Abstract
A 163 bp enhancer in the CYP2B2 5′ flank confers PB (phenobarbital) inducibility and constitutes a PBRU (PB response unit). The PBRU contains several transcription factor binding sites, including NR1, NR2 and NR3, which are direct repeats separated by 4 bp of the nuclear receptor consensus half-site AGGTCA, as well as an ER (everted repeat) separated by 7 bp (ER-7). Constitutive androstane receptor (CAR)–RXR (retinoic X receptor) heterodimers are known to bind to NR1, NR2 and NR3. Electrophoretic mobility-shift analysis using nuclear extracts from livers of untreated or PB-treated rats revealed binding of several other proteins to different PBRU elements. Using supershift analysis and in vitro coupled transcription and translation, the proteins present in four retarded complexes were identified as TRβ (thyroid hormone receptor β), LXR (liver X receptor), HNF-4 (hepatocyte nuclear factor 4) and heterodimers of PBX–PREP1 (pre-B cell homoeobox–Pbx regulatory protein 1). LXR–RXR heterodimers bound to NR3 and TRβ bound to NR3, NR1 and ER-7, whereas the PBX–PREP1 site is contained within NR2. The HNF-4 site overlaps with NR1. A mutation described previously, GRE1m1, which decreases PB responsiveness, increased the affinity of this site for HNF-4. The PBRU also contains a site for nuclear factor 1. The PBRU thus contains a plethora of transcription factor binding sites. The profiles of transcription factor binding to NR1 and NR3 were quite similar, although strikingly different from, and more complex than, that of NR2. This parallels the functional differences in conferring PB responsiveness between NR1 and NR3 on the one hand, and NR2 on the other.
Keywords: CYP2B, nuclear receptor, phenobarbital response unit binding protein, primary hepatocyte, rat liver
Abbreviations: apoB, apolipoprotein B; βRARE, retinoic acid β2 response element; CAR, constitutive androstane receptor; CMV, cytomegalovirus; CYP, cytochrome P450; DR, direct repeat; DTT, dithiothreitol; EMSA, electrophoretic mobility-shift analysis; ER, everted repeat; GRE, glucocorticoid response element; HNF, hepatocyte nuclear factor; LXR, liver X receptor; MEIS, myeloid ectopic viral integration site; NF1, nuclear factor 1; oligo, oligodeoxyribonucleotide; PB, phenobarbital; PBRU, PB response unit; PBREM, PB-responsive enhancer module; PBX, pre-B cell homoeobox; PREP, Pbx regulatory protein 1; RXR, retinoid X receptor; TALE, three-amino-acid extension; tk, thymidine kinase; TRα/β, thyroid hormone receptor α/β; UR, ubiquitous receptor
INTRODUCTION
Hepatic CYPs (cytochrome P450s) play a critical role in the metabolism of hydrophobic xenobiotics and endogenous hydrophobic metabolites [1,2]. The genes encoding these enzymes are either expressed constitutively or are induced by various chemicals [3]. Over the past decade, much progress has been made concerning the molecular mechanism whereby PB (phenobarbital) leads to the induction of the homologous rat CYP2B2 (and CYP2B1) and mouse Cyp2b10 genes, and of the chicken CYP2H1 gene [4]. Although the chicken CYP2H1 gene is PB-inducible in a chicken hepatoma cell line [5], in the rodent system generally the only cultured cells in which CYP2B genes respond normally to PB treatment are primary hepatocytes [6,7]. The PBRU (PB response unit), a 163 bp Sau3AI fragment located at nt −2317/−2155 in the CYP2B2 5′-flank, confers PB inducibility on heterologous promoters in primary rat hepatocytes and, following in situ transfection in rat liver, has the properties of a transcriptional enhancer [8–10]. The homologous region of the 5′-flank of the PB-inducible mouse Cyp2b10 gene contains a 162 bp segment with similar properties [11], which is 92% identical with the rat CYP2B2 PBRU [10].
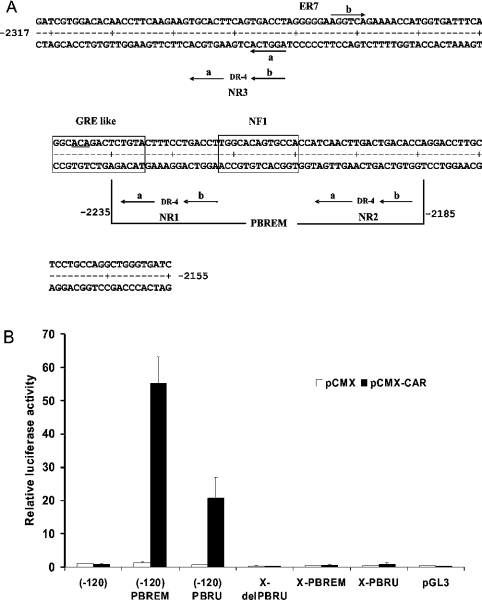
The rat CYP2B2 PBRU contains, among other putative transcription factor recognition sites, three DRs (direct repeats) separated by 4 bp (i.e. DR-4), as well as an ER (everted repeat) separated by 7 bp (i.e. ER-7), of the nuclear receptor consensus hexamer half-site motif AGGTCA (Figure 1A). Two of the DR-4 sites, NR1 and NR2, flank an NF1 (nuclear factor 1) site, and were recognized as putative nuclear-receptor-binding sites by Negishi and co-workers in the homologous mouse Cyp2b10 fragment [12]. The third DR-4 site, NR3, is upstream of NR1 and NR2 [13]. Negishi and co-workers [12] also defined a 51 bp PBREM (PB-responsive enhancer module) within the mouse Cyp2b10 PBRU sequence. The PBREM is limited to the NR1–NF1–NR2 elements (Figure 1A), and the mouse PBREM is essentially equivalent to the rat or mouse PBRU in conferring PB responsiveness in primary mouse and rat hepatocytes when placed directly adjacent to the heterologous tk (thymidine kinase) promoter [12,14]. However, replacing the PBRU with the PBREM in the CYP2B2 5′-flank in the natural sequence context reduces PB responsiveness in primary rat hepatocytes at least 4-fold [14]. This and other evidence [14,15] indicates that sequences outside the PBREM are required for maximal PB responsiveness. Possible candidates for such sequences are the upstream NR3 site [13] and the overlapping ER-7 site (Figure 1A). Indeed, mutational inactivation of the ER-7A half-site, which is the same sequence as the NR3B half-site (Figure 1A), reduces but does not abolish PB responsiveness in the natural sequence context; mutational inactivation of any NR1 or NR2 half-site has a similar effect [14].
Figure 1. DNA sequence of rat CYP2B2 and activation by CAR of PBRU- or PBREM-driven reporter gene transcription.
(A) DNA sequence of the rat CYP2B2 PBRU showing the positions of putative transcription factor binding sites. The vertical lines at nt positions −2235 and −2185 delimit the 51 bp PBREM sequence. The underlined bases in the GRE-like site are those mutated in the GRE1m1 mutation (ACA→GTG) [10,14]. (B) Activation by CAR of PBRU- or PBREM-driven reporter gene transcription. Human hepatoma HepG2 cells were cultured, and the experiments were conducted as described in the Experimental section. The corrected luciferase activity obtained for the (−120) construct co-transfected with pCMX was set at 1. The results shown are average values for three independent experiments. The error bars show the S.D. pGL3, the pBL3 basic vector; X-PBRU, a luc reporter derived from pGL3 carrying 2.5 kb of the CYP2B2 5′ flank including the PBRU in the natural sequence context; X-PBREM, identical with the X-PBRU, except that the PBRU was replaced by the PBREM; X del-PBRU, identical with X-PBRU, except that the PBRU was deleted; (−120) PBRU, the pGL3 basic vector carrying the PBRU directly upstream of the 120 bp CYP2B2 basic promoter; (−120) PBREM identical with (−120) PBRU, except that the PBRU was replaced by the PBREM; (−120), the pGL3 basic vector carrying the 120 bp CYP2B2 promoter.
Negishi and co-workers [16,17] have shown that treatment with PB or PB-type inducers leads to nuclear accumulation of the CAR (constitutive androstane receptor) in mouse liver. CAR, in the form of a heterodimer with the RXR (retinoid X receptor), binds both to the βRARE (retinoic acid β2 response element) [18,19] and to the NR1, NR2 and NR3 sites of the PBRU [13,20]. Co-transfection with a CAR expression vector activates transcription of reporter genes driven by the PBREM and by oligomerized βRARE and NR1 sequences in cultured cell lines in the absence of added ligand [16,20,21]. Given that PB fails to display agonist activity with respect to CAR [20,22], it is the ligand-independent transcriptional activation activity of CAR–RXR heterodimers that is thought to account for the PB-induced expression of CYP2B genes [4,16,17,21].
Although binding of CAR–RXR heterodimers to NR1, NR2 and NR3 has been reported from several groups [13,16,20,23], EMSA (electrophoretic mobility-shift analysis) and DNase I footprinting assays using nuclear extracts from livers of untreated or PB-treated rats or mice have revealed strong binding of a number of other proteins to different elements of the PBRU [8,10,11,24]. One such protein is NF1 [8,13,23], the role of which in conferring PB responsiveness is still not entirely clear [13]. We report here the identification, in rat liver nuclear extracts, of four other proteins or protein complexes binding to different PBRU elements. The proteins present in the retarded complexes were identified as HNF-4 (hepatocyte nuclear factor 4), TRβ (thyroid receptor-β), LXR (liver X receptor) and PBX (pre-B cell homoeobox)–PREP1 (Pbx regulatory protein 1) heterodimers.
EXPERIMENTAL
Materials and animals
Chee's medium for hepatocyte culture, as well as Lipofectin®, were from Invitrogen Canada (Burlington, ON, Canada). Oligodeoxyribonucleotides (oligos) were from Sigma Genosys (Oakville, ON, Canada). ITS (insulin, transferrin and selenium) was from Sigma–Aldrich Canada (Oakville, ON, Canada), Vitrogen 100 was from Cohesion (Palo Alto, CA, U.S.A.), collagenase Type IV was from Sigma–Aldrich, Percoll was from Amersham Biosciences (Baie d'Urfée, PQ, Canada), and [γ-32P]ATP (6000 Ci/mmol) was from PerkinElmer (Boston, MA, U.S.A.). Male Sprague–Dawley rats (200–250 g) were from Charles River Canada (Saint-Constant, PQ, Canada).
Isolation and culture of primary hepatocytes, cell culture, transfection, PB treatment and luciferase assays
The methods for hepatocyte isolation and culture in serum-free medium, essentially those of Waxman et al. [6], as well as those for liposome-mediated transfection [25] and plasmid purification, were as described previously [14]. Typically, for transfection of primary hepatocytes with the reporter construct, 1.8×106 cells were plated in six-well plates (10 cm2; Falcon, VWR International, Ville Mont-Royal, PQ, Canada) in 2 ml of serum-free modified Chee's medium [6] containing gentamicin (Invitrogen; 50 μg/ml) and incubated for 2 h to permit attachment. The cells were incubated for an additional 2.5 h in medium without antibiotic, and then were transfected and incubated for 16 h before adding PB (0.1 mM). After an additional 48 h of incubation (the medium was changed once, after 24 h), cells were harvested and luciferase activity (Promega Dual Luc; Fisher Scientific, Nepean, ON, Canada) was assayed by luminometry. For transfection, each well typically received 1 μg of firefly luc reporter construct [14], 3 μg of pBluescript carrier, and 5 ng of internal control plasmid (the Renilla luc gene under control of the cytomegalovirus (CMV) promoter/enhancer) in 15 μl of Lipofectin® reagent (Invitrogen).
HepG2 human hepatoma cells were cultured and maintained (5% CO2/37 °C) in medium B [26] supplemented with 10% (v/v) fetal-calf serum (Invitrogen). To perform transfections, 8× 106 cells in 2 ml of medium B without serum were first incubated overnight in six-well T75 Primaria culture dishes (VWR International). The medium was then removed, and each well received 200 ng of pCMX or pCMX-CAR, 10 ng of pRLSV40 Renilla internal control plasmid (Promega), 2 μg of luc reporter plasmid and 10 μl of SuperFect Reagent (Qiagen, Mississauga, ON, Canada) in 700 μl of medium B. After overnight incubation, the medium was removed, the cells were rinsed with Earle's balanced salts solution (1 ml per well), 2 ml of medium B was added and the incubation was continued for an additional 24 h. The medium was removed, 200 μl of lysis buffer was added and the cells were harvested by scraping, and lysed by freeze–thaw treatment. Luciferase activity (Promega Dual Luc) was assayed by luminometry.
Plasmids, plasmid constructs and site-directed mutagenesis
The luc reporter construct used for all assays of PB responsiveness in primary hepatocytes contained the rat CYP2B2 PBRU in the natural sequence context (the X-PBRU construct) [14], derived from the pGL3 basic vector (Promega). Plasmids carrying previously non-described mutations in PBRU sequence elements were obtained and characterized as described previously [14], using the PCR-based QuikChange® system (Stratagene, La Jolla, CA, U.S.A.) for site-directed mutagenesis. The mutations were generated in the X construct containing the CYP2B2 PBRU flanked by BglII sites as described previously [14]. The oligos used for the mutagenesis are shown in Table 1. The pGL3-derived luc reporter vectors used to transfect HepG2 cells have been described previously [14]. They include the X construct carrying the CYP2B2 PBRU (X-PBRU) or PBREM (X-PBREM) in the natural sequence context, and four vectors in which the enhancers were placed directly upstream of either the enhancerless tk promoter (tk-PBRU and tk-PBREM) or the 120 bp CYP2B2 basic promoter (−120-PBRU and −120-PBREM; the −120-PBREM construct carried the mouse PBREM). The pCMX-mCARβ expression vector for mouse CAR [19] (herein referred to as pCMX-CAR) was from Dr R. G. Evans via Dr Barry Foreman (City of Hope National Medical Center, Duarte, CA, U.S.A.). An expression vector for for HNF-4 [27] was from Dr F. Sladek (University of California, Riverside, CA, U.S.A.), and those for PBX1, PBX2 and PREP1 [28] were from Dr V. Zappavigna (DIBIT-H San Raffaele, Milan, Italy).
Table 1. Oligos used for PCR-based site-directed mutagenesis of the PBRU.
Only the sequence of the upper strand is shown. Mutated bases are shown in lower-case letters. The 5′ and 3′ coordinates are those of the rat CYP2B2 5′ flank.
| Mutation | 5′-end | Sequence | 3′-end |
|---|---|---|---|
| GRE scrambled | −2268 | GGTCAGAAAACCATGGTGATTTCAtttgggttggCTGTACTTTCCTGACCTTGG | −2215 |
| GREdel | −2268 | GGTCAGAAAACCATGGTGATTTCA–––––CTGTACTTTCCTGACCTTGG | −2215 |
| GRE1m1 | −2254 | GGTGATTTCAGGCgtgGACTCTGTACTTTC | −2225 |
| 5′NR1A | −2254 | GGTGATTTCAGGCACAGAaaaTGTACTTTCC | −2224 |
| GRE consensus | −2258 | CCATGGTGATTTCAGGtACAa-TCTGTACTTTCC | −2224 |
| TRβ consensus | −2258 | CCATGGTGATTTCAGGCtCAGgtcaTGTACTTTCCTGACCTTGGC | −2214 |
| NR1Abp1,2,3 | −2246 | CAGGCACAGACTCcacACTTTCCTGACC | −2219 |
| NR2-Sm | −2210 | GTGCCACCATCAACTTattTGACACCAGGACCTTGC | −2175 |
EMSA and supershift analysis and coupled in vitro transcription and translation
Nuclear extracts were prepared [29] from pooled livers of two or three untreated or PB-treated [30] rats. For EMSA analyses, oligos in single-stranded form were end-labelled to an initial specific activity of approx. 105 c.p.m./μg with [γ-32P]ATP using T4 polynucleotide kinase (Invitrogen) and subsequently annealed. Coupled in vitro transcription and translation (TNT T7; Promega) was performed according to the supplier's instructions.
Binding reaction mixtures for analysis using oligos corresponding to NR1 and NR3/ER-7, and for analyses involving in vitro coupled transcription and translation, were based on those of Handschin et al. [31]. They contained, in a final volume of 12 μl, 0.5 ng of labelled DNA, 10 mM Tris/HCl, pH 8.0, 40 mM KCl, 1 mM DTT (dithiothreitol), 0.05% Nonidet P40 (Sigma), 1 μg of poly(dI-dC) (Amersham Biosciences), 6% (v/v) glycerol, 100 ng of herring-sperm DNA (Invitrogen), and nuclear extract (3 μg of protein for ER-7 or 14 μg of protein for NR1 and NR3) or reaction mixtures for in vitro transcription and translation (3 μl). Reaction mixtures for analysis of binding of HNF-4 were on the basis of those of Honkakoski et al. [16]. They contained, in a final volume of 12 μl, 0.5 ng of labelled DNA, 10 mM Hepes, pH 7.9, 50 mM NaCl, 0.5 mM DTT, 0.05% Nonidet P40, 2 μg of poly(dI-dC), 15% glycerol, 100 ng of a non-specific double-stranded oligo (5′-CTAAAAGCTTTTAG-3′) and nuclear extract (3 μg of protein). Reaction mixtures for analysis using oligos corresponding to NR2 were on the basis of those of Bernier et al. [29]. They contained, in a final volume of 12 μl, 0.5 ng of labelled DNA, 17.5 mM Hepes, pH 7.6, 100 mM NaCl, 2.5 mM MgCl2, 0.7 mM ZnCl2, 0.7 mM Na2MoO4, 2H2O, 0.07 mM EGTA, 0.7 mM DTT, 0.07% Nonidet P40, 2 μg poly(dI-dC), 2 μg of BSA (Sigma–Aldrich), 3% Ficoll (Sigma–Aldrich), 100 ng of herring-sperm DNA and nuclear extract (7 μg of protein). The sequences of the oligos used for labelling, or as competitors, are provided in Tables 2–4.
Table 2. Oligos used for EMSA analysis of the HNF-4 site of the PBRU.
Only the sequence of the upper strand is shown. For CYP2B2 sequence, bases differing from wild-type are shown in lower-case letters. Where 5′ and 3′ coordinates are provided they are those of the rat CYP2B2 5′ flank.
| Oligo | 5′-end | Sequence | 3′-end |
|---|---|---|---|
| GRE-like | −2247 | TCAGGCACAGACTCTGTACTTTC | −2225 |
| GRE1m1 | −2247 | TCAGGCgtgGACTCTGTACTTTC | −2225 |
| HNF4(APOB) | CAAGGCGCCCTTTGGACCTTTCC |
Binding was initiated by addition of the labelled oligo (1 μl) followed by nuclear extract or coupled in vitro transcription and translation reaction mixtures. For supershift analyses, the antibodies were added (4 μg per assay, except 400 ng per assay for anti-HNF-4) with the protein extract prior to the addition of the labelled oligos. Reaction mixtures were incubated on ice for 30 min, except for supershift analysis using anti-TRβ, where protein-containing extracts and antibodies were incubated in the reaction mixture for 1 h on ice, following which the labelled oligos were added and incubation was continued for another 30 min. Incubations were conducted with or without various amounts of competitor DNA, and total DNA concentration was maintained constant by including in the binding reaction mixtures appropriate amounts of non-specific doubled-stranded oligos (5′-ACACTGAAGTGCACCTCTTGAAGGTTGG-3′ for NR1 and for studies of binding of in vitro-translated CAR–RXR; 5′-CTAAAAGCTTTTAG-3′ for NR2 and NR3/ER-7). All antibodies used for supershift analyses were from Santa Cruz Biotechnologies (Santa Cruz, CA, U.S.A.). They included anti-HNF-4α (sc-6556X), anti-RXR (sc-774), anti-PBX1,2,3 (sc-888X), anti-PBX1 (sc-889), anti-PBX2 (sc-890X), anti-Prep1 (sc-6245X), anti-MEIS1 [anti-(myeloid ectopic viral integration site 1); sc-10596X], anti-TRβ (sc-738X) and anti-UR [anti-(ubiquitous receptor); sc-1591X].
Binding reaction mixtures (8 μl) were loaded on to pre-run polyacrylamide (6–9%) gels and subjected to electrophoresis for 2–2.5 h at 10.5 V/cm. PhosphorImager screens (Molecular Dynamics, Amersham Biosciences) were exposed to the dried gels for appropriate periods and images were obtained using a Storm 860 PhosphorImager (Molecular Dynamics; Amersham Biosciences).
RESULTS
CAR activates PBRU- or PBREM-driven transcription from the basal CYP2B2 promoter, but is inactive when the enhancers are present in the natural sequence context
In HepG2 cells, exogenous CAR gives a 2–10-fold activation of reporter gene transcription driven by the mouse Cyp2b10 51 bp PBREM placed directly upstream of the enhancerless tk promoter (the tk context) [16,20]. Exogenous RXR is not required for this response [16,20], indicating that HepG2 cells already contain it. CAR also activated luc reporter gene transcription in HepG2 cells when the CYP2B2 PBRU or PBREM was placed directly adjacent to the 120 bp CYP2B2 basal promoter (−120 context) (Figure 1B), as well as when the enhancers were placed in the tk context (results not shown). However, when the CYP2B2 PBRU or PBREM was placed in the natural sequence context, essentially no activation was observed (Figure 1B). This result suggests that HepG2 cells may lack transcription factors required for CAR–RXR-mediated transcriptional activation via the CYP2B2 PBRU in the natural sequence context, and that a search for transcription factors in addition to, or other than, CAR that bind to the NR or other elements of the PBRU might be fruitful.
HNF-4 binds to the GRE (glucocorticoid response element)-like sequence of the PBRU, and the GRE1m1 mutation increases the affinity for HNF-4
Mutation of a GRE-like sequence immediately upstream of the NR1 site reduces PB responsiveness conferred by the PBRU to a similar extent to that of mutations that abolish the NR1 site [10,14]. The GRE-like site overlaps with the NR1 site, but the mutation (GRE1m1; ACA→GTG) is outside NR1 (Figure 1A).
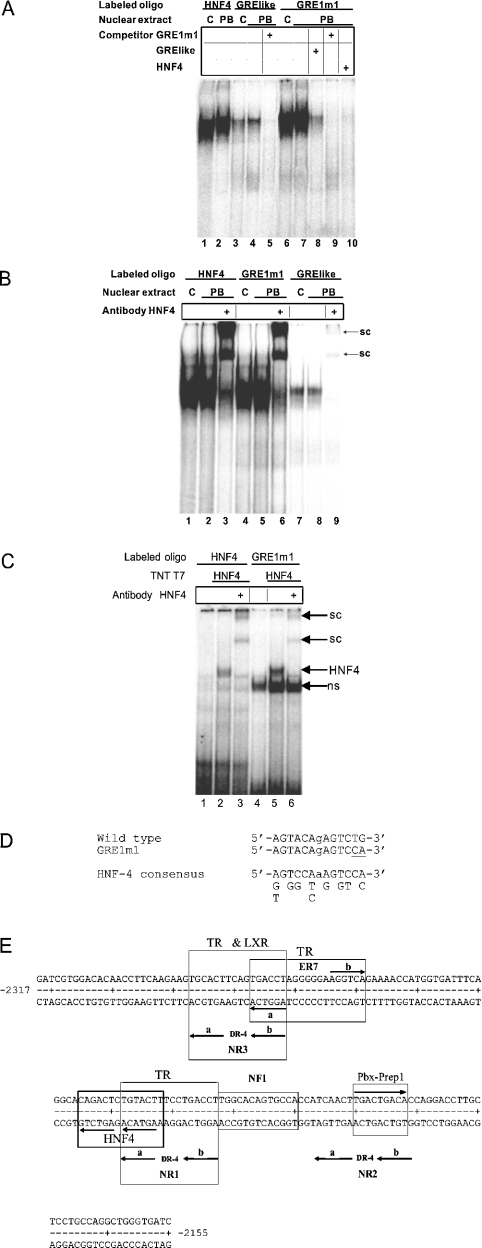
EMSA analysis using oligos related to the GRE-like sequence (Table 2) was undertaken to identify one or more proteins binding to this region that might explain the effect of the GRE1m1 mutation on PB responsiveness. When the putative GRE was the labelled oligo, a retarded complex was formed from a protein or proteins present at similar levels in nuclear extracts from untreated (Figure 2A, lane 3) and PB-treated (Figure 2A, lane 4) rats, which was absent in the presence of an unlabelled GRE1m1 competitor (Figure 2A, lane 5). When the labelled oligo contained the GRE1m1 sequence, the retarded complex was much more prominent (Figure 2A, lanes 6 and 7). It was undetectable in the presence of an unlabelled GRE1m1 competitor (Figure 2A, lane 9), and its level was reduced in the presence of the wild-type GRE competitor (Figure 2A, lane 8). The retarded complex formed using the labelled GRE1m1 oligo was of similar intensity to, and co-migrated with, a retarded complex formed with an oligo containing the HNF-4 site of the human apoB (apolipoprotein B) gene [32] (Figure 2A, lanes 1 and 2). Furthermore, the retarded complex was undetectable when the unlabelled apoB HNF-4 oligo was used as a competitor in EMSA analysis with the labelled GRE1m1 oligo (Figure 2A, lane 10). Supershift analysis using anti-HNF-4 antibody confirmed that the retarded complex formed with the labelled GRE1m1 oligo indeed contained HNF-4. In the presence of anti-HNF-4, the retarded complex formed with the labelled wild-type GRE oligo was eliminated, and barely detectable supershifted complexes were formed (Figure 2B, cf. lane 8 with lane 9). These supershifted complexes co-migrated with the much more prominent supershifted complexes formed in the presence of anti-HNF-4 with the labelled apoB HNF-4 oligo (Figure 2B, lane 3), and with the labelled GRE1m1 oligo (Figure 2B, lane 6). In all cases tested, similar results were obtained with nuclear extracts from livers of untreated and PB-treated rats (Figure 2A, cf. lane 1 with lane 2, lane 3 with lane 4, and lane 6 with 7; Figure 2B, cf. lane 1 with lane 2, lane 4 with and lane 5, and lane 7 with lane 8). The presence of an HNF-4 binding site in the GRE1m1 oligo was confirmed by coupled in vitro transcription and translation. When GRE1m1 was the labelled oligo, the presence of in vitro-translated HNF-4 led to the presence of a retarded complex (Figure 2C, cf. lane 4 with lane 5), which was disrupted and replaced by supershifted complexes in the presence of anti-HNF-4 (Figure 2C, lane 6). Similar results were obtained with the labelled apoB HNF-4 oligo (Figure 2C, lanes 1–3). Taken together, these results demonstrate that HNF-4 present in crude rat liver nuclear extracts binds to the GRE-like sequence of the PBRU, and that the GRE1m1 mutation increases affinity for HNF-4. The sequence of the high-affinity HNF-4 binding site present in the GRE1m1 sequence (5′-AGTACAgAGTCCA-3′) is a better fit than the corresponding wild-type sequence (5′-AGTACAgAGTCTG-3′) to the HNF-4 consensus of Rajas et al. [33] (Figure 2D). The relation of the HNF-4 site to the NR1 site with which it shares a half-site is shown in Figure 2(E).
Figure 2. HNF-4 binds to the putative GRE sequence adjacent to NR1 and the GRE1m1 mutant sequence has a higher affinity for the HNF-4 binding site.
The EMSA analysis using nuclear extract from livers of untreated (C) or PB-treated (PB) rats was conducted as described in the Experimental section. PB-treated rats were injected intraperitoneally with PB daily for 3 days at a dose of 75 mg/kg. (A) EMSA and competition analyses. Unlabelled competitor oligos, when present, were at 100 ng per assay. (B) Supershift assays were conducted as described in the Experimental section. sc, supershifted complex. (C) HNF-4 obtained by coupled in vitro transcription and translation of pBluescript-HNF-4α, conducted as described in the Experimental section, binds to the HNF-4 oligo and to the GRE1m1 oligo. sc, supershifted complex; ns, complex due to proteins present in the reticulocyte extract. (D) Comparison of the sequences of the wild-type HNF-4 site that overlaps the NR1A half-site with that of the GRE1m1 mutant sequence (the mutated bases are underlined) and with the consensus of Rajas et al. [33]. (E) DNA sequence of the rat CYP2B2 PBRU showing the positions of transcription factor binding sites identified in the present study. The HNF-4 site shares a half-site with NR1 as shown. The PBX–PREP1 binding site includes the NR2 spacer and the NR2B half-site, as shown. TRβ binds to the NR1 and NR3 DR-4 sites and to the ER-7 site as shown, but not to the NR2 DR-4 site. LXR binds to the NR3 DR-4 site as shown, but not detectably to NR1 or NR2.
The putative GRE is not required to maintain full PB responsiveness
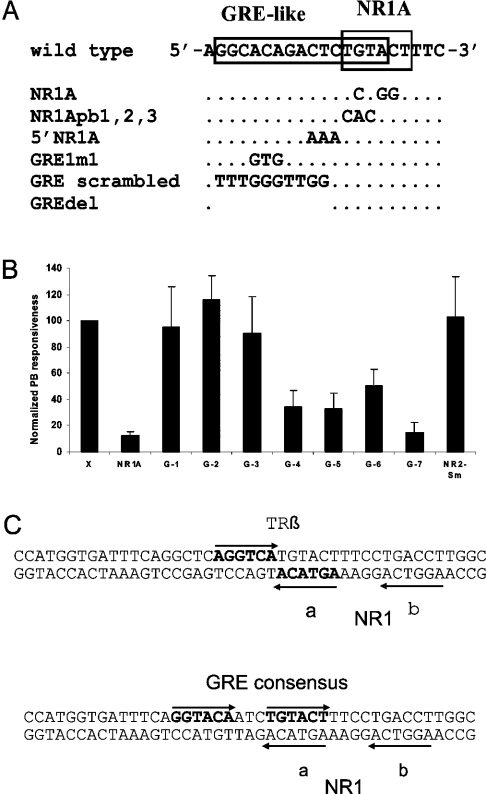
The HNF-4 binding site overlaps the NR1A half-site and extends upstream therefrom, and it thus contains sequences inside and outside of NR1 (Figures 1 and 2E). To define the sequence of the HNF-4 binding site, several oligos containing base changes at specific sites within the putative GRE (Figure 3A) were used as labelled oligos or competitors in EMSA analyses. The corresponding mutant sequences, as well as another mutant sequence, GREdel, in which GRE-like sequences outside of NR1A were deleted (Figure 3A), were also introduced into the PBRU in the natural sequence context, and their effects on PB responsiveness were determined by transfection analysis in primary rat hepatocytes. Mutations abolishing the NR1A site, such as the central substitution used previously to inactivate NR1A [14,16], abolish NR1 activity in transfection assays [14] (Figure 3B) and eliminate HNF-4 binding to the corresponding oligo (results not shown). Similar results were obtained with an additional NR1 mutation affecting three NR1A positions (NR1Abp1,2,3; results not shown). For mutations affecting the GRE-like sequence, but situated outside NR1A, affinity for HNF-4 tended to correlate inversely with PB responsiveness. Thus for the previously characterized GRE1m1 mutation, affinity for HNF-4 is increased (Figure 2), but PB responsiveness was reduced [10,14] (Figure 3B, bar G-5). (The residual PB responsiveness observed with the NR1A mutation, and presumably also for the NR1Abp1,2,3 and GRE1m1 mutations, depends upon an active NR2 sequence and upon other sequences within the PBRU [14]). However, for a mutation affecting 3 bp immediately upstream of NR1A (5′NR1A), and for a mutation in which every position but one upstream of NR1A in the putative GRE was changed (GRE scrambled), PB responsiveness was maintained at wild-type levels or above (Figure 3B, bars G-1 and G-2), but oligos containing them fail to compete for HNF-4 binding to GRE or GRE1m1 oligos in EMSA assays (results not shown). Furthermore, the GREdel mutant also retained full PB responsiveness (Figure 3B, bar G-3). Finally, when the GREdel mutation was combined with an NR1B half-site mutation, the level of PB responsiveness was reduced, but not abolished (Figure 3B, bar G-7), as is the case for simple half-site mutations [14] (Figure 3B, bar NR1A). Hence, in the natural sequence context, HNF-4 binding to its cognate recognition site is not required for PB responsiveness and the effect of increased HNF-4 binding, in the case of the GRE1m1 mutation, is to reduce PB responsiveness.
Figure 3. Assays of PB responsiveness in primary hepatocytes conferred by various mutants affected in the region of the overlapping HNF-4/NR1 site or in NR2.
(A) Sequences of the mutants affected in the region of the overlapping HNF-4/NR1 site. Only the upper strand is shown. NR1A, the inactivating half-site mutation used previously [14]; NR1Apb1,2,3, an additional NR1A half-site mutation; 5′NR1A, a mutation changing 3 bp on the upstream side of NR1; GRE1m1, the GRE mutant characterized previously [10,14]; GRE scrambled, a mutation in which the GRE-like sequence was scrambled; GREdel, a mutation in which 10 bp of the GRE-like sequence was deleted. (B) Assays for PB responsiveness by transfection of primary rat hepatocytes with mutant plasmids in which the PBRU was in the natural sequence context. X, the wild-type sequence; NR1A, the NR1A half-site mutation [14]; G-1, 5′NR1A; G-2, GRE scrambled; G-3, GREdel; G-4, the GRE-like sequence was converted into a GRE consensus sequence [see (C)]; G-5, GRE1m1; G-6, the GRE-like sequence was converted into a TRβ consensus sequence [see (C)]; G-7, a double mutant with GREdel and an NR1B half-site mutation [14]; NR2-Sm, a spacer mutation of NR2 (see Figure 4). The results were normalized by comparison with the fold-induction of the X construct in parallel assays with the same hepatocyte preparation. Results were derived from duplicate assays using hepatocytes of four to nine rats per construct. The average values for the fold induction by PB varied from 27 to 54 in these experiments, a similar range to that observed previously with this system [14]. The error bars show the S.D. By Student's unpaired two-tailed one sample t test assuming unequal variance, the X construct does not differ significantly from G-1, G-3 or NR2-Sm (P≥0.4) or from G-2 (P=0.051), but differs from all the others (P<0.001). By Student's unpaired two-tailed two sample t test assuming unequal variance, G-1, G-2 and G-3 are not significantly different from each other at the 95% confidence level; nor are G-4, G-5 and G-6 or NR1A and G-7; NR1A and G-7 are different from G-4 to G-6 (P<0.01) and from G-1 to G-3 (P<0.001); and G-4 to G-6 are different from G-1 to G-3 (P<0.01). (C) The sequences, in bold characters, of the GRE consensus and the TRβ consensus mutants. Both mutant sequences overlap the NR1A half-site as shown. The GRE consensus mutant sequence differs from the GRE consensus of Evans [60] at a single position. The TRβ consensus sequence differs from the perfect TREpal sequence [61] at two positions.
The results described immediately above suggest that increased HNF-4 binding to the overlapping NR1A site may inhibit PB responsiveness by competing for binding of an activating transcription factor (perhaps CAR–RXR) to NR1. In accordance with this, when the HNF-4 site was converted by mutation into a consensus GRE site or into a consensus TRβ site, overlapping but not modifying the NR1A half-site (Figure 3C), PB responsiveness was reduced to a level similar to that of the GRE1m1 mutant (Figure 3B, bars G-4 and G-6). Hence all three of these mutants may exhibit reduced PB responsiveness by a similar mechanism; that is, the creation of a binding site overlapping NR1A, which results in a competition for binding of an activating transcription factor.
Mutation of the NR2 spacer destroys a transcription factor recognition site
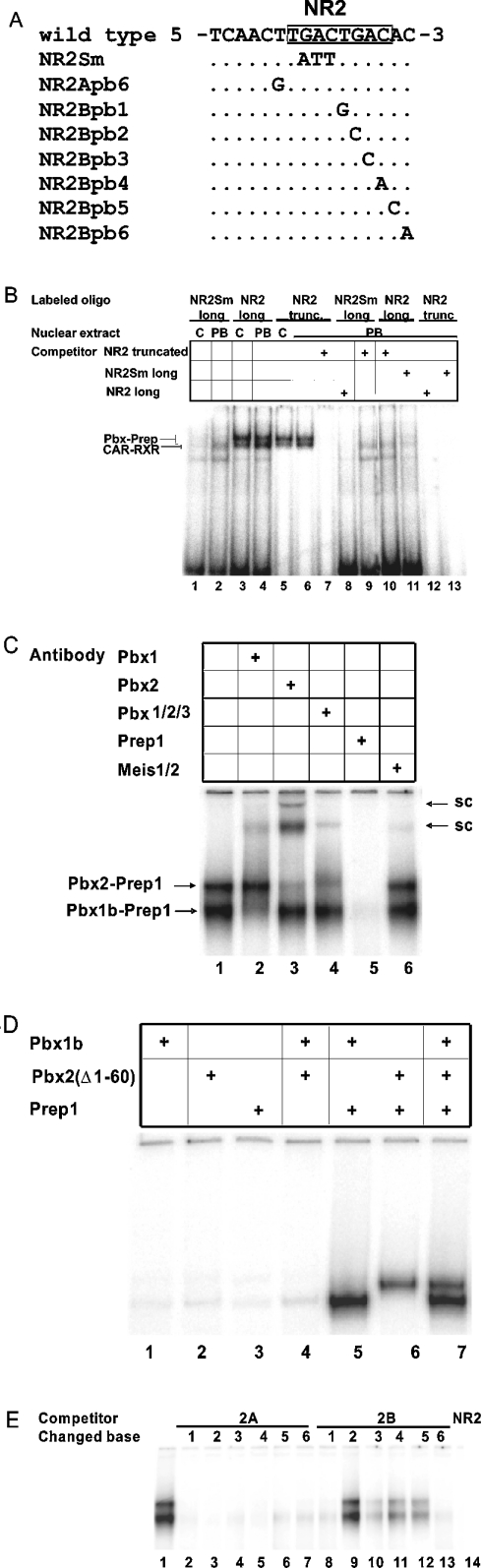
A 3 bp mutation in the NR2 spacer (GAC→ATT; NR2-Sm) (Figure 4A) had no discernable effect on PB responsiveness (Figure 3B, NR2-Sm). EMSA analysis using NR2- and NR2-Sm-related labelled oligos (Table 3) showed that the NR2-Sm mutation abolished a transcription factor recognition site (Figure 4B). A retarded complex, in the form of a characteristic doublet, was evident with the full NR2 sequence as labelled oligo (Figure 4B, lanes 3 and 4) and, at reduced intensity, with a truncated version of NR2 as labelled oligo (Figure 4B, lanes 5 and 6). This doublet was undetectable when NR2-Sm was the labelled oligo (Figure 4B, lanes 1 and 2). In the presence of an excess of unlabelled truncated NR2 oligo as competitor, the characteristic doublet was undetectable with the labelled truncated NR2 oligo (Figure 4B, lane 7), and with the labelled NR2 oligo (Figure 4B, lane 10). It was also undetectable with the NR2 oligo as competitor and truncated NR2 as the labelled oligo (Figure 4B, lane 12). In the presence of unlabelled NR2-Sm oligo as competitor, the signal of the characteristic doublet was dramatically reduced with NR2 as labelled oligo (Figure 4B, lane 11), and was undetectable with the truncated NR2 as labelled oligo (Figure 4B, lane 13). Hence the mutant NR2-Sm oligo retains only marginal affinity for the proteins responsible for the formation of the characteristic doublet. A faster-migrating, non-specific complex was present when NR2 or NR2-Sm was the labelled oligo (Figure 4B, cf. lanes 1–4 with lanes 8–11), but not when the truncated version of NR2 was the labelled oligo (Figure 4B, lanes 5–7 and 11–12).
Figure 4. PBX–PREP1 heterodimers bind to a recognition site within NR2.
(A) The wild-type sequence of part of the upper strand of the NR2 site showing the PBX–PREP consensus sequence (boxed), as well as the sequence of the NR2 spacer mutation (NR2Sm) and seven single-base changes introduced into NR2 oligo sequences as shown. (B) EMSA analysis using nuclear extracts from livers of untreated (C) or PB-treated (PB) rats. The long NR2 and NR2-Sm oligos had the full-length NR2 sequence plus several base-pairs on each side, and the NR2 truncated sequence was missing 1 bp of the NR2B half site (see Table 3). Competitors, when present, were at 100 ng per assay. (C) The proteins responsible for the characteristic doublet seen with NR2 were identified by supershift analysis as PBX2–PREP1 and PBX1b–PREP1. sc, supershifted complex. (D) PBX proteins and PREP1 obtained by coupled in vitro transcription and translation bound as heterodimers to the NR2 oligo. (E) Competition assays to delineate the PBX–PREP1 binding site. The sequences of the competitor oligos, present at 100 ng per assay, are provided in (A).
Table 3. Oligos used for EMSA analysis of the PBX-PREP site of the PBRU.
Only the sequence of the upper strand is shown. Bases differing from wild-type are shown in lower case letters. The 5′ and 3′ coordinates are those of the rat CYP2B2 5′ flank.
| Oligo | 5′-end | Sequence | 3′-end |
|---|---|---|---|
| NR2 | −2204 | CCATCAACTTGACTGACACCAG | −2183 |
| 2A1 | −2204 | CCAgCAACTTGACTGACACCAG | −2183 |
| 2A2 | −2204 | CCATgAACTTGACTGACACCAG | −2183 |
| 2A3 | −2204 | CCATCcACTTGACTGACACCAG | −2183 |
| 2A4 | −2204 | CCATCAcCTTGACTGACACCAG | −2183 |
| 2A5 | −2204 | CCATCAAaTTGACTGACACCAG | −2183 |
| 2A6 | −2204 | CCATCAACgTGACTGACACCAG | −2183 |
| 2Sm | −2204 | CCATCAACTTattTGACACCAG | −2183 |
| 2B1 | −2204 | CCATCAACTTGACgGACACCAG | −2183 |
| 2B2 | −2204 | CCATCAACTTGACTcACACCAG | −2183 |
| 2B3 | −2204 | CCATCAACTTGACTGcCACCAG | −2183 |
| 2B4 | −2204 | CCATCAACTTGACTGAaACCAG | −2183 |
| 2B5 | −2204 | CCATCAACTTGACTGACcCCAG | −2183 |
| 2B6 | −2204 | CCATCAACTTGACTGACAaCAG | −2183 |
| NR2 long | −2210 | GTGCCACCATCAACTTGACTGACACCAGGACCTTGC | −2175 |
| NR2Sm long | −2210 | GTGCCACCATCAACTTattTGACACCAGGACCTTGC | −2175 |
| NR2 truncated | −2200 | CAACTTGACTGACAC | −2186 |
With respect to the characteristic doublet, similar results were obtained with nuclear extracts of livers of untreated or PB-treated rats (Figure 4B, cf. lane 1 with lane 2, lane 3 with lane 4, and lane 5 with lane 6). However, a difference was evident with respect to another, slightly faster migrating, but much less prominent, retarded complex, which may be due to CAR–RXR heterodimers present in the nuclear extract from PB-treated rats bound to NR2-Sm (Figure 4B, lane 2). The putative CAR–RXR retarded complex was not detectable with NR2-Sm as labelled oligo and nuclear extract from untreated rats (Figure 4B, lane 1). It was also undetectable in the presence of a wild-type NR2 competitor with NR2-Sm as labelled oligo and nuclear extract from PB-treated rats (Figure 4B, lane 8), as was expected, given that CAR–RXR heterodimers bind to NR2 [20]. Finally, the putative CAR–RXR-retarded complex was not competed by the truncated NR2 oligo (Figure 4B, lane 9), also as expected, since the NR2 DR-4 sequence is incomplete in the truncated oligo (Table 3).
PBX1b–PREP1 and PBX2–PREP1 bind to NR2
Inspection of the NR2 spacer and NR2B half-site revealed the presence of a sequence (TGACTGAC, Figure 4A; see also Figure 2E) that fits with a single mismatch to the bipartite consensus binding site for PBX nuclear protein complexes (TGATTGAC) of Knoepfler and Kamps [34]. Heterodimerization of PBX and PREP1 proteins results in high-affinity DNA-binding complexes [28]. PBX and PREP proteins are members of the TALE (three-amino-acid extension) superclass of homoeodomain proteins [35–37]. The PBX proteins, PBX1, PBX2 and PBX3, belong to the PBC class of TALE proteins and are encoded by different genes [35,38]. Two forms of PBX1 and PBX3, designated a and b, are produced by alternative splicing near the 3′ end of their transcripts, such that the alternative b forms are C-terminally truncated and thus smaller than the full-length PBX1a, PBX2 and PBX3a forms [38]. PREP1 belongs to the MEINOX class of TALE proteins, which also includes the MEIS protein [28,35]. EMSA analysis using the wild-type labelled NR2 oligo along with antibody supershift experiments were thus undertaken to determine if the characteristic doublet complex was due to PBX–PREP1 heterodimers. Such doublets have been observed with PBX–PREP1 complexes associated with regulatory sequences of several other genes [28,39–41].
In the presence of anti-PBX1 antibody, the slower migrating retarded complex was undisturbed, but the faster moving complex was partially disrupted and a supershifted complex was generated (Figure 4C, cf. lane 2 with lane 1). In the presence of anti-PBX2 antibody on the other hand, the faster migrating retarded complex was undisturbed, but the slower moving complex was partially disrupted and a supershifted complex was generated (Figure 4C, cf. lane 3 with lane 1). A similar result was obtained with the anti-PBX1/2/3 antibody (Figure 4C, cf. lane 4 with lane 3). The anti-PBX1/2/3 antibody recognizes all three PBX3 proteins, PBX1, PBX2 and PBX3, but only the full-length and not the C-terminally truncated isoforms PBX1b and PBX2b. Hence the C-terminally truncated PBX1b isoform is present in the faster moving complex and PBX2 is present in the slower migrating complex. PREP1 is a commonly observed heterodimer partner of PBX proteins [28]. In the presence of anti-PREP1 antibody, both components of the doublet were absent (Figure 4C, lane 5). MEIS1 is another possible heterodimerization partner of PBX forms [42]. In the presence of the anti-MEIS1 antibody, the characteristic doublet was largely undisturbed, but a faint retarded complex was detected (Figure 4C, lane 6). Hence PBX1b–PREP1 and PBX2–PREP1 heterodimers bind to NR2. Although PREP1 is the major heterodimerization partner of the PBX proteins bound to the NR2 oligo, MEIS1 may also be present in the retarded complexes at a low level.
The binding of PBX1b–PREP1 and PBX2–PREP1 complexes to the NR2 oligo was confirmed by coupled in vitro transcription and translation. A control in vitro transcription and translation reaction mixture gave background levels of retarded complexes binding to the labelled NR2 oligo (Figure 4D, lane 1). PBX1b alone, PBX2 alone, PREP1 alone and PBX1b with PBX2 gave no increase over background (Figure 4D, lanes 2–4). However, PBX1b together with PREP1 gave a faster moving retarded complex (Figure 4D, lane 5), PREP2 together with PREP1 gave a slower moving complex (Figure 4D, lane 6), and PBX1b, PBX2 and PREP1 together gave a doublet (Figure 4D, lane 7). These results confirm that the NR2 oligo contains a binding site for PBX1b–PREP1 and PBX2–PREP1 heterodimers.
To delimit the extent of the PBX-PREP1 binding site, single bp changes were introduced into the A and B half-sites of the NR2 oligo (Figure 4A), and their effects on PBX–PREP binding to the labelled wild-type NR2 oligo were determined in competition assays. Changing NR2Bpb2 from G to C dramatically reduced competition (Figure 4E, cf. lane 1 with lane 9), single bp changes at NR2B positions 3, 4 and 5 caused a more modest reduction (Figure 4E, lanes 10–12), and changes at other NR2A and NR2B positions had little or no effect (Figure 4E, lanes 2–8 and lane 13). These results, along with the abolition of PBX–PREP binding by the NR2-Sm mutation, define the minimal critical region for PBX–PREP binding to the NR2 oligo as CTGACA, and thus confirm that the TGACTGAC sequence forms part of the PBX–PREP1 binding site (Figure 4A).
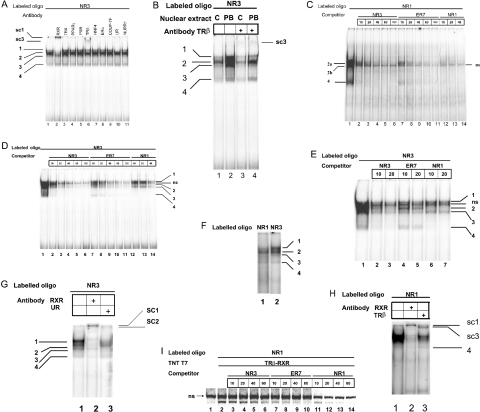
TRβ and LXR bind to NR3 and NR1
A complex pattern of at least four retarded complexes was observed when NR3 was used as the labelled oligo in EMSA analysis with rat liver nuclear extract (Figures 5A and 5B). A major retarded species, complex 2, and three minor species, complexes 1, 3 and 4, were observed (Figures 5A and 5B, lanes 1). Antibodies recognizing 10 different nuclear receptors were tested to determine whether the retarded complexes would be disrupted or whether supershifted complexes would be formed. Anti-RXR disrupted complexes 1 and 2, and led to the formation of a prominent supershifted complex (Figure 5A, lane 2). Anti-TRβ left complexes 1 and 4 largely intact and appeared to reduce the signal intensity of complexes 2 and 3 (Figure 5B, cf. lane 3 and with lanes 1 and 2). In any case the presence of anti-TRβ also led to the formation of a retarded complex (Figure 5A, lane 6 and Figure 5B, lane 4). An antibody recognizing both LXRα and LXRβ, anti-UR, left complexes 2 and 4 intact, but disrupted complex 1 and perhaps complex 3 (Figure 5A, lane 10). None of the other antibodies tested had detectable effects on the retarded complexes (Figure 5A, lanes 3–5, 7–9 and 10–11). The signal intensity for all retarded complexes was greater for extracts from PB-treated rats than for those from untreated rats (Figure 5B, cf. lane 1 with lane 2), suggesting that PB treatment may, directly or indirectly, increase nuclear levels of several transcription factors. This effect was not generalized, however, because it was not observed for HNF-4 or PBX–PREP (Figures 2 and 4).
Figure 5. EMSA analysis of rat liver nuclear proteins that bind to NR3 and to NR1.
(A) Supershift analysis of liver nuclear proteins from PB-treated rats that bind to NR3 using antibodies against 10 different nuclear receptors. Retarded complexes 1–4 are indicated. Supershifted complexes were visible with anti-RXR (sc1) and anti-TRβ (sc3). The anti-UR antibody disrupted the slowest moving retarded complex (complex 1), and perhaps complex 3 as well, but here it did not give a detectable supershifted complex. TR4, testicular orphan receptor 4; PPARα, peroxisome-proliferator-activated receptor α; ERα, oestrogen receptor α; COUP-TF, chicken albumin upstream-promoter transcription factor; NURR-1, Nur-related factor 1. (B) Supershift analysis of liver nuclear proteins from untreated or PB-treated rats that bind to NR3 using anti-TRβ. Complexes 1, 2, 3 and 4 are better resolved here than in (A). (C) Competition analysis of nuclear proteins from PB-treated rats binding to NR1 using 10–100 ng of unlabelled NR1, NR3 or ER-7 competitor, as shown. Retarded complexes 2a, 2b and 4 are indicated. ns, non-specific. (D) Competition analysis of liver nuclear proteins from PB-treated rats binding to NR3 using 10–100 ng of unlabelled NR1, NR3 or ER-7 competitor, as shown. Retarded complexes 1–4 are indicated. ns, non-specific. (E) Competition analysis of liver nuclear proteins from PB-treated rats binding to NR3 using 10 or 20 ng of unlabelled NR1, NR3 or ER-7 competitor as shown. Retarded complexes 1–4 are indicated. ns, non-specific. (F) Parallel EMSA analysis with NR1 and NR3 as labelled oligos using liver nuclear proteins from PB-treated rats. (G) Supershift analysis with NR3 as labelled oligo using liver nuclear proteins from PB-treated rats in the presence of anti-RXR and anti-UR, as shown. Retarded complexes 1–4 are indicated and sc1 and sc2 are the supershifted complexes generated by anti-RXR and anti-UR respectively. (H) Supershift analysis with NR1 as labelled oligo using liver nuclear proteins from PB-treated rats in the presence of anti-RXR and anti-TRβ antibodies, as shown. Retarded complex 4 is indicated and sc1 and sc3 are the supershifted complexes generated by anti-RXR and anti-TRβ respectively. (I) Competition analysis with NR1 as the labelled oligo for binding of in vitro-synthesized rat TRβ and RXR with 10–60 ng of unlabelled NR3, NR1 or ER-7 competitor, as shown. ns, retarded complex formed from proteins present in the in vitro transcription–translation reaction mixture.
When NR1 was used as the labelled oligo, a pattern of retarded complexes similar to that for NR3 was observed, except that for NR1 two major complexes, apparently corresponding to complexes 2 and 4 of NR3, were predominant (cf. Figure 5C, lane 1 with Figure 5A, lane 1 and Figure 5B, lane 2). Similar profiles of retarded complexes on NR1 derived from mouse or rat liver nuclear proteins have been observed previously [16,24]. These complexes were efficiently competed by the unlabelled oligos NR1 (Figure 5C, lanes 12–14), NR3 (Figure 5C, lanes 2–6) and ER-7 (Figure 5C, lanes 7–11). The competition by ER-7 strongly suggests that the specific retarded complexes were due to binding of one or more nuclear receptors. The competition analysis also revealed that complex 2 consisted of a doublet (Figure 5C, lanes 2 and 7), identified as 2a and 2b in Figure 5(C). Furthermore, complex 2b was seen to be superimposed upon a non-specific complex.
A similar competition analysis using NR3 as the labelled oligo (Figures 5D and 5E) revealed that the profile of retarded complexes was indeed more complex than that observed for NR1. Complexes 1 to 4 were clearly resolved, notably in the presence of 10 or 20 ng of ER-7 competitor (Figure 5D, lanes 7 and 8; Figure 5E, lanes 4 and 5) and an additional non-specific complex was also evident. Complex 1 was poorly competed by NR1 as compared with NR3 (Figure 5D, cf. lanes 13 and 14 with lanes 3 and 4; Figure 5E, cf. lanes 6 and 7 with lanes 2 and 3). On the other hand, complex 2 was efficiently competed by NR1 and NR3 (Figure 5E, lanes 2, 3, 6 and 7), and less so by ER-7 (Figure 5E, lanes 4 and 5). The results for complex 3 were similar to those for complex 1. Hence the proteins responsible for complex 2 recognize both NR1 and NR3, whereas those responsible for complexes 1 and 3 bind preferentially to NR3.
Parallel EMSA analysis with NR1 and NR3 as labelled oligos confirmed that complexes 1 and 3 were reduced or absent with NR1 as compared with NR3 (Figure 5F). High-resolution super-shift analysis with NR3 as labelled oligo confirmed that complexes 1 and 3 were disrupted by anti-UR with formation of a faint supershifted complex (Figure 5G, lane 3), indicating that they both contain LXR. This analysis using NR3 as labelled oligo also showed that anti-RXR disrupted complexes 1 and 2, but left complexes 3 and 4 largely intact, with formation of a super-shifted complex that did not enter the gel (Figure 5G, lane 2). Thus complex 1 appears to contain LXR–RXR heterodimers. Supershift analysis with NR1 as the labelled oligo gave results consistent with the conclusion that LXR does not form detectable complexes with NR1: anti-UR treatment did not lead to the formation of a detectable supershifted complex (results not shown), and anti-RXR produced a supershifted complex, but left no band at the position of complex 3 (Figure 5H, lane 2). For NR1, anti-TRβ produced a supershifted complex, and reduced the intensity of complex 2 (Figure 5H, lane 3).
The binding of TRβ–RXR heterodimers to the NR1 oligo was confirmed by coupled in vitro transcription and translation analysis. A retarded complex was formed with the NR1 oligo from proteins present in the in vitro transcription–translation mixture (Figure 5I, lane 1), and an additional TRβ–RXR complex was evident in the presence of in vitro-translated TRβ and RXR (Figure 5I, lane 2). The TRβ–RXR complex was reduced in the presence of increasing amounts of unlabelled NR3 (Figure 5I, lanes 3–6), and somewhat more efficiently by increasing amounts of unlabelled NR1 (Figure 5I, lanes 11–14) or ER-7 (Figure 5I, lanes 7–10). Similar results were obtained when ER-7 was used as the labelled oligo (results not shown). These results indicate that TBβ–RXR heterodimers bind to NR3, and, with higher affinity, to NR1 and ER-7 (see Figure 2E).
DISCUSSION
The orphan nuclear receptor CAR is essential for induction of CYP2B genes by PB and by PB-like inducers [43,44]. Although many details of the process remain to be clarified [4], CAR is thought to mediate PB responsiveness by binding to the NR1 element of the PBRU following PB-type inducer-dependent nuclear accumulation [16,17,21]. Supporting evidence for a role for CAR in activating CYP2B transcription is provided by the capacity of CAR to activate PBREM-driven transcription of reporter genes in the tk sequence context in HepG2 cells [16,20]. The results presented here confirm that CAR activates reporter gene transcription with both the heterologous tk and homologous CYP2B2 basal promoters in the tk and −120 sequence contexts. However, the failure of CAR to activate reporter gene transcription when the PBRU was placed in the natural context at nt −2317/−2155 in the CYP2B2 5′-flank suggests that other factors may be required to activate PBRU-driven transcription. Furthermore, there is reason to believe that something more than CAR binding to NR elements may be required for transcriptional activation via the PBRU. CAR–RXR heterodimers bind with similar affinities to NR1 and NR2 [20], and the quantitative contribution of NR1 and NR2 to PB responsiveness is similar [14,16]; yet NR1 and NR2 are not functionally equivalent in conferring PBRU-driven PB responsiveness in the natural sequence context: NR1 can replace NR2, but NR2 cannot replace NR1 [14]. Similarly, βRARE, a higher affinity site for CAR–RXR binding than NR1 or NR2 [20], cannot replace NR1, but can replace NR2 in conferring PBRU-driven PB responsiveness in the natural sequence context [14]. CAR–RXR heterodimers also bind to the upstream NR3 site of the CYP2B2 PBRU [13]. The half-sites of NR3 are identical with those of NR1, except for a single base-pair difference (Figure 1A), and mutational inactivation of a single NR3 half-site has a similar effect on PB responsiveness as mutational inactivation of any NR1 or NR2 half-site [14]. Furthermore, using the same assay system we have recently shown that NR3 is functionally equivalent to NR1 in conferring PBRU-driven PB responsiveness in the natural sequence context (M.-J. Beaudet, Y. Paquet, D. Claveau, M. Laizier and A. Anderson, unpublished work). Taken together, these considerations suggest that identification of additional transcription factors binding to NR1 and NR3, but not to NR2, might prove fruitful. On the other hand, they also suggest that any transcription factor binding to NR1 or NR3, but not both, is unlikely to be required for conferring PB responsiveness.
The results obtained here demonstrate that more transcription factors bind to the CYP2B2 NR elements than was previously thought [4,24]. An HNF-4 binding site overlaps the NR1 site and includes the NR1A half-site (Figure 2E). However, HNF-4 does not seem to play a positive role in activating PB-dependent CYP2B transcription. If anything, it plays a negative role, given that the GRE1m1 mutation, which increases affinity for HNF-4, reduces PB responsiveness. It remains to be seen whether, under certain physiological conditions, HNF-4 binding plays a regulatory role in controlling the level of PB induction. The properties of the GRE1m1 mutation had suggested that a positive element is present immediately adjacent to NR1 in the PBRU. The results presented here suggest rather that the lowered PB responsiveness of the GRE1m1 mutant is due to interference by HNF-4 with the binding of a positively acting factor, perhaps CAR–RXR, to the NR1 site. This conclusion is consistent with the results of Rivera-Rivera et al. [23], who concluded from deletion analysis of the mouse Cyp2b10 PBRU using tail-vein injection of DNA that the GRE does not contribute to PB-dependent activation.
The binding of PBX–PREP heterodimers to NR2 (Figure 2E) is clearly not required for activity of the NR2 site, given that the NR2-Sm mutation abolishes PBX–PREP binding, but does not affect PB responsiveness. However, PBX–PREP1 heterodimers often interact with other factors to activate transcription [45–47], and it remains to be determined whether PBX–PREP binding may, under some conditions, interact with CAR–RXR or other nuclear receptors that bind to NR2 to modulate PB responsiveness.
Muangmoonchai et al. [24] analysed rat liver nuclear proteins binding to the NR1 and NR2 sites of the CYP2B1 gene, and the EMSA profiles they observed were very similar to those seen here. They found that unlabelled NR1 did not compete for proteins bound to NR2 and vice versa, and concluded, in agreement with our results, that NR1 and NR2 bind different rat liver nuclear proteins. However, they also observed that the characteristic doublet obtained with NR2 was competed, albeit inefficiently, by an oligo corresponding to an RXRα binding site, an observation difficult to reconcile with our conclusion that the doublet represents PBX–PREP heterodimers bound to NR2. A possible resolution of this apparent paradox may be provided by the presence of two tandem -TGAC- half sites separated by 3 bp in the RXR competitor oligo used by Muangmoonchai et al. [24]. Although PBX–MEIS1 and PBX–PREP1 heterodimers typically bind to two contiguous half-sites with the consensus 5′TGACTGAT-3′ [34,48], PBX–MEIS1 can bind to tandem half-sites separated by 3, 6 or 7 bp [46,47], raising the possibility that the tandem 5′-TGAC-3′ half-sites separated by 3 bp in the RXR competitor oligo of Muangmoonchai et al. [24] may bind PBX–PREP1 heterodimers as well.
The profiles of transcription factor binding to NR1 and NR3 were similar, but they were strikingly different from, and more complex than, that of NR2. This parallels the functional differences in conferring PB responsiveness between NR1 and NR3 on the one hand, and NR2 on the other. There were at least four specific retarded complexes when NR3 was the labelled oligo (complexes 1–4), two of which were disrupted by antibodies against RXR (complexes 1 and 2), and two of which were disrupted by antibodies against LXR (complexes 1 and 3). Thus complex 1 presumably contains LXR–RXR heterodimers, and, indeed, it co-migrated with LXRα–RXR and LXRβ–RXR heterodimers obtained by coupled in vitro transcription and translation (M.-J. Beaudet and A. Anderson, unpublished work). Complex 3 may represent a heterodimer of LXR with another nuclear receptor, such as the atypical LXRα–PPARα (peroxisome-proliferator-activated receptor α) heterodimer that binds to a site in the mouse Cyp7a promoter [49]. Treatment with antibodies against TRβ reduced the intensities of complexes 2 and 3, and generated a supershifted complex. Hence TRβ seems to be present in more than one complex, perhaps reflecting its ability to bind to DNA as monomers, homodimers and heterodimers [50]. Binding of LXR and TRβ to NR3 (Figure 2E) is not unexpected, given that both can bind to DR-4 elements [50,51] and, in some cases, to the same element [52]. TRα fails to bind to NR1 [16], but heterodimers formed from in vitro-translated chicken TRα and RXR have been reported to bind to it [53].
Retarded complex 2 formed with NR3 as the labelled oligo was efficiently competed by NR1 (Figures 5D and 5E). Thus the same complex would be formed with NR1 as the labelled oligo. However, when NR1 was the labelled oligo, complex 2 appeared as a doublet, identified as 2a and 2b in Figure 5(C). Hence either complex 2a or 2b on NR1 is presumably identical with complex 2 on NR3. Another possibility is that complex 2 on NR3 is also a doublet, one component of which co-migrates with the non-specific complex seen in Figures 5(D) and 5(E). In any case, all these complexes contain RXR, as they are all efficiently supershifted by anti-RXR (Figures 5G and 5H; see also [16,24]). One dimerization partner for RXR in these complexes is doubtless TRβ. The other(s) remain to be identified, but one may be CAR.
Given that CAR–RXR heterodimers obtained by mixed in vitro translation bind to NR1, NR2 and NR3, they would be expected to bind to these sites when nuclear extracts are used a source of transcription factors as well. However, except when NR2-Sm was used as the labelled oligo, we detected no complex that could be identified as CAR–RXR heterodimers, and even then it was barely detectable. Presumably, CAR–RXR heterodimers were present when NR1 and NR3 were used as labelled oligos, perhaps as we have just seen as part of complex 2, but the band representing them may have been superimposed on another. This raises the possibility that still other complexes exist that may co-migrate with the complexes we have identified, and particularly with those designated 1–3. Candidates for such complexes include TRβ homodimers and TRβ monomers, as we have observed that TRβ–RXR heterodimers and TRβ homodimers or monomers obtained from coupled in vitro transcription and translation can bind to NR3 (M.-J. Beaudet, A. Lachaud and A. Anderson, unpublished work).
The failure, in the present study and in others, to observe identifiable CAR–RXR heterodimers bound to wild-type NR1, NR2 or NR3 sequences when nuclear extracts are used as a source of protein is striking, given the widely held view that CAR–RXR heterodimers activate PB-dependent CYP2B transcription in rat and mouse liver and in cultured hepatocytes. This observation is particularly noteworthy given that abundant retarded complexes are identifiable by EMSA analysis under these conditions, as demonstrated in the present study and by others [16,24]. Also noteworthy is the failure of CAR to activate reporter gene transcription in HepG2 cells when the PBRU or the PBREM was placed in the natural sequence context (Figure 1B). This observation raised the possibility that HepG2 cells may lack transcription factors necessary for CAR-mediated transcriptional activation. Although this may be the case, HepG2 cells have been reported to contain HNF-4 [54,55], PBX–PREP1 [45] and LXR [56–58]. TRβ is also present in HepG2 cells, albeit at low levels as compared with other hepatoma cell lines [59]. Hence the four transcription factors binding to NR-1, NR-2 or NR-3 identified here are not good candidates for factors that might stimulate CAR-mediated transcriptional activation in HepG2 cells. The proteins in the as-yet-unidentified complexes (see below) remain candidates, however.
Taken together, the results of our EMSA analysis using NR1 and NR3 as labelled oligos indicate that complex 1, which forms on NR3 but is undetectable on NR1, consists of LXR–RXR heterodimers. Complex 2 forms on both NR1 and NR3, and consists of RXR with TRβ and other as-yet-unidentified heterodimerization partner(s). Complex 3 forms largely, if not exclusively, on NR3, and contains TRβ as well as LXR, apparently with an atypical heterodimerization partner. Complex 4, which forms on both NR1 and NR3, was neither disrupted nor supershifted by any of the antibodies tested, and its identity is unknown at present. Since LXR binds to NR3, but not detectably to NR1, LXR is not likely to be required for conferring PB responsiveness.
The possible roles of TRβ–RXR and the as-yet-unidentified complexes in mediating transcriptional activation by the CYP2B2 PBRU in the natural sequence context, and more generally in conferring PB responsiveness, remain to be determined. However, the proteins of complexes 2 and 4, which bind to NR1 and NR3, but not to NR2, are potential candidates for transcription factors that mediate such activities.
Table 4. Oligos used for EMSA analysis of the NR1 and NR3 elements of the PBRU.
Only the sequence of the upper strand is shown. The 5′ and 3′ coordinates are those of the rat CYP2B2 5′ flank.
| Oligo | 5′-end | Sequence | 3′-end |
|---|---|---|---|
| NR1 | −2236 | CTCTGTACTTTCCTGACCTTGG | −2215 |
| NR3 | −2295 | AAGTGCACTTCAGTGACCTAGG | −2274 |
| ER7 | −2285 | CAGTGACCTAGGGGGAAGGTCAGAA | −2261 |
Acknowledgments
We thank Normand Marceau and members of his laboratory for advice on isolating rat hepatocytes; Sophie Tremblay-Paquet for her participation in preliminary EMSA experiments; Marie-Josée Landry for contributing to site-specific mutagenesis; and Dr R. G. Evans, Dr Barry Foreman, Dr F. Sladek, Dr J. Puymirmat and Dr V. Zapavigna for generously providing expression vectors. This work was supported by a grant from the Instituts de recherche en santé du Canada.
References
- 1.Guengerich F. P. Boca Raton: CRC Press; 1987. Mammalian Cytochromes P-450, vol. 1. [Google Scholar]
- 2.Nebert D. W., Russell D. W. Clinical importance of the cytochromes P450. Lancet. 2002;360:1155–1162. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- 3.Okey A. B. Enzyme induction in the cytochrome P-450 system. Pharmacol. Ther. 1990;45:241–298. doi: 10.1016/0163-7258(90)90030-6. [DOI] [PubMed] [Google Scholar]
- 4.Handschin C., Meyer U. A. Induction of drug metabolism: the role of nuclear receptors. Pharmacol. Rev. 2003;55:649–673. doi: 10.1124/pr.55.4.2. [DOI] [PubMed] [Google Scholar]
- 5.Handschin C., Meyer U. A. A conserved nuclear receptor consensus sequence (DR-4) mediates transcriptional activation of the chicken CYP2H1 gene by phenobarbital in a hepatoma cell line. J. Biol. Chem. 2000;275:13362–13369. doi: 10.1074/jbc.275.18.13362. [DOI] [PubMed] [Google Scholar]
- 6.Waxman D. J., Morrissey J. J., Naik S., Jauregui H. O. Phenobarbital induction of cytochromes P-450: high-level long-term responsiveness of primary rat hepatocyte cultures to drug induction, and glucocorticoid dependence of the phenobarbital response. Biochem. J. 1990;271:113–119. doi: 10.1042/bj2710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinclair P. R., Bement W. J., Haugen S. A., Sinclair J. F., Guzelian P. S. Induction of cytochrome P-450 and 5-aminolevulinate synthase activities in cultured rat hepatocytes. Cancer Res. 1990;50:5219–5224. [PubMed] [Google Scholar]
- 8.Trottier E., Belzil A., Stoltz C., Anderson A. Localization of a phenobarbital responsive element (PBRE) in the 5′-flanking region of the rat CYP2B2 gene. Gene. 1995;158:263–268. doi: 10.1016/0378-1119(94)00916-g. [DOI] [PubMed] [Google Scholar]
- 9.Park Y. K., Li H., Kemper B. Phenobarbital induction mediated by a distal CYP2B2 sequence in rat liver transiently transfected in situ. J. Biol. Chem. 1996;271:23725–23728. doi: 10.1074/jbc.271.39.23725. [DOI] [PubMed] [Google Scholar]
- 10.Stoltz C., Vachon M.-H., Trottier E., Dubois S., Paquet Y., Anderson A. The CYP2B2 phenobarbital response unit contains an accessory factor element and a putative glucocorticoid response element essential for conferring maximal phenobarbital responsiveness. J. Biol. Chem. 1998;273:8528–8536. doi: 10.1074/jbc.273.14.8528. [DOI] [PubMed] [Google Scholar]
- 11.Honkakoski P., Negishi M. Characterization of a phenobarbital-responsive enhancer module in mouse P450 Cyp2b10 gene. J. Biol. Chem. 1997;272:14943–14949. doi: 10.1074/jbc.272.23.14943. [DOI] [PubMed] [Google Scholar]
- 12.Honkakoski P., Moore R., Washburn K. A., Negishi M. Activation by diverse xenochemicals of the 51-base pair phenobarbital-responsive enhancer module in the CYP2B10 gene. Mol. Pharmacol. 1998;53:597–601. doi: 10.1124/mol.53.4.597. [DOI] [PubMed] [Google Scholar]
- 13.Kim J., Min G., Kemper B. Chromatin assembly enhances binding to the CYP2B1 PBRU of NF-1, which binds simultaneously with CAR/RXR and enhances CAR/RXR-mediated activation of the PBRU. J. Biol. Chem. 2001;276:7559–7567. doi: 10.1074/jbc.M008090200. [DOI] [PubMed] [Google Scholar]
- 14.Paquet Y., Trottier E., Beaudet M. J., Anderson A. Mutational analysis of the CYP2B2 phenobarbital response unit and inhibitory effect of the constitutive androstane receptor on phenobarbital responsiveness. J. Biol. Chem. 2000;275:38427–38436. doi: 10.1074/jbc.M005776200. [DOI] [PubMed] [Google Scholar]
- 15.Liu S., Rivera-Rivera I., Bredemeyer A. J., Kemper B. Functional analysis of the phenobarbital-responsive unit in rat CYP2B2. Biochem. Pharmacol. 2001;62:21–28. doi: 10.1016/s0006-2952(01)00635-9. [DOI] [PubMed] [Google Scholar]
- 16.Honkakoski P., Zelko I., Sueyoshi T., Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol. Cell. Biol. 1998;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamoto T., Sueyoshi T., Zelko I., Moore R., Washburn K., Negishi M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol. Cell. Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baes M., Gulick T., Choi H. S., Martinoli M. G., Simha D., Moore D. D. A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol. Cell. Biol. 1994;14:1544–1552. doi: 10.1128/mcb.14.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forman B. M., Tzameli I., Choi H. S., Chen L., Simha D., Seol W., Evans R. M., Moore D. D. Androstane metabolites bind to and deactivate the nuclear receptor CAR-β. Nature (London) 1998;395:612–615. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- 20.Tzameli I., Pissios P., Schuetz E. G., Moore D. D. The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol. Cell. Biol. 2000;20:2951–2958. doi: 10.1128/mcb.20.9.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Min G., Kemper J. K., Kemper B. Glucocorticoid receptor interacting protein-1 mediates ligand-independent nuclear translocation and activation of constitutive androstane receptor (CAR) in vivo. J. Biol. Chem. 2002;277:26356–26363. doi: 10.1074/jbc.M200051200. [DOI] [PubMed] [Google Scholar]
- 22.Moore L. B., Parks D. J., Jones S. A., Bledsoe R. K., Consler T. G., Stimmel J. B., Goodwin B., Liddle C., Blanchard S. G., Willson T. M., et al. Orphan nuclear receptors CAR and PXR share xenobiotic and steroid ligands. J. Biol. Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- 23.Rivera-Rivera I., Kim J., Kemper B. Transcriptional analysis in vivo of the hepatic genes, Cyp2b9 and Cyp2b10, by intravenous administration of plasmid DNA in mice. Biochim. Biophys. Acta. 2003;1619:254–262. doi: 10.1016/s0304-4165(02)00484-1. [DOI] [PubMed] [Google Scholar]
- 24.Muangmoonchai R., Smirlis D., Wong S. C., Edwards M., Phillips I. R., Shephard E. A. Xenobiotic induction of cytochrome P450 2B1 (CYP2B1) is mediated by the orphan nuclear receptor constitutive androstane receptor (CAR) and requires steroid co-activator 1 (SRC-1) and the transcription factor Sp1. Biochem. J. 2001;355:71–78. doi: 10.1042/0264-6021:3550071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacoby D. B., Zilz N. D., Towle H. C. Sequences within the 5′-flanking region of the S14 gene confer responsiveness to glucose in primary hepatocytes. J. Biol. Chem. 1989;264:17623–17626. [PubMed] [Google Scholar]
- 26.Adeli K., Sinkevitch C. Secretion of apolipoprotein B in serum-free cultures of human hepatoma cell line, HepG2. FEBS Lett. 1990;263:345–348. doi: 10.1016/0014-5793(90)81410-p. [DOI] [PubMed] [Google Scholar]
- 27.Sladek F. M., Zhong W., Lai E., Darnell J. E., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid receptor superfamily. Genes Dev. 1990;4:2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- 28.Berthelsen J., Zappavigna V., Mavilio F., Blasi F. Prep1, a novel functional partner of Pbx proteins. EMBO J. 1998;17:1423–1433. doi: 10.1093/emboj/17.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernier D., Thomassin H., Allard D., Guertin M., Hamel D., Blaquière M., Beauchemin M., LaRue H., Estable-Puig M., Bélanger L. Functional analysis of developmentally regulated chromatin-hypersensitive domains carrying the α1-fetoprotein gene promoter and the albumin/α1-fetoprotein intergenic enhancer. Mol. Cell. Biol. 1993;13:1619–1633. doi: 10.1128/mcb.13.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labbé D., Jean A., Anderson A. A constitutive member of the rat cytochrome P450IIB subfamily: full-length coding sequence of the P450IIB3 cDNA. DNA. 1988;7:253–260. doi: 10.1089/dna.1988.7.253. [DOI] [PubMed] [Google Scholar]
- 31.Handschin C., Podvinec M., Meyer U. A. CXR, a chicken xenobiotic-sensing orphan nuclear receptor, is related to both mammalian pregnane X receptor (PXR) and constitutive androstane receptor (CAR) Proc. Natl. Acad. Sci. U.S.A. 2000;97:10769–10774. doi: 10.1073/pnas.97.20.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metzger S., Halaas J. L., Breslow J. L., Sladek F. M. Orphan receptor HNF-4 and bZip protein C/EBPα bind to overlapping regions of the apolipoprotein B gene promoter and synergistically activate transcription. J. Biol. Chem. 1993;268:16831–16838. [PubMed] [Google Scholar]
- 33.Rajas F., Gautier A., Bady I., Montano S., Mithieux G. Polyunsaturated fatty acyl coenzyme A suppress the glucose-6-phosphatase promoter activity by modulating the DNA binding of hepatocyte nuclear factor 4α. J. Biol. Chem. 2002;277:15736–15744. doi: 10.1074/jbc.M200971200. [DOI] [PubMed] [Google Scholar]
- 34.Knoepfler P. S., Kamps M. P. The highest affinity DNA element bound by Pbx complexes in t(1;19) leukemic cells fails to mediate cooperative DNA-binding or cooperative transactivation by E2a-Pbx1 and class I Hox proteins – evidence for selective targetting of E2a-Pbx1 to a subset of Pbx-recognition elements. Oncogene. 1997;14:2521–2531. doi: 10.1038/sj.onc.1201097. [DOI] [PubMed] [Google Scholar]
- 35.Burglin T. R. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997;25:4173–4180. doi: 10.1093/nar/25.21.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mann R. S., Chan S. K. Extra specificity from extradenticle: the partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 1996;12:258–262. doi: 10.1016/0168-9525(96)10026-3. [DOI] [PubMed] [Google Scholar]
- 37.Mann R. S. The specificity of homeotic gene function. BioEssays. 1995;17:855–863. doi: 10.1002/bies.950171007. [DOI] [PubMed] [Google Scholar]
- 38.Monica K., Galili N., Nourse J., Saltman D., Cleary M. L. PBX2 and PBX3, new homeobox genes with extensive homology to the human proto-oncogene PBX1. Mol. Cell. Biol. 1991;11:6149–6157. doi: 10.1128/mcb.11.12.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herzig S., Fuzesi L., Knepel W. Heterodimeric Pbx-Prep1 homeodomain protein binding to the glucagon gene restricting transcription in a cell type-dependent manner. J. Biol. Chem. 2000;275:27989–27999. doi: 10.1074/jbc.M003345200. [DOI] [PubMed] [Google Scholar]
- 40.Penkov D., Tanaka S., Di Rocco G., Berthelsen J., Blasi F., Ramirez F. Cooperative interactions between PBX, PREP, and HOX proteins modulate the activity of the α2(V) collagen (COL5A2) promoter. J. Biol. Chem. 2000;275:16681–16689. doi: 10.1074/jbc.M909345199. [DOI] [PubMed] [Google Scholar]
- 41.Goudet G., Delhalle S., Biemar F., Martial J. A., Peers B. Functional and cooperative interactions between the homeodomain PDX1, Pbx, and Prep1 factors on the somatostatin promoter. J. Biol. Chem. 1999;274:4067–4073. doi: 10.1074/jbc.274.7.4067. [DOI] [PubMed] [Google Scholar]
- 42.Bischof L. J., Kagawa N., Moskow J. J., Takahashi Y., Iwamatsu A., Buchberg A. M., Waterman M. R. Members of the Meis1 and Pbx homeodomain protein families cooperatively bind a cAMP-responsive sequence (CRS1) from bovine CYP17. J. Biol. Chem. 1998;273:7941–7948. doi: 10.1074/jbc.273.14.7941. [DOI] [PubMed] [Google Scholar]
- 43.Wei P., Zhang J., Egan-Hafley M., Liang S., Moore D. D. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature (London) 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 44.Ueda A., Hamadeh H. K., Webb H. K., Yamamoto Y., Sueyoshi T., Afshari C. A., Lehmann J. M., Negishi M. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol. Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- 45.Gregory P. A., Mackenzie P. I. The homeodomain Pbx2–Prep1 complex modulates hepatocyte nuclear factor 1α-mediated activation of the UDP-glucuronosyltransferase 2B17 gene. Mol. Pharmacol. 2002;62:154–161. doi: 10.1124/mol.62.1.154. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs Y., Schnabel C. A., Cleary M. L. Trimeric association of Hox and TALE homeodomain proteins mediates Hoxb2 hindbrain enhancer activity. Mol. Cell. Biol. 1999;19:5134–5142. doi: 10.1128/mcb.19.7.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y., Yin L., Hillgartner F. B. The homeodomain proteins PBX and MEIS1 are accessory factors that enhance thyroid hormone regulation of the malic enzyme gene in hepatocytes. J. Biol. Chem. 2001;276:23838–23848. doi: 10.1074/jbc.M102166200. [DOI] [PubMed] [Google Scholar]
- 48.Chang C. P., Jacobs Y., Nakamura T., Jenkins N. A., Copeland N. G., Cleary M. L. Meis proteins are major in vivo DNA binding partners for wild-type but not chimeric Pbx proteins. Mol. Cell Biol. 1997;17:5679–5687. doi: 10.1128/mcb.17.10.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gbaguidi G. F., Agellon L. B. The atypical interaction of peroxisome proliferator-activated receptor α with liver X receptor α antagonizes the stimulatory effect of their respective ligands on the murine cholesterol 7α-hydroxylase gene promoter. Biochim. Biophys. Acta. 2002;1583:229–236. doi: 10.1016/s1388-1981(02)00217-2. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J., Lazar M. A. The mechanism of action of thyroid hormones. Annu. Rev. Physiol. 2000;62:439–466. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]
- 51.Song C., Kokontis J. M., Hiipakka R. A., Liao S. S. Ubiquitous receptor: a receptor that modulates gene activation by retinoic acid and thyroid hormone receptors. Proc. Natl. Acad. Sci. U.S.A. 1994;91:10809–10813. doi: 10.1073/pnas.91.23.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawai K., Sasaki S., Morita H., Ito T., Suzuki S., Misawa H., Nakamura H. Unliganded thyroid hormone receptor β1 represses liver X receptor α/oxysterol-dependent transactivation. Endocrinology. 2004;145:5515–5524. doi: 10.1210/en.2004-0382. [DOI] [PubMed] [Google Scholar]
- 53.Makinen J., Frank C., Jyrkkarinne J., Gynther J., Carlberg C., Honkakoski P. Modulation of mouse and human phenobarbital-responsive enhancer module by nuclear receptors. Mol. Pharmacol. 2002;62:366–378. doi: 10.1124/mol.62.2.366. [DOI] [PubMed] [Google Scholar]
- 54.Lausen J., Thomas H., Lemm I., Bulman M., Borgschulze M., Lingott A., Hattersley A. T., Ryffel G. U. Naturally occurring mutations in the human HNF4α gene impair the function of the transcription factor to a varying degree. Nucleic Acids Res. 2000;28:430–437. doi: 10.1093/nar/28.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stolze I., Berchner-Pfannschmidt U., Freitag P., Wotzlaw C., Rossler J., Frede S., Acker H., Fandrey J. Hypoxia-inducible erythropoietin gene expression in human neuroblastoma cells. Blood. 2002;100:2623–2628. doi: 10.1182/blood-2001-12-0169. [DOI] [PubMed] [Google Scholar]
- 56.Liang Y., Jiang X. C., Liu R., Liang G., Beyer T. P., Gao H., Ryan T. P., Dan Li S., Eacho P. I., Cao G. Liver X receptors (LXRs) regulate apolipoprotein AIV-implications of the antiatherosclerotic effect of LXR agonists. Mol. Endocrinol. 2004;18:2000–2010. doi: 10.1210/me.2003-0477. [DOI] [PubMed] [Google Scholar]
- 57.Gbaguidi G. F., Agellon L. B. The inhibition of the human cholesterol 7α-hydroxylase gene (CYP7A1) promoter by fibrates in cultured cells is mediated via the liver X receptor α and peroxisome proliferator-activated receptor α heterodimer. Nucleic Acids Res. 2004;32:1113–1121. doi: 10.1093/nar/gkh260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaplan R., Zhang T., Hernandez M., Gan F. X., Wright S. D., Waters M. G., Cai T. Q. Regulation of the angiopoietin-like protein 3 gene by LXR. J. Lipid Res. 2003;44:136–143. doi: 10.1194/jlr.m200367-jlr200. [DOI] [PubMed] [Google Scholar]
- 59.Lin K. H., Shieh H. Y., Hsu H. C. Negative regulation of the antimetastatic gene Nm23-H1 by thyroid hormone receptors. Endocrinology. 2000;141:2540–2547. doi: 10.1210/endo.141.7.7570. [DOI] [PubMed] [Google Scholar]
- 60.Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glass C. K., Holloway J. M., Devary O. V., Rosenfeld M. G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988;54:313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]