1 plate and their corresponding well number.
Figures S1-S8.
Tables S1-S3. Strains, plasmids, primers.