Abstract
We have used the Friend virus model to determine the basic mechanisms by which the immune system can control persistent retroviral infections. Previously we showed that CD4+ T cells play an essential role in keeping persistent retrovirus in check. The present in vitro experiments with a Friend virus-specific CD4+ T-cell clone revealed that these cells produce gamma interferon (IFN-γ), which acts with two distinct mechanisms of antiviral activity. First, IFN-γ had a direct inhibitory effect on virus production. This inhibitory effect was noncytolytic and, interestingly, was not associated with decreased cell surface expression of viral antigens. The second mechanism of IFN-γ-mediated antiviral activity was an enhancement of CD4+ T-cell-mediated cytolytic activity. We also found an in vivo role for IFN-γ in the control of persistent Friend virus infections. Neutralization of IFN-γ in persistently infected mice resulted in significantly increased levels of virus in the spleen, and a significant percentage of IFN-γ-deficient mice were unable to maintain long-term control over Friend virus infections.
The most serious problems caused by many retroviruses often result from the effects of persistent infection rather than the initial acute phases of infection. For example, the acute phase of infection with human immunodeficiency virus (HIV) is generally resolved relatively quickly, but the virus persists and after several years results in diminished CD4+ T-cell levels, loss of immune functions, and the onset of AIDS. As a model for studying persistent retrovirus infections, we have used Friend virus complex (FV) in strains of mice which generally recover from acute disease but develop lifelong, low-level persistent infections (6, 28). Many types of cells including erythroid precursors, monocytes, and lymphocytes are initially infected with FV during the acute phase of disease (28). Recovery from the acute phase is dependent on genes of the major histocompatibility complex (MHC) and complex immune responses including cytotoxic T-lymphocyte (CTL), CD4+ T-helper, and antibody responses (reviewed in references 30 and 31). Following recovery from FV-induced splenomegaly and viremia, the infection in the spleen is reduced by over 1,000-fold and appears to be primarily restricted to a very small population of B cells (28).
Most persistently infected mice live a normal life span, although for unknown reasons, approximately 5% of the mice eventually relapse with FV-induced erythroleukemia (6). Unlike HIV, FV infection does not deplete CD4+ T-cell levels, but if mice with persistent FV are experimentally depleted of their CD4+ T cells, a large percentage of the mice relapse with acute disease and develop erythroleukemia (28). Thus, CD4+ T cells play a critical role in containing persistent FV infections. In contrast, depletion of CD8+ T cells does not induce relapse or increased levels of persistent virus, nor is there an additive effect from dual depletion of both CD4+ and CD8+ T cells (28). Furthermore, CD4 depletions do not induce the loss of virus-neutralizing antibodies (28). These results suggest that CD4+ T cells may have direct antiviral effects during the persistent phase of infection, rather than simply providing classical helper functions for CD8+ CTL and/or antibody-producing B cells.
Attempts to isolate sufficient numbers of virus-specific CD4+ T cells from persistently infected mice to do ex vivo analyses have so far been unsuccessful. Therefore, we have established an FV-specific CD4+ T-cell clone to study possible mechanisms by which these cells may control persistent FV infections. The current experiments reveal two separate mechanisms of CD4+ T-cell antiviral activity in vitro. CD4+ T cells can lyse infected target cells, and they can also suppress virus replication by production of gamma interferon (IFN-γ). In vivo, neutralization of IFN-γ using monoclonal antibodies (MAb) increased the levels of virus in persistently infected mice, and mice with genetic inactivation of the IFN-γ gene were susceptible to late-onset FV-induced splenomegaly whereas normal mice were not.
MATERIALS AND METHODS
Mice.
The mouse strains used in this study were age- and sex-matched (C57BL/10 × A.BY)F1, C57BL/6 (B6), B6 IFN-γ knockout (B6.G/KO) (provided by Genetech, Inc., South San Francisco, Calif.), and B6 interleukin-12 (IL-12) knockout (provided by Hoffmann-LaRoche Inc., Nutley, N.J.) mice. These mice are of the Fv1b/b genotype and so are susceptible to B-tropic FV. All mice were 12 to 24 weeks of age at experimental onset. The parental strains used to produce F1 mice were obtained from Jackson Laboratory. All animals were treated in accordance with the regulations and guidelines of the National Institutes of Health and the Animal Care and Use Committee of Rocky Mountain Laboratories.
Virus.
The Friend virus used in the challenge experiments was FV complex (B-tropic Friend murine leukemia helper virus (F-MuLV) and polycythemia-inducing spleen focus-forming virus) (37) obtained from a 10% spleen cell homogenate from BALB/c mice infected 14 days previously with 3,000 spleen focus-forming units (SFFU) of virus as described previously (9). For virus challenge experiments, mice were infected by intravenous injection of 0.5 ml of phosphate-buffered balanced salt solution containing 1% normal mouse serum and 3,000 SFFU of FV. To test for relapse of splenomegaly, the mice were palpated as previously described (28, 29).
Cell lines.
FT-5 and FBL-3 are Friend virus-induced tumor lines from C57BL/6 mice and were described previously (33). Dunni is a Mus dunni cell line (35) and Dunni-FB29 is chronically infected with the FB29 strain of F-MuLV (42).
Antibodies.
Fluorescein isothiocyanate (FITC)-labeled anti-CD3 (145-2C11), anti-CD4 (GK1.5), and anti-CD8 (169.4) MAb and purified anti-CD3 MAb (145-2C11) were purchased from Pharmingen (San Diego, Calif.). FITC-labeled anti-mouse immunoglobulin (Ig) was purchased from Cappel (West Chester, Pa.). The anti-F-MuLV envelope gp70 MAb 720 was described previously (43). For the blocking experiment and in vivo administration, supernatants from Cellmax microcapillary cultures (Cellco, Germantown, Md.) were used: 169.4 for anti-CD8 MAb, 191.1 for anti-CD4 MAb, and XMG1.2 for anti-IFN-γ (5).
Antigenic peptides.
A synthetic peptide with the sequence from amino acids 122 to 141 of F-MuLV Env (F-MuLVenv122–141, DEPLTSLTPRCNTAWNRLKL) was used for CD4+ T-cell stimulation. This peptide is recognized by CD4+ T cells in an H-2Ab-restricted manner (33, 45). The F-MuLVenv462–479 peptide (HPPSYVYSQFEKSYRHKR), which is recognized by CD4+ T cells in an I-Eb/d-restricted manner (33, 45), was used as a negative control.
Generation of FV-specific T-cell clones.
Female (B10 × A.BY)F1 mice were immunized with 106 FV-induced FBL-3 tumor cells by subcutaneous injection into the dorsal region. After complete tumor regression, mixed lymphocyte-tumor cell cultures (MLTC) and limiting-dilution cultures were performed by previously reported methods (39). In brief, 105 irradiated (100 Gy) FBL-3 tumor cells were mixed with 5 × 106 immune spleen cells in complete medium (RPMI 1640 medium supplemented with 10% fetal bovine serum and 5 × 10−5 M 2-mercaptoethanol) and incubated for 7 days at 37°C in 5% CO2. CD4+ cells in MLTC cultures were enriched using the MidiMACS separation system (Miltenyi Biotech, Bergisch Gladbach, Germany). Limiting dilution of the CD4+ cells (one cell per well) was done in the presence of 2.5 ng of recombinant human IL-2 (R & D Systems, Minneapolis, Minn.) per ml. All growing colonies were expanded and tested to determine specificity. FV antigen-specific CD4+ colonies were recloned at 0.2 cell per well, and one clone, BF1-9, was maintained by passage every 7 days with irradiated syngeneic spleen cells (10 Gy) and FBL-3 tumor cells (100 Gy) in the presence of recombinant human IL-2. The surface phenotype of BF1-9 was Thy-1.2+ CD4+ CD8− CD3+ surface Ig−.
Proliferation assay.
A total of 105 T-cells were cocultured with stimulator cells under one of the following conditions: (i) 104 irradiated (100 Gy) FBL-3 tumor cells, (ii) 5 × 105 irradiated (10 Gy) FV-infected spleen cells; or (iii) synthetic peptide antigen. All wells also contained 5 × 105 irradiated (10 Gy) syngeneic spleen cells as antigen-presenting cells (APC). Cultures were done in 96-well flat-bottom tissue culture plates at 37°C for 48 h. Then 8.5 kBq of [3H]thymidine (NEN, Boston, Mass.) was added to each well 4 h before the termination of the cultures. The cells were then transferred onto UniFilter plates by using a Filter Mate harvester. The filters were dried, 50 μl of Micro-Scint-O was added per well, and the radioactivity was counted in a TopCount counter. All radioactivity-counting supplies and equipment were from Packard (Meridian, Conn.).
F-MuLV inhibition assays.
BF1-9 cells stimulated for 12 to 14 days with irradiated FBL-3 cells and APC (syngeneic irradiated spleen cells) were passed through nylon wool columns to deplete long-lived APC. To test for inhibition of virus production, 106 nylon wool-nonadherent BF1-9 cells were cocultured for 3 days with F-MuLV-producing cells, either 104 Dunni-FB29 cells per well or 105 FT-5 cells per well, plus 106 irradiated (10 Gy) spleen cells as APC in a total volume of 2 ml per well of 24-well plates. The culture supernatants were subjected to titer determination by focal infectivity assays (46) on susceptible M. dunni cells (104 cells/well in 24-well plates) that had been pretreated with 4 μg of Polybrene per ml 1 day earlier. The cultures were incubated for 3 days, fixed with ethanol, stained with F-MuLV envelope-specific MAb 720, and developed with goat anti-mouse peroxidase-conjugated antisera (Cappel) and aminoethylcarbazol to detect foci. For inhibition by supernatants, the supernatants from BF1-9 T cells (106/well) stimulated for 2 days with APC (106/well) and 0.1 μM F-MuLVenv122–141 peptide antigen were harvested, pooled, aliquoted, and frozen at −70°C. A 1-ml volume of supernatant was added per well of Dunni-FB29 cultures seeded 1 day previously with 1.0 ml of medium containing 104 cells/well in 24-well plates. For the kinetics study, each sample supernatant was frozen at −70°C and thawed before being used for viral titer determination. All samples went through only a single freeze-thaw cycle. In the anti-IFN-γ blocking experiments, the supernatants from Cellmax microcapillary cultures were titrated for the optimal concentration needed to neutralize recombinant murine IFN-γ-mediated inhibition of virus production (data not shown). A 200-fold dilution of the supernatant was sufficient to neutralize 5 ng of recombinant IFN-γ per ml, and 20-fold-diluted supernatant was used for the blocking experiment in vitro.
Insert cultures.
Tissue culture inserts (no. 3495; Becton Dickinson, Franklin Lakes, N.J.) were used for transwell studies, where the Dunni-FB29 and BF1-9 cells were separated by a permeable membrane. Dunni-FB29 (104/well), APC (106/well), F-MuLVenv122–142 (1 μM), and MAb (final concentration, 5%) were cultured in the wells, and APC (106/well), F-MuLVenv122–142 (1 μM), and BF1-9 T cells (106/well) were cultured in the inserts. Negative control wells were the same except that they contained no T cells. Supernatants taken from wells after 3 days of culture were subjected to titer determination.
Cytotoxicity assay.
Cytotoxic activity was measured using a 51Cr release assay. Target cells were labeled with 51Cr by incubation of 2 × 106 cells for 60 min at 37°C with 200 μCi of Na251CrO2 (Amersham, Arlington Heights, Ill.) in 0.1 ml of RPMI 1640 medium plus 20% fetal bovine serum. After incubation, the cells were washed three times and resuspended in RPMI 1640 medium plus 10% fetal bovine serum. Graded numbers of effector cells were mixed with 104 51Cr-labeled target cells in the presence or absence of syngeneic irradiated spleen cells (105 cells per well) and 0.1 μM antigenic peptide F-MuLVenv122–141 in a total volume of 200 μl in the wells of a flat-bottom 96-well plate. The cultures were centrifuged at 200 × g for 1 min and incubated in 5% CO2 at 37°C for 8 or 18 h as indicated. After incubation, the plates were centrifuged at 200 × g for 5 min and 50 μl of supernatant was harvested on Lummaplate (Packard, Meridian, Conn.) and measured for radioactivity using a Topcount (Packard) bench top microplate scintillation counter. For FT-5 targets, which have a high spontaneous release of 51Cr, dead cells were determined by trypan blue dye exclusion. Discrimination between effectors and targets was possible because FT-5 cells are four- to fivefold larger than BF1-9 cells. When the FT-5 cells were used, the assay mixture was cultured for 9 h.
In vivo administration of anti-IFN-γ MAb and anti-CD4 MAb.
CD4+ T-cell depletions in mice persistently infected with FV were performed as described previously (28). Supernatants from anti-CD4 MAb hybridoma 191.1 Cellmax microcapillary cultures were injected by the intraperitoneal route twice per week for 1 month. At 7 to 10 days following the last injection of antibody, CD4+ T-cell levels in mononuclear blood ranged from <1 to 3% of the nucleated peripheral blood cells. Neutralization of IFN-γ for the relapse experiments was done by intraperitoneal injections of 0.5 ml of artificial capillary culture supernatants containing approximately 0.5 mg of XMG1.2 antibody per ml (5). The mice were injected twice a week for 1 month.
Infectious-center assays.
Titrations of single-cell suspensions from persistently infected mouse spleens were plated onto susceptible Dunni cells, cocultivated for 5 days, fixed with ethanol, stained with F-MuLV envelope-specific MAb 720 (43), and developed with peroxidase-conjugated goat anti-mouse IgG and substrate (Cappel) to detect foci.
Flow cytometric analyses.
Cell suspensions were analyzed by flow cytometry using a FACSCalibur (Becton Dickinson, San Jose, Calif.). A total of 10,000 cells were analyzed for expression of envelope using MAb 720 (43) followed by FITC-labeled goat anti-mouse Ig. Glycogag was stained with MAb 34 (8), which was developed using FITC-labeled goat anti-mouse IgG2b-specific antisera (Caltag, Burlingame, Calif.). Dead cells were gated out by propidium iodide staining.
RESULTS
In vitro model of CD4+ T-cell-mediated antiviral effects.
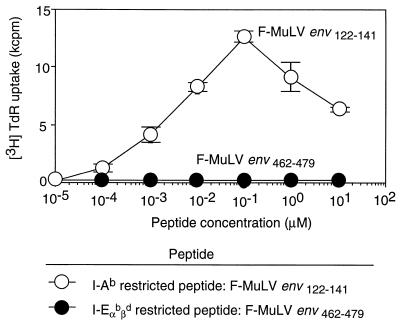
To investigate possible mechanisms of CD4+ T-cell-mediated suppression of FV production, we established an FV-specific CD4+ T-cell clone, BF1-9, that could be used for in vitro studies. The BF1-9 clone was isolated from a (B10 × A.BY)F1 (H-2b) mouse immunized with an FV-induced tumor line, FBL-3. BF1-9 T cells proliferated on stimulation with either FBL-3 tumor cells or FV-infected spleen cells but not uninfected spleen cells (data not shown). The specificity of BF1-9 was determined by demonstrating dose-dependent proliferation upon stimulation with a Friend virus peptide, F-MuLVenv122–141, in the presence of syngeneic APC (Fig. 1). F-MuLVenv122–141 is an immunodominant peptide previously shown to be restricted by major histocompatibility complex (MHC) class II H-2Ab molecules (33). In contrast, BF1-9 T cells did not proliferate in response to F-MuLVenv462–479, an MHC class II H-2Eb/d-restricted peptide (33) (Fig. 1).
FIG. 1.
Specificity of BF1-9 CD4+ T-cell clone for F-MuLVenv122–141 peptide. A total of 105 BF1-9 cloned cells were cultured with irradiated, syngeneic, uninfected mouse spleen cells as APC and antigen in the form of either H-2Ab-restricted synthetic peptide F-MuLVenv122–141 (○) or H-2E-restricted peptide F-MuLVenv462–479 (●) at the indicated concentrations (4). Proliferative responses were measured as described in Materials and Methods. This experiment was repeated twice with similar results. The data shown are from triplicate samples, and the error bars show standard deviations. TdR, thymidine.
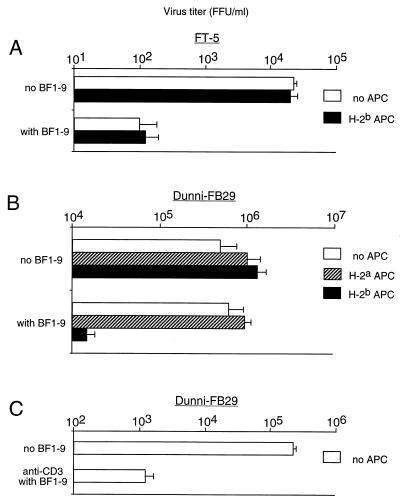
The BF1-9 clone was tested for antiviral effects in vitro by coculture with an MHC-matched, FV-induced, monocytic tumor line (FT-5) that expressed H-2Ab molecules and produced FV (33). The production of virus by FT-5 cells dropped by approximately 100-fold in the presence of BF1-9 T cells, and this effect did not require APC (Fig. 2A). To determine if direct recognition of H-2Ab molecules on the virus-producing cells was required for the suppression of virus production, BF1-9 T cells were cocultured with an M. dunni cell line chronically infected with the FB29 strain of F-MuLV (Dunni-FB29 cells). Dunni-FB29 cells do not express H-2Ab molecules, and no suppression of virus production was observed in the absence of APC (Fig. 2B). However, addition of APC to the cultures produced an approximately 100-fold reduction in virus production when, and only when, the MHC type of the APC matched that of the BF1-9 T cells (Fig. 2B). In addition, the requirement for syngeneic APC could be replaced by stimulating the BF1-9 T cells with immobilized anti-T-cell receptor (CD3) antibodies (Fig. 2C). Thus, suppression of virus production did not require direct recognition of H-2Ab molecules on the virus-producing cells. The data indicated that the primary requirement for BF1-9 T-cell-mediated suppression of virus production was activation of the T cells through the T-cell receptor.
FIG. 2.
BF1-9-mediated suppression of FV production by FT-5 and Dunni-FB29 cells. F-MuLV-producing cells were cocultured either with or without 106 BF1-9 cells and 106 APC to test for effects on virus production. H-2b APC were spleen cells from (B10 × A.BY)F1 mice, and H-2a APC were spleen cells from (B10.A × A/Wy)F1 mice. After 3 days, the virus in the culture supernatants was titrated by the focal infectivity assay as described in Materials and Methods. All results are from triplicate wells, and the error bars indicate standard deviations. (A) A total of 105 FT-5 (Friend virus-induced monocytic leukemia, H-2b, class II positive) cells were used as virus-producing cells. There was no requirement for APC to observe BF1-9-mediated suppression of virus production in this cell line. (B) To test for BF1-9-mediated effects on an MHC-mismatched class II-negative cell type, an M. dunni-derived cell line (35) was chronically infected with the FB29 strain of F-MuLV (Dunni-FB29) and used as a virus-producing cell line. A total of 104 Dunni-FB29 cells were cultured in the absence of APC, in the presence APC that were MHC matched (H-2b) with the BF1-9 cells, or in the presence of MHC-mismatched (H-2a) APC as indicated. BF1-9-mediated suppression of virus production occurred only in the presence of APC that were MHC matched with the BF1-9 cells. (C) In this experiment the BF1-9 cells were cultured for 3 days, in the absence of APC, with Dunni-FB29 in tissue culture plates coated with 10 μg of anti-CD3 MAb per ml. Similar results were obtained from four separate experiments.
Soluble antiviral factors in the supernatants of stimulated BF1-9 cells.
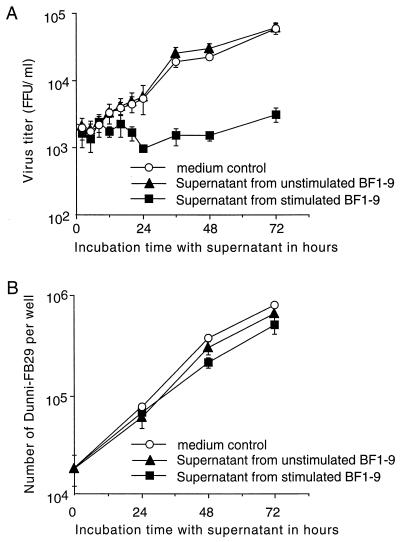
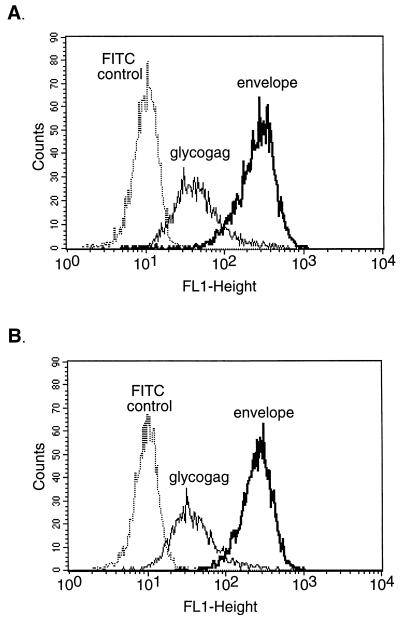
To determine whether T-cell-secreted soluble factors were involved in the suppression of virus production, supernatants from activated BF1-9 cells were tested for antiviral activity against Dunni-FB29 cells at various time points. By 24 h after addition of supernatants from activated BF1-9 cells, there was a significant reduction in virus production by Dunni-FB29 cells compared to that observed following the addition of supernatants from unstimulated BF1-9 cultures or medium alone (Fig. 3A). This reduction was not due to growth inhibition or cytotoxicity of the Dunni-FB29 cells since there were no significant differences in cell growth regardless of treatment (Fig. 3B). Addition of activated BF1-9 culture supernatants to FT-5 cells also suppressed virus production (data not shown). Thus, stimulated BF1-9 T cells produced a soluble antiviral factor which suppressed FV production through a mechanism that did not inhibit cell growth. Interestingly, flow cytometric analyses of the supernatant-treated cells showed that inhibition of virus production was also not associated with any major loss of cell surface expression of viral envelope or glycogag proteins (Fig. 4). Thus, there was not a general downregulation of viral antigen expression associated with loss of infectivity.
FIG. 3.
Suppression of virus production by supernatants from stimulated BF1-9 CD4+ T cells. A total of 104 Dunni-FB29 cells were cultured for 24 h before addition of either medium (○), supernatant from unstimulated BF1-9 cells (▴), or supernatant from BF1-9 cells stimulated with APC plus antigenic peptide (■). (A) Samples were taken at the indicated time points following addition of the supernatants. The samples were frozen at −70°C, thawed, and titrated for virus infectivity by focal immunoassays. (B) The Dunni-FB29 cells from the cultures were counted by the trypan blue dye exclusion method to determine viable-cell numbers at the indicated time points. This experiment was repeated twice with similar results. All samples in both panels are from triplicate wells, and error bars indicate standard deviations.
FIG. 4.
Flow cytometric analyses of F-MuLV antigen expression on Dunni-FB29 cells before and after incubation with activated BF1-9 supernatants. Dunni-FB29 cells were analyzed before (A) and after (B) incubation with supernatant from activated BF1-9 cells by staining for cell surface expression of F-MuLV envelope and Glycogag proteins using MAbs 720 (43) and 34 (8), respectively. The mean fluorescence intensity for envelope before incubation was 249, compared to 247 after incubation. The mean fluorescence intensity for Glycogag was 43 before incubation, compared to 38 after incubation. FITC control is the level of fluorescence following incubation with FITC-labeled secondary antibody in the absence of primary antibody.
Role of cell-to-cell contact in suppression of virus production.
The suppression of virus production by activated BF1-9 culture supernatants was not as potent as when BF1-9 T cells and APC were added to the Dunni-FB29 cultures (compare Fig. 3A and 2B). Further experiments were done to determine if cell-to-cell contact was necessary for a portion of the antiviral activity of BF1-9 T cells. Transwell cultures were studied in which the virus-producing Dunni-FB29 cells were either separated from the BF1-9 T cells by a permeable membrane which prevented cell-to-cell contact or mixed with the BF1-9 T cells on the same side of the membrane. APC and antigenic peptide were added to both sides of the transwell cultures to control for conditions. Consistent with the presence of a soluble antiviral factor, BF1-9 cells were able to significantly reduce virus production by Dunni-FB29 cells when the T-cell effectors were separated from the Dunni-FB29 cells by the transwell membrane (Fig. 5). However, a significantly higher level of suppression was observed when both the effector cells and target cells were on the same side of the membrane (Fig. 5). Thus, it appeared that both soluble factors and cell-to-cell contact were involved in the mechanisms by which BF1-9 suppressed virus production.
FIG. 5.
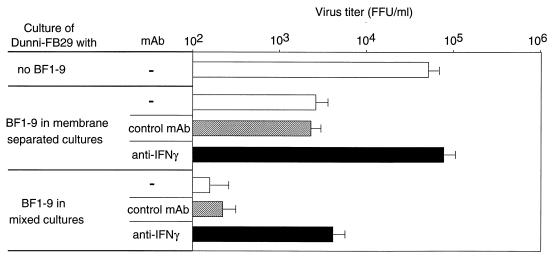
Effects of cell-to-cell contact on T-cell-mediated suppression of virus production. Transwell culture plates were used to test for the effects of cell-to-cell contact on the suppression of virus production in BF1-9/Dunni-FB29 cocultures. The BF1-9 cells and virus-producing cells either were or were not separated by the permeable membrane in the transwell plates as indicated. The supernatants were subjected to titer determination for virus infectivity following 3 days of culture. Both sides of the cultures contained APC and antigenic peptide F-MuLVenv122–141 (1.0 μM). Anti-IFN-γ antibody (solid bars) or anti-CD8 control antibody (hatched bars) was added at a final concentration of 5% as indicated. This experiment was repeated twice with similar results. Error bars indicate standard deviations.
Identification of IFN-γ as the predominant soluble antiviral factor.
RNase protection assays showed that activation of BF1-9 cells with APC plus peptide resulted in slightly increased expression of tumor necrosis factor alpha (TNF-α), TNF-β, and lymphotoxin-β and a strong increase in IFN-γ expression (data not shown). To follow up on the possible involvement of IFN-γ, an IFN-γ-neutralizing MAb was used to test whether neutralization of IFN-γ would reduce the antiviral activity in the transwell culture system. Neutralization of IFN-γ in the membrane-separated cultures completely abolished the antiviral activity and restored virus production to the level in cultures with no BF1-9 cells (Fig. 5). Thus, the main soluble antiviral factor appeared to be IFN-γ. In contrast, neutralization of IFN-γ in the mixed cultures only partially reversed the antiviral activity and did not completely restore virus production. Therefore, production of IFN-γ did not account for all of the antiviral activity present in mixed cultures, where cell-to-cell contact was possible.
CD4+ T-cell cytotoxicity.
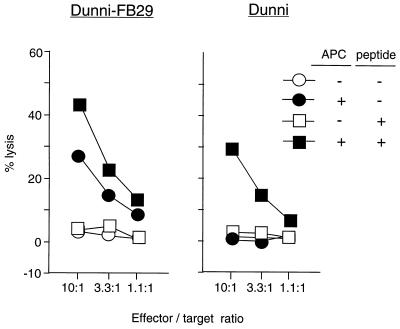
Microscopic examination of the mixed cultures revealed that Dunni-FB29 cells appeared to be dying after overnight contact with BF1-9 T cells but not after overnight incubation in membrane-separated cultures. It has been reported that CD4+ T cells can have cytolytic activity which is MHC class II restricted (51). As a reminder, Dunni-FB29 cells do not express the MHC class II restriction elements for recognition by BF1-9 T cells (H-2Ab molecules), but the cultures also contained syngeneic APC. To assess the killing of Dunni-FB29 by BF1-9 T cells, the Dunni-FB29 cells were labeled with 51Cr, mixed with BF1-9 T cells, and tested for 51Cr release. No cell death was observed at 8 h after culture, but at 18 h there was low but significant lysis of Dunni-FB29 (Fig. 6). Cytolysis was dependent on the presence of APC in the cultures. Increased stimulation of the BF1-9 cells by addition of antigenic peptide enhanced the cell killing but was not required (Fig. 6). Furthermore, even uninfected Dunni cells were killed by BF1-9 T cells, but in that case, both APC and antigenic peptide were required (Fig. 6). Thus, cytolysis was dependent on the activation of the BF1-9 T cells, but no direct recognition of the target cells themselves was necessary. These results are relevant to the in vivo model of FV infection, where infected cells may or may not express MHC class II antigens.
FIG. 6.
BF1-9 cytotoxicity against Dunni-FB29 cells and uninfected Dunni cells. To test the ability of BF1-9 cells to kill infected cells, cytotoxicity was measured in standard 51Cr release assays. 51Cr-labeled Dunni-FB29 or uninfected Dunni cells (104/well) were cultured for 18 h with BF1-9 cells at the indicated effector/target ratio in the presence (solid symbols) or absence (open symbols) of 105 syngeneic, irradiated, uninfected spleen cells per well. Antigenic peptide F- MuLVenv122–141 (0.1 μM) was added as indicated. Cytotoxicity against Dunni-FB29 cells by BF1-9 cells required only APC. Uninfected Dunni cells were killed only when both APC and peptide were present. The spontaneous release from labeled Dunni-FB29 and Dunni cells was 24 and 26%, respectively. The data shown are from duplicate wells. Results from two additional experiments gave similar results.
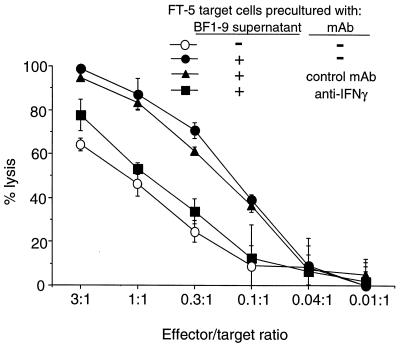
To determine if direct recognition of target cells could also result in cytolysis, FV-infected FT-5 tumor cells which expressed H-2Ab molecules were tested as targets in the absence of added APC. Since FT-5 cells had high spontaneous release of 51Cr, the numbers of dead FT-5 cells were determined by the trypan blue dye exclusion method rather than 51Cr release. Coculturing of FT-5 cells with BF1-9 T cells produced significant FT-5 cell death in the absence of APC (Fig. 7). Interestingly, preincubation of the FT-5 target cells with supernatants from activated BF1-9 T cells made them more susceptible to lysis by BF1-9 T cells (Fig. 7). This sensitization to lysis was mostly reversed by addition of IFN-γ-neutralizing MAb to the preincubation media, indicating a predominant role for IFN-γ (Fig. 7). Sensitization to killing by preincubation with activated BF1-9 supernatant was associated with increased expression of MHC class II molecules on the cell surface of the FT-5 targets, possible increasing their sensitivity to cytolysis (data not shown). Thus, cytolysis via direct recognition in the absence of APC was possible, and IFN-γ sensitized infected FT-5 target cells for CD4+ T-cell-mediated killing.
FIG. 7.
BF1-9 cytotoxicity against FT-5 cells. MHC class II-positive (H-2b) FT-5 cells were tested as targets for lysis by BF1-9 cells (○). Also tested were FT-5 targets that were precultured for 2 days with 5% supernatant from activated BF1-9 cells (●). This preincubation with activated BF1-9 supernatant sensitized the targets for killing. Addition of 5% anti-IFN-γ MAb to the supernatant prior to culture neutralized the capacity of the supernatant to sensitize for killing (■), while addition of anti-CD8 control MAb did not (▴). The assays were done in triplicate, and error bars indicate standard deviations. Five independent experiments gave similar results.
In vivo studies of IFN-γ.
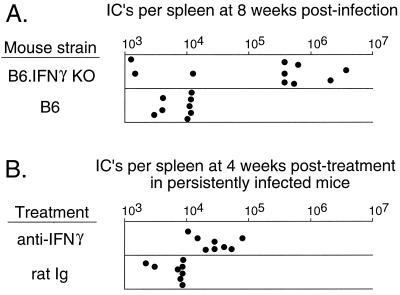
As an approach to study the effects of IFN-γ in vivo, B6 mice with genetic inactivation at the IFN-γ locus (B6.G/KO mice) were analyzed. Normal B6 mice are infectable with FV but are not susceptible to FV-induced splenomegaly or erythroleukemia due to the genetic resistance gene, Fv2 (1, 2, 27, 36, 48). At 2 weeks after infection with FV, no splenomegaly was observed in control B6 mice or B6.G/KO mice (Table 1). However, after 12 weeks, 7 of 22 B6.G/KO mice were splenomegalic while none of the B6 control mice were splenomegalic (Table 1). In addition, levels of infectious centers in the spleen were tested at 8 weeks postinfection. Of 10 B6.G/KO mice, 7 failed to keep FV infections under 5 × 105 infectious centers (IC) per spleen (Fig. 8A) and the group had a geometric mean titer of greater than 105 IC per spleen (Table 1). By contrast, the highest level in any of the B6 control mice was 1.6 × 104 IC per spleen, with a geometric mean titer of less than 104 IC per spleen (Fig. 8A; Table 1). Since IL-12 is known to be an important regulator of IFN-γ production, we also tested for splenomegaly in IL-12 knockout mice. No splenomegaly was observed in mice over the 12-week observation period (Table 1). These results indicated that production of IFN-γ played an important role in the long-term control of FV infections in vivo and that the production of IFN-γ was not dependent on IL-12.
TABLE 1.
Measures of FV-induced disease
| Mouse strain | No. of mice with splenomegaly/total no. at:
|
No. of IC at 8 wk (mean log10 ± SD) | |
|---|---|---|---|
| 2 wk | 12 wk | ||
| B6 | 0/24 | 0/24 | 3.96 ± 0.12b |
| B6.G/KO | 0/22 | 7/22a | 5.31 ± 1.27b |
| B6.IL-12 KO | 0/20 | 0/20a | NTc |
The difference between the incidence of splenomegaly in the B6.G/KO group and the B6.IL-12 KO group at 12 weeks postinfection is statistically significant (P = 0.0063 by Fisher's exact test). B6.G/KO mice with splenomegaly progressed to erythroleukemia.
At 8 weeks postinfection, the difference between the number of IC in the B6 group (n = 8) and the B6.G/KO group (n = 10) is statistically significant (P = 0.0092 by the unpaired t test).
NT, not tested.
FIG. 8.
Increased persistent FV infection in B6.G/KO and mice treated with anti-IFN-γ antibody. Each dot represents the number of F-MuLV IC per spleen from individual mice. Spleens were assayed for IC formation by the focal infectivity assay described in Materials and Methods. (A) Levels of IC in individual normal B6 mice versus B6.G/KO mice at 8 weeks after infection with FV. (B) Persistent FV infections were established in (B10 × A.BY)F1 mice as described previously 2. The mice were then treated with IFN-γ-neutralizing MAb as described in Materials and Methods. Controls were age- and sex-matched mice treated with rat Ig at the same concentration as the IFN-γ-neutralizing rat MAb. IFN-γ-neutralizing MAb-treated mice had significantly larger numbers of IC as determined by comparing the geometric means between the two groups using an unpaired t test (two-tailed P < 0.0001). The mouse in the IFN-γ-neutralizing MAb group with the largest number of IC had a grossly enlarged spleen, indicating relapse of disease.
Since depletions of CD4+ T cells from mice persistently infected with FV have previously been shown to induce the reactivation of virus and relapses of erythroleukemia at an incidence of approximately 50% (28), it was of interest to determine if this effect occurred due to loss of IFN-γ production. IFN-γ was neutralized in persistently infected (B10 × A.BY)F1 mice by injections of IFN-γ-neutralizing MAb twice a week for 1 month. At that time point, their spleens were removed to determine the levels of infection. The anti-IFN-γ treated group had significantly larger numbers of IC than the control group did (Fig. 8B; Table 1), and one of the treated mice had a grossly enlarged spleen, indicative of relapse of erythroleukemia. These results confirm the results from the B6.G/KO indicating involvement of IFN-γ in the control of persistent FV.
DISCUSSION
Numerous antiviral activities for CD4+ T cells and IFN-γ have previously been described, but to our knowledge, this is the first direct evidence for their in vivo role in the control of a persistent retroviral infection. The mechanism by which CD4+ T cells control persistent FV infections in mice is of particular interest since immunological help for either CD8+ T cells or B cells did not appear to be critical (28). These findings implicated a direct mechanism of CD4+ T-cell control, and the present results suggest that IFN-γ may be a key component, although not the only component. CD4+ T cells also have antiviral activity in the transgenic-mouse model for hepatitis B virus (20). In that model, IFN-γ also has antiviral activity but CD8+ T cells appear to be the predominant producers of IFN-γ (24). In HIV infection of humans, CD4+ T cells have also been associated with antiviral activities, and mechanisms including the production of IFN-γ and chemokines have been implicated (21, 44). The neutralization of IFN-γ in our model significantly increased the numbers of spleen IC in persistently infected mice, but in contrast to CD4+ T-cell depletions, this was not sufficient to induce a relapse of splenomegaly in a significant proportion of the mice. In addition, knockout mice deficient in CD4+ T cells have an 80% incidence of late-onset splenomegaly (27) while IFN-γ-deficient mice have only a 32% incidence (Table 1). Thus, it appears that CD4+ T-cell-mediated mechanisms in addition to IFN-γ work to control persistent FV infection.
One additional mechanism that might account for the control of persistent FV by CD4+ T cells is cytolytic activity such as we demonstrated in vitro (Fig. 6 and 7). CD4+ cytolytic activity specific for HIV antigens has previously been reported (12, 34), but its relevance in vivo is not known. In the FV model, the main reservoir for persistent virus is splenic B cells (28), and since B cells express MHC class II molecules, they are potentially capable of activating CD4+ T cells for cytolytic activity. The results of the present experiments indicate that such activated CD4+ T cells could also kill nearby MHC class II-negative cells through a bystander effect, as shown in Fig. 6. Such bystander killing could be critical in the control of virus spread from B cells to other cells in the vicinity since the receptor for FV is ubiquitously expressed (50, 52) and all dividing cells are potential targets for infection. It is probably especially important to keep FV from spreading to erythroblasts which are the primary cells in adult animals susceptible to FV-induced transformation and leukemia.
It is interesting that although CD4+ T cells and IFN-γ maintain immunological control over persistently infected cells, mice are never able to completely clear the virus. The virus manages to escape the strong immunological pressures from virus-neutralizing antibodies, CD8+ CTL, and CD4+ T-cell responses that are generated during acute infection (7, 30). Evidence indicates that this escape occurs very early in infection and is probably related to the infection of a small population of B cells (15, 16, 28). Persistent FV appears relatively quiescent in B cells, since we have not been able to detect viral antigen expression on freshly isolated splenic B cells by flow cytometric analyses, even though such cells can produce infectious centers after in vitro culture (data not shown). It has previously been shown that resting B cells are resistant to CD4+ T-cell-mediated killing while activated B cells are good targets (17, 25). Thus, resting B cells might be the reservoir for persistent FV and reactivated virus is in a dynamic equilibrium with the immune system. The present results show that we can perturb this equilibrium by neutralizing IFN-γ as well as by depleting CD4+ T cells. Some variability was observed in the in vivo studies, suggesting that undefined compensatory mechanisms may be able to overcome IFN-γ deficiency, at least in some mice, for limited periods.
There are relatively few models available for the study of persistent viral infections, but there is some evidence that IFN-γ may be a key component in the control of other persistent viruses. IFN-γ, in combination with TFN-α, works in a noncytopathic manner to control hepatitis B virus in a transgenic-mouse model (4, 23). Likewise, we found no evidence for a direct cytopathic effect from IFN-γ in our model (Fig. 3B). In addition, IFN-γ is involved in the control of persistent lymphocytic choriomenigitis virus in mice (22, 49) and in the control of reactivated herpes simplex virus infections in mice (3). In most studies, it was thought that CD8+ T cells were the most relevant sources of IFN-γ. The data presented here support results from the hepatitis B virus model (20), suggesting that CD4+ T cells may also be important producers of antiviral IFN-γ in some situations.
There are some interesting analogies between the control of persistent FV in mice and HIV in humans that should be considered. For example, both HIV-specific CD4+ CTL (26, 38, 41, 47) and antiviral effects for IFN-γ have been described (11, 13, 14, 18, 40). Persistent HIV hides in resting CD4+ T cells rather than B cells (10, 19), but, unlike mouse T cells, human T cells are MHC class II positive and can serve as targets for CD4+ CTL (41). Vigorous CD4+ T-cell responses have been associated with control of viremia in some long-term-nonprogressive HIV-1 infections (44), and individuals who maintain normal CD4+ T cell levels following infection are able to keep HIV loads at low or undetectable levels (32). Thus, it is possible that CD4+ T cells may contribute directly to the control of HIV rather than simply provide immunological help for other cells such as CD8+ CTL.
Some interesting questions remain in the persistent FV model. For example, CD4 depletions result in relapse in only about half of the mice in any given experiment. We have yet to determine how the virus is controlled in the remaining mice, whether nonrelapsing mice have distinct control mechanisms from the beginning or whether they are somehow capable of compensating for the loss of CD4+ T cells. We are also interested in finding the mechanism of IFN-γ-mediated control of virus production. The evidence indicating that the decrease in virus production by Dunni-FB29 cells was not associated with a decrease in the expression of either viral capsid or envelope proteins (Fig. 4) suggests that packaging or particle formation might be defective. We are currently investigating this and other issues such as identification of the relevant sources of IFN-γ in vivo. In the future, we hope to discover ways to bring persistent FV out of hiding and make it susceptible to clearance by the immune system or drug-based therapies.
REFERENCES
- 1.Behringer R R, Dewey M J. Cellular site and mode of Fv-2 gene action. II. Conditional protection of Fv-2ss cells by admixture with Fv-2rr cells. Exp Hematol. 1989;17:330–334. [PubMed] [Google Scholar]
- 2.Ben-David Y, Bernstein A. Friend virus-induced erythroleukemia and the multistage nature of cancer. Cell. 1991;66:831–834. doi: 10.1016/0092-8674(91)90428-2. [DOI] [PubMed] [Google Scholar]
- 3.Cantin E, Tanamachi B, Openshaw H. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J Virol. 1999;73:3418–3423. doi: 10.1128/jvi.73.4.3418-3423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavanaugh V J, Guidotti L G, Chisari F V. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J Virol. 1997;71:3236–3243. doi: 10.1128/jvi.71.4.3236-3243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherwinski H M, Schumacher J H, Brown K D, Mosmann T R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesebro B, Bloom M, Wehrly K, Nishio J. Persistence of infectious Friend virus in spleens of mice after spontaneous recovery from virus-induced erythroleukemia. J Virol. 1979;32:832–837. doi: 10.1128/jvi.32.3.832-837.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesebro B, Miyazawa M, Britt W J. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu Rev Immunol. 1990;8:477–499. doi: 10.1146/annurev.iy.08.040190.002401. [DOI] [PubMed] [Google Scholar]
- 8.Chesebro B, Wehrly K, Cloyd M, Britt W, Portis J, Collins J, Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: Friend-specific and FMR-specific antigens. Virology. 1981;112:131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- 9.Chesebro B, Wehrly K, Stimpfling J. Host genetic control of recovery from Friend leukemia virus-induced splenomegaly. Mapping of a gene within the major histocompatibility complex. J Exp Med. 1974;140:1457–1467. doi: 10.1084/jem.140.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun T W, Engel D, Berrey M M, Shea T, Corey L, Fauci A S. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coccia E M, Krust B, Hovanessian A G. Specific inhibition of viral protein synthesis in HIV-infected cells in response to interferon treatment. J Biol Chem. 1994;269:23087–23094. [PubMed] [Google Scholar]
- 12.Curiel T J, Wong J T, Gorczyca P F, Schooley R T, Walker B D. CD4+ human immunodeficiency virus type 1 (HIV-1) envelope-specific cytotoxic T lymphocytes derived from the peripheral blood cells of an HIV-1-infected individual AIDS Res. Hum Retroviruses. 1993;9:61–68. doi: 10.1089/aid.1993.9.61. [DOI] [PubMed] [Google Scholar]
- 13.Dhawan S, Heredia A, Lal R B, Wahl L M, Epstein J S, Hewlett I K. Interferon-gamma induces resistance in primary monocytes against human immunodeficiency virus type-1 infection. Biochem Biophys Res Commun. 1994;201:756–761. doi: 10.1006/bbrc.1994.1765. [DOI] [PubMed] [Google Scholar]
- 14.Dhawan S, Wahl L M, Heredia A, Zhang Y, Epstein J S, Meltzer M S, Hewlett I K. Interferon-gamma inhibits HIV-induced invasiveness of monocytes. J Leukoc Biol. 1995;58:713–716. doi: 10.1002/jlb.58.6.713. [DOI] [PubMed] [Google Scholar]
- 15.Dittmer U, Brooks D M, Hasenkrug K J. Protection against establishment of retroviral persistence by vaccination with a live attenuated virus. J Virol. 1999;73:3753–3757. doi: 10.1128/jvi.73.5.3753-3757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dittmer U, Brooks D M, Hasenkrug K J. Requirement for multiple lymphocyte subsets in protection against retroviral infection by a live attenuated vaccine. Nat Med. 1999;5:189–193. doi: 10.1038/5550. [DOI] [PubMed] [Google Scholar]
- 17.Erb P, Grogg D, Troxler M, Kennedy M, Fluri M. CD4+ T cell-mediated killing of MHC class II-positive antigen-presenting cells. I. Characterization of target cell recognition by in vivo or in vitro activated CD4+ killer T cells. J Immunol. 1990;144:790–795. [PubMed] [Google Scholar]
- 18.Fan S X, Turpin J A, Aronovitz J R, Meltzer M S. Interferon-gamma protects primary monocytes against infection with human immunodeficiency virus type 1. J Leukoc Biol. 1994;56:362–368. doi: 10.1002/jlb.56.3.362. [DOI] [PubMed] [Google Scholar]
- 19.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 20.Franco A, Guidotti L G, Hobbs M V, Pasquetto V, Chisari F V. Pathogenetic effector function of CD4-positive T helper 1 cells in hepatitis B virus transgenic mice. J Immunol. 1997;159:2001–2008. [PubMed] [Google Scholar]
- 21.Furci L, Scarlatti G, Burastero S, Tambussi G, Colognesi C, Quillent C, Longhi R, Loverro P, Borgonovo B, Gaffi D, Carrow E, Malnati M, Lusso P, Siccardi A G, Lazzarin A, Beretta A. Antigen-driven C-C chemokine-mediated HIV-1 suppression by CD4(+) T cells from exposed uninfected individuals expressing the wild-type CCR-5 allele. J Exp Med. 1997;186:455–460. doi: 10.1084/jem.186.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidotti L G, Borrow P, Brown A, McClary H, Koch R, Chisari F V. Noncytopathic clearance of lymphocytic choriomeningitis virus from the hepatocyte. J Exp Med. 1999;189:1555–1564. doi: 10.1084/jem.189.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 24.Guidotti L G, Rochford R, Chung J, Shapiro M, Purcell R, Chisari F V. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 25.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 26.Hammond S A, Bollinger R C, Stanhope P E, Quinn T C, Schwartz D, Clements M L, Siliciano R F. Comparative clonal analysis of human immunodeficiency virus type 1 (HIV-1)-specific CD4+ and CD8+ cytolytic T lymphocytes isolated from seronegative humans immunized with candidate HIV-1 vaccines. J Exp Med. 1992;176:1531–1542. doi: 10.1084/jem.176.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasenkrug K J. Lymphocyte deficiencies increase susceptibility to friend virus-induced erythroleukemia in Fv-2 genetically resistant mice. J Virol. 1999;73:6468–6473. doi: 10.1128/jvi.73.8.6468-6473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasenkrug K J, Brooks D M, Dittmer U. Critical role for CD4+ T cells in controlling retrovirus replication and spread in persistently infected mice. J Virol. 1998;72:6559–6564. doi: 10.1128/jvi.72.8.6559-6564.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasenkrug K J, Brooks D M, Robertson M N, Srinivas R V, Chesebro B. Immunoprotective determinants in Friend murine leukemia virus envelope protein. Virology. 1998;248:66–73. doi: 10.1006/viro.1998.9264. [DOI] [PubMed] [Google Scholar]
- 30.Hasenkrug K J, Chesebro B. Immunity to retroviral infection: The Friend virus model. Proc Natl Acad Sci USA. 1997;94:7811–7816. doi: 10.1073/pnas.94.15.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasenkrug K J, Dittmer U. The role of CD4 and CD8 T cells in recovery and protection from retroviral infection: lessons from the Friend virus model. Virology. 2000;272:244–249. doi: 10.1006/viro.2000.0387. [DOI] [PubMed] [Google Scholar]
- 32.Haynes B F, Pantaleo G, Fauci A S. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 33.Iwashiro M, Kondo T, Shimizu T, Yamagishi H, Takahashi K, Matsubayashi Y, Masuda T, Otaka A, Fujii N, Ishimoto A, Miyazawa M, Robertson M J, Chesebro B, Kuribayashi K. Multiplicity of virus-encoded helper T-cell epitopes expressed on FBL-3 tumor cells. J Virol. 1993;67:4533–4542. doi: 10.1128/jvi.67.8.4533-4542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kundu S K, Katzenstein D, Moses L E, Merigan T C. Enhancement of human immunodeficiency virus (HIV)-specific CD4+ and CD8+ cytotoxic T-lymphocyte activities in HIV-infected asymptomatic patients given recombinant gp160 vaccine. Proc Natl Acad Sci USA. 1992;89:11204–11208. doi: 10.1073/pnas.89.23.11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lander M R, Chattopadhyay S K. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ecotropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J Virol. 1984;52:695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970;45:163–169. [PubMed] [Google Scholar]
- 37.Lilly F, Steeves R A. B-tropic Friend virus: a host-range pseudotype of spleen focus-forming virus (SFFV) Virology. 1973;55:363–370. doi: 10.1016/0042-6822(73)90176-1. [DOI] [PubMed] [Google Scholar]
- 38.Liu A Y, Miskovsky E P, Stanhope P E, Siliciano R F. Production of transmembrane and secreted forms of tumor necrosis factor (TNF)-alpha by HIV-1-specific CD4+ cytolytic T lymphocyte clones. Evidence for a TNF-alpha-independent cytolytic mechanism. J Immunol. 1992;148:3789–3798. [PubMed] [Google Scholar]
- 39.Matsubayashi Y, Hirama T, Morioka A, Iwashiro M, Masuda T, Uchino H, Takeshita S, Yamagishi H, Udono H, Mieno M, et al. Participation of a dominant cytotoxic T cell population defined by a monoclonal antibody in syngeneic anti-tumor responses. Eur J Immunol. 1990;20:2095–2103. doi: 10.1002/eji.1830200931. [DOI] [PubMed] [Google Scholar]
- 40.Meylan P R, Guatelli J C, Munis J R, Richman D D, Kornbluth R S. Mechanisms for the inhibition of HIV replication by interferons-alpha, -beta, and -gamma in primary human macrophages. Virology. 1993;193:138–148. doi: 10.1006/viro.1993.1110. [DOI] [PubMed] [Google Scholar]
- 41.Orentas R J, Hildreth J E, Obah E, Polydefkis M, Smith G E, Clements M L, Siliciano R F. Induction of CD4+ human cytolytic T cells specific for HIV-infected cells by a gp160 subunit vaccine. Science. 1990;248:1234–1237. doi: 10.1126/science.2190315. [DOI] [PubMed] [Google Scholar]
- 42.Perryman S, Nishio J, Chesebro B. Complete nucleotide sequence of Friend murine leukemia virus, strain FB29. Nucleic Acids Res. 1991;19:6950. doi: 10.1093/nar/19.24.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson M N, Miyazawa M, Mori S, Caughey B, Evans L H, Hayes S F, Chesebro B. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and Western blotting. J Virol Methods. 1991;34:255–271. doi: 10.1016/0166-0934(91)90105-9. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu T, Uenishi H, Teramura Y, Iwashiro M, Kuribayashi K, Tamamura H, Fujii N, Yamagishi H. Fine structure of a virus-encoded helper T-cell epitope expressed on FBL-3 tumor cells. J Virol. 1994;68:7704–7708. doi: 10.1128/jvi.68.12.7704-7708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sitbon M, Nishio J, Wehrly K, Lodmell D, Chesebro B. Use of a focal immunofluorescence assay on live cells for quantitation of retroviruses: distinction of host range classes in virus mixtures and biological cloning of dual-tropic murine leukemia viruses. Virology. 1985;141:110–118. doi: 10.1016/0042-6822(85)90187-4. [DOI] [PubMed] [Google Scholar]
- 47.Stanhope P E, Liu A Y, Pavlat W, Pitha P M, Clements M L, Siliciano R F. An HIV-1 envelope protein vaccine elicits a functionally complex human CD4+ T cell response that includes cytolytic T lymphocytes. J Immunol. 1993;150:4672–4686. [PubMed] [Google Scholar]
- 48.Suzuki S, Axelrad A A. FV-2 locus controls the proportion of erythropoietic progenitor cells (BFU-E) synthesizing DNA in normal mice. Cell. 1980;19:225–236. doi: 10.1016/0092-8674(80)90404-3. [DOI] [PubMed] [Google Scholar]
- 49.Tishon A, Lewicki H, Rall G, Von Herrath M, Oldstone M B. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology. 1995;212:244–250. doi: 10.1006/viro.1995.1477. [DOI] [PubMed] [Google Scholar]
- 50.Tite J P, Janeway C A., Jr Cloned helper T cells can kill B lymphoma cells in the presence of specific antigen: Ia restriction and cognate vs. noncognate interactions in cytolysis. Eur J Immunol. 1984;14:878–886. doi: 10.1002/eji.1830141004. [DOI] [PubMed] [Google Scholar]
- 51.Tite J P, Jones B, Katz M E, Janeway C A., Jr Generation, propagation, and variation in cloned, antigen-specific, Ia-restricted cytolytic T-cell lines. Curr Top Microbiol Immunol. 1986;126:93–100. doi: 10.1007/978-3-642-71152-7_12. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Kavanaugh M P, North R A, Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991;352:729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]