Abstract
COVID-19 can lead to various complications, including severe respiratory symptoms. Both viral infections and total thyroidectomy are known to cause hypocalcemia, making a history of thyroidectomy a potential risk factor for hypocalcemia in COVID-19 patients. We present the case of a 34-year-old woman with Graves’ disease who developed hypocalcemia due to COVID-19 following a total thyroidectomy. The patient underwent an uneventful total thyroidectomy, with preservation of at least three of the four parathyroid glands. Postoperatively, her parathyroid hormone (PTH) levels were normal, and she was discharged without tetany. However, on postoperative day 90, she experienced mild hypocalcemia during a COVID-19 infection, although it was asymptomatic. By postoperative day 127, she presented with severe tetany and general malaise. Testing confirmed a reinfection with SARS-CoV-2 and hypocalcemia, while PTH levels remained normal. Treatment with intravenous calcium gluconate, oral calcium lactate, and alfacalcidol effectively resolved the hypocalcemia and tetany. The patient was subsequently discharged without tetany and has since been monitored without the need for calcium or vitamin D supplementation. This case highlights that the COVID-19 infection following a total thyroidectomy can cause hypocalcemia. Postoperative hypocalcemia is a common issue in head and neck surgery, and viral infections like COVID-19 should be considered in the differential diagnosis of hypocalcemia.
Keywords: vitamine d, covid-19, parathyroid hormone, thyroidectomy, hypocalcemia
Introduction
The outbreak of COVID-19, caused by SARS-CoV-2, has profoundly impacted global medicine. Despite the development of vaccines, many individuals continue to experience COVID-19, which can lead to various serious complications. Beyond respiratory issues, endocrine disturbances, particularly hypocalcemia, have been documented as complications of COVID-19 [1]. Additionally, total thyroidectomy is known to cause hypocalcemia, with reported incidence rates ranging from 2% to 83% [2]. Here, we present a case of hypocalcemia following a total thyroidectomy, attributed to COVID-19.
Case presentation
A 34-year-old woman with Graves’ disease underwent medical treatment for a year at a private clinic. She was referred to our department for a total thyroidectomy due to inadequate control of thyroid function with thiamazole (20 mg/day) and potassium iodide (50 mg/day). The patient had a history of asthma and had not been vaccinated against SARS-CoV-2. At her initial visit to our department, the laboratory data were as follows: albumin: 4.5 g/dL; calcium: 9.2 mg/dL; phosphate: 3.4 mg/dL; free triiodothyronine: 2.58 pg/mL; free thyroxine: 0.78 ng/mL; thyroid-stimulating hormone: 14 μIU/mL; intact parathyroid hormone (PTH): 46.5 pg/mL; thyrotropin receptor antibody: 10.6 IU/L; anti-thyroglobulin antibody: 12.3 IU/mL; and anti-thyroid peroxidase antibody: 9 IU/mL (Table 1).
Table 1. Preoperative laboratory data.
| Parameter | Measured value | Normal range |
| Albumin | 4.5 g/dL | 4.1-5.1 g/dL |
| Calcium | 9.2 mg/dL | 8.8-10.1 mg/dL |
| Phosphate | 3.4 mg/dL | 2.7-4.6 mg/dL |
| Free triiodothyronine | 2.58 pg/mL | 2.30-4.00 pg/mL |
| Free thyroxine | 0.78 ng/dL | 0.90-1.7 ng/dL |
| Thyroid-stimulating hormone | 14 μIU/mL | 0.50-5.00 μIU/mL |
| Intact parathyroid hormone | 46.5 pg/mL | 15.00-65.00 pg/mL |
| Thyrotropin receptor antibody | 10.6 IU/L | 0.00-1.99 IU/L |
| Anti‐thyroglobulin antibody | 12.3 IU/mL | <28 IU/mL |
| Anti-thyroid peroxidase antibody | 9 IU/mL | <16 IU/mL |
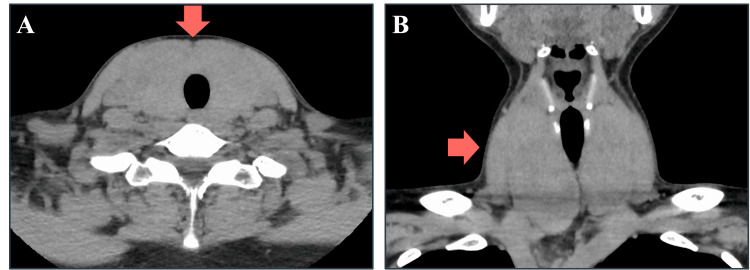
CT revealed a diffusely enlarged thyroid gland with a maximum width of approximately 7 cm (Figure 1A, 1B).
Figure 1. CT image of the thyroid.
The thyroid gland was enlarged to a maximum width of approximately 7 cm. (A) Axial CT scan showing a diffusely enlarged thyroid gland. (B) Coronal CT scan displaying the same diffuse enlargement of the thyroid gland.
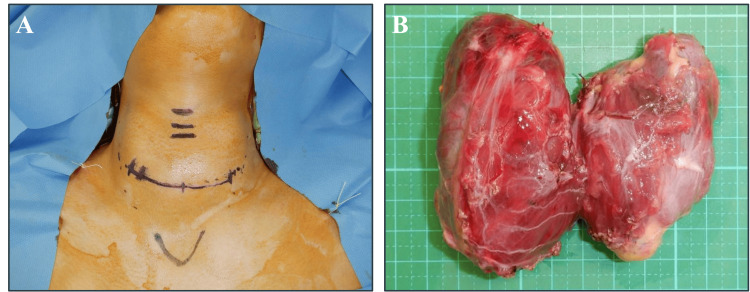
We performed a total thyroidectomy through a transverse cervical incision under general anesthesia (Figure 2A). At least three out of four parathyroid glands were preserved, and the surgery was completed without complications. The excised thyroid gland weighed 125 g (Figure 2B). The patient received a calcium gluconate infusion for a few days post-surgery and was discharged with oral calcium lactate (3 g/day), alfacalcidol (2 μg/day), and levothyroxine. Follow-up blood tests showed normal levels of PTH and calcium without any signs of tetany, leading to the discontinuation of calcium lactate and alfacalcidol.
Figure 2. Intraoperative findings.
(A) Total thyroidectomy performed through a 7-cm transverse cervical incision. (B) Removed thyroid gland, weighing 125 grams.
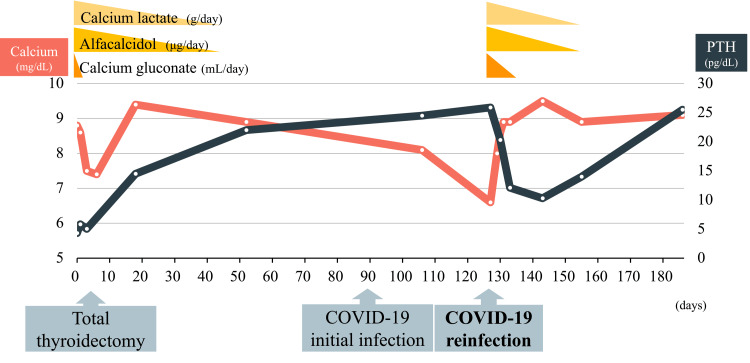
The patient experienced an initial SARS-CoV-2 infection on postoperative day 90, with mild symptoms that resolved spontaneously. Although there was a slight decrease in calcium levels to 8.1 mg/dL, the PTH level remained normal, and no tetany was observed (Figure 3).
Figure 3. Postoperative course of corrected calcium and PTH levels.
The PTH level gradually increased following total thyroidectomy, with calcium supplementation concluding one month after surgery. Despite normal PTH levels, severe hypocalcemia occurred following the COVID-19 reinfection. Calcium administration resolved the symptoms of reactive PTH downregulation. After recovery from COVID-19, both calcium and PTH levels remained within the normal range without supplementation.
PTH: parathyroid hormone
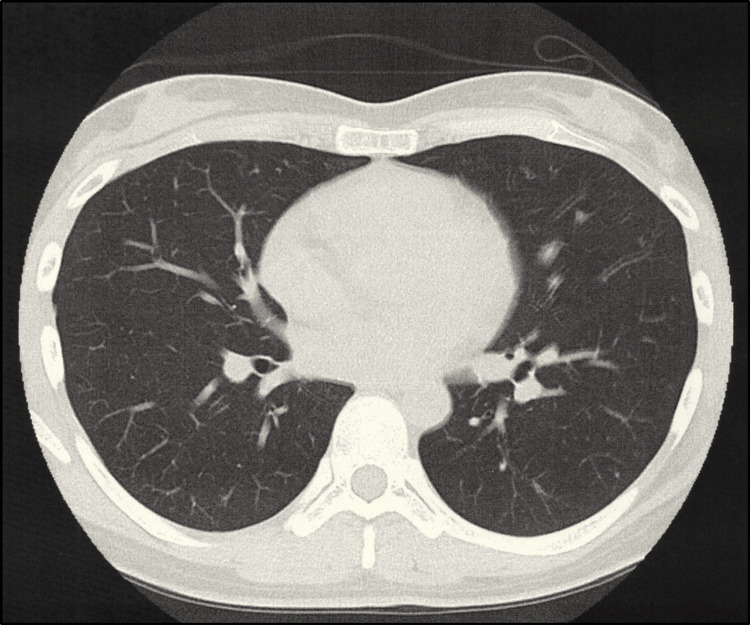
The patient was reinfected with SARS-CoV-2 on postoperative day 127, presenting with severe tetany, difficulty walking, general malaise, cough, and nasal discharge. Both SARS-CoV-2 antigen and PCR tests were positive, with a low PCR threshold cycle value of 23 indicating reinfection. A chest CT revealed no evidence of pneumonia (Figure 4).
Figure 4. CT scan of the chest.
The chest CT scan revealed no evidence of pneumonia.
The complete blood count was normal. Other laboratory results showed: albumin: 4.3 g/dL, calcium: 6.6 mg/dL, phosphate: 3.2 mg/dL, sodium: 138 mmol/L, potassium: 3.3 mmol/L, magnesium: 1.8 mg/dL, 1,25-(OH) vitamin D: 37 pg/mL, blood sugar: 110 mg/dL, blood urea nitrogen: 7.5 mg/dL, creatinine: 0.62 mg/dL, and PTH: 25.9 pg/mL (Table 2).
Table 2. Laboratory data during COVID-19 reinfection.
| Parameter | Measured value | Normal range |
| Albumin | 4.3 g/dL | 4.1-5.1 g/dL |
| Calcium | 6.6 mg/dL | 8.8-10.1 mg/dL |
| Phosphate | 3.2 mg/dL | 2.7-4.6 mg/dL |
| Free triiodothyronine | 1.69 pg/mL | 2.30-4.00 pg/mL |
| Free thyroxine | 1.36 ng/dL | 0.90-1.7 ng/dL |
| Thyroid-stimulating hormone | 1.5 μIU/mL | 0.50-5.00 μIU/mL |
| Intact parathyroid hormone | 25.9 pg/mL | 15.00-65.00 pg/mL |
| Sodium | 138 mmol/L | 138-145 mmol/L |
| Potassium | 3.3 mmol/L | 2.7-4.6 mg/dL |
| Magnesium | 1.8 mg/dL | 1.7-2.6 mg/dL |
| 1.25-(OH) Vitamin D | 37 pg/mL | 20-60 pg/ml |
| Blood sugar | 110 mg/dL | 73-110 mg/dL |
| Blood urea nitrogen | 7.5 mg/dL | 8.0-20.0 mg/dL |
| Creatinine | 0.62 mg/dL | 0.46-0.79 mg/dL |
Thyroid function remained normal with oral levothyroxine administration. Given the patient’s hypocalcemia with normal PTH levels, renal function, and vitamin D levels, COVID-19 was considered the cause of hypocalcemia. Hypocalcemia and tetany improved immediately with intravenous calcium gluconate, oral calcium lactate (3 g/day), and alfacalcidol (3 μg/day). Her malaise gradually resolved, and she was discharged 17 days after admission. The doses of calcium lactate and alfacalcidol were tapered off, and PTH levels, which had decreased in response to calcium supplementation, normalized. One year after the COVID-19 infection, the patient showed no signs of tetany and maintained normal calcium and PTH levels without medication.
Discussion
Hypoparathyroidism, pseudohypoparathyroidism, vitamin D deficiency, and renal failure are the primary causes of hypocalcemia. Since Bossoni et al. reported the first case of severe hypocalcemia associated with COVID-19 in 2020 [3], the virus has been recognized as a potential inducer of hypocalcemia. The incidence of hypocalcemia among hospitalized COVID-19 patients ranges from 62.6% to 87.2% [4,5]. Given that hypocalcemia may serve as a prognostic marker for ventilator requirements and mortality in COVID-19 patients [6], normalization of calcium levels is crucial for effective management.
Virus-dependent calcium influx into cells, vitamin D deficiency, functional hypoparathyroidism, and PTH resistance are considered causes of hypocalcemia in COVID-19 [5]. Viruses utilize calcium signaling to enhance replication, with calcium playing a critical role throughout the viral life cycle, including viral structure formation, entry, gene expression, protein synthesis, fusion with host cells, and release of mature viruses [6]. In enveloped viruses such as SARS-CoV and Ebola, calcium directly interacts with viral fusion peptides to promote replication [7]. During the 2003 SARS and 2016 Ebola epidemics, hypocalcemia was observed in approximately 60% of affected patients [7]. Additionally, patients with COVID-19 often experience vitamin D deficiency, which can contribute to hypocalcemia [8]; however, vitamin D levels in our patient were normal. Functional hypoparathyroidism and PTH resistance, which have been reported in critically ill patients, may also be relevant in COVID-19 cases [9].
To date, 13 cases of COVID-19 with hypocalcemia have been reported, including the current case (Table 3) [3,10-20].
Table 3. Summary of reported cases of hypocalcemia associated with COVID-19.
Calcium levels are adjusted by albumin (4 - albumin + calcium).
PTH: parathyroid hormone
| Case | Year | Author | Age/sex | Calcium (mg/dL) | PTH (pg/mL) | Phosphate (mg/dL) | Vitamin D (pg/mL) | Outcome of COVID-19 | History |
| 1 | 2020 | Bossoni et al. [3] | 72/F | 4.75 | 10 | 5.2 | 8 | Recovered | Total thyroidectomy |
| 2 | 2020 | Demir et al. [10] | 68/F | 6.2 | 2.8 | 7.8 | 5.3 | Died | |
| 3 | 2021 | Bonnet et al. [11] | 82/F | 6.9 | 8.9 | 2.9 | 44 | Recovered | |
| 4 | 2021 | Dianatfar et al. [12] | 44/F | 6.3 | 3 | 5.7 | 33 | Recovered | |
| 5 | 2021 | Heidarpour et al. [13] | 22/M | 5 | 145 | - | 32 | Died | |
| 6 | 2021 | Puca et al. [14] | 81/F | 5.7 | 107 | - | 4.5 | Recovered | |
| 7 | 2022 | Azanjac et al. [15] | 62/M | 5 | 4.2 | - | 10 | Died | |
| 8 | 2022 | Georgakopoulou et al. [16] | 53/M | 6.9 | 11.7 | 4.7 | 38.4 | Recovered | |
| 9 | 2022 | Grigoravičius et al. [17] | 39/M | 4.4 | 3 | 6.5 | 15 | Recovered | |
| 10 | 2022 | Irisson-Mora et al. [18] | 63/F | 4.8 | 2.1 | 8.3 | 31 | Died | |
| 11 | 2023 | Bitew et al. [19] | 48/M | 2.6 | 12 | 11.2 | 7 | Recovered | |
| 12 | 2024 | Selva et al. [20] | 14/M | 3.5 | 14.7 | 10 | 21 | Recovered | |
| 13 | 2024 | Present case | 34/F | 6.6 | 25.9 | 3.2 | 37 | Recovered | Total thyroidectomy |
Of these patients, 46% (n = 6) were women. The median age was 53 years (range: 14-82 years). The median calcium level, adjusted for albumin, was 5.0 mg/dL (range: 2.6-6.9 mg/dL). Nine patients recovered, while four died from the infection. Notably, hypocalcemia with normal PTH levels (10-65 pg/mL) was observed in seven cases. It is possible that PTH levels were actually low but appeared normal due to the hypocalcemia. An additional six patients were diagnosed with hypoparathyroidism.
In Bossoni et al.’s case report, a patient who had undergone total thyroidectomy 19 years prior to the COVID-19 infection had normal calcium levels before the infection but developed hypocalcemia afterward [3], similar to our case. Our patient, who also underwent a total thyroidectomy, experienced hypocalcemia triggered by COVID-19. We observed that the initial COVID-19 infection caused mild hypocalcemia, which was exacerbated by a subsequent infection. Although most parathyroids were preserved and PTH levels seemed normal, it is plausible that parathyroid function was lower compared to patients who had not undergone total thyroidectomy. Hypocalcemia with normal PTH may be attributed to increased calcium demand due to virus-dependent calcium influx or PTH resistance.
A limitation of this study is the use of 1,25-(OH) vitamin D as a marker of vitamin D deficiency. While 1,25-(OH) vitamin D measures activated vitamin D, 25-(OH) vitamin D, which reflects the total amount of vitamin D, should also be assessed for a complete diagnosis of vitamin D deficiency.
Clinicians should be aware that a history of thyroid surgery combined with viral infections, including COVID-19, may increase the risk of hypocalcemia, even when PTH levels are normal. Further research is needed to determine whether residual parathyroid function after thyroidectomy is adequate to maintain serum calcium levels during high calcium demands, such as during viral infections.
Conclusions
We report the case of a patient who developed hypocalcemia due to a COVID-19 infection following a total thyroidectomy. Despite normal vitamin D and PTH levels, COVID-19 was identified as the cause of hypocalcemia. While hypocalcemia is commonly observed after head and neck treatments, including total thyroidectomy, head and neck surgeons should be aware of viral infections as a potential contributing factor.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. Asahikawa Medical University Institutional Review Board issued approval 24077. The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Approval for data collection was obtained from the Asahikawa University Institutional Review Board (#24077).
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Takumi Kumai, Takahiro Inoue, Miki Takahara
Acquisition, analysis, or interpretation of data: Takumi Kumai, Takahiro Inoue, Kenzo Ohara
Drafting of the manuscript: Takumi Kumai, Takahiro Inoue, Kenzo Ohara
Critical review of the manuscript for important intellectual content: Takumi Kumai, Kenzo Ohara, Miki Takahara
References
- 1.High prevalence of hypocalcemia in non-severe COVID-19 patients: a retrospective case-control study. Pal R, Ram S, Zohmangaihi D, et al. Front Med (Lausanne) 2020;7:590805. doi: 10.3389/fmed.2020.590805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assessing symptomatic hypocalcemia risk after total thyroidectomy: a prospective study. Košec A, Gašić A, Hergešić F, Rašić I, Košec V, Bedeković V. Int Arch Otorhinolaryngol. 2024;28:0–21. doi: 10.1055/s-0043-1777450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Severe hypocalcemia in a thyroidectomized woman with Covid-19 infection. Bossoni S, Chiesa L, Giustina A. Endocrine. 2020;68:253–254. doi: 10.1007/s12020-020-02326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prevalence and predictive value of hypocalcemia in severe COVID-19 patients. Liu J, Han P, Wu J, Gong J, Tian D. J Infect Public Health. 2020;13:1224–1228. doi: 10.1016/j.jiph.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hypocalcemia in COVID-19: prevalence, clinical significance and therapeutic implications. di Filippo L, Doga M, Frara S, Giustina A. Rev Endocr Metab Disord. 2022;23:299–308. doi: 10.1007/s11154-021-09655-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Association between hypocalcemia and outcome in COVID-19 patients: a retrospective study. Patidar BS, Mukhopadhyay T, Subramanian A, et al. J Lab Physicians. 2023;15:187–193. doi: 10.1055/s-0042-1757415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endocrine and metabolic aspects of the COVID-19 pandemic. Marazuela M, Giustina A, Puig-Domingo M. Rev Endocr Metab Disord. 2020;21:495–507. doi: 10.1007/s11154-020-09569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitamin D status in hospitalized patients with SARS-CoV-2 infection. Hernández JL, Nan D, Fernandez-Ayala M, et al. J Clin Endocrinol Metab. 2021;106:0–53. doi: 10.1210/clinem/dgaa733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hypocalcemia in hospitalized patients with COVID-19: roles of hypovitaminosis D and functional hypoparathyroidism. Hashemipour S, Kiani S, Shahsavari P, et al. J Bone Miner Metab. 2022;40:663–669. doi: 10.1007/s00774-022-01330-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahr's syndrome presenting with seizures in SARS-CoV-2 (COVID-19) pneumonia-a case report. Demir G, Balaban O, Tekeci MH, Issı Z, Erdem AF. Neurol Sci. 2020;41:3063–3065. doi: 10.1007/s10072-020-04733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decompensated primary hypoparathyroidism in a patient with COVID-19. Bonnet JB, Berchoux E, Sultan A. Ann Endocrinol (Paris) 2021;82:123–124. doi: 10.1016/j.ando.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hypoparathyroidism after COVID-19 pneumonia. Dianatfar M, Sanjari M, Dalfardi B. Shiraz E-Med J. 2021;22:0. [Google Scholar]
- 13.Rhabdomyolysis plus hypocalcemia and diabetic ketoacidosis as concurrent rare COVID-19 manifestations. Heidarpour M, Vakhshoori M, Haghighatpanah MA, Ashrafi L, Khorvash F, Iraj B. Case Rep Med. 2021;2021:6625086. doi: 10.1155/2021/6625086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Severe hypocalcaemia in a COVID-19 female patient. Puca E, Puca E, Pipero P, Kraja H, Como N. Endocrinol Diabetes Metab Case Rep. 2021;2021 doi: 10.1530/EDM-20-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Incidental diagnosis of Fahr’s syndrome after coronavirus disease 2019 infection with the fatal outcome. Azanjac Arsic A, Petrovic M, Vesic K. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10367947/ Hippokratia. 2022;26:161. [PMC free article] [PubMed] [Google Scholar]
- 16.COVID-19 induced hypoparathyroidism: a case report. Georgakopoulou VE, Avramopoulos P, Papalexis P, et al. Exp Ther Med. 2022;23:346. doi: 10.3892/etm.2022.11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unrecognized primary hypoparathyroidism with severe hypocalcemia in the presence of COVID-19 infection. Grigoravičius D, Šiaulienė L, Visockienė Ž. Acta Med Litu. 2022;29:136–143. doi: 10.15388/Amed.2021.29.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahr's syndrome for primary hypoparathyroidism in a patient with COVID-19. Irisson-Mora I, Rodríguez-Hernández LA, Balcázar-Padrón JC, Peralta Luzon J, Portocarrero-Ortiz L. Cureus. 2022;14:0. doi: 10.7759/cureus.26342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahr syndrome presenting with status epilepticus after COVID-19 infection. Bitew HY, Kambutse I, Tuyizere A, Claude G. JCEM Case Rep. 2023;1:0. doi: 10.1210/jcemcr/luad072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.COVID-19 infection with severe hypocalcaemia and superior mesenteric artery syndrome-a case report. Selva Raj SR, Han GH, Karupiah M, Nagendram SV, Kang WH. AME Case Rep. 2024;8:54. doi: 10.21037/acr-23-106. [DOI] [PMC free article] [PubMed] [Google Scholar]