Abstract
Biomaterials have potential applications in the treatment of myocardial infarction (MI). These biomaterials have the ability to mechanically support the ventricular wall and to modulate the inflammatory, metabolic, and local electrophysiological microenvironment. In addition, they can play an equally important role in promoting angiogenesis, which is the primary prerequisite for the treatment of MI. A variety of biomaterials are known to exert pro-angiogenic effects, but the pro-angiogenic mechanisms and functions of different biomaterials are complex and diverse, and have not yet been systematically described. This review will focus on the pro-angiogenesis of biomaterials and systematically describe the mechanisms and functions of different biomaterials in promoting angiogenesis in MI.
1. Introduce
Myocardial infarction (MI), also known as a heart attack, occurs when the blood supply to the heart muscle is severely and persistently restricted, causing irreparable harm.1 Typically, the degradation of the coronary endothelium or the rupture of weak atherosclerotic plaques is the primary cause of MI. Thrombogenic chemicals are released upon plaque rupture, which leads to platelet activation, a series of coagulation processes, and the development of a thrombus that attaches to the artery wall. This may lead to the embolization of downstream atherosclerotic fragments. Additionally, this hypercoagulable state has the potential to rupture other weak fibroatheromas, which can result in myocardial cell necrosis.2 Also, coronary artery spasm, which can restrict blood flow or even completely block it, leading to myocardial ischemia and necrosis, thus causing MI. MI can also be exacerbated by underlying diseases such as myocarditis, hypertension, diabetes, high cholesterol, and smoking.3
MI, a common cardiovascular disease, affects millions of people every year. MI is considerably common in developed countries and increasingly dangerous for emerging countries.4 MI occurs in people over the age of 40, and is especially common in people over the age of 50.5 Men are more likely to have MIs, although women are also at higher risk after menopause.6 A family history of MI has been linked to an increased risk of the condition in individuals, according to substantial research on genetic variables. Additionally, lifestyle decisions are quite important. Smoking, high cholesterol, hypertension, diabetes, sedentary lifestyle, and obesity are risk factors linked to an increased risk of MI.7 Diet is also a significant contributing factor.8 Cardiovascular health is promoted by a diet high in fiber, low in fat and sodium, and lots of fruits and vegetables.9 Symptoms that appear after a MI include pain in the chest, upper arms, lower jaw, or upper abdomen during physical activity or at rest. Other ischemia symptoms, such as dyspnea or fatigue, are possible. MI may be accompanied by atypical symptoms, such as palpitations or cardiac arrest, and in some cases without symptoms, known as a “silent heart attack”.10
MI occurs when one or more blood vessels that supply the heart muscle are blocked, resulting in a shortage of blood flow and subsequent ischemia. This condition may lead to severe necrosis of cardiac muscle cells, known as myocardial cells, and may result in cardiac dysfunction or heart failure.11 The current effective strategy to save ischemic myocardium in the treatment of myocardial infarction is timely reperfusion to save the dying myocardium, reduce the infarct size, and improve cardiac function. Traditional treatments include thrombolytic therapy,12 surgical treatments13 (such as percutaneous coronary intervention and coronary artery bypass grafting), medication-based therapies,14 and heart transplantation. Surgical or thrombolytic interventions aim to restore effective blood flow to the infarcted area as soon as possible, while pharmacological treatment or combined surgery or thrombolytic therapy can effectively improve the prognosis and clinical outcome of MI. Although these treatments have greatly lowered mortality rates, they have drawbacks such as dependence on external equipment, drug toxicity issues, limited donor organ availability, inherent invasiveness, and other associated harm.15 Furthermore, the process of reperfusion might cause further myocardial cell death.16 As a result, finding novel and more effective ways to retain myocardial function and develop new tactics for treating MI is an important topic in cardiovascular disease research.
Various prospective therapeutic techniques are currently being researched to treat MI and restore heart function, with some showing promising results. Cell transplantation,17,18 exosomes,19 nucleic acids,20 and myocardial patches21 are examples. Luo et al.22 used poly(lactic coglycolic acid) (PLGA) particles to encapsulate secretory factors from human bone marrow MSCs, which were subsequently wrapped with the MSC membrane to form “Synthetic MSC” (or synMSC). This novel method addressed the issues of stem cell stability and exosome metabolism. Direct injection of synMSC increased angiogenesis, reduced left ventricular remodeling, and had a synergistic effect in lowering infarct area via anti-inflammatory and angiogenic activities in a rat model of acute MI. Another study23 used monodisperse silica to produce a Gel@MSN/miR-21–5p delivery system that significantly suppressed inflammatory reactions by reducing M1 macrophage polarization in the infarcted myocardium. This approach also delivered microRNA-21–5p to endothelial cells (ECs), greatly increasing local neovascularization and preserving high-risk cardiac cells. As an excellent target molecule, matrix metalloproteinase (MMP) can enhance angiogenesis by targeting MMP-2/9 up-regulation following MI.24 Chen et al.25 created a responsive hydrogel based on MMP-2/9 and loaded with a composite gene nanocarrier (CTL4) to achieve two goals: MMP clearance and macrophage function regulation. This method successfully reduced the early inflammatory response of MI while also promoting angiogenesis. Tissue engineering and regenerative medicine techniques have shown promising results in repairing and replacing injured myocardial tissue, and biomaterials may have applications in the treatment of MI. This review aims to summarize therapeutic approaches that promote angiogenesis, with a particular emphasis on the application of biomaterial strategies in the treatment of MI. These strategies enhance blood supply to the ischemic myocardium, thereby preserving and restoring damaged cardiac function.
2. Biomaterial Application in MI
Various biomaterials have recently emerged as effective new solutions for cardiac tissue regeneration, prevention of ventricular remodeling and scar tissue formation, and reduction of heart failure. Biomaterial-based cardiac tissue engineering has long been considered a promising therapy option for MI. These biomaterials are designed to address specific challenges associated with MI, such as the acidic microenvironment,26 cardiac biomechanical alterations,27 excessive production of reactive oxygen species (ROS),28 sustained overexpression of matrix metalloproteinases (MMPs),29 monocyte-regulated microenvironment,30 and inadequate angiogenesis.31 The following are the primary functions of biomaterials in this context:
2.1. Supporting
Following MI, the ischemia and hypoxic circumstances cause necrosis and apoptosis of myocardial cells, resulting in decreased cell density and extracellular matrix (ECM) composition. This process is followed by increased production of matrix metalloproteinases (MMPs), which accelerates ECM degradation and causes progressive ventricular wall weakening and enlargement in the infarcted area.31 These changes contribute to heart function impairment.32 Therefore, optimizing the postinfarction biomechanical environment is critical for MI treatment. The Young’s modulus of normal myocardium ranges from 20 to 500 kPa, while its shear modulus is approximately 6 kPa.33,34 In material design for myocardial applications, it is essential that the mechanical properties of the materials closely match those of the myocardium. Discrepancies in mechanical properties can lead to several complications. Specifically, if the elastic modulus and mechanical strength of the material do not correspond with those of cardiac tissue, it can adversely affect the integration of the material with the surrounding myocardium. Such a mismatch may result in stress concentrations at the interface between the implanted material and the myocardium, potentially leading to inflammation or tissue damage at the implantation site. Moreover, inappropriate mechanical properties may compromise the material’s functional performance, as they can hinder cell survival and proliferation within the material, thus impairing myocardial tissue repair and regeneration. Researches indicates that a mechanically suitable environment enhances cardiac cell function and facilitates cardiac tissue repair.35 Materials that are excessively stiff or too soft may fail to replicate the natural mechanical properties of cardiac tissue, thereby affecting the heart’s overall function. This could result in the repaired myocardium being unable to synchronize effectively, thereby diminishing the heart’s pumping efficiency.36 Consequently, ensuring that hydrogels or biomaterials used in myocardial infarction treatment possess mechanical properties aligned with those of myocardial tissue is critical for treatment efficacy and patient safety.
Hydrogels, which are known for their biocompatibility, biodegradability, contractility, and elasticity, provide mechanical support to the damaged myocardium, limit pathological remodeling, and aid in the maintenance of contractile function. As a result, they are widely used in the treatment of MI.15 McLaughlin et al.37 used recombinant human collagen type I (rHCI) and type III (rHCIII), which are ECM components found in the healthy heart, to improve cardiac function after MI in a mouse model during the proliferative stage of the infarction. The findings showed that the clinical-grade rHCI matrix reduced longitudinal endocardial strain, prevented unfavorable cardiac remodeling, and improved cardiac function during the later stages of proliferation. Alginate, an anionic polysaccharide derived from sea algae, is nonthrombotic and has a structure similar to the injured ECM in MI. It can temporarily replace damaged ECM and reverse left ventricular remodeling following a heart attack. Studies on its application in post-MI heart treatment have revealed that it enhances scar thickness, inhibits unfavorable cardiac remodeling, and reduces dysfunction in both recent and chronic MIs.38 Hydrogels produced from acellular tissue or ECM have been studied for MI treatment in order to better imitate the natural cellular milieu. Injectable hydrogels generated from swine cardiac ECM have shown favorable biocompatibility and blood compatibility, suggesting that they offer significant promise for heart repair following MI.39
2.2. Inflammatory Regulation
Following MI, necrotic cardiomyocytes release damage-associated molecular patterns (DAMPs), which trigger an innate immune response and initiate the cardiac repair process.40 This repair process can be roughly divided into three stages: the inflammatory phase, the proliferative phase, and the maturation phase.41 In the early stages of MI, a rapid inflammatory response is crucial for the removal of necrotic cardiomyocytes and tissue debris in the infarcted area. This response involves the recruitment of neutrophils through chemokines and the migration of a large number of macrophages from the bone marrow/spleen. These immune cells, which accumulate in the damaged cardiac tissue, contribute to local clearance by phagocytosing necrotic tissue and releasing pro-inflammatory cytokines, thereby activating further inflammatory responses. Although this stage of the inflammatory response is necessary for subsequent tissue repair, excessive inflammatory activity may exacerbate cardiac damage by increasing injury to surviving cardiomyocytes at the periphery and promoting protease activity.42 Meanwhile, resident cardiac macrophages that survive partially counteract the inflammation mediated by recruited macrophages.43 The transition to the proliferative phase occurs around days 3–7 postinfarction, during which inflammatory cells begin to express anti-inflammatory and repair-related factors. For example, macrophages undergo metabolic reprogramming, leading to enhanced expression of anti-inflammatory cytokines and tissue repair factors, thereby promoting the proliferation and activation of myofibroblasts within the infarcted area. This process facilitates collagen deposition, extracellular matrix remodeling, and scar formation.44,45 The apoptosis of the majority of repair cells signifies the end of the proliferative phase. During the maturation phase, newly synthesized matrix gradually cross-links and reorganizes into more stable and structured scar tissue, contributing to the long-term stability and mechanical strength of the cardiac tissue.46 The success of cardiac remodeling following myocardial infarction hinges on effective inflammation control, appropriate extracellular matrix construction, and timely reduction of cellular activity. These factors are crucial to ensuring optimal recovery of the heart postmyocardial infarction.45
In order to mitigate the postinfarct inflammatory response, a common strategy is to modulate the macrophage phenotype. Changing the phenotype of macrophages to increase M2 polarization may facilitate cardiac tissue healing.47 Neutrophil release via neutrophil gelatinase-related lipoproteins can influence macrophage polarization toward a “repair” phenotype, thereby improving heart healing results.48 Graphene oxide, a natural antioxidant, has been shown to reduce inflammatory polarization of M1 macrophages by lowering reactive oxygen species (ROS) levels, and it can also act as a gene carrier to further polarize M1 macrophages toward the M2 phenotype, addressing inflammation and treating MI synergistically.49 Bayuan Viscous Ionic Hydrogel has antioxidant and ROS scavenging characteristics and can respond to ROS abundant in the infarcted heart. It modulates macrophage polarization levels and facilitates MI healing.50 Excessive inflammatory response and early intervention can cause long-term tissue damage, increased scar tissue, and increased cell loss, resulting in infarct expansion and poor remodeling.51 For the treatment of MI in rats, composite hydrogels containing V1-Cal and Nap-Phe-Phe-Tyr (NapFFY) have been created. In vivo studies have shown that continuous release of V1-Cal efficiently lowers TRPV1 expression and activation in a rat model of MI, reducing cell death and the release of inflammatory cytokines.52 To achieve long-term improvement in heart function after MI, inflammation must be appropriately and properly regulated to create a physiological balance between inflammatory promotion and repair, ultimately maximizing myocardial repair outcomes.
2.3. Metabolic Regulation
Extensive studies have indicated that mitochondria play an important role in ventricular remodeling after a heart attack. Maintaining mitochondrial activity and homeostasis can improve myocardial metabolic function, minimize the severity of MI, and limit ventricular remodeling. Endogenous miR-210, for example, targets mitochondrial energy metabolism to preserve myocardial cells and improve cardiac performance.53 As the principal cause of oxidative stress, mitochondrial reactive oxygen species (ROS) cause irreparable damage to cardiac and vascular cells. The removal of ROS is critical for boosting myocardial cell healing. Melanin, a widely distributed biopolymer, is biocompatible, biodegradable, and has powerful free radical scavenging and chelating properties. Jin Zhou and colleagues isolated melanin nanoparticles (MNPs) from cuttlefish ink sac and mixed them with alginate to generate an MNPs/alginate (MNPs/Alg) hydrogel. In vitro investigations revealed that the capacity of MNPs/Alg hydrogel to scavenge hydroxyl radicals (OH) and 1′-diphenyl-2-picrylhydraryl radicals (DPPH) varied with MNP concentration. When compared to the pure Alg hydrogel group, rats treated with MNPs/Alg hydrogel had considerably lower DHE fluorescence, showing that MNPs play a key role in regulating the ROS microenvironment. This leads to increased cardiac cell survival and remodeling of the local microenvironment.54 Fullerene alcohol, a carbon nanomaterial with high water solubility and minimal cytotoxicity, has found widespread use in tissue engineering. More interestingly, ROS rapidly attach to electron-deficient locations on the surface of fullerene alcohol nanoparticles, analogous to the quenching of superoxide dismutase. ROS in nearby electron-deficient spots may transfer electrons to fullerene alcohol cages, resulting in ROS removal. As a result, fullerene derivatives effectively prevent ROS-induced oxidative stress-induced cellular damage. Wang’s research team created a fullerenol/alginate hydrogel injectable with high antioxidant activity. In comparison to the control group and pure alginate hydrogel, fullerenol effectively removes ROS post-MI, protects brown adipose-derived stem cells (BadSCs) from oxidative stress damage, and increases their viability in the MI area.55
2.4. Local Microenvironment Regulation
Researchers have focused on the electrophysiological milieu when creating biomaterials to improve heart repair after MI, in addition to the pathological microenvironment caused by ROS injury. The myocardium is a tissue that contracts and relaxes in response to electrical impulses. The electrical conductivity of normal myocardium is 0.16 S/cm.56 The creation of fibrous scar tissue in the infarcted region, however, impedes electrical transmission in the heart by disrupting synchronous contraction between healthy myocardial tissue and scar tissue, eventually leading to ventricular dysfunction.57 A critical goal in heart regeneration is to rebuild the conductive milieu in the infarcted myocardial to facilitate electrical conduction and integration. Mihic Anton et al.58 were the first to use conductive biomaterials in heart treatment, with the goal of improving electrical transmission between infarcted areas. They created a conductive hydrogel by combining the conductive biomaterial Polypyrrole (PPy) with the chitosan side chain, resulting in the PPy-chitosan hydrogel. The PPy-chitosan-treated group displayed longer QRS duration and enhanced cardiac function in a rat MI model. The lateral conduction velocity of the heart treated with PPy-chitosan was quicker along the epicardial surface, according to optical mapping. This conductive polymer increased the conductive milieu in the infarcted myocardium, offering a unique strategy for the treatment of MI. Similarly, due to their excellent electrical conductivity and mechanical rigidity, materials such as gold nanorods (GNRs),59 reduced graphene oxide (rGO),60 poly(vinyl alcohol) (PVA),61 polyaniline (PANI),62 and carbon fibers (CFs)63 have been widely used to promote tissue regeneration after MI. Strong conductivity has also been reported for the TA-PEG/HA-SH/ADSCs/gene hydrogel-based holographic system (TA-PEG).64 Wang’s research team created Au@Pt nanoparticles/Alginate (Au@Pt/Alg) hydrogels by combining conductive gold nanoparticles (AuNPs) with Pt nanoparticles with catalytic and antioxidant capabilities. These bimetallic nanoparticles were used to increase electrical conduction velocity in the MI area, hence promoting heart healing and managing the ROS microenvironment.65
2.5. Promote Angiogenesis
Blood arteries are important in disease situations because they serve as conduits for gas exchange, nutrient diffusion, and waste elimination. They not only meet the high metabolic needs of inflammatory areas, but they also aid in the prevention of cellular malfunction and death. In the field of heart tissue engineering and regeneration, timely and effective angiogenesis is critical for implant survival and healing. Endogenous angiogenesis after a MI has been shown in animal experiments to minimize scar formation and improve left ventricular remodeling. Increased neovascularization in the infarcted region expands clinical therapy options.66 Therapeutic angiogenesis, which includes controlling new blood vessel activities to treat diseases, was first postulated by JM Inser.67 Numerous studies have since used this method to treat ischemia diseases.68 Various initiatives have been made in recent years to increase angiogenesis and boost microvascular system healing. Exosomes, a powerful tool for MSCs therapy, have demonstrated encouraging results in stimulating angiogenesis in a variety of animal models. Exosomes derived from MSCs, for example, have been shown to modify the milieu of the infarcted myocardium by boosting angiogenesis and suppressing inflammatory responses.69 Extracellular vesicles (EVs) derived from MSCs have also been found to induce angiogenesis via miR-210, resulting in better function in the infarcted heart.70 A gas transmitter, hydrogen sulfide (H2S), rapidly diffuses through cell membranes and promotes intercellular signal transmission to trigger angiogenesis. It has been used in the treatment of cardiovascular disorders. Liang et al. created a composite hydrogel based on 2-aminopyridine-5-thiocarboxamide, a small molecule H2S donor, to address the issue of H2S’s short half-life and inefficient release. The hydrogel released sulfide after cardiac injection, resulting in elevated quantities of heart-related mRNA (Cx43, α-SMA, and cTnT) and angiogenic factors (VEGFA and Ang-1). This indicated that the H2S-releasing hydrogel effectively promoted cardiovascular development.71 The following chapter will go into greater detail on biomaterials that induce angiogenesis.
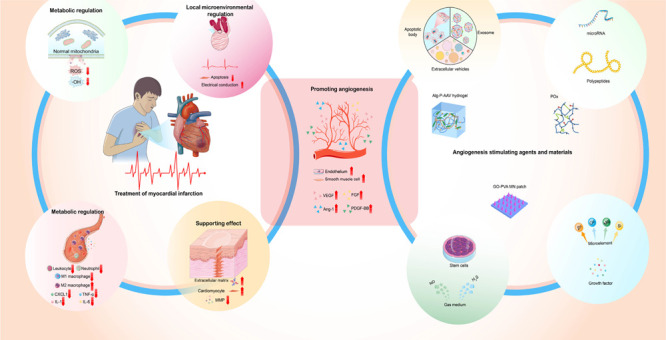
In the preceding paragraph, we have explored the therapeutic potential of several biomaterials in myocardial infarction, including stem cells, growth factors, exosomes, PEG, metal nanoparticles, hydrogen sulfide, chitosan, and collagen. Each of these materials offers unique benefits and mechanisms for improving myocardial repair and regeneration. For an integrated overview of these biomaterials and their contributions to myocardial infarction therapy, please refer to Figure 1. Biomaterials have multifunctional qualities that make them a promising tool for encouraging tissue regeneration and repair, notably in the setting of MI heart repair. The reduced blood flow to the affected area, which can result in tissue damage and compromised cardiac function, is a key challenge in MI. Promoting angiogenesis, or the development of new blood vessels, is thus an important first step in restoring blood flow to the injured region of the heart and enabling tissue repair. The primary focus of this section will be on the angiogenic properties of biomaterials.
Figure 1.
Roles of different biomaterials in the treatment of myocardial infarction. Stem cells, growth factors, exosomes, polyethylene glycol, metal nanoparticles, hydrogen sulfide, chitosan, and collagen are among the biomaterials available for the therapy of myocardial infarction. In the context of a myocardial infarction, these biomaterials serve several purposes. They promote the repair and regeneration of cardiac tissue by providing structural support via their scaffolding characteristics. Furthermore, they have anti-inflammatory properties, lowering inflammatory reactions and suppressing inflammatory cell infiltration as well as inflammatory factor release. Furthermore, biomaterials can regulate metabolism, changing the metabolic processes of cardiac cells to improve energy supply and boost cellular function recovery. They can also improve the electrical conductivity of cardiac tissue by modulating the local microenvironment. Importantly, biomaterials promote angiogenesis by increasing vascular density and improving cardiac perfusion, so supplying the blood supply required for myocardial regeneration. The images in Figure 1 are original and created by the authors, free from third-party copyright restrictions.
3. Angiogenesis-Promoting Biological Materials Improve MI Regeneration and Repair
As the earliest functioning organ to develop in the embryo, the vascular system is crucial in the transfer of nutrients and oxygen throughout the body. Angiogenesis is the formation of new blood vessels via vascular sprouting or intussusception. Vascular sprouting is a multistage, highly regulated process. ECs respond to signals such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) throughout this process. To degrade the ECM, MMPs are involved. Endothelial tip cells direct the growth of newly generated vascular sprouts in response to a gradient of growth stimuli. This migration is facilitated by the signaling of ECs surface proteins such as Notch and VEGF receptors. Stalk cells are ECs that are located behind the leading tip cells. They divide to lengthen the nascent blood vessels and form lumens. Smooth muscle cells and/or pericytes respond to signals such as transforming growth factor beta (TGF-β) and platelet-derived growth factor B (PDGF-B) after new and immature blood vessels are created. These signals aid in the stabilization of ECs and the development of new and mature blood vessels.72
Angiogenesis is an important process in both physiological and pathological circumstances, and it has received a lot of interest in the medical world. Promoting angiogenesis has emerged as a frequent technique for treating ischemia disorders in recent years.73 When tissues are subjected to ischemia and hypoxia, the body responds with a stress response aimed at alleviating ischemia by boosting angiogenesis and generating collateral circulation. In ischemic myocardium, for example, the expression of hypoxia-inducible factor (HIF-1) increases, stimulating the expression of VEGF, FGF, and their corresponding receptors. This enhances angiogenesis in ischemic areas, increases capillary density, and establishes effective collateral circulation, alleviating the effects of ischemia to some extent.74 This physiological compensatory action, however, is insufficient in severely ischemic tissues. Therapeutic angiogenesis has been acknowledged as a viable technique for improving MI based on this pathological development. Exogenous vascular growth factors can be delivered to ischemic tissue to generate collateral blood flow, thereby circumventing damaged blood vessels, boosting blood perfusion in the ischemic myocardium, and ultimately achieving therapeutic goals.66 It is critical to understand that angiogenesis is a complex physiological process involving numerous cells and chemicals. It includes the destruction of the vascular endothelium matrix, EC migration and proliferation, the creation of vascular sprouts, and the establishment of new basement membranes by EC tubes.75 Angiogenesis is tightly controlled in healthy tissues by a fine balance of stimulating and inhibiting signals. Any disturbance in this balance might result in aberrant blood vessel growth and, as a result, imbalance. As a result, the creation of biomaterials that stimulate angiogenesis has emerged as an important research avenue way to promote MI regeneration and repair. With ongoing biomaterials breakthroughs, it is possible to achieve balanced management of neovascularization and improve treatment outcomes.
3.1. Biomaterials that Promote Angiogenesis
Angiogenesis is important in several physiological processes, such as bone regeneration, wound healing, and tissue repair.76 Tissue engineering procedures for fostering the formation and growth of new blood vessels rely on three critical components: biological scaffold materials, stimulating agents, and bioreactors. Scaffold materials not only enable cell attachment and growth, but they also provide structural support for the generation of new blood vessels. Cells, growth factors,77 EVs,78 noncoding RNA,79 peptides,80 and other biological materials that affect the in vivo repair/regeneration milieu to promote angiogenesis are examples of stimulating factors. Bioreactors produce stable conditions and deliver nutrients to help new blood vessels form and grow. When treating a MI, equal emphasis should be placed on controlling ischemia remodeling and boosting myocardial regeneration. As a result, future research in this area should concentrate on rebuilding the ischemic core while maintaining the functional integrity of the border zone.81 Finally, research on biomaterials that stimulate angiogenesis is critical for restoring cardiac function and enhancing patient quality of life.
3.1.1. Materials for Biological Scaffolds
Emerging research suggests that cell-free scaffold materials may facilitate in vivo blood supply restoration. Reduced graphene oxide, a new nanobiomaterial, has been found to induce angiogenesis by encouraging bone marrow MSCs differentiation via ROS activation.82 Macrophages secrete cytokines and chemical mediators that attract other cells such as ECs and fibroblasts while also degrading scaffold materials, encouraging angiogenesis. Furthermore, the pore size of the scaffold materials influences the development and perfusion of vascular cavities. The application of stents with bigger holes (90–160 μm) may result in increased fibrosis and decreased angiogenesis. Implants with hole diameters of 30–40 μm, however, can enhance vascularization while minimizing fibrous encapsulation.83 Although EVs have shown potential in stimulating angiogenesis, direct injection at the target site may result in rapid clearance. The addition of clinical-grade hyaluronic acid (HA) can allow for continuous release, preserving EV biological activity and increasing angiogenesis while decreasing apoptosis and fibrosis.84 Similarly, recombinant humanized type III collagen (rhCol III) has been studied in the treatment of MI for its role in stimulating cell proliferation, migration, and angiogenesis.85 Biological scaffold materials are critical in tissue engineering procedures aimed at promoting angiogenesis. As a result, investigating the biological properties of various scaffold materials and their applications in stimulating angiogenesis is critical in the field.
3.1.1.1. Single Material
Collagen, the most prevalent protein in the ECM, is an important component of connective tissues such as skin, bone, muscle, ligament, tendon, and the heart.86 Collagen not only contributes to the stability and structural integrity of tissues and organs in living creatures, but it also plays an important role in the cellular milieu, assisting in the storage and release of cell mediators such as growth factors.87 Furthermore, because of its innate biocompatibility and biodegradability, collagen is regarded as an ideal biological scaffold material, providing necessary support and signals for cell attachment and proliferation.88 In the process of articular cartilage regeneration, for example, collagen, which has tissue-forming properties and promotes cell proliferation, is widely used as a scaffold material, particularly type II collagen, which is abundant in chondrocytes. Type II collagen scaffolds are used to aid in cartilage healing and regeneration.89
Collagen scaffolds are important in the process of vascular regeneration because they promote cell migration and adhesion. Type IV collagen is mostly found in ECs, which line the basement membrane of blood vessels. Bonanno et al.90 used a rat aorta model to explore the effect of type IV collagen on angiogenesis. They discovered that high concentrations of type IV collagen (300 ug/mL) increased microvascular length by 119% when compared to untreated type I collagen cultures. Type IV collagen activates heart vascular cells in a dose-dependent manner, enhancing angiogenesis elongation and survival. EC migration during angiogenesis is dependent on collagen remodeling. ECs, interstitial cells, and inflammatory cells have all been found to contribute to the production of matrix proteases, which regulate the destruction of the collagen scaffold and the synthesis of new ECM. This mechanism is critical for the formation of new blood vessels. MMP-1, for example, promotes type I collagen degradation, whereas MMP-2 exposes integrin binding sites on collagen and stimulates signaling molecules such as TGF-β. Membrane-bound matrix metalloproteinases (MT-MMPs) produced on tip cells may provide an additional mechanism for collagen breakdown and sprout elongation.91 Furthermore, physical characteristics of scaffold materials, such as gaps and channels, can influence cell movement. ECs interact more intimately with scaffold materials when the pore size is less than 80 μm. Collagen scaffolds with tunable pore diameters have increased angiogenic potential.92
Emerging evidence suggests that collagen breakdown products play a role in regulating numerous activities during heart repair and in steady-state settings. The breakdown product p1158/p1159, which is produced from type I collagen and promoted by MMP2 and MMP9, has been demonstrated to enhance angiogenesis and minimize scar formation following a MI. Canstatin, a byproduct of MMP2-mediated type IV collagen degradation, controls voltage-dependent calcium channel activity in rat cardiomyocytes and decreases hypoxia-induced apoptosis in rat H9C2 cardiomyocytes. Tumstatin protects cardiomyocytes from apoptosis caused by ROS, while endostatin promotes myofibroblast proliferation and migration.93 Collagen, with its rather stiff structure that interacts intimately with other cellular and noncellular components of the myocardium, also plays an important role in preserving the shape, size, and function of the heart.94 However, collagen has a procoagulant action, and there are potential risks associated with its usage in the treatment of cardiovascular disease. Professor Shulamit Levenberg’s team used tropoelastin to create a composite porous scaffold with exceptional angiogenesis-promoting properties. Confocal photographs of the implantation location in the experimental group after 14 days demonstrated its extraordinary angiogenesis ability in a nude mouse abdominal white line defect model.95 Marcy Zenobi-Wong of the Swiss Federal Institute of Technology in Zurich recently proposed a new collagen derivative comprising multiple recognition peptides to improve vascular network creation. Rapid formation of blood arteries, lymphatic vessels, and mesoscale capillaries can be aided by modulating the gel hardness and using Sortase A (SrtA)-mediated cross-linking. Furthermore, the addition of the secondary cross-linking enzyme factor XIII (FXIII) allows for the in situ coupling of VEGF QK peptide to collagen, promoting blood vessel and lymphatic vessel creation without the use of exogenous VEGF.96 This study shows that enzyme cross-linking methods can add specific enzyme recognition peptides while keeping collagen structural features, giving intriguing prospects for the treatment of cardiovascular disorders. Collagen, despite being a high-quality biomaterial, has limitations in its application. These limitations include low mechanical strength and stability, a rapid rate of degradation, and difficulties in managing its quality. The quality of collagen scaffolds is determined by the source of collagen and the methods used to process it, posing difficulties in attaining exact findings in research and clinical applications.97
Silk fibroin is produced from silk via a number of processing procedures including degumming, dissolving, separation, and drying. Noncytotoxicity, minimal immunogenicity, and great biocompatibility are among the benefits of the extracted silk fibroin.98 Furthermore, silk fibroin is a biomacromolecule material with exceptional processability. Highly porous and extensible micro/nano structures can be formed using processes such as freeze-drying and ethanol treatment, making it suitable as a scaffold material.99 Silk fibroin biomaterials have been approved by the US Food and Drug Administration because of their remarkable performance in clinical trials.100 Previous research has shown that silk fibroin can have different mechanical properties by controlling β-fold formation, influencing cellular activity and tissue regeneration.101 Its possible mechanisms include stimulation of the mitogen-activated protein kinase and PI3K signaling pathways, both of which increase cell migration.102 Silk fibroin modulates the paracrine signaling of MSCs, resulting in the release of anti-inflammatory substances such as IGF-1, VEGF, and collagen. This, in turn, promotes cell proliferation, angiogenesis, and the polarization of macrophages toward an anti-inflammatory phenotype via integrin/PI3K/Akt signaling. As a result, silk fibroin has an effect on wound healing by altering the activity of resident cells within the injured skin milieu.103 Kambe et al. explored the use of silk fibroin hydrogels with varying rates of degradation in the treatment of MI.104 Their findings demonstrated that a 12-week injection of unmodified silk fibroin hydrogel resulted in a moderate breakdown rate and could reduce left ventricular hypertrophy. According to the researchers, this effect could be related to the dense and random arrangement of collagen fibers within the silk fibroin hydrogel, which provides enhanced resistance to left ventricular pressure while inhibiting ventricular dilation. However, the mechanical strength and overall stability of pure silk fibroin scaffolds limit their applicability, necessitating modifications to increase their bioavailability.105
Chitosan offers a variety of benefits, including reduced immunogenicity, allowing it to be used in medical implants and devices. It also has good mechanical properties, antimicrobial capabilities, and biodegradability, all of which are important when constructing membrane or porous carrier scaffolds.106 According to research, chitosan’s breakdown products, d-Glu and N-AC-Glu, can protect adipose-derived stem cells from reactive oxygen species (ROS) damage. Chitosan can also attract chemokines such as stromal cell-derived factor 1 (SDF-1), which promotes the homing of stem cells to damaged tissues. This procedure improves the microenvironment of MI by increasing the recruitment of endothelial progenitor cells(EPCs) from the circulation.107 Furthermore, chitosan hydrogel has been shown to boost type I collagen expression in BADSCs (brown adipose-derived stem cells) and promote the development of cardiac cells. BADSCs can also directly differentiate into vascular cells, aiding in the production of new blood vessels.108 These findings imply that chitosan could be useful in cardiac tissue engineering and regenerative medicine.109 Chitosan biomaterials, however, create difficulties due to their insolubility in water and other common solvents.110 Overcoming this barrier may necessitate the employment of irritant chemicals and high temperatures, which may raise toxicity issues. As a result, while using chitosan to treat MI, these restrictions must be carefully considered.
The glycosaminoglycan HA is present in cartilage, connective tissue, the vascular system, and the ECM. It regulates a variety of tissue remodeling activities, including embryonic development, wound healing, angiogenesis, and cancer.111 HA is a linear polysaccharide made up of alternating units of N-acetylglucosamine (GlcNAc) and glucuronic acid (GlcA). Previous research has shown that the molecular weight of HA is related to its function. HA is normally found in healthy tissues as a high molecular weight polymer (>106 kDa), but low molecular weight HA can cause inflammation and angiogenesis.112 HA fragments conjugate with CD44 and activate CXCL1/GRO12, inducing capillary EC sprouting.113 Furthermore, HA affects vascular EC barrier function via the interaction of the CD44v10 subtype and the S1P receptor. The activation of the S1P receptor and the transfer of the RhoA/Rac1 signal to the cytoskeleton of ECs are involved in this process,114 emphasizing the critical role of HA in tissue repair and regeneration. HA is found in the ECM of the heart and plays an important function in maintaining structural integrity and regulating cellular activity. In rat models, HA hydrogel has shown promise in improving heart function after a MI. HA promotes the production of growth factors and cytokines involved in tissue repair and regeneration, such as VEGF and bFGF, in addition to enhancing collagen deposition and decreasing fibrosis.115 Surprisingly, degraded HA oligosaccharides (o-HA) with less than 10 disaccharide units can stimulate the production of the chemokines Ccl2 and Cxcl5. This activation enhances M2-type macrophage polarization, reduces neutrophil-induced inflammation, stimulates MAPK and JAK/STAT signaling pathways, speeds myocardial function reconstruction, and promotes compensatory cardiac function.116 These findings emphasize the role of HA in cardiac tissue healing and its potential as a treatment for MI.
Gelatin scaffold materials have various advantages, including minimal immunogenicity, cost-effectiveness, and the existence of cell adhesion sites and matrix metalloproteinase hydrolysis sites, which promote cell proliferation and migration. Furthermore, gelatin has bioactive components such as cell-binding motifs such as Arg-Gly-Asp (RGD) that provide excellent biodegradability and water solubility, making it a popular material for constructing biological scaffold materials.117 However, the physiological temperature required for most cell culture research is lower than the physical gelation temperature of gelatin, necessitating the use of methacrylated gelatin (GelMA).118 GelMA, which is made by methacrylating gelatin with methacrylic anhydride (MA), is photosensitive and can cross-link when exposed to UV or visible light. Three-dimensional structures with mechanical qualities suited for cell development and differentiation can be formed by altering the MA/gelatin ratio, enabling adaptability in terms of physical and chemical properties. Furthermore, GelMA can be built into unique morphological forms utilizing various production processes such as 3D printing and electrospinning to satisfy specific application needs,119 such as angiogenesis stimulation. Moderate bioelectrical stimulation has been shown to improve signal transmission in myocardial cells, hasten maturation, and aid in the repair or regeneration of more uniform and mature myocardial microstructures. GelMA hydrogel is commonly used as a scaffold for cardiac repair to stimulate tissue regeneration since it is an effective vehicle of conductive components. GelMA-based hydrogel systems, such as GelMA-O5/rGO, have been shown to increase the expression of cardiac troponin I (cTnI) and Cx43 in myocardial cells while decreasing caspase-3 expression levels, thereby improving damaged myocardial tissue and restoring myocardial function.120 Furthermore, Tang et al. used the photocuring properties of GelMA to encapsulate EVs within the GelMA hydrogel via physical capture. This photocuring approach lowers secondary tissue damage to the heart, enhances EV delivery efficiency, and proposes a novel strategy for MI repair and treatment.121
Alginate, a polysaccharide derived from seaweed, has significant biological activity and is used widely in medicine and the food industry.122 Notably, alginate is important in tissue engineering and regeneration. Its nonthrombogenic nature and moderate physical gelation process make it easier to work with in a variety of applications. Furthermore, alginate hydrogel matrices have a texture and hardness that are similar to the ECM, making them excellent for tissue growth and regeneration.123 The use of alginate scaffolds as biological grafts in MI rat models has been found to reduce remodeling and dysfunction. Alginate, a biocompatible natural polymer, has been extensively used in combination with substances such as VEGF and basic fibroblast growth factor 1 (bFGF 1) to stimulate angiogenesis and endothelial differentiation.124 As a result, alginate-based hydrogels can be used as scaffold materials for transporting cells that have been transformed with the RGDfK peptide, enhancing neovascularization and heart function.125 Despite its numerous benefits, alginate has certain drawbacks, including poor dimensional stability and low rip strength. It may distort if not adequately supported, reducing its usefulness as a scaffold material for tissue restoration. In addition, it is crucial to ensure that the alginate is well mixed to avoid air bubbles, which can compromise its potential to induce tissue regeneration.126
As previously stated, natural biomaterials such as collagen, gelatin, and chitosan have high cell compatibility. However, due to variations in raw materials, processing methods, and other factors, replicating their consistent characteristics can be challenging.. Furthermore, the mechanical properties of these materials frequently fall short of the requirements of practical applications. Table 1 provides a summary of the mechanical properties, conductivity, degradation rate, and biocompatibility of several monomer materials previously mentioned in this paper. It can be seen that sodium alginate exhibits superior mechanical properties, while gelatin displays greater conductivity. However, no single material can fulfill more than one of these properties simultaneously. Synthetic polymer materials, however, are well-known for their regulated release rates and excellent flexibility, making them preferred alternatives for tissue engineering scaffold materials. Polyethylene glycol (PEG) hydrogels, for example, have been shown to enhance angiogenesis in vivo by upregulating the expression of angiogenic factors such as VEGF and basic fibroblast growth factor (bFGF).133 PEG hydrogels also promote the recruitment of EPCs, aiding in the development of new blood vessels.134 By providing growth factors such as VEGF, polylactic acid-glycolic acid (PLGA) enhances tissue vascular regeneration.135 Similarly, poly(ethylene oxide) (PEO) is a synthetic polymer that has been used as a coating material to improve EC adhesion and proliferation.136 Through interactions with numerous proteins, heparin, a highly sulfated glycosaminoglycan (GAG), affects various physiological and pathological processes, including angiogenesis. However, its anticoagulant properties preclude its use in stimulating angiogenesis. To address this issue, Liu et al. created a heparin-like polysaccharide with a high affinity for VEGF, semisynthetic chitosan sulfate (SCS). SCS generated an abundance of blood vessels and arteries expressing CD31hi/Emcnhi in a mouse model of hindlimb ischemia. Furthermore, SCS affected macrophage polarization toward the M2 phenotype by increasing endogenous VEGF secretion, causing angiogenesis in ischemic circumstances via the VEGF–VEGFR2 signaling pathway.137
Table 1. Comparison of Single Material Properties for MI Scaffold Requirements.
| name | modulus | conductivity | biocompatibility | degradation |
|---|---|---|---|---|
| collagen127 | ≈5 kPa (elastic modulus) | yes | ||
| gelatin128 | ≈0.5 kPa (Young’s modulus) | 0.6 × 10–5 S cm–1 | yes | |
| chitosan129 | 6.73 ± 1.14 MPa (Young’s modulus) | 2.4 ± 0.9 × 10–2 S cm–1 (with phytic-acid-doped PANI) | yes | |
| silk fibroin130 | ∼8 kPa (Young’s modulus) | yes | 3.1 ± 0.6 weeks | |
| HA131 | Young’s modulus low: ∼7 kPa high: ∼35–40 kPa | yes | ∼3 and 10 weeks | |
| Alg132 | 29 kPa Young’s modulus/14 kPa dynamic modulus | yes | >40 days | |
| normal myocardium | Young’s modulus 20–500 kPa | 0.16 S/m | yes |
3.1.1.2. Composite Material
Collagen, silk fibroin, chitosan, HA, gelatin, alginic acid, polyethylene glycol, and other biodegradable and biocompatible polymer compounds have received a lot of interest and use in tissue engineering. Scaffolds made of a single material component, however, sometimes exhibit limitations such as poor stability and low mechanical qualities under physiological settings, restricting their future practical application. Novel materials can be created by combining two or more polymers and modifying key properties of the tissue engineering scaffold to better mimic the natural ECM. Shi et al., for example, created a cartilage regeneration scaffold by combining silk fibroin and gelatin in a mass ratio of 1:2 (6.9% w/v).138 This combination strikes a balance between mechanical capabilities and disintegration rate, yielding a scaffold that closely resembles the attributes of newly created cartilage. The results show that adding HA lowers the swelling properties of the nanofunctionalized hydrogels while improving their mechanical qualities. Although the mechanical qualities of these systems may not exceed clinical application criteria, differentiation results in better cell proliferation and greater gene expression related to osteogenesis and angiogenesis, highlighting their potential as customizable constructions for tissue regeneration. Furthermore, Zheng et al. discovered that incorporating hydroxyapatite into nanofunctionalized hydrogels improved their mechanical capabilities while decreasing swelling qualities when compared to GelMA alone. Following differentiation, the modified composite hydrogel demonstrated favorable cell proliferation and enhanced gene expression related with osteogenesis and angiogenesis. These findings emphasize the potential for future tissue regeneration applications of these customizable structures.139
Traditional material qualities such as mechanical strength are insufficient in the field of MI healing. Electrical responsiveness is also a crucial factor to consider. While chitosan can regulate cell attachment, metabolism, and proliferation, it falls short of meeting the needs of MI treatment. Because natural myocardial tissue has specific anisotropy and mechanical strength, a corresponding electrical response between materials and cardiac tissue is required. To solve this issue, researchers used excimer laser microarray techniques to create a chitosan-polyaniline composite with a foldable honeycomb design.140 This composite material not only improves electrical conductivity but also has tunable mechanical properties, bringing up new possibilities for cardiac biomaterial design. Similarly, the polypyrrole-chitosan hydrogel reduces tissue resistance by 30%, improves fibrotic scar tissue conductivity by 33%, increases field potential amplitude, and allows synchronous heart contraction due to its low resistance.141 Composite biomaterials provide many advantages, including improved mechanical and biological properties, adjustable functionalities, and design flexibility. However, presents several challenges. The inclusion of numerous materials makes it difficult to adequately characterize and anticipate the behavior of the materials. Additionally, managing the distribution and orientation of various components during the production process can be challenging. Furthermore, separate components may degrade at various rates, which complicates maintaining consistent material properties over time. It is vital to highlight that the usage of numerous materials and sophisticated production processes frequently leads in higher composite biomaterial costs. Determining the compatibility of various scaffold material components with surrounding tissues is also crucial, as this can result in adverse in vivo reactions. To summarize, careful consideration of these factors is essential when designing and selecting composite biomaterials.
3.1.2. Angiogenesis Factors
3.1.2.1. Stem Cell
Researchers have been drawn to stem cells because of their unique ability to self-renew, replicate, proliferate, and differentiate in multiple directions. Over the last few decades, significant progress has been made in both basic research and therapeutic applications of stem cells. Notable achievements include elucidating the epigenetic patterns of stem cells during asymmetric division,142 unraveling the fundamental molecular regulatory mechanisms underlying cell differentiation,143 investigating novel mechanisms of genome homeostasis in pluripotent stem cells,144 and using stem cells to treat a variety of diseases such as heart disease,145 Alzheimer’s disease,146 Parkinson’s syndrome,147 and dialysis.148 Similarly, researchers have employed stem cells to promote angiogenesis in ischemic disorders, aiming to improve tissue function and/or increase blood perfusion.
Human bone marrow-derived mononuclear cells (BM-MNCs), EPCs, and pluripotent stem cell-derived endothelial cells (PSC-ECs) have all been researched extensively for their ability to promote angiogenesis and wound healing.149 Because they are widely available, safe, and efficacious, BM-MNCs are frequently employed in experimental treatments for ischemia disorders.150 The mechanism behind their angiogenic characteristics remains a topic of debate, with some researchers suggesting that the differentiation of BM-MNCs into endothelial cells contributes to their angiogenic activities.151 Kikuchi-Taura et al., however, propose that BM-MNCs connect with ECs via gap junction-mediated signal transduction, hence increasing angiogenesis and promoting the survival of wounded ECs during ischemia.152 Certainly, the release of exosomes by cells and the secretion of various angiogenic factors by BM-MNCs play crucial roles in promoting angiogenesis. EPCs are hematopoietic cells found in bone marrow that have the ability to develop into ECs and contribute to angiogenesis.153 In the case of myocardial ischemia, EPCs not only directly support the creation of a vascular network required for the provision of nutrients to new myocardial cells, but they also emit paracrine signals that enhance myocardial cell survival.154 Pluripotent stem cells, particularly induced pluripotent stem cells (iPSCs), have received a lot of attention because of their abundance, lack of immunological rejection, and lack of ethical problems. These cells have been extensively utilized in research on vascular regeneration.155 Kim found that iPSC-derived vascular cells have greater angiogenic capacities when compared to primary somatic cells in a comparative research.156 Lee et al.157 also explored the ability of iPSC-derived lymphoECs to promote angiogenesis. They implanted lymphoendothelial cells (lymphoECs) produced from iPSCs into skin lesions on the backs of naked mice and observed their absorption into the lymphatic network as well as the subsequent rise in lymphatic angiogenesis and lymphangiogenesis. Furthermore, spheroids composed of cardiomyocytes derived from human-induced pluripotent stem cells are more prone to vascularization through the natural circulatory system. Notably, 4 weeks after being implanted at the site of a MI in rats, these spheroids demonstrated significant vascularization.158 IPSCs have shown exceptional therapeutic effects in cardiovascular disorders, including MI, making them a promising cell type for increasing angiogenesis.159 Overall, these findings indicate the ability of stem cells to promote angiogenesis. However, different studies use different cell kinds, application methods, cell numbers, patient characteristics, research strategies, and objectives. Moreover, the use of foreign cells poses inherent risks such as immunological rejection, tumorigenicity, and transfusion-related toxicity, which limit their clinical applicability.160 Furthermore, stem cell infusion may result in transfusion-related acute lung injury (TRALI) or transfusion-associated circulatory overload (TACO), which are adverse events that must be meticulously managed and prevented in the clinical setting. Additionally, large-scale production of stem cells for clinical use faces significant technical and economic challenges. Maintaining cell quality and functionality requires sophisticated bioreactors and rigorous monitoring, which are both costly and time-consuming. The financial burden of cell isolation, culture, expansion, differentiation, and quality control highlights the need for cost-effective manufacturing processes. Furthermore, delivering viable stem cells to clinical sites involves challenges such as storage, transportation, and scheduling. Cryopreservation and careful handling are essential to preserve therapeutic properties, adding to logistical complexity. As a result, more extensive research is needed to enhance our understanding of how to effectively boost angiogenesis using stem cell-based techniques.
3.1.2.2. Extracellular Vesicle
Stem cells, as previously stated, have the ability to stimulate angiogenesis. However, there are several challenges associated with clinical therapies based on stem cells. For example, the low survival rate of transplanted cells within the ischemic environment of heart tissue restricts their clinical efficacy. The paracrine effect of stem cells on the heart, along with their ability to improve microvascular dysfunction, are crucial components of stem cell therapy.161 EVs have emerged as an important tool for intercellular communication in the treatment of ischemia illnesses under both normal physiological and pathological situations. These vesicles, which include vesicles, exosomes, and apoptotic bodies, allow information transfer between cells by transporting various biomolecules and exerting biological effects.162 Notably, EVs produced from stem cells have similar biological properties while providing advantages such as reduced immunogenicity, decreasing the potential hazards associated with allogeneic implantation. Additionally, these EVs are not replicable and have enhanced safety profiles.
While some cell therapies try to increase angiogenesis directly through cell survival or differentiation, other cells can exert impact via paracrine signaling. EVs produced from stem cells have garnered significant attention in the context of ischemic disorders. CDCs-EXO163 and CPCs-EXO164 are two examples. EVs generated from induced pluripotent stem cells (iPSC-EVs) have been found to be high in miRNA and proteins, which promote angiogenesis, motility, and antiapoptotic properties in mouse ECs. iPSC-EVs improve left ventricular function, induce angiogenesis, inhibit apoptosis, and alleviate myocardial hypertrophy in animal models of myocardial infarction (MI) and reperfusion. These findings suggest they may offer a safer therapeutic option for patients with ischemic MI.165 EVs generated by cardiovascular progenitor cells (CVPCs) developed from human pluripotent stem cells (hPSCs), according to Wu et al., contain a long noncoding RNA known as Metastasis-Associated Lung Adenocarcinoma Transcript 1 (MALAT1). By targeting miR-497, these EVs reduce myocardial cell death and enhance EC tube formation, highlighting the potential application of hCVPC-EVs in supporting healing in infarcted hearts.166 Similarly, cardiosphere-derived cells extracellular vesicles (CDC-EVs) produced from cardiosphere-derived cells (CDCs) can polarize M1 macrophages toward an angiogenic phenotype in the early stages of ischemia cardiac damage by upregulating arginase 1.167 Metal stents are commonly used to prevent and treat MI. Professor Cheng Ke’s research group developed a novel approach to restore the biological function of ischemia-injured tissues: creating exosome-eluting stents based on the properties of exosomes released by mesenchymal stem cells (MSCs), which stimulate EC repair and angiogenesis. Exosome-eluting stents outperformed metal stents and drug-eluting stents in animal tests using ApoE–/– mice, in terms of promoting angiogenesis and muscle regeneration in ischemic areas.168
While the therapeutic approach based on EVs offers great potential, it is critical to recognize the current limitations. Overcoming these obstacles is critical to ensuring the efficacy of extracellular vesicle-based therapeutics. For example, concerns such as the half-life of EVs in vivo, lack of targeting selectivity, and potential off-target effects must be addressed. To address these problems, researchers have been investigating the development of formulations that replicate the properties of stem cell-derived EVs, with the goal of achieving targeted homing and, to some extent, increased repair capacities.169 The clinical translation and large-scale production of exosomes for pro-angiogenesis present a multitude of challenges. First, the development of efficient isolation and purification techniques is of paramount importance. The current methods are inadequate and difficult to maintain consistency and high purity. Second, the implementation of standardized sample characterization methodologies is essential to guarantee that exosomes from disparate batches exhibit identical biological properties and therapeutic effects. It is imperative that rigorous clinical trials be conducted to validate the safety and efficacy of these treatments, a process that requires significant resources and time. Furthermore, logistical and storage considerations must be addressed, as exosomes frequently necessitate cryopreservation, which introduces additional complexities pertaining to transportation and storage.170 Addressing these roadblocks and assuring the reproducibility and scalability of extracellular vesicle-based therapeutics is critical for their successful clinical translation. More research and development efforts are clearly needed before the full potential of EVs can be realized in the clinical context.
3.1.2.3. Growth Factor
The stimulation of exogenous angiogenic factor has been shown in studies to efficiently promote angiogenesis in ischemic peripheral tissues. This procedure results in the creation of new compensatory collateral circulation and an increase in blood flow to ischemic tissues.171 As a result, angiogenic factors are regarded as promising therapeutic agents for the treatment of ischemic heart disease and improving arterial endothelial protection. Extensive research into growth factor-induced angiogenesis has indicated that treatment of exogenous growth factors produces beneficial results in animal trials. However, in clinical trials, virtually all angiogenesis treatments based on growth factors have failed to demonstrate significant improvements in functional angiogenesis in patients. One explanation for these experimental failures is that the human body may have suffered significant damage throughout the course of disease, rendering the compensatory systems insufficient to meet the demand.172 Despite this, researchers are nonetheless excited about the prospective applications of growth factors.
FGF is a regulatory protein that promotes the growth of epithelial and mesenchymal cells. FGF has been found to help to myocardial preservation in the context of MI by increasing the number of arterioles and capillaries in the infarcted area, consequently lowering the extent of damage to the canine myocardium.173 Hepatocyte growth factor (HGF) has strong angiogenic and antiapoptotic capabilities, making it a promising therapy option for ischemic heart failure. In one investigation, Vasant Jayasankar et al. administered replication-deficient recombinant adenovirus carrying the HGF gene directly into the myocardium of rats with heart failure after MI. The findings revealed a considerable increase in angiogenesis, underlining HGF’s therapeutic potential.174 VEGF, a well-known vascular development factor, is essential for angiogenesis. It increases vascular permeability, promotes vascular EC migration, modifies the ECM, and promotes the development of new blood vessels.175 Angiopoietin,176 platelet-derived growth factor-BB,177 and TGF-β178 are all key players in angiogenesis. These numerous growth factors all play a role in the intricate process of vascular formation and hold great promise for increasing angiogenesis and tissue repair.
The manner of administration for growth factors presents challenges that limit their use. Traditional methods of drug delivery include local injection and systemic drug delivery, but both have several issues. Local injection of VEGF at the target region, for example, may result in uneven drug distribution as well as poor retention and penetration. Furthermore, because VEGF has a limited half-life in circulation, it is ineffective when injected systemically.179 Furthermore, when administered systemically, growth factors may elicit nontargeted effects at the systemic level, potentially leading to adverse reactions and safety concerns. Additionally, growth factors may stimulate aberrant angiogenesis, thereby elevating the risk of cancer and other pathological conditions. As a result, increasing the efficacy of growth factors has emerged as a key focus of contemporary research. East China University of Science and Technology’s research team has made strides in this area by using semisynthetic chitosan sulfate (SCS) as a carrier to efficiently deliver natural growth factors. They were able to enhance angiogenesis, regulate anti-inflammatory macrophages, and boost endogenous VEGF secretion by employing an extremely low dose of exogenous VEGF. This technique stimulates angiogenesis in ischemic conditions via the VEGF–VEGFR2 signaling pathway, achieving the goal of biomaterials designed to improve the microenvironment of local ischemia and promote in situ angiogenesis.91 Ma et al. also created a shell–core fiber scaffold material that incorporates VEGF and perfluorotributylamine (PFTBA). PFTBA facilitates oxygen release, which protects Schwann cells from hypoxia-induced cell death. Exogenous VEGF promotes the formation of a new microvascular network, thereby assuming responsibility for oxygen transport. This technique enhanced angiogenesis during nerve regeneration and encouraged axonal regrowth and nerve function recovery in a rat model of long-segment nerve injury.180 Exogenous growth factors have been shown in numerous investigations to promote angiogenesis. The clinical translation and large-scale production of growth factors for pro-angiogenesis present a multitude of challenges. First, the development of efficient and stable delivery systems is required. The existing methods are inefficient and difficult to maintain consistency and high purity in large-scale production. The potential of semisynthetic carriers and novel nanotechnologies to provide solutions to this problem is evident; however, further research and optimization are required. Second, the standardization of sample characterization is essential to guarantee the consistency of the biological properties and therapeutic effects of different batches of growth factors. The technologies employed in large-scale production are not yet sufficiently mature, and processes to maintain the quality and functionality of growth factors must be developed. Moreover, rigorous clinical trials are necessary to validate the safety and efficacy of these treatments, which require significant resources and time. The limited scale of existing studies makes it challenging to fully assess their potential. Although the anticipated benefits of this therapy have yet to be realized, it is critical to maintain these research efforts in order to expand our understanding and optimize their clinical uses.
3.1.2.4. Noncoding RNA
Stem cell therapy works predominantly through a paracrine mechanism, with exosome-carried microRNAs (miRNAs) playing an important role in controlling angiogenesis.181 As a result, the utilization of noncoding RNA has piqued the interest of scientists. miRNAs and long noncoding RNAs are examples of noncoding RNA. As tiny noncoding regulatory RNAs, miRNAs provide a powerful mechanism for influencing post-transcriptional gene expression. They are required for cell survival, proliferation, apoptosis, immune response, insulin secretion, neurotransmitter synthesis, circadian rhythm, angiogenesis, viral replication, and other functions.182 A growing body of evidence demonstrates that a number of miRNAs regulate the onset, development, maturation, and cardiovascular illnesses.183 MiRNAs have the ability to modulate several gene expression levels at the same time as well as induce the release of various endogenous molecules. However, in vascular disorders, microRNA expression frequently fluctuates, leading to harmful effects. This demonstrates the potential role of miRNAs in cardiovascular healing, making them attractive targets for angiogenesis therapy.
Ana Eulalio demonstrated the effectiveness of externally delivering particular miRNAs in boosting myocardial cell proliferation and enabling cardiac healing.184 Extensive screening investigations have revealed that miRNA-21 is highly expressed in vascular ECs and has an important role in tissue fibrosis.185 According to new research, miRNA-21 has the highest expression level in cardiac macrophages and exerts paracrine effects on cardiac fibroblasts. This modulation includes the conversion of cardiac macrophages into pro-inflammatory macrophages, which promotes fibrotic signal transduction186 and stimulates angiogenesis to support tissue regeneration.187 Similarly, miR-126, the most abundant and specific miRNA in ECs, has the ability to inhibit SPRED1, VCAM1, and PIK3R2. When miR-126 is inhibited, it increases VEGF signaling, which is necessary for maintaining vascular structure in vivo.188 Previous research has shown that miR-126 can be induced not just by ECs but also by EPCs, vascular smooth muscle cells, and myocardial cells. MiR-126 generates pro-survival and pro-angiogenesis signals in cardiomyocytes by activating the PI3K/Akt pathway, inhibiting histone deacetylase (HDACs), and stimulating myogenesis and proliferation without changing VEGF-A levels.189 Furthermore, through inhibiting HIF-1an, miR-31–5p improves EC function, and exogenous administration of miR-31–5p dramatically stimulates angiogenesis and improves vascular network formation in diabetic mice.190
Long noncoding RNAs (lncRNAs) can control gene expression at multiple levels, including chromatin, DNA, transcription, and post-transcription. They accomplish this via interfering with coding gene translation, limiting polymerase II activity, encouraging post-transcriptional alterations, binding to functional proteins, functioning as precursors for small molecular RNAs, and attaching to chromosomes.191 As a result, they have a significant impact the development and outcomes of several diseases.192 Numerous research in recent years have found that lncRNAs can act as endogenous sponges, influencing the expression and function of miRNAs. For example, lnc-H19 acts as a molecular sponge for endogenous miR-106a, negatively inhibiting Angpt1 expression. In turn, angiogenesis is induced in a mouse model of metabolic osteoporosis (CBS) via the lncRNAH19-Angpt1-Tie2/NO axis.193 MALAT1 upregulates its expression and modifies the 15-LOX1/STAT3 signaling pathway in hypoxic circumstances, boosting the proliferation and migration of vascular endothelial cells (VECs). Chen et al. found that Mfn1 overexpression successfully corrected microvascular dysfunction and alleviated cardiac microvascular EC injury by suppressing excessive mitochondrial fragmentation and mitochondrial-dependent apoptosis. In contrast, MALAT1 knockdown exacerbated cardiac function in mice with MI via the miR-26b-5p/Mfn1 pathway.194 MALAT1 produced by EVs originating from M1 macrophages, however, competitively binds to miR-25–3p, increasing the expression of CDC42 and activating the MEK/ERK pathway in a MI model. This impedes angiogenesis and myocardial regeneration after a heart attack, but inhibiting MALAT1 can enhance angiogenesis.195 Currently, noncoding RNA research faces several obstacles. First, target specificity and off-target effects are significant concerns, potentially leading to unintended biological responses. Second, delivery and stability issues hinder clinical application. ncRNAs are prone to degradation, and developing protective delivery vehicles like lipid nanoparticles or polymer-based systems can enhance stability and targeted delivery. Additionally, immune response may arise from exogenous ncRNAs, causing inflammatory reactions or reducing efficacy. Solutions include using autologous ncRNAs or optimizing formulations to minimize immunogenicity.196
Upscaling noncoding RNA therapies presents several hurdles. Production scalability is a major issue, as producing ncRNAs in large quantities with consistent quality is technically challenging and costly. Ensuring quality control at scale is also critical, as variability in RNA sequences and structures can impact performance.
3.1.2.5. Polypeptide
Polypeptides, as particular hormones or protein fragments. QHREDGS, generated from angiopoietin-1 that can bind to integrin, is one example of an angiogenic peptide. It is a new angiogenic peptide with the ability to enhance the overall number of blood vessels in wounds.197 Guan et al.198 used 3D bioprinting and chemical coupling to create hydrogel patches containing QHREDGS, with the goal of increasing loading rate and slowing release. In vitro angiogenesis investigations revealed a considerable increase in the creation of tubular networks in the peptide-containing group, while animal experiments validated QHREDGS’ critical involvement in enhancing angiogenesis rates. Histin-1, a functional peptide that promotes EC adhesion, motility, and angiogenesis, has been used to treat diabetic ulcers. Cao et al.’s199 study demonstrated that Histin-1 can stimulate angiogenesis in human microvascular endothelial cells (HMECs) and increase the total number of blood vessels in wounds. Histin-1 may stimulate the Ras and Rab interacting factor 2/Rab 5/Rac1 signaling axis to enhance angiogenesis in vascular ECs. According to research, the osteogenic peptide KP and the angiogenic peptide QK work together to improve the osteogenic differentiation ability of bone marrow mesenchymal stem cells (BMSCs) and the angiogenesis ability of human umbilical vein endothelial cells (HUVECs), resulting in increased new bone formation in the rat cranium.200 Furthermore, RADA16 D-peptide modified by collagen hydrogel (AP)201 and RGD (Arg-Gly-Asp) self-assembled peptide202 have been shown to induce angiogenesis.
The clinical applications and large-scale production of peptides for pro-angiogenic therapies face several challenges. Clinically, peptides are unstable in vivo, prone to enzymatic degradation, and may provoke immune responses, impacting their safety and efficacy. Additionally, their effects can vary by pathology and patient. For large-scale production, developing stable and efficient delivery systems remains difficult, with existing methods like nanoparticles and hydrogels needing further refinement. Standardizing peptide characterization is essential to ensure consistent quality and therapeutic effects. Rigorous clinical trials are necessary but resource-intensive, with current studies often too limited to fully evaluate potential. Addressing these issues is crucial for successful clinical translation and scalability of peptide therapies.
3.1.2.6. Others
Nutritional metal ions (such as Mn2+, Zn2+, and Ca2+) have been revealed to have cytokine-like qualities in immune modulation in recent years.203 Zn2+, for example, can be used to make Zn-based immunomodulatory adjuvants (Zn-LDH), which successfully target in situ, distant, and metastatic cancers. In addition, they significantly prevent recurrent tumor development and metastasis.204 Furthermore, copper, magnesium, zinc, and strontium have been demonstrated to increase angiogenesis by activating pathways like Wnt, PI3K/Akt, MAPK, and hypoxia-inducible factor 1. Copper (Cu), a trace metal element that is vital to the human body, is primarily involved in catalytic metabolic activities. Cu is frequently used in biomaterials for a variety of applications due to its powerful antibacterial activities and low cytotoxicity.205 Cu interacts with various angiogenic growth factors, including considerably raising VEGF expression, modulating angiopoietin transcription, and promoting matrix metalloproteinase activity.206 Romero-Sánchez Lilian B et al., for example, found that 5% Cu-doped mesoporous bioactive glass (MBG-5Cu) significantly increased the number and thickness of blood vessels in the zebrafish subintestinal venous plexus (SIVP) compared to Cu-free MBG.207 Xiao et al. created copper-based metal–organic frameworks (Cu-MOFs) that store Cu2+ ions in another investigation. Folic acid was used as a stabilizer to regulate the rate of Cu2+ release. Cu2+ efficiently encouraged the migration of red blood cells and fibroblasts, as well as the development of new blood vessels by upregulating VEGF.208 Pneg et al. created a CuO-containing composite hydrogel that enhanced angiogenesis and protected vascular ECs from high-level inflammation and oxidative. Mg was also observed to increase the amount and speed of angiogenesis at the implantation site. Mg2+ activated HIF-1 by boosting the activity of the magnesium transporter subtype 1 (MagT1), resulting in enhanced production of VEGF transcription factors and better angiogenesis.209 Furthermore, Si ions produced from biocompatible and biodegradable materials have shown biological activity in vitro in stimulating cell migration and angiogenesis gene expression. Wu’s R&D team created a collaborative medication loading and delivery platform using porous silicon particles that electrostatically adsorbed VEGF. This method increased the efficiency of angiogenesis and efficiently promoted angiogenesis in vivo.210 However, more research is needed to discover the appropriate metal ion concentration to stimulate angiogenesis.
NO is a multifunctional signal molecule that plays critical functions in vasodilation, angiogenesis, signal transmission and integration, infection elimination, and immunological modulation, especially in angiogenesis and heart protection.211 Exogenous NO donors have shown potential in cardiovascular disease studies as a treatment option. For example, Zhao et al.212 created a comb-shaped polymer (CS-NO) that can release NO stably in the presence of glycosidase. By compensating for NO in vivo, CS-NO increased the mobilization and angiogenesis of EPCs in a mouse model of diabetic hind limb ischemia. Champeau et al.213 found that releasing NO from a PAA:F127/GSNO hydrogel for 5 days not only promoted angiogenesis but also raised the expression of TGF-β, insulin-like growth factor-I, SDF-1, and IL-10 genes in injured tissues. However, the insecurity of NO donors and the lack of target specificity continue to be issues. Yao et al. proposed adsorption of NO onto Copper-Benzene-1,3,5-tricarboxylate (HKust-1) and encapsulation with photothermal graphene oxide to allow regulated NO delivery via near-infrared (NIR) radiation to address this. The experimental results showed a considerable improvement in vascular density in the NO-releasing group, reaching a maximum of 2.9 ± 0.4%.214 Similarly, H2S stimulates angiogenesis.215 Exogenous H2S acts on ECs via KATP channels, activating the MAPK pathway and causing the development of new blood vessels.216 Sanchez-Aranguren et al.217 used liposomes to encapsulate sodium thiosulfate (STS), developing a controlled delivery nanoparticle system, to overcome the fast release and clearance of H2S. The results demonstrated that liposome-encapsulated STS improved VEGF mRNA and protein expression, as well as capillary development. To summarize, gas transmitters-induced therapeutic angiogenesis intelligent materials have a lot of potential for the future.
3.1.3. Bioreactor
Bioreactors are important in tissue engineering because they provide a regulated environment for cell proliferation and tissue formation. By delivering physical and biochemical cues to cells, they are vital for replicating in vivo circumstances and encouraging cell differentiation and ECM synthesis. Bioreactors are widely divided into two types: static and dynamic. Static bioreactors maintain a motionless culture environment, whereas dynamic bioreactors stimulate cells mechanically via fluid flow and pressure gradients. Mechanical stimulation promotes ECM deposition while simultaneously modulating cell behavior, proliferation, and differentiation.
Traditional static cell culture procedures rely significantly on diffusion to distribute nutrients and oxygen, as well as to remove waste. However, when producing large-scale tissue engineering grafts, the utilization of bioreactors provides a substantial advantage in terms of increasing the supply of oxygen and nutrients. Bioreactors have enormous potential for overcoming diffusion constraints in this process. Jakob Schmid et al.218 created a perfusion microbial reactor system that allows for oxygen-controlled development of 3D cell cultures on scaffolds. This arrangement can assess the oxygen concentration (OC) in the geometric center of the 3D cell culture and maintain it at a preset level by independently adjusting the perfusion rate of each bioreactor using an autonomous feedback system. Additionally, automatic cell inoculation processes have been established to aid in the formation of homogeneous tissues. The bioreactor has several benefits such as bubble-free operation, homogeneous flow distribution, and a variable manufacturing method, making it a novel option for researching 3D cell cultures on solid scaffolds. Nakazato et al. created the Rotating Wall Container (RWV) bioreactor to enable the application of mechanical stimulation in dynamic cultures. This bioreactor maintains a steady glucose concentration around the cultured tissue and effectively distributes nourishment and oxygen to cells or tissues. Notably, the RWV system subjects cultured cells or tissues to mild shear stress. The RWV group dramatically enhances cell survival rate, contraction period, electrical characteristics of mature myocardial cells, and elevation of mechanical stress-related mediators when compared to the control group. This bioreactor provides an optimal growth environment for human-induced pluripotent stem cell-derived cardiac tissues (hiPSC-CTs) and a method for creating functional myocardial tissue for treating heart failure caused by myocardial cell loss.219 Bioreactors can also be modified to match the specific requirements of different tissue types, making them useful for scaling up tissue production for therapeutic usage.220 Bioreactors are important in tissue engineering because they provide a regulated and ideal environment for cell proliferation and tissue formation. In this section, we have summarized the role of various angiogenesis stimulatory agents, such as stem cell types, extracellular vesicles, growth factors, noncoding RNA, peptides, and other bioactive compounds. Figure 2 offers a visual summary of these agents in promoting angiogenesis, providing a comprehensive overview of their contributions to blood vessel formation.
Figure 2.
Angiogenesis stimulatory agents include stem cell types, extracellular vesicles, growth factors, noncoding RNA, peptides, and other bioactive compounds. These factors are critical in the regulation of angiogenesis. Stem cells have the ability to develop into endothelial cells and hence contribute directly to blood vessel creation. Extracellular vesicles influence signaling pathways involved in angiogenesis via their payload of functional molecules such as miRNA and proteins. Growth factors promote the proliferation and migration of endothelial cells, resulting in the formation of blood vessels. Noncoding RNA regulates gene expression and plays a role in the regulation of angiogenesis. Peptide molecules interact with cell surface receptors, triggering angiogenesis-related signaling cascades. Furthermore, other bioactive compounds, such as extracellular matrix components and biomaterials, have the ability to stimulate angiogenesis. The images are original and created by the authors, free from third-party copyright restrictions.
3.2. Mechanisms and Materials to Promote Angiogenesis in MI Regeneration and Repair
Inadequate angiogenesis post-MI leads to insufficient oxygen and nutrient supply to the myocardium, potentially causing myocardial cell hypertrophy and progressing to heart failure.209 Stimulating angiogenesis is crucial for minimizing cell necrosis, promoting tissue repair, and improving treatment outcomes. Thus, enhancing angiogenesis to restore blood supply is a key therapeutic strategy for rescuing ischemic myocardial cells. Understanding the molecular pathways of angiogenesis is vital for developing novel MI treatments. Angiogenesis, the formation of new blood vessels from existing capillaries or postcapillary venules, involves endothelial cell proliferation and migration, vascular lumen formation, and vessel maturation. Numerous cytokines and signaling molecules play a role in this process, and the balance between stimulatory and inhibitory factors is critical.210 Under normal conditions, angiogenic factors maintain blood supply and tissue metabolism. However, they also play significant roles in diseases like tumors, inflammation, and psoriasis.211
The heart, a highly vascularized organ, requires steady blood flow for proper function. MI damages blood vessels, disrupting nutrient and oxygen supply to heart cells, thus affecting myocardial viability and systolic performance. Promoting angiogenesis and integrating with the host vascular system are crucial for MI regeneration and repair.246 Current research indicates that no single biomaterial can meet these requirements effectively. Consequently, angiogenesis-based biomaterials for MI treatment often involve multiple materials, potentially enhancing postinfarction cardiac function, preventing heart failure, and improving patient prognosis.
MSCs have emerged as a possible therapy option for MI, with the goal of promoting heart repair. To optimize their potential, biomaterials that can keep donor cells on the heart surface and manage their phenotypic must be developed. Gautrot et al.221 from the University of London proposed using poly(2-alkyl-2-oxazoline) (POx) based on 2-ethyl-2-oxazoline and 2-butenyl-2-oxazoline for this purpose. In vitro microfluidic tests have shown that a partially degradable POx hydrogel can boost the upregulation of angiogenic factors and improve angiogenesis. The implantation of an epicardial membrane made of POx hydrogel loaded with MSCs in a rat model of MI aided cardiac function and structure recovery, as well as the production of new blood vessels. Wu et al. described another strategy, which involves the development of an injectable conductive alginate gel containing polyaniline (PANI) and adeno-associated virus (AAV9-VEGF) for the treatment of MI.222 This gel successfully transduced cardiomyocytes, resulting in VEGF overexpression, which increased HUVECs proliferation, migration, and tube formation. Fan et al. created a microneedle patch made of graphene oxide and poly(vinyl alcohol) to generate controlled and sustained VEGF release.223 The microneedle patch significantly encouraged new blood vessel development, reduced myocardial fibrosis, and restored heart function, demonstrating its potential in the treatment of MI.
The pro-angiogenic mechanism that is most frequently associated with these materials is dependent on the presence of VEGF,VEGF is an important positive regulator that promotes EC proliferation and vascular development.224 VEGF functions as a signal sensor in angiogenesis, influencing both healthy and pathological angiogenesis.225 VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and PIGF are all members of the VEGF family. VEGF-A has received particular attention due to its critical role in controlling angiogenesis.226 According to research, VEGF-A enhances vascular EC mitosis and permeability by activating VEGFR1 and VEGFR2.227 VEGFR2 is the major VEGF-A signaling receptor, with two tyrosine residues exerting distinct effects on angiogenesis and vascular permeability.228 Downstream signal transduction pathways of VEGFR2 include the PI3K/AKT pathway,229 the ERK1/2 pathway,230 the SRC kinase pathway,231 the NF-kB pathway,232 the JAK/STAT pathway,233 and others. In angiogenesis, VEGFR2 activation activates the PI3K signaling pathway, which leads to AKT activation and promotes the proliferation and survival of vascular ECs. The ERK1/2 signaling pathway predominantly promotes vascular EC proliferation and migration, regulating blood vessel development and repair. VEGF-A can also work with EGF and platelet-derived growth factor (PDGF) to increase the release of nitric oxide (NO) from ECs and surrounding cells. The NO have the ability to stimulate angiogenesis has been proved, and administering exogenous NO to the site of infarction has proven to be a successful therapeutic method for MI. Hao et al.109 developed a method that used locally created ROS following ischemia-reperfusion to stimulate selective cleavage of borate groups, resulting in the release of NO. This method not only eliminated ROS but also enhanced the release of NO. The number of -SMA arterioles and CD31 capillaries in the border area of MI increased significantly in the CS-B-NO group, indicating accelerated angiogenesis. The long-term treatment increased heart repair and function. As a result, VEGF-A is essential for angiogenesis and tissue healing.234
In addition to the previously mentioned VEGF, numerous other mechanisms have been identified that can promote neovascularization. Endothelial and smooth muscle cells are the primary cell types that make up the vascular wall, and their proliferation and differentiation are critical in angiogenesis. FGF has been shown in studies to stimulate the proliferation and differentiation of both ECs235 and smooth muscle cells.236 FGF binds to high-affinity receptors on the surface of ECs, including FGFR1, FGFR2, FGFR3, and FGFR4, activating signal transduction pathways such as MAPK, PI3K/AKT, and JAK/STAT. These mechanisms aid in EC proliferation, migration, and lumen creation.237 The ECM, a complex molecular network that forms the basement wall of blood arteries, regulates EC migration and directed proliferation. FGF stimulates the development and activity of MMP, which destroy the ECM, allowing ECs and smooth muscle cells to facilitate angiogenesis.238 FGF also interacts with VEGF to promote EC proliferation and migration. FGFR regulates the MAPK signaling pathway, which controls VEGF production, and VEGF, in turn, modulates its own function by upregulating FGF expression. Furthermore, FGF stimulates VEGFR2 expression via the ERK1/2 pathway.239 A significant influx of inflammatory cells occurs during the tissue repair process after a MI, and FGF can induce macrophages and T lymphocytes to secrete inflammatory cytokines such as tumor necrosis factor (TNF-α) and interleukin 1 (IL-1), thereby promoting the proliferation, migration, and neovascularization of vascular ECs and smooth muscle cells.240 Overall, FGF can induce angiogenesis via a variety of pathways, highlighting its critical biological role in this process. As a result, FGF has tremendous therapeutic promise in vascular illnesses such as MI and ischemic stroke.
A class of cell growth factors known as platelet-derived growth factors (PDGFs) are released by platelets and other cells. They are very important in controlling cell survival, differentiation, and proliferation.241 PDGFs perform a variety of tasks, including as encouraging EC growth, causing vascular smooth muscle cells to migrate, and assisting in the gathering and differentiation of perivascular cells to form a perivascular outer membrane. These activities aid in the stability and effective operation of blood vessels.242 Platelet-derived growth factor-BB (PDGF-BB) is a crucial element in encouraging angiogenesis. In order to promote angiogenesis and tissue regeneration, PDGF-BB increases the migration of BMSCs and EPCs toward ischemic or wounded tissue.177 It has been noted that mononuclear preosteoblasts release a large amount of PDGF-BB in the subchondral bone throughout the process of subchondral bone angiogenesis. The development of new blood vessels is triggered by this release, which increases the signal of the platelet-derived growth factor receptor (PDGFR) in the surrounding cells.243 Additionally, it has been discovered that PDGF-BB regulates VEGF-R2 signal transduction, which in turn inhibits the proliferation of ECs. This control prevents aberrant angiogenesis brought on by VEGF and restricts excessive blood vessel formation. As a result, blood vessels can efficiently create a functional capillary network.244 In conclusion, PDGF-BB is essential for promoting the organization and differentiation of vascular cells, which preserves the normal form and function of blood arteries. Accurate PDGF control is critical for tissue growth and repair because it supplies vital nutrients and oxygen. By knowing and effectively controlling the effects of PDGFs, it becomes possible to facilitate tissue healing and maintain healthy vascular function.
The TGF-β signaling pathway is complex, and many factors can alter the link between vascular inhibition and angiogenesis. The angiogenesis pathway is activated by TGF β receptor-II (TβRII), which attracts the TGF type I receptor, which is largely present in ECs, and then activates ALK-1. ALK1 then activates the transcription factors Smad 1, 5, and 8, resulting in the formation of a pro-angiogenic phenotype.178 TGF-β may also enhance EC migration by beginning cytoskeletal remodeling and down-regulating and dismantling tight junctions. TGF-β stimulation promotes TGF-β receptor type II translocation into tight junctions, leading in their breakdown.245 Because TGFβ’s role in angiogenesis is influenced by its interaction with numerous other growth factors and signaling pathways such as VEGF, FGF, and Wnt, the interaction and regulation of these factors and pathways with TGF-β, add to the complexity and challenges associated with TGF-β’s role in angiogenesis. Furthermore, TGF-β appears to have a paradoxical effect in ECs, and this integrated effect is mostly dependent on the interaction between TGF-β and the ALK1 and ALK5 receptors. When TGF-β levels are low, it interacts with the ALK1 receptor, causing MMP-2 and MMP-9 gene production to increase and EC migration and invasion to enhance. TGF-β, however, interacts with the ALK5 receptor and may block angiogenesis at high concentrations.246 As a result, TGF-β regulation in angiogenesis is a difficult task that necessitates careful evaluation of its interactions and effects.
EGF is a polypeptide molecule found in mammalian tissues that plays an important function in cell proliferation. EGF works by binding to its receptor, which is known as the epidermal growth factor receptor (EGFR) or ErbB receptor. The ErbB receptor family starts multiple downstream signal transduction pathways, including the Ras-Raf-MAP-kinase,247 PI3K/Akt,248 and stress-activated protein kinase.249 When the Ras pathway is activated, a phosphorylation cascade occurs, which results in the activation of MAPKs, notably ERK1 and ERK2. These proteins play a role in the transcription of molecules related to cell proliferation, survival, and transformation. Cell growth, proliferation, survival, and motility are all regulated by the PI3K/Akt pathway. Protein kinase pathways stimulated by stress, such as protein kinase C and Jak/Stat, and transform these signals into distinct transcription programs within the nucleus. Cell division, survival, migration, invasion, adhesion, and repair are all mediated by these processes.250 The tissue is subjected to ischemia and hypoxia during ischemic disorders, resulting in the production of EGF, which stimulates angiogenesis via numerous processes. EGF, for example, works directly on ECs, boosting proliferation and migration,251 increasing the number and length of new blood vessels. EGF also enhances the development of EPCs in bone marrow into ECs, providing precursor cells for the construction of new blood vessels.252 EGF also activates signaling pathways in ECs, promoting their proliferation, migration, and the creation of functional lumens.253 Furthermore, EGF stimulates the production and activity of MMPs,254 which lowers matrix adherence and increases EC movement and lumen creation.
Angiogenic genes and proteins are important substances that encourage the creation of new blood vessels and play important roles in a variety of biological processes. They include a wide variety of chemicals, including as cytokines, growth factors, and ECM components, each having unique structures and functions that can influence cellular and tissue activity via multiple signaling pathways. MMPs, for example, have an impact on the formation and function of the vascular wall via a variety of ways. MMPs can alter EC function, enhance vascular smooth muscle cell migration and proliferation, and regulate smooth muscle cell contraction within blood arteries.255 By dissolving the ECM, MMPs can promote the release of VEGF, bFGF, and ECM. VEGF and bFGF, in turn, can promote MMP production and activity, resulting in a mutual encouragement of the angiogenesis process.256 Furthermore, MMPs can influence the angiogenesis process by modulating the biological activities of VEGF and bFGF. Angiogenic factors are involved in physiological and pathological processes such as inflammation, immunological responses, and cancer in addition to angiogenesis. They influence a variety of biological processes, including cell proliferation, differentiation, survival, migration, and transformation, as well as other biological processes. Numerous factors influence the role of angiogenic factors, including cell surface receptors, signaling pathways, gene expression, and environmental factors. These variables regulate angiogenic factor synthesis, secretion, receptor binding, and signal transduction, eventually influencing their biological effects. As a result of their numerous kinds, extensive range of effects, and multiple regulation mechanisms, angiogenic factors are complex. Understanding the complexities of angiogenic factors will help us better understand their function in MI and provide useful insights for therapy and prevention.
These researchers revealed that their multifunctional patch had improved qualities when compared to patches that merely provided mechanical support in a rat model of MI. It successfully decreased cell death, controlled local inflammatory responses, reduced fibrosis, and promoted angiogenesis. Furthermore, the patch was found to be effective in preserving cardiac function and maintaining left ventricular architecture. These findings highlight the benefits of comprehensive and coordinated treatment methods for MI therapy.
And Figure 3 summarizes these mechanisms and materials, offering a visual representation of their contributions to myocardial repair and regeneration processes.
Figure 3.
Mechanisms and materials to promote angiogenesis in MI regeneration and repair. Several materials used for repair and regeneration after MI are mentioned in the figure, with emphasis on their mechanisms in promoting angiogenesis. Vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), transforming growth factor (TGF), and epidermal growth factor (EGF) are just a few of the growth factors that are essential to the angiogenesis process. Through a number of signaling pathways, including MAPK, JAK/STAT, PI3K/AKT, and others, these growth factors promote the proliferation, migration, and development of blood vessels in endothelial and smooth muscle cells. Additionally, growth factors reduce the impact of inflammatory elements, encouraging macrophage and T lymphocyte engagement in the development of new blood vessels. Furthermore, they influence pericytes, endothelial progenitor cells, and bone marrow mesenchymal stem cells to increase angiogenesis. The images are original and created by the authors, free from third-party copyright restrictions.
4. Summary and Prospect
Several hurdles must be overcome in order to improve biomaterials that stimulate angiogenesis for the treatment of MI and improve patient outcomes. These obstacles include the necessity for quick vascularization, correct blood vessel organization, long-term efficacy assessment, safety assurance, and consistency. Rapid vascularization is critical, yet current approaches frequently fail. To avoid difficulties, blood vessel organization must follow the natural growth pattern. More research is needed to determine the long-term efficacy of newly formed blood vessels. Immune rejection, foreign body responses, and cellular instability are all safety concerns. Manufacturing process standardization and quality control are also required to provide consistent performance. Addressing these issues will help to promote biomaterial-based angiogenesis stimulation, allowing for more effective treatments for MI and better patient outcomes.
Future work should focus on optimization and improvement to increase the therapeutic application value of biomaterials. This includes standardizing the manufacturing process to ensure stability and consistency, as well as undertaking long-term effects and safety research. Furthermore, during MI treatment, the combination of mechanical parameters, inflammatory response control, and angiogenesis must be examined. Biomaterials can be effectively integrated with various treatment modalities by advancing research and innovation in creating an optimum healing environment. Designing individualized, precise, and diversified therapeutic techniques that enable accurate targeting, regulated healing rates, coordinated timing, and quick and stable vascularization is part of this. These advances not only boost clinical translation and improve patient prognosis, but they also help to ensure the long-term development of MI research.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 32000967), Sichuan Cadre Health Research Project (No. Chuanganyan ZH2022-201), Chengdu Key R&D Support Program Major Technology Application Demonstration Project (No. 2021-YF09-00033-SN), Sichuan Provincial Science and Technology Program (2023YFS0036), and Natural Science Foundation of Sichuan Province (No. 2022NSFSC0817; No. 2022NSFSC0811; No. 23NSFSC1540; No. 23NSFSC1541).
Author Contributions
# These authors contributed equally to this work.
The authors declare no competing financial interest.
References
- Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nature reviews. Cardiology 2020, 17 (12), 773–789. 10.1038/s41569-020-0403-y. [DOI] [PubMed] [Google Scholar]
- Reed G.; Rossi J.; Cannon C. Acute myocardial infarction. Lancet (London, England) 2017, 389 (10065), 197–210. 10.1016/S0140-6736(16)30677-8. [DOI] [PubMed] [Google Scholar]
- Alzahrani T.; Pena I.; Temesgen N.; Glantz S. Association Between Electronic Cigarette Use and Myocardial Infarction. American journal of preventive medicine 2018, 55 (4), 455–461. 10.1016/j.amepre.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leancă S.; Crişu D.; Petriş A.; Afrăsânie I.; Genes A.; Costache A.; Tesloianu D.; Costache I. Left Ventricular Remodeling after Myocardial Infarction: From Physiopathology to Treatment. Life 2022, 12 (8), 1111. 10.3390/life12081111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tea V.; Danchin N.; Puymirat E. [Myocardial infarction in young patient: Epidemiological specificities and risk factors]. Presse medicale (Paris, France: 1983) 2019, 48 (12), 1383–1386. 10.1016/j.lpm.2019.06.001. [DOI] [PubMed] [Google Scholar]
- Rørholm Pedersen L.; Frestad D.; Mide Michelsen M.; Dam Mygind N.; Rasmusen H.; Elena Suhrs H.; Prescott E. Risk Factors for Myocardial Infarction in Women and Men: A Review of the Current Literature. Curr. Pharm. Des. 2016, 22 (25), 3835–52. 10.2174/1381612822666160309115318. [DOI] [PubMed] [Google Scholar]
- Teo K.; Rafiq T. Cardiovascular Risk Factors and Prevention: A Perspective From Developing Countries. Canadian journal of cardiology 2021, 37 (5), 733–743. 10.1016/j.cjca.2021.02.009. [DOI] [PubMed] [Google Scholar]
- Delgado-Lista J.; Alcala-Diaz J.; Torres-Peña J.; Quintana-Navarro G.; Fuentes F.; Garcia-Rios A.; Ortiz-Morales A.; Gonzalez-Requero A.; Perez-Caballero A.; Yubero-Serrano E.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet (London, England) 2022, 399 (10338), 1876–1885. 10.1016/S0140-6736(22)00122-2. [DOI] [PubMed] [Google Scholar]
- Ushula T.; Mamun A.; Darssan D.; Wang W.; Williams G.; Whiting S.; Najman J. Dietary patterns explaining variations in blood biomarkers in young adults are associated with the 30-year predicted cardiovascular disease risks in midlife: A follow-up study. Nutrition, metabolism, and cardiovascular diseases: NMCD 2023, 33, 1007. 10.1016/j.numecd.2023.02.019. [DOI] [PubMed] [Google Scholar]
- Thygesen K.; Alpert J.; Jaffe A.; Chaitman B.; Bax J.; Morrow D.; White H. Fourth Universal Definition of Myocardial Infarction (2018). Journal of the American College of Cardiology 2018, 72 (18), 2231–2264. 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- Xin M.; Olson E.; Bassel-Duby R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nature reviews. Molecular cell biology 2013, 14 (8), 529–41. 10.1038/nrm3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold A.; Simoons M. Thrombolytic therapy for evolving myocardial infarction needs an approach that integrates benefit and risk. European heart journal 1995, 16 (11), 1502–9. 10.1093/oxfordjournals.eurheartj.a060770. [DOI] [PubMed] [Google Scholar]
- Vogel B.; Claessen B. E.; Arnold S. V.; Chan D.; Cohen D. J.; Giannitsis E.; Gibson C. M.; Goto S.; Katus H. A.; Kerneis M.; Kimura T.; Kunadian V.; Pinto D. S.; Shiomi H.; Spertus J. A.; Steg P. G.; Mehran R. ST-segment elevation myocardial infarction. Nat. Rev. Dis. Primers 2019, 5 (1), 39. 10.1038/s41572-019-0090-3. [DOI] [PubMed] [Google Scholar]
- Braunwald E. Unstable angina and non-ST elevation myocardial infarction. American journal of respiratory and critical care medicine 2012, 185 (9), 924–32. 10.1164/rccm.201109-1745CI. [DOI] [PubMed] [Google Scholar]
- Zheng Z.; Tan Y.; Li Y.; Liu Y.; Yi G.; Yu C.; Wei H. Biotherapeutic-loaded injectable hydrogels as a synergistic strategy to support myocardial repair after myocardial infarction. Journal of controlled release: official journal of the Controlled Release Society 2021, 335, 216–236. 10.1016/j.jconrel.2021.05.023. [DOI] [PubMed] [Google Scholar]
- Hausenloy D.; Yellon D. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 2013, 123 (1), 92–100. 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantalis V.; Hare J. Use of mesenchymal stem cells for therapy of cardiac disease. Circulation research 2015, 116 (8), 1413–30. 10.1161/CIRCRESAHA.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers V.; Lee R. Stem-cell therapy for cardiac disease. Nature 2008, 451 (7181), 937–42. 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- Khan M.; Nickoloff E.; Abramova T.; Johnson J.; Verma S.; Krishnamurthy P.; Mackie A.; Vaughan E.; Garikipati V.; Benedict C.; et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circulation research 2015, 117 (1), 52–64. 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabisonia K.; Prosdocimo G.; Aquaro G.; Carlucci L.; Zentilin L.; Secco I.; Ali H.; Braga L.; Gorgodze N.; Bernini F.; et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 2019, 569 (7756), 418–422. 10.1038/s41586-019-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K.; Ozpinar E. W.; Su T.; Tang J.; Shen D.; Qiao L.; Hu S.; Li Z.; Liang H.; Mathews K.; Scharf V.; Freytes D. O.; Cheng K. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci. Transl. Med. 2020, 12 (538), eaat9683 10.1126/scitranslmed.aat9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L.; Tang J.; Nishi K.; Yan C.; Dinh P.; Cores J.; Kudo T.; Zhang J.; Li T.; Cheng K. Fabrication of Synthetic Mesenchymal Stem Cells for the Treatment of Acute Myocardial Infarction in Mice. Circulation research 2017, 120 (11), 1768–1775. 10.1161/CIRCRESAHA.116.310374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Chen X.; Jin R.; Chen L.; Dang M.; Cao H.; Dong Y.; Cai B.; Bai G.; Gooding J. J.; Liu S.; Zou D.; Zhang Z.; Yang C. Injectable hydrogel with MSNs/microRNA-21–5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci. Adv. 2021, 7 (9), 6740. 10.1126/sciadv.abd6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C.; Shi J.; Zhuang Y.; Zhang L.; Huang L.; Yang W.; Chen B.; Chen Y.; Xiao Z.; Shen H.; Zhao Y.; Dai J. Myocardial-Infarction-Responsive Smart Hydrogels Targeting Matrix Metalloproteinase for On-Demand Growth Factor Delivery. Adv. Mater. 2019, 31 (40), e1902900 10.1002/adma.201902900. [DOI] [PubMed] [Google Scholar]
- Chen W.; Wang C.; Liu W.; Zhao B.; Zeng Z.; Long F.; Wang C.; Li S.; Lin N.; Zhou J. A Matrix-Metalloproteinase-Responsive Hydrogel System for Modulating the Immune Microenvironment in Myocardial Infarction. Adv. Mater. 2023, 35 (13), e2209041 10.1002/adma.202209041. [DOI] [PubMed] [Google Scholar]
- Khabbaz K.; Zankoul F.; Warner K. Intraoperative metabolic monitoring of the heart: II. Online measurement of myocardial tissue pH. Annals of thoracic surgery 2001, 72 (6), S2227–33. 10.1016/S0003-4975(01)03284-2. [DOI] [PubMed] [Google Scholar]
- Pesce M.; Duda G.; Forte G.; Girao H.; Raya A.; Roca-Cusachs P.; Sluijter J.; Tschöpe C.; Van Linthout S. Cardiac fibroblasts and mechanosensation in heart development, health and disease. Nature reviews. Cardiology 2023, 20 (5), 309–324. 10.1038/s41569-022-00799-2. [DOI] [PubMed] [Google Scholar]
- Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free radical biology & medicine 2018, 117, 76–89. 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Spinale F. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol. Rev. 2007, 87 (4), 1285–342. 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M.; Pittet M.; Swirski F. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 2010, 121 (22), 2437–45. 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A.; Khattab A.; Islam M.; Hweij K.; Zeitouny J.; Waters R.; Sayegh M.; Hossain M.; Paul A. Injectable Hydrogels for Cardiac Tissue Repair after Myocardial Infarction. Adv. Sci. 2015, 2 (11), 1500122 10.1002/advs.201500122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower G.; Gardner J.; Forman M.; Murray D.; Voloshenyuk T.; Levick S.; Janicki J. The relationship between myocardial extracellular matrix remodeling and ventricular function. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery 2006, 30 (4), 604–10. 10.1016/j.ejcts.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Patel R.; Patel D. Injectable Hydrogels in Cardiovascular Tissue Engineering. Polymers 2024, 16 (13), 1878. 10.3390/polym16131878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.; Kim M. C.; Lee J. Y. Nanomaterial-Based Electrically Conductive Hydrogels for Cardiac Tissue Repair. International journal of nanomedicine 2022, 17, 6181–6200. 10.2147/IJN.S386763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro J.; Miguel S.P.; Galván-Chacón V.; Patrocinio D.; Pagador J.B.; Sánchez-Margallo F. M.; Ribeiro M.P.; Coutinho P. Three-Dimensionally Printed Hydrogel Cardiac Patch for Infarct Regeneration Based on Natural Polysaccharides. Polymers 2023, 15 (13), 2824. 10.3390/polym15132824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahemi N. H.; Mahat M. M.; Asri N. A. N.; Amir M. A.; Ab Rahim S.; Kasri M. A. Application of Conductive Hydrogels on Spinal Cord Injury Repair: A Review. ACS biomaterials science & engineering 2023, 9 (7), 4045–4085. 10.1021/acsbiomaterials.3c00194. [DOI] [PubMed] [Google Scholar]
- McLaughlin S.; McNeill B.; Podrebarac J.; Hosoyama K.; Sedlakova V.; Cron G.; Smyth D.; Seymour R.; Goel K.; Liang W.; Rayner K. J.; Ruel M.; Suuronen E. J.; Alarcon E. I. Injectable human recombinant collagen matrices limit adverse remodeling and improve cardiac function after myocardial infarction. Nat. Commun. 2019, 10 (1), 4866. 10.1038/s41467-019-12748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa N.; Miller L.; Feinberg M.; Holbova R.; Shachar M.; Freeman I.; Cohen S.; Leor J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 2008, 117 (11), 1388–96. 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- Seif-Naraghi S. B.; Singelyn J. M.; Salvatore M. A.; Osborn K. G.; Wang J. J.; Sampat U.; Kwan O. L.; Strachan G. M.; Wong J.; Schup-Magoffin P. J.; Braden R. L.; Bartels K.; DeQuach J. A.; Preul M.; Kinsey A. M.; DeMaria A. N.; Dib N.; Christman K. L. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci. Transl. Med. 2013, 5 (173), 173ra25 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Shao Y.-h.; Zhang J.-m.; Wang Y.; Zhou M.; Li H.-q.; Zhang C.-c.; Yu P.-j.; Gao S.-j.; Wang X.-r.; Jia L.-x.; Piao C.-m.; Du J.; Li Y.-l. Macrophage CARD9 mediates cardiac injury following myocardial infarction through regulation of lipocalin 2 expression. Sig. Transduct. Target Ther. 2023, 8 (1), 394. 10.1038/s41392-023-01635-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis N. G. The inflammatory response in myocardial injury, repair, and remodelling. Nature reviews. Cardiology 2014, 11 (5), 255–65. 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzaroma E.; Toldo S.; Farkas D.; Seropian I. M.; Van Tassell B. W.; Salloum F. N.; Kannan H. R.; Menna A. C.; Voelkel N. F.; Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl. Acad. Sci. U.S.A. 2011, 108 (49), 19725–30. 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap J.; Irei J.; Lozano-Gerona J.; Vanapruks S.; Bishop T.; Boisvert W. A. Macrophages in cardiac remodelling after myocardial infarction. Nature reviews. Cardiology 2023, 20 (6), 373–385. 10.1038/s41569-022-00823-5. [DOI] [PubMed] [Google Scholar]
- Honold L.; Nahrendorf M. Resident and Monocyte-Derived Macrophages in Cardiovascular Disease. Circulation research 2018, 122 (1), 113–127. 10.1161/CIRCRESAHA.117.311071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H.; Song Y.; Chen J.; Zhang N.; Wang Q.; Li Q.; Gao J.; Yang H.; Dong Z.; Weng X.; Wang Z.; Sun D.; Yakufu W.; Pang Z.; Huang Z.; Ge J. Platelet-Like Fusogenic Liposome-Mediated Targeting Delivery of miR-21 Improves Myocardial Remodeling by Reprogramming Macrophages Post Myocardial Ischemia-Reperfusion Injury. Adv. Sci. 2021, 8 (15), e2100787 10.1002/advs.202100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K.; Li Y. Y.; Jin J. A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair. Sig. Transduct. Target Ther. 2021, 6 (1), 79. 10.1038/s41392-020-00455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M.; Ma Y.; Iyer R.; DeLeon-Pennell K.; Yabluchanskiy A.; Garrett M.; Lindsey M. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res. Cardiol. 2017, 112 (3), 33. 10.1007/s00395-017-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horckmans M.; Ring L.; Duchene J.; Santovito D.; Schloss M.; Drechsler M.; Weber C.; Soehnlein O.; Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur. Heart J. 2016, 38 (3), 187–197. 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- Han J.; Kim Y.; Lim M.; Kim H.; Kong S.; Kang M.; Choo Y.; Jun J.; Ryu S.; Jeong H.; et al. Dual Roles of Graphene Oxide To Attenuate Inflammation and Elicit Timely Polarization of Macrophage Phenotypes for Cardiac Repair. ACS Nano 2018, 12 (2), 1959–1977. 10.1021/acsnano.7b09107. [DOI] [PubMed] [Google Scholar]
- He Y.; Li Q.; Chen P.; Duan Q.; Zhan J.; Cai X.; Wang L.; Hou H.; Qiu X. A smart adhesive Janus hydrogel for non-invasive cardiac repair and tissue adhesion prevention. Nat. Commun. 2022, 13 (1), 7666. 10.1038/s41467-022-35437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu S.; Frangogiannis N. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circulation research 2016, 119 (1), 91–112. 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.; Wu C.; He S.; Wang Y.; Wang D.; Tao H.; Wang C.; Pang X.; Li F.; Yuan Y.; Gross E. R.; Liang G.; Zhang Y. V1-Cal hydrogelation enhances its effects on ventricular remodeling reduction and cardiac function improvement post myocardial infarction. Chem. Eng. J. 2022, 433, 134450. 10.1016/j.cej.2021.134450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R.; Dasgupta C.; Mulder C.; Zhang L. MicroRNA-210 Controls Mitochondrial Metabolism and Protects Heart Function in Myocardial Infarction. Circulation 2022, 145 (15), 1140–1153. 10.1161/CIRCULATIONAHA.121.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.; Liu W.; Zhao X.; Xian Y.; Wu W.; Zhang X.; Zhao N.; Xu F.; Wang C. Natural Melanin/Alginate Hydrogels Achieve Cardiac Repair through ROS Scavenging and Macrophage Polarization. Adv. Sci. 2021, 8 (20), e2100505 10.1002/advs.202100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao T.; Li J.; Yao F.; Dong D.; Wang Y.; Yang B.; Wang C. Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair. ACS Nano 2017, 11 (6), 5474–5488. 10.1021/acsnano.7b00221. [DOI] [PubMed] [Google Scholar]
- Raghavan K.; Porterfield J. E.; Kottam A. T.; Feldman M. D.; Escobedo D.; Valvano J. W.; Pearce J. A. Electrical conductivity and permittivity of murine myocardium. IEEE transactions on bio-medical engineering 2009, 56 (8), 2044–53. 10.1109/TBME.2009.2012401. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Luo Y.; Zhu R.; Huang X.; Bai S. Advanced bioactive hydrogels for the treatment of myocardial infarction. Journal of materials chemistry. B 2022, 10 (41), 8375–8385. 10.1039/D2TB01591A. [DOI] [PubMed] [Google Scholar]
- Mihic A.; Cui Z.; Wu J.; Vlacic G.; Miyagi Y.; Li S.; Lu S.; Sung H.; Weisel R.; Li R. A Conductive Polymer Hydrogel Supports Cell Electrical Signaling and Improves Cardiac Function After Implantation into Myocardial Infarct. Circulation 2015, 132 (8), 772–84. 10.1161/CIRCULATIONAHA.114.014937. [DOI] [PubMed] [Google Scholar]
- Navaei A.; Saini H.; Christenson W.; Sullivan R.; Ros R.; Nikkhah M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta biomaterialia 2016, 41, 133–46. 10.1016/j.actbio.2016.05.027. [DOI] [PubMed] [Google Scholar]
- Zhao G.; Feng Y.; Xue L.; Cui M.; Zhang Q.; Xu F.; Peng N.; Jiang Z.; Gao D.; Zhang X. Anisotropic conductive reduced graphene oxide/silk matrices promote post-infarction myocardial function by restoring electrical integrity. Acta biomaterialia 2022, 139, 190–203. 10.1016/j.actbio.2021.03.073. [DOI] [PubMed] [Google Scholar]
- Wu F.; Gao A.; Liu J.; Shen Y.; Xu P.; Meng J.; Wen T.; Xu L.; Xu H. High Modulus Conductive Hydrogels Enhance In Vitro Maturation and Contractile Function of Primary Cardiomyocytes for Uses in Drug Screening. Adv. Healthcare Mater. 2018, 7 (24), e1800990 10.1002/adhm.201800990. [DOI] [PubMed] [Google Scholar]
- Feng J.; Shi H.; Yang X.; Xiao S. Self-Adhesion Conductive Sub-micron Fiber Cardiac Patch from Shape Memory Polymers to Promote Electrical Signal Transduction Function. ACS Appl. Mater. Interfaces 2021, 13 (17), 19593–19602. 10.1021/acsami.0c22844. [DOI] [PubMed] [Google Scholar]
- Meng J.; Xiao B.; Wu F.; Sun L.; Li B.; Guo W.; Hu X.; Xu X.; Wen T.; Liu J.; et al. Co-axial fibrous scaffolds integrating with carbon fiber promote cardiac tissue regeneration post myocardial infarction. Materials today. Bio 2022, 16, 100415 10.1016/j.mtbio.2022.100415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.; Tan B.; Chen J.; Bao R.; Zhang X.; Liang S.; Shang Y.; Liang W.; Cui Y.; Fan G.; et al. An injectable conductive hydrogel encapsulating plasmid DNA-eNOs and ADSCs for treating myocardial infarction. Biomaterials 2018, 160, 69–81. 10.1016/j.biomaterials.2018.01.021. [DOI] [PubMed] [Google Scholar]
- Liu W.; Zhao N.; Yin Q.; Zhao X.; Guo K.; Xian Y.; Li S.; Wang C.; Zhu M.; Du Y.; et al. Injectable Hydrogels Encapsulating Dual-Functional Au@Pt Core-Shell Nanoparticles Regulate Infarcted Microenvironments and Enhance the Therapeutic Efficacy of Stem Cells through Antioxidant and Electrical Integration. ACS Nano 2023, 17 (3), 2053–2066. 10.1021/acsnano.2c07436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Reboll M.; Korf-Klingebiel M.; Wollert K. Angiogenesis after acute myocardial infarction. Cardiovascular research 2021, 117 (5), 1257–1273. 10.1093/cvr/cvaa287. [DOI] [PubMed] [Google Scholar]
- Takeshita S.; Zheng L.; Brogi E.; Kearney M.; Pu L.; Bunting S.; Ferrara N.; Symes J.; Isner J. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J. Clin. Invest. 1994, 93 (2), 662–70. 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.; Zhao L.; Zhu D.; Wang Y.; Xiao Y.; Oguljahan B.; Zhao X.; Kirlin W.; Yin L.; Chilian W.; et al. Exosomes derived from induced vascular progenitor cells promote angiogenesis in vitro and in an in vivo rat hindlimb ischemia model. American journal of physiology. Heart and circulatory physiology 2019, 317 (4), H765–H776. 10.1152/ajpheart.00247.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng X.; Chen L.; Chen W.; Yang J.; Yang Z.; Shen Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 2015, 37 (6), 2415–24. 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- Wang N.; Chen C.; Yang D.; Liao Q.; Luo H.; Wang X.; Zhou F.; Yang X.; Yang J.; Zeng C.; et al. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochimica et biophysica acta. Molecular basis of disease 2017, 1863 (8), 2085–2092. 10.1016/j.bbadis.2017.02.023. [DOI] [PubMed] [Google Scholar]
- Liang W.; Chen J.; Li L.; Li M.; Wei X.; Tan B.; Shang Y.; Fan G.; Wang W.; Liu W. Conductive Hydrogen Sulfide-Releasing Hydrogel Encapsulating ADSCs for Myocardial Infarction Treatment. ACS Appl. Mater. Interfaces 2019, 11 (16), 14619–14629. 10.1021/acsami.9b01886. [DOI] [PubMed] [Google Scholar]
- Sun X.; Altalhi W.; Nunes S. Vascularization strategies of engineered tissues and their application in cardiac regeneration. Advanced drug delivery reviews 2016, 96, 183–94. 10.1016/j.addr.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Kocher A.; Schuster M.; Szabolcs M.; Takuma S.; Burkhoff D.; Wang J.; Homma S.; Edwards N.; Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nature medicine 2001, 7 (4), 430–6. 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- Semenza G. Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochemical pharmacology 2000, 59 (1), 47–53. 10.1016/S0006-2952(99)00292-0. [DOI] [PubMed] [Google Scholar]
- Adams R.; Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nature reviews. Molecular cell biology 2007, 8 (6), 464–78. 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Ma B.; Wang T.; Li J.; Wang Q. Extracellular matrix derived from Wharton’s Jelly-derived mesenchymal stem cells promotes angiogenesis via integrin αVβ3/c-Myc/P300/VEGF. Stem Cell Res. Ther. 2022, 13 (1), 327. 10.1186/s13287-022-03009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A.; Hasan A.; Kindi H.; Gaharwar A.; Rao V.; Nikkhah M.; Shin S.; Krafft D.; Dokmeci M.; Shum-Tim D.; et al. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano 2014, 8 (8), 8050–62. 10.1021/nn5020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorius J.; Wang C.; Stambouli O.; Hussner T.; Qi Y.; Tertel T.; Borger V.; Mohamud Yusuf A.; Hagemann N.; Yin D.; Dittrich R.; Mouloud Y.; Mairinger F. D.; Magraoui F. E.; Popa-Wagner A.; Kleinschnitz C.; Doeppner T. R.; Gunzer M.; Meyer H. E.; Giebel B.; Hermann D. M. Small extracellular vesicles obtained from hypoxic mesenchymal stromal cells have unique characteristics that promote cerebral angiogenesis, brain remodeling and neurological recovery after focal cerebral ischemia in mice. Basic Res. Cardiol. 2021, 116 (1), 40. 10.1007/s00395-021-00881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J.; Qiao B.; Duan S.; Xu C.; Chen B.; Hao W.; Yu B.; Li Y.; Du J.; Xu F. Unlockable Nanocomplexes with Self-Accelerating Nucleic Acid Release for Effective Staged Gene Therapy of Cardiovascular Diseases. Adv. Mater. 2018, 30 (31), e1801570 10.1002/adma.201801570. [DOI] [PubMed] [Google Scholar]
- Firoozi S.; Pahlavan S.; Ghanian M.; Rabbani S.; Tavakol S.; Barekat M.; Yakhkeshi S.; Mahmoudi E.; Soleymani M.; Baharvand H. A Cell-Free SDKP-Conjugated Self-Assembling Peptide Hydrogel Sufficient for Improvement of Myocardial Infarction. Biomolecules 2020, 10 (2), 205. 10.3390/biom10020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contessotto P.; Pandit A. Therapies to prevent post-infarction remodelling: From repair to regeneration. Biomaterials 2021, 275, 120906 10.1016/j.biomaterials.2021.120906. [DOI] [PubMed] [Google Scholar]
- Jiao D.; Wang J.; Yu W.; Zhang K.; Zhang N.; Cao L.; Jiang X.; Bai Y. Biocompatible reduced graphene oxide stimulated BMSCs induce acceleration of bone remodeling and orthodontic tooth movement through promotion on osteoclastogenesis and angiogenesis. Bioactive materials 2022, 15, 409–425. 10.1016/j.bioactmat.2022.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden L.; Mortisen D.; Sussman E.; Dupras S.; Fugate J.; Cuy J.; Hauch K.; Laflamme M.; Murry C.; Ratner B. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. U.S.A. 2010, 107 (34), 15211–6. 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzana C.; Cras A.; Simelière F.; Guesdon R.; Desgres M.; Correa B.; Peuffier A.; Bellamy V.; Gouarderes S.; Alberdi A.; et al. Biomaterial-embedded extracellular vesicles improve recovery of the dysfunctional myocardium. Biomaterials 2022, 291, 121877 10.1016/j.biomaterials.2022.121877. [DOI] [PubMed] [Google Scholar]
- Hu C.; Liu W.; Long L.; Wang Z.; Zhang W.; He S.; Lu L.; Fan H.; Yang L.; Wang Y. Regeneration of infarcted hearts by myocardial infarction-responsive injectable hydrogels with combined anti-apoptosis, anti-inflammatory and pro-angiogenesis properties. Biomaterials 2022, 290, 121849 10.1016/j.biomaterials.2022.121849. [DOI] [PubMed] [Google Scholar]
- Eyre D. Collagen: molecular diversity in the body’s protein scaffold. Science (New York, N.Y.) 1980, 207 (4437), 1315–22. 10.1126/science.7355290. [DOI] [PubMed] [Google Scholar]
- Gelse K.; Pöschl E.; Aigner T. Collagens--structure, function, and biosynthesis. Advanced drug delivery reviews 2003, 55 (12), 1531–46. 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Huynh T.; Abraham G.; Murray J.; Brockbank K.; Hagen P.; Sullivan S. Remodeling of an acellular collagen graft into a physiologically responsive neovessel. Nature biotechnology 1999, 17 (11), 1083–6. 10.1038/15062. [DOI] [PubMed] [Google Scholar]
- Makris E.; Gomoll A.; Malizos K.; Hu J.; Athanasiou K. Repair and tissue engineering techniques for articular cartilage. Nature reviews. Rheumatology 2015, 11 (1), 21–34. 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno E.; Iurlaro M.; Madri J.; Nicosia R. Type IV collagen modulates angiogenesis and neovessel survival in the rat aorta model. In vitro cellular & developmental biology. Animal 2000, 36 (5), 336–40. . [DOI] [PubMed] [Google Scholar]
- Minor A.; Coulombe K. Engineering a collagen matrix for cell-instructive regenerative angiogenesis. Journal of biomedical materials research. Part B, Applied biomaterials 2020, 108 (6), 2407–2416. 10.1002/jbm.b.34573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien F.; Harley B.; Yannas I.; Gibson L. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26 (4), 433–41. 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- Silva A.; Pereira C.; Fonseca A.; Pinto-do-Ó P.; Nascimento D. Bearing My Heart: The Role of Extracellular Matrix on Cardiac Development, Homeostasis, and Injury Response. Front. Cell Dev. Biol. 2021, 8, 621644 10.3389/fcell.2020.621644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J.; Laurent G. Collagen turnover and its regulation in the normal and hypertrophying heart. European heart journal 1995, 16, 38–44. 10.1093/eurheartj/16.suppl_C.38. [DOI] [PubMed] [Google Scholar]
- Landau S.; Szklanny A.; Yeo G.; Shandalov Y.; Kosobrodova E.; Weiss A.; Levenberg S. Tropoelastin coated PLLA-PLGA scaffolds promote vascular network formation. Biomaterials 2017, 122, 72–82. 10.1016/j.biomaterials.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Rütsche D.; Nanni M.; Rüdisser S.; Biedermann T.; Zenobi-Wong M. Enzymatically Crosslinked Collagen as a Versatile Matrix for In Vitro and In Vivo Co-Engineering of Blood and Lymphatic Vasculature. Adv. Mater. 2023, 35 (16), e2209476 10.1002/adma.202209476. [DOI] [PubMed] [Google Scholar]
- Wright N.; Humphrey J. Denaturation of collagen via heating: an irreversible rate process. Annu. Rev. Biomed. Eng. 2002, 4, 109–28. 10.1146/annurev.bioeng.4.101001.131546. [DOI] [PubMed] [Google Scholar]
- Chen J.; Zhan Y.; Wang Y.; Han D.; Tao B.; Luo Z.; Ma S.; Wang Q.; Li X.; Fan L.; et al. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta biomaterialia 2018, 80, 154–168. 10.1016/j.actbio.2018.09.013. [DOI] [PubMed] [Google Scholar]
- Lee J.; Jang E.; Kim J.; Park S.; Kang Y.; Park S.; Lee K.; Kim J.; Youn Y.; Ryu W. Highly flexible and porous silk fibroin microneedle wraps for perivascular drug delivery. Journal of controlled release: official journal of the Controlled Release Society 2021, 340, 125–135. 10.1016/j.jconrel.2021.10.024. [DOI] [PubMed] [Google Scholar]
- Holland C.; Numata K.; Rnjak-Kovacina J.; Seib F. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthcare Mater. 2019, 8 (1), e1800465 10.1002/adhm.201800465. [DOI] [PubMed] [Google Scholar]
- Hu X.; Shmelev K.; Sun L.; Gil E.; Park S.; Cebe P.; Kaplan D. Regulation of silk material structure by temperature-controlled water vapor annealing. Biomacromolecules 2011, 12 (5), 1686–96. 10.1021/bm200062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Mora C.; Mrowiec A.; García-Vizcaíno E.; Alcaraz A.; Cenis J.; Nicolás F. Fibroin and sericin from Bombyx mori silk stimulate cell migration through upregulation and phosphorylation of c-Jun. PloS one 2012, 7 (7), e42271 10.1371/journal.pone.0042271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Sheng R.; Chen J.; Wang H.; Zhu Y.; Cao Z.; Zhao X.; Wang Z.; Liu C.; Chen Z.; Zhang P.; Kuang B.; Zheng H.; Shen C.; Yao Q.; Zhang W. Silk Fibroin and Sericin Differentially Potentiate the Paracrine and Regenerative Functions of Stem Cells Through Multiomics Analysis. Adv. Mater. 2023, 35, e2210517 10.1002/adma.202210517. [DOI] [PubMed] [Google Scholar]
- Kambe Y.; Yamaoka T. Biodegradation of injectable silk fibroin hydrogel prevents negative left ventricular remodeling after myocardial infarction. Biomaterials science 2019, 7 (10), 4153–4165. 10.1039/C9BM00556K. [DOI] [PubMed] [Google Scholar]
- Janani G.; Kumar S.; Mandal B. Fiber-Reinforced Silk Composite for Enhanced Urokinase Production Using High-Density Perfusion Culture and Bioactive Molecule Supplementation. ACS biomaterials science & engineering 2019, 5 (11), 6137–6151. 10.1021/acsbiomaterials.9b01162. [DOI] [PubMed] [Google Scholar]
- Beleño Acosta B.; Advincula R.; Grande-Tovar C. Chitosan-Based Scaffolds for the Treatment of Myocardial Infarction: A Systematic Review. Molecules 2023, 28 (4), 1920. 10.3390/molecules28041920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Wang H.; Wang Y.; Lin Q.; Yao A.; Cao F.; Li D.; Zhou J.; Duan C.; Du Z.; et al. The influence of chitosan hydrogel on stem cell engraftment, survival and homing in the ischemic myocardial microenvironment. Biomaterials 2012, 33 (11), 3093–106. 10.1016/j.biomaterials.2011.12.044. [DOI] [PubMed] [Google Scholar]
- Wang H.; Shi J.; Wang Y.; Yin Y.; Wang L.; Liu J.; Liu Z.; Duan C.; Zhu P.; Wang C. Promotion of cardiac differentiation of brown adipose derived stem cells by chitosan hydrogel for repair after myocardial infarction. Biomaterials 2014, 35 (13), 3986–98. 10.1016/j.biomaterials.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Hao T.; Qian M.; Zhang Y.; Liu Q.; Midgley A.; Liu Y.; Che Y.; Hou J.; Zhao Q. An Injectable Dual-Function Hydrogel Protects Against Myocardial Ischemia/Reperfusion Injury by Modulating ROS/NO Disequilibrium. Adv. Sci. 2022, 9 (15), e2105408 10.1002/advs.202105408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y.; Li P.; Guo Z. Cationic chitosan derivatives as potential antifungals: A review of structural optimization and applications. Carbohydr. Polym. 2020, 236, 116002 10.1016/j.carbpol.2020.116002. [DOI] [PubMed] [Google Scholar]
- Sironen R.; Tammi M.; Tammi R.; Auvinen P.; Anttila M.; Kosma V. Hyaluronan in human malignancies. Experimental cell research 2011, 317 (4), 383–91. 10.1016/j.yexcr.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Maloney F.; Kuklewicz J.; Corey R.; Bi Y.; Ho R.; Mateusiak L.; Pardon E.; Steyaert J.; Stansfeld P.; Zimmer J. Structure, substrate recognition and initiation of hyaluronan synthase. Nature 2022, 604 (7904), 195–201. 10.1038/s41586-022-04534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.; Lee Y.; Hahn J.; Choe J.; Kwon H.; Ro J.; Jeoung D. Hyaluronic acid targets CD44 and inhibits FcepsilonRI signaling involving PKCdelta, Rac1, ROS, and MAPK to exert anti-allergic effect. Molecular immunology 2008, 45 (9), 2537–47. 10.1016/j.molimm.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Jiang D.; Liang J.; Noble P. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91 (1), 221–64. 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q.; Torres J.; Hakim M.; Babiak P.; Pal P.; Battistoni C.; Nguyen M.; Panitch A.; Solorio L.; Liu J. Collagen- and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R 2021, 146, 100641. 10.1016/j.mser.2021.100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N.; Liu C.; Wang X.; He T.; Li L.; Liang X.; Wang L.; Song L.; Wei Y.; Wu Q.; et al. Hyaluronic Acid Oligosaccharides Improve Myocardial Function Reconstruction and Angiogenesis against Myocardial Infarction by Regulation of Macrophages. Theranostics 2019, 9 (7), 1980–1992. 10.7150/thno.31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertlein S.; Brown G.; Lim K.; Jungst T.; Boeck T.; Blunk T.; Tessmar J.; Hooper G.; Woodfield T.; Groll J. Thiol-Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Adv. Mater. 2017, 29 (44), e1703404 10.1002/adma.201703404. [DOI] [PubMed] [Google Scholar]
- Klotz B.; Gawlitta D.; Rosenberg A.; Malda J.; Melchels F. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34 (5), 394–407. 10.1016/j.tibtech.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian A.; Singh R.; Patel K.; Lee J.; Kim H. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioactive materials 2022, 8, 267–295. 10.1016/j.bioactmat.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S.; Yu C.; Liu N.; Zhao M.; Chen Z.; Liu J.; Li G.; Huang H.; Guo H.; Sun T.; et al. Injectable conductive gelatin methacrylate/oxidized dextran hydrogel encapsulating umbilical cord mesenchymal stem cells for myocardial infarction treatment. Bioactive materials 2022, 13, 119–134. 10.1016/j.bioactmat.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.; Cui X.; Zhang Z.; Xu Y.; Guo J.; Soliman B. G; Lu Y.; Qin Z.; Wang Q.; Zhang H.; Lim K. S; Woodfield T. B F; Zhang J. Injection-Free Delivery of MSC-Derived Extracellular Vesicles for Myocardial Infarction Therapeutics. Adv. Healthcare Mater. 2022, 11 (5), e2100312 10.1002/adhm.202100312. [DOI] [PubMed] [Google Scholar]
- Lee K.; Mooney D. Hydrogels for tissue engineering. Chem. Rev. 2001, 101 (7), 1869–79. 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- Ruvinov E.; Cohen S. Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook: From ocean algae to patient bedside. Advanced drug delivery reviews 2016, 96, 54–76. 10.1016/j.addr.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Jay S.; Saltzman W. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. Journal of controlled release: official journal of the Controlled Release Society 2009, 134 (1), 26–34. 10.1016/j.jconrel.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J.; Lee M.; Ko E.; Jeong J.; DiPietro L.; Kong H. Alginate Sulfates Mitigate Binding Kinetics of Proangiogenic Growth Factors with Receptors toward Revascularization. Mol. Pharmaceutics 2016, 13 (7), 2148–54. 10.1021/acs.molpharmaceut.5b00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia A.; Sajjadi S. Transformable bubble-filled alginate microfibers via vertical microfluidics. Lab Chip 2019, 19 (5), 851–863. 10.1039/C8LC01081A. [DOI] [PubMed] [Google Scholar]
- Serpooshan V.; Zhao M.; Metzler S. A.; Wei K.; Shah P. B.; Wang A.; Mahmoudi M.; Malkovskiy A. V.; Rajadas J.; Butte M. J.; et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials 2013, 34 (36), 9048–55. 10.1016/j.biomaterials.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.; Hsieh M. H.; Li S. H.; Wu J.; Weisel R. D.; Chang Y.; Sung H. W.; Li R. K. A conductive cell-delivery construct as a bioengineered patch that can improve electrical propagation and synchronize cardiomyocyte contraction for heart repair. Journal of controlled release: official journal of the Controlled Release Society 2020, 320, 73–82. 10.1016/j.jconrel.2020.01.027. [DOI] [PubMed] [Google Scholar]
- Mawad D.; Mansfield C.; Lauto A.; Perbellini F.; Nelson G. W.; Tonkin J.; Bello S. O.; Carrad D. J.; Micolich A. P.; Mahat M. M.; Furman J.; Payne D.; Lyon A. R.; Gooding J. J.; Harding S. E.; Terracciano C. M.; Stevens M. M. A conducting polymer with enhanced electronic stability applied in cardiac models. Sci. Adv. 2016, 2 (11), e1601007 10.1126/sciadv.1601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambe Y.; Yamaoka T. Biodegradation of injectable silk fibroin hydrogel prevents negative left ventricular remodeling after myocardial infarction. Biomaterials science 2019, 7 (10), 4153–4165. 10.1039/C9BM00556K. [DOI] [PubMed] [Google Scholar]
- Tous E.; Ifkovits J. L.; Koomalsingh K. J.; Shuto T.; Soeda T.; Kondo N.; Gorman J. H. 3rd; Gorman R. C.; Burdick J. A. Influence of injectable hyaluronic acid hydrogel degradation behavior on infarction-induced ventricular remodeling. Biomacromolecules 2011, 12 (11), 4127–35. 10.1021/bm201198x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley C. J.; Dolan E. B.; Otten M.; Hinderer S.; Duffy G. P.; Murphy B. P. An injectable alginate/extra cellular matrix (ECM) hydrogel towards acellular treatment of heart failure. Drug delivery and translational research 2019, 9 (1), 1–13. 10.1007/s13346-018-00601-2. [DOI] [PubMed] [Google Scholar]
- Su Z.; Goldstein N.; Jiang H.; Wang M.; Duigou G.; Young C.; Fisher P. PEG-3, a nontransforming cancer progression gene, is a positive regulator of cancer aggressiveness and angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 1999, 96 (26), 15115–20. 10.1073/pnas.96.26.15115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel I.; Noiri M.; Yamauchi Y.; Kato K.; Chung U.; Teramura Y. Enhancement of intercellular interaction between iPSC-derived neural progenitor cells and activated endothelial cells using cell surface modification with functional oligopeptides. Biomaterials science 2022, 10 (4), 925–938. 10.1039/D1BM01503F. [DOI] [PubMed] [Google Scholar]
- Su Y.; Zhang B.; Sun R.; Liu W.; Zhu Q.; Zhang X.; Wang R.; Chen C. PLGA-based biodegradable microspheres in drug delivery: recent advances in research and application. Drug delivery 2021, 28 (1), 1397–1418. 10.1080/10717544.2021.1938756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi D.; Wu S.; Kuss M.; Shi W.; Chung S.; Deegan P.; Kamenskiy A.; He Y.; Duan B. Mechanically robust cryogels with injectability and bioprinting supportability for adipose tissue engineering. Acta biomaterialia 2018, 74, 131–142. 10.1016/j.actbio.2018.05.044. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Dai K.; Gao Z.; Tang W.; Shen T.; Yuan Y.; Wang J.; Liu C. Sulfated polysaccharide directs therapeutic angiogenesis via endogenous VEGF secretion of macrophages. Sci. Adv. 2021, 7 (7), 8217. 10.1126/sciadv.abd8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W.; Sun M.; Hu X.; Ren B.; Cheng J.; Li C.; Duan X.; Fu X.; Zhang J.; Chen H.; Ao Y. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv. Mater. 2017, 29 (29), e1701089 10.1002/adma.201701089. [DOI] [PubMed] [Google Scholar]
- Zheng J.; Zhao F.; Zhang W.; Mo Y.; Zeng L.; Li X.; Chen X. Sequentially-crosslinked biomimetic bioactive glass/gelatin methacryloyl composites hydrogels for bone regeneration. Materials science & engineering. C, Materials for biological applications 2018, 89, 119–127. 10.1016/j.msec.2018.03.029. [DOI] [PubMed] [Google Scholar]
- Kapnisi M.; Mansfield C.; Marijon C.; Guex A. G.; Perbellini F.; Bardi I.; Humphrey E. J.; Puetzer J. L.; Mawad D.; Koutsogeorgis D. C.; Stuckey D. J.; Terracciano C. M.; Harding S. E.; Stevens M. M. Auxetic Cardiac Patches with Tunable Mechanical and Conductive Properties toward Treating Myocardial Infarction. Adv. Funct. Mater. 2018, 28 (21), 1800618 10.1002/adfm.201800618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S.; Wu J.; Li S.; Wang L.; Sun Y.; Xie J.; Ramnath D.; Weisel R.; Yau T.; Sung H.; et al. The conductive function of biopolymer corrects myocardial scar conduction blockage and resynchronizes contraction to prevent heart failure. Biomaterials 2020, 258, 120285 10.1016/j.biomaterials.2020.120285. [DOI] [PubMed] [Google Scholar]
- Tran V.; Lim C.; Xie J.; Chen X. Asymmetric division of Drosophila male germline stem cell shows asymmetric histone distribution. Science (New York, N.Y.) 2012, 338 (6107), 679–82. 10.1126/science.1226028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosic J.; Kim G.; Pavlovic M.; Schröder C.; Mersiowsky S.; Barg M.; Hofherr A.; Probst S.; Köttgen M.; Hein L.; et al. Eomes and Brachyury control pluripotency exit and germ-layer segregation by changing the chromatin state. Nature cell biology 2019, 21 (12), 1518–1531. 10.1038/s41556-019-0423-1. [DOI] [PubMed] [Google Scholar]
- Wang L.; Li J.; Zhou H.; Zhang W.; Gao J.; Zheng P. A novel lncRNA Discn fine-tunes replication protein A (RPA) availability to promote genomic stability. Nat. Commun. 2021, 12 (1), 5572. 10.1038/s41467-021-25827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanelidis A.; Premer C.; Lopez J.; Balkan W.; Hare J. Route of Delivery Modulates the Efficacy of Mesenchymal Stem Cell Therapy for Myocardial Infarction: A Meta-Analysis of Preclinical Studies and Clinical Trials. Circulation research 2017, 120 (7), 1139–1150. 10.1161/CIRCRESAHA.116.309819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.; Ke W.; Zhou X.; Qian Y.; Feng S.; Wang R.; Cui G.; Tao R.; Guo W.; Duan Y.; et al. Human Neural Stem Cells Reinforce Hippocampal Synaptic Network and Rescue Cognitive Deficits in a Mouse Model of Alzheimer’s Disease. Stem cell reports 2019, 13 (6), 1022–1037. 10.1016/j.stemcr.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara T.; Kameda M.; Sasaki T.; Tajiri N.; Date I. Cell Therapy for Parkinson’s Disease. Cell transplantation 2017, 26 (9), 1551–1559. 10.1177/0963689717735411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Badawy A.; El-Badri N. Clinical Efficacy of Stem Cell Therapy for Diabetes Mellitus: A Meta-Analysis. PloS one 2016, 11 (4), e0151938 10.1371/journal.pone.0151938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L.; Kim J.; Woo Y.; Huang N. Stem cell-based therapies to promote angiogenesis in ischemic cardiovascular disease. American journal of physiology. Heart and circulatory physiology 2016, 310 (4), H455–65. 10.1152/ajpheart.00726.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi-Yuyama E.; Matsubara H.; Murohara T.; Ikeda U.; Shintani S.; Masaki H.; Amano K.; Kishimoto Y.; Yoshimoto K.; Akashi H.; et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet (London, England) 2002, 360 (9331), 427–35. 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- Asahara T.; Murohara T.; Sullivan A.; Silver M.; van der Zee R.; Li T.; Witzenbichler B.; Schatteman G.; Isner J. Isolation of putative progenitor endothelial cells for angiogenesis. Science (New York, N.Y.) 1997, 275 (5302), 964–7. 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Kikuchi-Taura A.; Okinaka Y.; Takeuchi Y.; Ogawa Y.; Maeda M.; Kataoka Y.; Yasui T.; Kimura T.; Gul S.; Claussen C.; et al. Bone Marrow Mononuclear Cells Activate Angiogenesis via Gap Junction-Mediated Cell-Cell Interaction. Stroke 2020, 51 (4), 1279–1289. 10.1161/STROKEAHA.119.028072. [DOI] [PubMed] [Google Scholar]
- Asahara T.; Masuda H.; Takahashi T.; Kalka C.; Pastore C.; Silver M.; Kearne M.; Magner M.; Isner J. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circulation research 1999, 85 (3), 221–8. 10.1161/01.RES.85.3.221. [DOI] [PubMed] [Google Scholar]
- Rehman J.; Li J.; Orschell C.; March K. Peripheral blood ″endothelial progenitor cells″ are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 2003, 107 (8), 1164–9. 10.1161/01.CIR.0000058702.69484.A0. [DOI] [PubMed] [Google Scholar]
- Leeper N.; Hunter A.; Cooke J. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation 2010, 122 (5), 517–26. 10.1161/CIRCULATIONAHA.109.881441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.; Song S.; Choi K.; Suh W. Cooperation of endothelial and smooth muscle cells derived from human induced pluripotent stem cells enhances neovascularization in dermal wounds. Tissue engineering. Part A 2013, 19, 2478–85. 10.1089/ten.tea.2012.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.; Park C.; Lee J.; Kim S.; Kwon P.; Kim W.; Jeon Y.; Lee E.; Yoon Y. Generation of pure lymphatic endothelial cells from human pluripotent stem cells and their therapeutic effects on wound repair. Sci. Rep. 2015, 5, 11019. 10.1038/srep11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattapally S.; Zhu W.; Fast V.; Gao L.; Worley C.; Kannappan R.; Borovjagin A.; Zhang J. Spheroids of cardiomyocytes derived from human-induced pluripotent stem cells improve recovery from myocardial injury in mice. American journal of physiology. Heart and circulatory physiology 2018, 315 (2), H327–H339. 10.1152/ajpheart.00688.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L.; Gregorich Z.; Zhu W.; Mattapally S.; Oduk Y.; Lou X.; Kannappan R.; Borovjagin A.; Walcott G.; Pollard A.; et al. Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine. Circulation 2018, 137 (16), 1712–1730. 10.1161/CIRCULATIONAHA.117.030785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B.; Li H.; Zhang Z.; Chen P.; Wang L.; Fan X.; Ning X.; Pan Y.; Zhou F.; Hu X.; Chang J.; Ou C. Extracellular vesicles engineering by silicates-activated endothelial progenitor cells for myocardial infarction treatment in male mice. Nat. Commun. 2023, 14 (1), 2094. 10.1038/s41467-023-37832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo S.; Klychko E.; Thorne T.; Misener S.; Schultz K.; Millay M.; Ito A.; Liu T.; Kamide C.; Agrawal H.; et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circulation research 2011, 109 (7), 724–8. 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G.; D’Angelo G.; Raposo G. Shedding light on the cell biology of extracellular vesicles. Nature reviews. Molecular cell biology 2018, 19 (4), 213–228. 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- Gallet R.; Dawkins J.; Valle J.; Simsolo E.; de Couto G.; Middleton R.; Tseliou E.; Luthringer D.; Kreke M.; Smith R. R.; Marban L.; Ghaleh B.; Marban E. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2016, 38 (3), 201–211. 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol E.; Goumans M.; Sluijter J. Cardiac Progenitor-Cell Derived Exosomes as Cell-Free Therapeutic for Cardiac Repair. Advances in experimental medicine and biology 2017, 998, 207–219. 10.1007/978-981-10-4397-0_14. [DOI] [PubMed] [Google Scholar]
- Adamiak M.; Cheng G.; Bobis-Wozowicz S.; Zhao L.; Kedracka-Krok S.; Samanta A.; Karnas E.; Xuan Y.; Skupien-Rabian B.; Chen X.; et al. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circulation research 2018, 122 (2), 296–309. 10.1161/CIRCRESAHA.117.311769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q.; Wang J.; Tan W. L. W.; Jiang Y.; Wang S.; Li Q.; Yu X.; Tan J.; Liu S.; Zhang P.; Tiang Z.; Chen Z.; Foo R. S.-Y.; Yang H.-T. Extracellular vesicles from human embryonic stem cell-derived cardiovascular progenitor cells promote cardiac infarct healing through reducing cardiomyocyte death and promoting angiogenesis. Cell Death Dis. 2020, 11 (5), 354. 10.1038/s41419-020-2508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentkowski K.; Mursleen A.; Snitzer J.; Euscher L.; Lang J. CDC-derived extracellular vesicles reprogram inflammatory macrophages to an arginase 1-dependent proangiogenic phenotype. American journal of physiology. Heart and circulatory physiology 2020, 318 (6), H1447–H1460. 10.1152/ajpheart.00155.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S.; Li Z.; Shen D.; Zhu D.; Huang K.; Su T.; Dinh P.; Cores J.; Cheng K. Exosome-eluting stents for vascular healing after ischaemic injury. Nature biomedical engineering 2021, 5 (10), 1174–1188. 10.1038/s41551-021-00705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N.; Song Y.; Huang Z.; Chen J.; Tan H.; Yang H.; Fan M.; Li Q.; Wang Q.; Gao J.; et al. Monocyte mimics improve mesenchymal stem cell-derived extracellular vesicle homing in a mouse MI/RI model. Biomaterials 2020, 255, 120168 10.1016/j.biomaterials.2020.120168. [DOI] [PubMed] [Google Scholar]
- Cheng L.; Hill A. Therapeutically harnessing extracellular vesicles. Nature reviews. Drug discovery 2022, 21 (5), 379–399. 10.1038/s41573-022-00410-w. [DOI] [PubMed] [Google Scholar]
- Carmeliet P.; Jain R. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473 (7347), 298–307. 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.; Singh G.; Baker A. Overcoming disease-induced growth factor resistance in therapeutic angiogenesis using recombinant co-receptors delivered by a liposomal system. Biomaterials 2014, 35 (1), 196–205. 10.1016/j.biomaterials.2013.09.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa-Miwa A.; Uchida Y.; Nakamura F.; Tomaru T.; Kido H.; Kamijo T.; Sugimoto T.; Kaji K.; Utsuyama M.; Kurashima C.; Ito H. Salvage of infarcted myocardium by angiogenic action of basic fibroblast growth factor. Science 1992, 257 (5075), 1401–3. 10.1126/science.1382313. [DOI] [PubMed] [Google Scholar]
- Jayasankar V.; Woo Y.; Bish L.; Pirolli T.; Chatterjee S.; Berry M.; Burdick J.; Gardner T.; Sweeney H. Gene transfer of hepatocyte growth factor attenuates postinfarction heart failure. Circulation 2003, 108, II230–6. 10.1161/01.cir.0000087444.53354.66. [DOI] [PubMed] [Google Scholar]
- Melincovici C.; Boşca A.; Şuşman S.; Mărginean M.; Mihu C.; Istrate M.; Moldovan I.; Roman A.; Mihu C. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59 (2), 455–467. [PubMed] [Google Scholar]
- Jarajapu Y.; Bhatwadekar A.; Caballero S.; Hazra S.; Shenoy V.; Medina R.; Kent D.; Stitt A.; Thut C.; Finney E.; et al. Activation of the ACE2/angiotensin-(1–7)/Mas receptor axis enhances the reparative function of dysfunctional diabetic endothelial progenitors. Diabetes 2013, 62 (4), 1258–69. 10.2337/db12-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H.; Cui Z.; Wang L.; Xia Z.; Hu Y.; Xian L.; Li C.; Xie L.; Crane J.; Wan M.; et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nature medicine 2014, 20 (11), 1270–8. 10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Abraham S.; McKenzie J.; Jeffs N.; Swire M.; Tripathi V.; Luhmann U.; Lange C.; Zhai Z.; Arthur H.; et al. LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature 2013, 499 (7458), 306–11. 10.1038/nature12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature medicine 1995, 1 (1), 27–31. 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Ma T.; Hao Y.; Li S.; Xia B.; Gao X.; Zheng Y.; Mei L.; Wei Y.; Yang C.; Lu L.; et al. Sequential oxygen supply system promotes peripheral nerve regeneration by enhancing Schwann cells survival and angiogenesis. Biomaterials 2022, 289, 121755 10.1016/j.biomaterials.2022.121755. [DOI] [PubMed] [Google Scholar]
- Lou R.; Chen J.; Zhou F.; Wang C.; Leung C.; Lin L. Exosome-cargoed microRNAs: Potential therapeutic molecules for diabetic wound healing. Drug discovery today 2022, 27 (10), 103323 10.1016/j.drudis.2022.07.008. [DOI] [PubMed] [Google Scholar]
- Lee Y.; Dutta A. MicroRNAs in cancer. Annual review of pathology 2009, 4, 199–227. 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latronico M.; Catalucci D.; Condorelli G. Emerging role of microRNAs in cardiovascular biology. Circulation research 2007, 101 (12), 1225–36. 10.1161/CIRCRESAHA.107.163147. [DOI] [PubMed] [Google Scholar]
- Eulalio A.; Mano M.; Ferro M. D.; Zentilin L.; Sinagra G.; Zacchigna S.; Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 2012, 492 (7429), 376–381. 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- Ramanujam D.; Sassi Y.; Laggerbauer B.; Engelhardt S. Viral Vector-Based Targeting of miR-21 in Cardiac Nonmyocyte Cells Reduces Pathologic Remodeling of the Heart. Molecular therapy: the journal of the American Society of Gene Therapy 2016, 24 (11), 1939–1948. 10.1038/mt.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanujam D.; Schön A.; Beck C.; Vaccarello P.; Felician G.; Dueck A.; Esfandyari D.; Meister G.; Meitinger T.; Schulz C.; et al. MicroRNA-21-Dependent Macrophage-to-Fibroblast Signaling Determines the Cardiac Response to Pressure Overload. Circulation 2021, 143 (15), 1513–1525. 10.1161/CIRCULATIONAHA.120.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T.; Waisman A.; Ranjan R.; Roes J.; Krieg T.; Müller W.; Roers A.; Eming S. Differential roles of macrophages in diverse phases of skin repair. Journal of immunology (Baltimore, Md.: 1950) 2010, 184 (7), 3964–77. 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- Fish J.; Santoro M.; Morton S.; Yu S.; Yeh R.; Wythe J.; Ivey K.; Bruneau B.; Stainier D.; Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Developmental cell 2008, 15 (2), 272–84. 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov D.; Orekhov A.; Bobryshev Y. The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease. Journal of molecular and cellular cardiology 2016, 97, 47–55. 10.1016/j.yjmcc.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Yan C.; Chen J.; Wang C.; Yuan M.; Kang Y.; Wu Z.; Li W.; Zhang G.; Machens H.; Rinkevich Y.; et al. Milk exosomes-mediated miR-31–5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug delivery 2022, 29 (1), 214–228. 10.1080/10717544.2021.2023699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.; Sun W.; Guo Z.; Zhang J.; Yu H.; Liu B. Mechanisms of lncRNA/microRNA interactions in angiogenesis. Life sciences 2020, 254, 116900 10.1016/j.lfs.2019.116900. [DOI] [PubMed] [Google Scholar]
- Thum T.; Fiedler J. LINCing MALAT1 and angiogenesis. Circulation research 2014, 114 (9), 1366–8. 10.1161/CIRCRESAHA.114.303896. [DOI] [PubMed] [Google Scholar]
- Behera J.; Kumar A.; Voor M.; Tyagi N. Exosomal lncRNA-H19 promotes osteogenesis and angiogenesis through mediating Angpt1/Tie2-NO signaling in CBS-heterozygous mice. Theranostics 2021, 11 (16), 7715–7734. 10.7150/thno.58410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Li S.; Zhang Y.; Wang M.; Li X.; Liu S.; Xu D.; Bao Y.; Jia P.; Wu N.; et al. The lncRNA Malat1 regulates microvascular function after myocardial infarction in mice via miR-26b-5p/Mfn1 axis-mediated mitochondrial dynamics. Redox biology 2021, 41, 101910 10.1016/j.redox.2021.101910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.; Luo L.; Wei X.; Gong D.; Li Z.; Li S.; Tang W.; Jin L. M1 Bone Marrow-Derived Macrophage-Derived Extracellular Vesicles Inhibit Angiogenesis and Myocardial Regeneration Following Myocardial Infarction via the MALAT1/MicroRNA-25–3p/CDC42 Axis. Oxid. Med. Cell. Longevity 2021, 2021, 9959746 10.1155/2021/9959746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle M.; El-Daly S.; Fabbri M.; Calin G. Noncoding RNA therapeutics - challenges and potential solutions. Nature reviews. Drug discovery 2021, 20 (8), 629–651. 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y.; Reis L. A.; Feric N.; Knee E. J.; Gu J.; Cao S.; Laschinger C.; Londono C.; Antolovich J.; McGuigan A. P.; Radisic M. Diabetic wound regeneration using peptide-modified hydrogels to target re-epithelialization. Proc. Natl. Acad. Sci. U.S.A. 2016, 113 (40), E5792–E5801. 10.1073/pnas.1612277113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan G.; Qizhuang Lv; Liu S.; Jiang Z.; Zhou C.; Liao W. 3D-bioprinted peptide coupling patches for wound healing. Materials Today Bio. 2022, 13, 100188 10.1016/j.mtbio.2021.100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.; Shi X.; Zhao X.; Chen B.; Li X.; Li Y.; Chen Y.; Chen C.; Lu H.; Liu J. Acellular dermal matrix decorated with collagen-affinity peptide accelerate diabetic wound healing through sustained releasing Histatin-1 mediated promotion of angiogenesis. International journal of pharmaceutics 2022, 624, 122017 10.1016/j.ijpharm.2022.122017. [DOI] [PubMed] [Google Scholar]
- Li R.; Zhou C.; Chen J.; Luo H.; Li R.; Chen D.; Zou X.; Wang W. Synergistic osteogenic and angiogenic effects of KP and QK peptides incorporated with an injectable and self-healing hydrogel for efficient bone regeneration. Bioactive materials 2022, 18, 267–283. 10.1016/j.bioactmat.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J.; Kim S.; Seo Y.; Jeon J.; Davaa G.; Hyun J.; Kim S. Self-assembling peptide gels promote angiogenesis and functional recovery after spinal cord injury in rats. J. Tissue Eng. 2022, 13, 204173142210864 10.1177/20417314221086491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi H.; Abolmaali S.; Heidari R.; Valizadeh H.; Tamaddon A.; Azarpira N. Integrin receptor-binding nanofibrous peptide hydrogel for combined mesenchymal stem cell therapy and nitric oxide delivery in renal ischemia/reperfusion injury. Stem Cell Res. Ther. 2022, 13 (1), 344. 10.1186/s13287-022-03045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Zhang R.; Wei X.; Lv M.; Jiang Z. Metalloimmunology: The metal ion-controlled immunity. Advances in immunology 2020, 145, 187–241. 10.1016/bs.ai.2019.11.007. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Zhao J.; Hu X.; Wang C.; Jia Y.; Zhu C.; Xie S.; Lee J.; Li F.; Ling D. A Peritumorally Injected Immunomodulating Adjuvant Elicits Robust and Safe Metalloimmunotherapy against Solid Tumors. Adv. Mater. 2022, 34 (41), e2206915 10.1002/adma.202206915. [DOI] [PubMed] [Google Scholar]
- Yang R.; Li G.; Zhuang C.; Yu P.; Ye T.; Zhang Y.; Shang P.; Huang J.; Cai M.; Wang L.; Cui W.; Deng L. Gradient bimetallic ion-based hydrogels for tissue microstructure reconstruction of tendon-to-bone insertion. Sci. Adv. 2021, 7 (26), 3816. 10.1126/sciadv.abg3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A.; Renaudin G.; Forestier C.; Nedelec J.; Descamps S. Biological properties of copper-doped biomaterials for orthopedic applications: A review of antibacterial, angiogenic and osteogenic aspects. Acta biomaterialia 2020, 117, 21–39. 10.1016/j.actbio.2020.09.044. [DOI] [PubMed] [Google Scholar]
- Romero-Sánchez L.; Marí-Beffa M.; Carrillo P.; Medina M.; Díaz-Cuenca A. Copper-containing mesoporous bioactive glass promotes angiogenesis in an in vivo zebrafish model. Acta biomaterialia 2018, 68, 272–285. 10.1016/j.actbio.2017.12.032. [DOI] [PubMed] [Google Scholar]
- Xiao J.; Zhu Y.; Huddleston S.; Li P.; Xiao B.; Farha O.; Ameer G. Copper Metal-Organic Framework Nanoparticles Stabilized with Folic Acid Improve Wound Healing in Diabetes. ACS Nano 2018, 12 (2), 1023–1032. 10.1021/acsnano.7b01850. [DOI] [PubMed] [Google Scholar]
- Gao P.; Fan B.; Yu X.; Liu W.; Wu J.; Shi L.; Yang D.; Tan L.; Wan P.; Hao Y.; et al. in vitroBiofunctional magnesium coated Ti6Al4V scaffold enhances osteogenesis and angiogenesis and for orthopedic application. Bioactive materials 2020, 5 (3), 680–693. 10.1016/j.bioactmat.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W.; Jin Y.; Cui Y.; Xi F.; Liu X.; Wo F.; Wu J. A co-delivery platform for synergistic promotion of angiogenesis based on biodegradable, therapeutic and self-reporting luminescent porous silicon microparticles. Biomaterials 2021, 272, 120772 10.1016/j.biomaterials.2021.120772. [DOI] [PubMed] [Google Scholar]
- Liu X.; Huang Y.; Pokreisz P.; Vermeersch P.; Marsboom G.; Swinnen M.; Verbeken E.; Santos J.; Pellens M.; Gillijns H.; et al. Nitric oxide inhalation improves microvascular flow and decreases infarction size after myocardial ischemia and reperfusion. Journal of the American College of Cardiology 2007, 50 (8), 808–17. 10.1016/j.jacc.2007.04.069. [DOI] [PubMed] [Google Scholar]
- Zhao Q.; Zhang J.; Song L.; Ji Q.; Yao Y.; Cui Y.; Shen J.; Wang P.; Kong D. Polysaccharide-based biomaterials with on-demand nitric oxide releasing property regulated by enzyme catalysis. Biomaterials 2013, 34 (33), 8450–8. 10.1016/j.biomaterials.2013.07.045. [DOI] [PubMed] [Google Scholar]
- Champeau M.; Póvoa V.; Militão L.; Cabrini F.; Picheth G.; Meneau F.; Jara C.; de Araujo E.; de Oliveira M. Supramolecular poly(acrylic acid)/F127 hydrogel with hydration-controlled nitric oxide release for enhancing wound healing. Acta biomaterialia 2018, 74, 312–325. 10.1016/j.actbio.2018.05.025. [DOI] [PubMed] [Google Scholar]
- Yao S.; Wang Y.; Chi J.; Yu Y.; Zhao Y.; Luo Y.; Wang Y. Porous MOF Microneedle Array Patch with Photothermal Responsive Nitric Oxide Delivery for Wound Healing. Adv. Sci. 2022, 9 (3), e2103449 10.1002/advs.202103449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W.; Wang M.; Moore P.; Jin H.; Yao T.; Zhu Y. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovascular research 2007, 76 (1), 29–40. 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A.; Pyriochou A.; Altaany Z.; Yang G.; Marazioti A.; Zhou Z.; Jeschke M.; Branski L.; Herndon D.; Wang R.; et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 2009, 106 (51), 21972–7. 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Aranguren L.; Grubliauskiene M.; Shokr H.; Balakrishnan P.; Wang K.; Ahmad S.; Marwah M. Sodium Thiosulphate-Loaded Liposomes Control Hydrogen Sulphide Release and Retain Its Biological Properties in Hypoxia-like Environment. Antioxidants 2022, 11 (11), 2092. 10.3390/antiox11112092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid J.; Schwarz S.; Meier-Staude R.; Sudhop S.; Clausen-Schaumann H.; Schieker M.; Huber R. A Perfusion Bioreactor System for Cell Seeding and Oxygen-Controlled Cultivation of Three-Dimensional Cell Cultures. Tissue engineering. Part C, Methods 2018, 24 (10), 585–595. 10.1089/ten.tec.2018.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato T.; Kawamura T.; Uemura T.; Liu L.; Li J.; Sasai M.; Harada A.; Ito E.; Iseoka H.; Toda K.; et al. Engineered three-dimensional cardiac tissues maturing in a rotating wall vessel bioreactor remodel diseased hearts in rats with myocardial infarction. Stem cell reports 2022, 17 (5), 1170–1182. 10.1016/j.stemcr.2022.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.; Ji Y.; Chi B.; Xiao T.; Li C.; Yan X.; Xiong X.; Mao L.; Cai D.; Zou A.; Wang Y.; Zhang L.; Tang L.; Wang Q. A 3D culture system improves the yield of MSCs-derived extracellular vesicles and enhances their therapeutic efficacy for heart repair. Biomed. Pharmacother. 2023, 161, 114557 10.1016/j.biopha.2023.114557. [DOI] [PubMed] [Google Scholar]
- You Y.; Kobayashi K.; Colak B.; Luo P.; Cozens E.; Fields L.; Suzuki K.; Gautrot J. Engineered cell-degradable poly(2-alkyl-2-oxazoline) hydrogel for epicardial placement of mesenchymal stem cells for myocardial repair. Biomaterials 2021, 269, 120356 10.1016/j.biomaterials.2020.120356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.; Zhang Y.; Xu Y.; Long L.; Hu X.; Zhang J.; Wang Y. Injectable polyaniline nanorods/alginate hydrogel with AAV9-mediated VEGF overexpression for myocardial infarction treatment. Biomaterials 2023, 296, 122088 10.1016/j.biomaterials.2023.122088. [DOI] [PubMed] [Google Scholar]
- Fan Z.; Wei Y.; Yin Z.; Huang H.; Liao X.; Sun L.; Liu B.; Liu F. Near-Infrared Light-Triggered Unfolding Microneedle Patch for Minimally Invasive Treatment of Myocardial Ischemia. ACS Appl. Mater. Interfaces 2021, 13 (34), 40278–40289. 10.1021/acsami.1c09658. [DOI] [PubMed] [Google Scholar]
- Ferrara N.; Gerber H.; LeCouter J. The biology of VEGF and its receptors. Nature medicine 2003, 9 (6), 669–76. 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nature medicine 2003, 9 (6), 653–60. 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Ferrara N.; Adamis A. Ten years of anti-vascular endothelial growth factor therapy. Nature reviews. Drug discovery 2016, 15 (6), 385–403. 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- Apte R.; Chen D.; Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176 (6), 1248–1264. 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y.; Ohgimoto K.; Kataoka Y.; Yoshida N.; Shibuya M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc. Natl. Acad. Sci. U.S.A. 2005, 102 (4), 1076–81. 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T.; Suzuki J.; Yamawaki H.; Sharma V.; Sheu S.; Berk B. Losartan metabolite EXP3179 activates Akt and endothelial nitric oxide synthase via vascular endothelial growth factor receptor-2 in endothelial cells: angiotensin II type 1 receptor-independent effects of EXP3179. Circulation 2005, 112 (12), 1798–805. 10.1161/CIRCULATIONAHA.104.509760. [DOI] [PubMed] [Google Scholar]
- Arulanandam R.; Batenchuk C.; Angarita F.; Ottolino-Perry K.; Cousineau S.; Mottashed A.; Burgess E.; Falls T.; De Silva N.; Tsang J.; et al. VEGF-Mediated Induction of PRD1-BF1/Blimp1 Expression Sensitizes Tumor Vasculature to Oncolytic Virus Infection. Cancer cell 2015, 28 (2), 210–24. 10.1016/j.ccell.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Arif A.; Gong Y.; Jia J.; Eswarappa S.; Willard B.; Horowitz A.; Graham L.; Penn M.; Fox P. Stimulus-dependent phosphorylation of profilin-1 in angiogenesis. Nature cell biology 2012, 14 (10), 1046–56. 10.1038/ncb2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainson R.; Johnston D.; Chu H.; Holderfield M.; Nakatsu M.; Crampton S.; Davis J.; Conn E.; Hughes C. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood 2008, 111 (10), 4997–5007. 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.; Lu B.; Zhang X.; Zhang J.; Lai L.; Li D.; Wu Y.; Song Y.; Luo J.; Pang X.; et al. Cucurbitacin E, a tetracyclic triterpenes compound from Chinese medicine, inhibits tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling pathway. Carcinogenesis 2010, 31 (12), 2097–104. 10.1093/carcin/bgq167. [DOI] [PubMed] [Google Scholar]
- Semenza G. HIF-1: using two hands to flip the angiogenic switch. Cancer metastasis reviews 2000, 19, 59–65. 10.1023/A:1026544214667. [DOI] [PubMed] [Google Scholar]
- Schweigerer L.; Neufeld G.; Friedman J.; Abraham J.; Fiddes J.; Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature 1987, 325 (6101), 257–9. 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto T.; Leconte I.; Swain J.; Fox J. Autocrine FGF signaling is required for vascular smooth muscle cell survival in vitro. Journal of cellular physiology 1998, 177 (1), 58–67. . [DOI] [PubMed] [Google Scholar]
- Murakami M.; Elfenbein A.; Simons M. Non-canonical fibroblast growth factor signalling in angiogenesis. Cardiovascular research 2008, 78 (2), 223–31. 10.1093/cvr/cvm086. [DOI] [PubMed] [Google Scholar]
- Hanneken A.; Maher P.; Baird A. High affinity immunoreactive FGF receptors in the extracellular matrix of vascular endothelial cells--implications for the modulation of FGF-2. J. Cell Biol. 1995, 128 (6), 1221–8. 10.1083/jcb.128.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.; Chen T.; Ding Z.; Wang Y.; Wei Y.; Wei X. Inhibition of FGF-FGFR and VEGF-VEGFR signalling in cancer treatment. Cell Proliferation 2021, 54 (4), e13009 10.1111/cpr.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten N.; Verbruggen S.; Gijbels M.; Post M.; De Winther M.; Donners M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17 (1), 109–18. 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- Tian Y.; Zhan Y.; Jiang Q.; Lu W.; Li X. Expression and function of PDGF-C in development and stem cells. Open biology 2021, 11 (12), 210268 10.1098/rsob.210268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen V.; Paulis Y.; Nowak-Sliwinska P.; Deumelandt K.; Hosaka K.; Soetekouw P.; Cimpean A.; Raica M.; Pauwels P.; van den Oord J.; et al. Targeting PDGF-mediated recruitment of pericytes blocks vascular mimicry and tumor growth. Journal of pathology 2018, 246 (4), 447–458. 10.1002/path.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W.; Liu G.; Liu X.; Zhou Y.; Sun Q.; Zhen G.; Wang X.; Hu Y.; Gao P.; Demehri S.; Cao X.; Wan M. Angiogenesis stimulated by elevated PDGF-BB in subchondral bone contributes to osteoarthritis development. JCI Insight 2020, 5 (8), e135446 10.1172/jci.insight.135446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni-Barrera R.; Butschkau A.; Uccelli A.; Certelli A.; Valente P.; Bartolomeo M.; Groppa E.; Burger M.; Hlushchuk R.; Heberer M.; et al. PDGF-BB regulates splitting angiogenesis in skeletal muscle by limiting VEGF-induced endothelial proliferation. Angiogenesis 2018, 21 (4), 883–900. 10.1007/s10456-018-9634-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaykumar A.; Plumber S.; Barry D.; Binns D.; Wichaidit C.; Grzemska M.; Earnest S.; Goldsmith E.; Cleaver O.; Cobb M. WNK1 collaborates with TGF-β in endothelial cell junction turnover and angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 2022, 119 (30), e2203743119 10.1073/pnas.2203743119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque S.; Morris J. Transforming growth factor-β: A therapeutic target for cancer. Human vaccines & immunotherapeutics 2017, 13 (8), 1741–1750. 10.1080/21645515.2017.1327107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G.; Robinson F.; Beers Gibson T.; Xu B.; Karandikar M.; Berman K.; Cobb M. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 2001, 22 (2), 153–83. 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Chan T.; Rittenhouse S.; Tsichlis P. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annual review of biochemistry 1999, 68, 965–1014. 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J.; Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2003, 21 (14), 2787–99. 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- Roskoski R. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacological research 2014, 79, 34–74. 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Peng D.; Ando K.; Hußmann M.; Gloger M.; Skoczylas R.; Mochizuki N.; Betsholtz C.; Fukuhara S.; Schulte-Merker S.; Lawson N. D; Koltowska K. Proper migration of lymphatic endothelial cells requires survival and guidance cues from arterial mural cells. eLife 2022, 11, e74094 10.7554/eLife.74094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himburg H.; Doan P.; Quarmyne M.; Yan X.; Sasine J.; Zhao L.; Hancock G.; Kan J.; Pohl K.; Tran E.; et al. Dickkopf-1 promotes hematopoietic regeneration via direct and niche-mediated mechanisms. Nature medicine 2017, 23 (1), 91–99. 10.1038/nm.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V. B.; Besner G. E.; Mehta V. B.; Besner G. E. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors 2007, 25 (4), 253–63. 10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- Kim S.; Choi J.; Lim H.; Lee S.; Kim W.; Cho S.; Kim J.; Kim J.; Choe J.; Nam S.; et al. EGF-induced MMP-9 expression is mediated by the JAK3/ERK pathway, but not by the JAK3/STAT-3 pathway in a SKBR3 breast cancer cell line. Cellular signalling 2009, 21 (6), 892–8. 10.1016/j.cellsig.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Cui N.; Hu M.; Khalil R. Biochemical and Biological Attributes of Matrix Metalloproteinases. Progress in molecular biology and translational science 2017, 147, 1–73. 10.1016/bs.pmbts.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.; Huang Y.; Cunningham C.; Han K.; Chang M.; Seiki M.; Zhou Z.; Azar D. Matrix metalloproteinase 14 modulates signal transduction and angiogenesis in the cornea. Survey of ophthalmology 2016, 61 (4), 478–97. 10.1016/j.survophthal.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]





