Abstract
In the high-stakes realm of critical care, where daily decisions are crucial and clear communication is paramount, comprehending the rationale behind Artificial Intelligence (AI)-driven decisions appears essential. While AI has the potential to improve decision-making, its complexity can hinder comprehension and adherence to its recommendations. “Explainable AI” (XAI) aims to bridge this gap, enhancing confidence among patients and doctors. It also helps to meet regulatory transparency requirements, offers actionable insights, and promotes fairness and safety. Yet, defining explainability and standardising assessments are ongoing challenges and balancing performance and explainability can be needed, even if XAI is a growing field.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-05005-y.
Keywords: Explainable artificial intelligence, Interpretability, Clinical decision-making, Regulatory compliance, Algorithmic bias, Patient autonomy, Fairness, Transparency, Generative artificial intelligence
Introduction
The healthcare sector has witnessed a surge in Artificial Intelligence (AI) models, particularly in crucial areas such as medical imaging, perioperative, and critical care, where extensive volumes of data are constantly generated. In these fields, the rapid development of AI-based models holds significant potential for enhancing medical decision-making and improving patient outcomes [1].
However, a recent survey of intensive care unit (ICU) professionals sheds light on their doubts regarding AI [2]. Seventy-one percent of participants were either unsure or disagreed that AI can be used reliably in ICU decision-making. The usual diffidence in a novelty may be at least partially responsible. However, this lack of confidence could also come from distrust of decisions based on algorithms that resemble "black boxes". This prompts the question: should AI models be made explainable to clinicians?
Background
The AI literature offers varied interpretations of explainability, underscoring the absence of a formal definition. Sometimes, explainability is mistakenly used interchangeably with interpretability and transparency [3]. Interpretability may refer to the degree to which a human can understand the internal mechanisms and decision-making processes of an AI model [4]. Interpretable models are designed to be easily understood and straightforward, enabling users to trace and grasp how inputs are transformed into outputs, sometimes through an identifiable pathophysiologic rationale. Examples of inherently interpretable models include decision trees and linear regression, where the logic and rules governing the model's decisions are clear and easy to follow.
Explainability, in contrast, involves techniques and methods used to make the decisions of more complex, often opaque models (like deep neural networks) understandable to humans. This typically involves post hoc explanations, which are generated after the model has made its decisions. Hence, techniques such as Local Interpretable Model-agnostic Explanations (LIME) and Shapley Additive Explanations (SHAP) are commonly used to clarify which factors influenced the model’s predictions and why they did so, without necessarily simplifying the model itself or understanding the underlying biochemical mechanism (See Additional file 1).
Models should be explainable for clinicians: yes!
A right to explanation?
The European General Data Protection Regulation (GDPR) requires that individuals be informed about automated decision-making processes. This includes their underlying mechanisms, significance, and potential consequences of their application for the individual. The information provided should be sufficiently comprehensive to ensure the understanding of the decision's rationale and, potentially, their right to challenge the algorithm's outcomes (Articles 13, 14, 15, 22 and Recital 71 [5, 6]).
While the GDPR does not explicitly define a "right to explanation," some experts interpret these requirements as effectively establishing one [7]. Nonetheless, there is considerable debate about the extent to which the regulation genuinely provides this right [8–10].
The recent Artificial Intelligence Act emphasises the necessity of transparency and human oversight in high-risk AI systems. Specifically, it mandates that these systems—including many AI-powered medical devices [11]—must be designed and developed to ensure "sufficient transparency to enable users to interpret the system’s output" and "use it appropriately" (Article 13 [12]). This emphasis on transparency aims to build trust and accountability by making AI systems understandable and open to scrutiny. However, the Act does not provide specific level for explainability [10].
Facilitating AI acceptance in decision-making
In the high-pressure environment of the ICU, doctors need clarity when making decisions, especially when using AI-based support systems [13]. The lack of transparency in AI models can impede trust in their diagnostic, therapeutic, and prognostic suggestions, leading to potential "decision paralysis". This is further exacerbated by accountability concerns: how can one take responsibility for decisions based on AI models that are not fully understood [14]?
Critical care doctors frequently encounter syndrome-based diseases, such as acute kidney injury (AKI) and sepsis, as well as events like the need for mechanical support, all marked by notable heterogeneity. Their partially understood nature poses challenges for many AI models to promptly identify effective interventions for treatment or prevention. Explainable AI (XAI) models can be more actionable (for definitions and descriptions of explainable AI (XAI) terminology, consult the Additional file 1). For instance, the models developed by Lauritsen et al. [15], provide early warnings for various critical illnesses, while pinpointing the specific factors driving their predictions for each patient. These models not only offer state-of-the-art, real-time predictions for critical illnesses like sepsis, AKI, or acute lung injury (ALI), but also provide insights into the electronic health records underpinning these predictions that would otherwise have remained unidentified. Such an approach enables practitioners to respond more effectively and personally, focusing on modifiable factors.
From a patient's perspective, the opacity of AI systems can also impair their comprehension, impacting their informed consent and autonomy. This lack of clarity could unintentionally shift decision-making power from patients and doctors to less transparent algorithms, potentially fostering a new kind of medical paternalism where it is assumed that "computers know the best" [16]. To navigate these challenges effectively, clinicians could benefit from understanding the rationale behind the outcomes produced by AI-based models. The paramount focus should be on deciphering why a particular AI model arrives at specific results and the underlying factors influencing its decision-making process. This approach parallels the collaborative mental models that clinicians establish with their colleagues, akin to seeking a second opinion [17]. In addition, with this knowledge in hand, clinicians should communicate more effectively with patients and their families, facilitating informed decisions about their healthcare [18].
Ensuring safety, clinical relevance, and fairness
Engineers and clinicians have distinct expectations about model explainability. Engineers typically focus on the interpretability of the model’s inner workings, such as for debugging purposes, whereas clinicians emphasise the clinical relevance of its outputs [19]. Hence, drawing a parallel with their role in pharmacovigilance, clinicians should play a central role in evaluating AI models throughout their lifecycle.
In this context, explainable models may help identifying spurious correlations that could lead to iatrogenic harm. For example, Deasy et al. [20] proposed an AI model that predicts in-hospital mortality for ICU patients using numerous variables derived from the MIMIC-III database [21], a comprehensive collection of critical care data, without prior variable selection. A closer look into its functioning revealed that certain features, such as a priest's visit, were strong predictors of imminent mortality. In a scenario where this model is applied practically, if religious visit patterns change, the model might wrongly predict how likely patients are to survive. This could cause medical teams to either act too slowly or take unnecessary actions.
Similarly, during the COVID-19 pandemic, researchers harnessed AI-driven models to analyse X-rays and CT scans for quick identification of COVID-related pneumonia. DeGrave et al. used post-hoc explainability methods such as saliency maps and generative adversarial networks (GANs) to study their trustworthiness. Saliency maps highlight the most influential image regions for model predictions, while GANs transform images to reveal key features differentiating classes (See Additional file 1). They demonstrated that some deep-learning models took 'shortcuts' by relying on features like laterality markers (e.g., the "R" letter adjacent to the right side of the radiograph) or patient positioning to draw their conclusions, rather than focusing on medically relevant pathology [22], rendering their predictions less reliable.
To ensure the transparent use of AI in healthcare, a thorough examination of potential biases and disparities arising from the inclusion or exclusion of certain variables is essential. An important example is the historical use of racial or ethnic data in calculations of glomerular filtration rates, a practice that has led to increased diagnostic disparities in kidney disease among marginalised groups [23]. Consequently, when AI is used for purposes such as predicting AKI, it is imperative for clinicians to clearly understand how the algorithm incorporates sensitive demographic data. They need to be keenly aware of the effects of such data on both the accuracy and fairness of the model's predictions, in order to avoid reinforcing existing healthcare inequalities [24]. This is not only ethically prudent, but in some instances has become a governmental priority [25].
Models should be explainable for clinicians: no!
The proof is in the pudding?
When a model has no significant impact or has proven its performance sufficiently, the cost of explanation may outweigh the benefit [26]. If an AI model consistently outperforms a clinician, even without being explainable, it could be considered ethically justifiable to use it. In such cases, employing the AI as a co-pilot becomes a viable option, provided the clinician can independently verify and confirm the accuracy of the AI's decisions [27].
It is sometimes suggested that there may be a trade-off between accuracy and explainability when incorporating an explanation mechanism in AI systems [28]. A study [29] found that in medical scenarios (e.g., stroke diagnosis), the general public prioritised accuracy over explainability, emphasising the need for accurate and timely decisions for better outcomes. Conversely, in non-healthcare scenarios (e.g., criminal justice), explainability was valued more for ensuring fairness and transparency. Although post-hoc explainability can help mitigate the trade-off between accuracy and explainability, the difference in priorities across different sectors of society underscores the need for context-specific AI policy development and public engagement.
Likewise, it can be argued that even in intensive care, especially in predictive models, there are areas where understanding the associations behind an algorithm matters less than its efficiency and promptness. For instance, the Hypotension Prediction Index (HPI) from Edwards Lifesciences Corp. (Irvine, USA) uses a machine learning algorithm to forecast hypotension by analysing physiological alterations in the artery waveform. By employing variables selected from millions of individual and combinatorial ones, derived from invasive arterial line waveform analysis, it efficiently predicts and prevents intraoperative hypotension, despite lacking a straightforward physiological explanation for its output [30, 31].
Is explainability reliable?
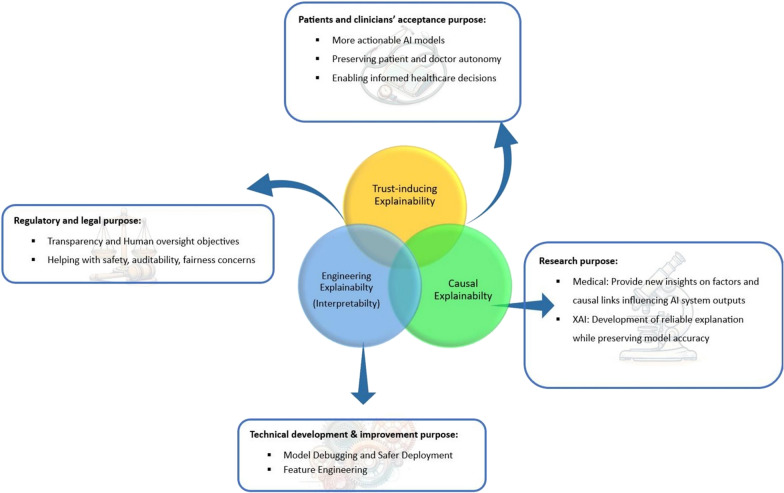
Explainability, as previously mentioned, can have multiple meanings, and can vary according to stakeholders’ unique expectations (Fig. 1). Additionally, numerous XAI methods exist (Additional file 1: Fig. S1), yet standardised methods for assessing their accuracy and comprehensiveness are deficient [32, 33].
Fig. 1.
Which explainability for which audience?
Even state-of-the-art XAI methods often provide erroneous, misleading, or incomplete explanations, especially as the complexity of models increases [10]. For example, post-hoc methods, which use external tools to clarify an algorithm's operations often without deeply examining its core workings, are inherently prone to approximations [34]. When attempting to emulate the predictions of black-box models, they might rely on different features for their explanations, potentially leading to a misinterpretation of the model's true processes. Moreover, identifying an AI model's key features does not ensure their effective or expected use, particularly from a clinical perspective [28]. For instance, saliency maps can indicate where the model is “looking,” but not what the model actually “sees” [34].
These caveats partly explain why there is still no consensus on whether AI models, as seen in decision support systems, should inherently possess explainability as a core attribute [35]. In this context, recent advancements in generative AI, such as OpenAI's ChatGPT, present significant challenges to reliable explainability. These challenges notably include model complexity, limited access to the internal workings of proprietary systems, and the difficulty of evaluating explanations without clear benchmarks [36].
Recognising our own cognitive biases
We should recognise the ubiquity of black boxes in various domains. In many medical practices, clinicians commonly use numerous medications such as paracetamol, as well as diagnostic tools like as lab tests and magnetic resonance imaging (MRI), without fully understanding their inner workings. This prevailing lack of transparency mirrors the concept of black boxes, where the intricacies of interventions remain elusive. Similarly, the human body remains an enigma in many respects [37].
Furthermore, human clinicians are often not held to the same stringent standards of explainability as AI systems [38]. Everyday crucial decisions made by an intensivist, such as admitting patients to the intensive care unit, often involve elements of inexplicability due to intuition or implicit biases [39]. AI systems, on the other hand, can be held to a higher standard of explainability, which may not always be realistic or necessary. This double standard has led some authors to argue that the explainability requirements for AI should be considered relative to those of human decision-makers [40] for a fair and practical evaluation of decision-making in medical contexts.
Healthcare practitioners might place unwarranted confidence in models that highlight explainability. In fact, when using these models, their capacity to identify and correct major model errors seems reduced. Authors have suggested that this overconfidence may, in part, arise from an "information overload" effect [32, 41], which might also induce data fatigue.
Similarly, it is essential not to consider the workings of AI models strictly through an "anthropomorphic" perspective or to insist on just causal explanations. AI models can integrate factors that significantly improve predictive accuracy, even if these factors do not have a clear causal link to the model's outcomes [42]. While it is vital to steer clear of spurious correlations, it is worth noting that not all diseases are entirely understood in causal terms. Some might be influenced by unpredictable external factors, rather than being purely deterministic.
From explainable AI to trustworthy AI
Ensuring the trustworthiness of AI systems is essential for promoting their widespread adoption in high-stakes ICU environments and for their routine use in decision-making. While explainability plays a role, it is neither fully sufficient [43] nor strictly indispensable for cultivating acceptance of AI systems. Trust does not arise merely from meeting a single criterion; it emerges from a combination of AI system attributes, including reliability, safety, fairness, and auditability [44]. These principles should act as a framework for evaluating AI systems throughout various stages of their lifecycle, from data collection and preprocessing to model training, evaluation, and deployment [3, 45].
Transparency of AI systems, as advocated by regulations, appears here as a cornerstone to foster trust in AI technologies. In a holistic approach to system opacity, it refers to the degree to which appropriate information about a device—including its intended use, development, performance, and underlying logic—is clearly communicated to stakeholders. [46]. The recent AI Act emphasises the need for transparency and human oversight in high-risk AI systems. Instead of mandating the use of XAI tools, it ensures users receive pertinent documentation and information [47].
In the ICU context, this information could be presented via user-friendly graphical interfaces, complemented by a robust documentation approach. This could include "model facts" sheets [37], specifically designed to provide essential model information to clinical end users. Table 1 summarises the essential aspects clinicians need to focus on when implementing AI Models in the healthcare environment.
Table 1.
Top 10 must-knows for clinicians using AI models
| 1. Objective & Scope |
|---|
| Purpose: Model’s primary goal (e.g., prediction, diagnosis, recommendation) |
| Target population: The patient demographic the model caters to |
| 2. Model insights |
| Structure: A concise description of the model's design |
| Explainability: Clarity of the model outputs for clinicians and patients |
| Key variables: Main features the model use, and their medical relevance |
| 3. Data source |
| Data origin: Where training and validation data comes from, ensuring relevance to clinician's patient base |
| Adaptability: Ability to retrain the model using local datasets |
| Open access: Accessibility to data/code for replication (e.g., on platforms like GitHub) |
| 4. Evaluation & Validation |
| Performance metrics: Measures of model accuracy |
| Benchmarking: Comparison to simpler, more interpretable models |
| Practical validation: Testing in real clinical settings, beyond just retrospective data |
| 5. Model limitations |
| Performance concerns: Situations or conditions where model efficacy may diminish |
| Reliability: Model’s expression of confidence and uncertainty in its results |
| Error management: Approaches for handling and correcting inaccurate outputs |
| 6. Clinical integration |
| Human oversight: Human involvement in model-driven decisions |
| Workflow integration: Model's fit into existing clinical processes |
| User experience: Interface design and clarity of information |
| Training & education: learning resources provided for staff and clinicians |
| 7. Ethical considerations |
| Demographic equity: Performance consistency across diverse patient groups |
| Fairness audit: Efforts to identify and rectify potential biases |
| 8. Regulatory aspects |
| Data privacy & security: Protocols for patient data management and protection |
| Legal adherence: Compliance with regulations like GDPR, AI Act |
| Clinician liability: Responsibilities when using the model |
| 9. Maintenance & Audit |
| Safety checks: Monitoring model safety and efficiency |
| Updates & evolution: Keeping the model current line with new data and insights |
| 10. Feedback & Reporting |
| Feedback channels: Systems for collecting and addressing user feedback |
| Adverse event: Procedures to handle and report any negative outcomes associated with the model's deployment |
Conclusion
Over the past decade, research in AI and machine learning applications in medicine has witnessed an impressive 20-fold increase [48]. However, the practical integration of these advanced methodologies into healthcare can be hindered by trust issues [19]. Increased transparency is deemed essential, and explainability is considered a crucial component of this endeavour, even though questions persist about determining the appropriate level of explainability for a specific audience (Fig. 1). This implies facing challenges across legal, ethical, technical, and economic dimensions [47].
The notion that a necessary trade-off exists between accuracy and explainability in AI models is being re-evaluated with the expansion of the field of XAI research [34, 49, 50]; (Additional file 1: Fig. S2). In medical AI, where models are typically based on detailed, structured data grounded in physiopathology, the performance difference between interpretable and more complex models often turns out to be minimal [34].
However, explainability alone does not guarantee effective AI application. It remains pivotal to grasp the implications of employing AI models, as well as to understand when and how to integrate them into clinical judgement while preserving patient autonomy in shared decision-making [16].
Supplementary Information
Additional file 1: Fig. S1. This document provides a comprehensive overview of explainable artificial intelligence (XAI), detailing definitions, the difference between explainability and interpretability, and the classification of explanations as global or local. It includes a taxonomy of XAI methods. Fig. S2. It also addresses the balance between model complexity and the necessity for explainability in healthcare.
Acknowledgements
Not applicable.
Abbreviations
- AI
Artificial Intelligence
- AKI
Acute Kidney Injury
- ALI
Acute Lung Injury
- ARDS
Acute Respiratory Distress Syndrome
- CT
Computed Tomography
- GANs
Generative adversarial networks
- GDPR
General Data Protection Regulation
- HPI
Hypotension Prediction Index
- ICU
Intensive Care Unit
- LIME
Local Interpretable Model-agnostic Explanations
- MIMIC
Medical Information Mart for Intensive Care
- MRI
Magnetic Resonance Imaging
- SHAPE
Shapley Additive Explanations
- USA
United States of America
- XAI
Explainable AI
Author contributions
G.A. prepared the first draft and figures. Each author listed contributed original content focused on particular segments of the debate, and collectively reviewed and endorsed the final manuscript.
Funding
Gwénolé Abgrall, MD was supported the Fondation pour la Recherche Médicale (Grant Number FDM202306017126) and the Société de Réanimation de Langue Française.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Andre L. HOLDER, MD, MSc, has received speaker fees from Baxter International and has served as a consultant for Philips Medical. He also has funding from the NIH (NIGMS) for developing a sepsis algorithm. The other authors have no conflict of interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saqib M, Iftikhar M, Neha F, Karishma F, Mumtaz H. Artificial intelligence in critical illness and its impact on patient care: a comprehensive review. Front Med. 2023;20(10):1176192. 10.3389/fmed.2023.1176192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van De Sande D, Van Genderen ME, Braaf H, Gommers D, Van Bommel J. Moving towards clinical use of artificial intelligence in intensive care medicine: business as usual? Intensive Care Med. 2022;48(12):1815–7. 10.1007/s00134-022-06910-y [DOI] [PubMed] [Google Scholar]
- 3.Markus AF, Kors JA, Rijnbeek PR. The role of explainability in creating trustworthy artificial intelligence for health care: a comprehensive survey of the terminology, design choices, and evaluation strategies. J Biomed Inform. 2021;113: 103655. 10.1016/j.jbi.2020.103655 [DOI] [PubMed] [Google Scholar]
- 4.Grote T. Allure of simplicity: on interpretable machine learning models in healthcare. Philod Med. 2023;4:1.36640751 [Google Scholar]
- 5.Article 29 Data Protection Working Party, ‘Guidelines on Automated individual decision-making and Profiling For the purposes of Regulation 2016/679’ [2017].
- 6.Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation). 2016.
- 7.Goodman B, Flaxman S. European Union regulations on algorithmic decision-making and a ‘right to explanation’. 2016 [cited 2023 Oct 26]; Available from: https://arxiv.org/abs/1606.08813
- 8.Kaminski ME. The Right to Explanation, Explained. 2019 [cited 2024 May 22]; Available from: https://lawcat.berkeley.edu/record/1128984
- 9.Casey, Bryan; Farhangi, Ashkon; Vogl, Roland. Rethinking Explainable Machines: The GDPR’s Right to Explanation Debate and the Rise of Algorithmic Audits in Enterprise. 2019 [cited 2024 May 22]; Available from: https://lawcat.berkeley.edu/record/1128983
- 10.Chung NC, Chung H, Lee H, Chung H, Brocki L, Dyer G. False Sense of Security in Explainable Artificial Intelligence (XAI) [Internet]. arXiv; 2024 [cited 2024 May 23]. Available from: https://arxiv.org/abs/2405.03820
- 11.Quaranta M, Amantea IA, Grosso M. Obligation for AI Systems in healthcare: Prepare for trouble and make it double? Rev Socionetwork Strat. 2023. 10.1007/s12626-023-00145-z. 10.1007/s12626-023-00145-z [DOI] [Google Scholar]
- 12.European Commission. 2021. Proposal for a Regulation laying down harmonised rules on Artificial Intelligence and amending certain union legislative acts. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A52021PC0206.
- 13.Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. npj Digit Med. 2020;3(1):17. 10.1038/s41746-020-0221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sauerbrei A, Kerasidou A, Lucivero F, Hallowell N. The impact of artificial intelligence on the person-centred, doctor-patient relationship: some problems and solutions. BMC Med Inform Decis Mak. 2023;23(1):73. 10.1186/s12911-023-02162-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lauritsen SM, Kristensen M, Olsen MV, Larsen MS, Lauritsen KM, Jørgensen MJ, et al. Explainable artificial intelligence model to predict acute critical illness from electronic health records. Nat Commun. 2020;11(1):3852. 10.1038/s41467-020-17431-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenzini G, Arbelaez Ossa L, Shaw DM, Elger BS. Artificial intelligence and the doctor–patient relationship expanding the paradigm of shared decision making. Bioethics. 2023;37(5):424–9. 10.1111/bioe.13158 [DOI] [PubMed] [Google Scholar]
- 17.Cai CJ, Winter S, Steiner D, Wilcox L, Terry M. ‘Hello AI’: uncovering the onboarding needs of medical practitioners for human-ai collaborative decision-making. Proc ACM Hum-Comput Interact. 2019;7(3):1–24. 10.1145/3359206 [DOI] [Google Scholar]
- 18.Matulionyte R, Nolan P, Magrabi F, Beheshti A. Should AI-enabled medical devices be explainable? Int J Law Inf Technol. 2022;30(2):151–80. 10.1093/ijlit/eaac015 [DOI] [Google Scholar]
- 19.Bienefeld N, Boss JM, Lüthy R, Brodbeck D, Azzati J, Blaser M, et al. Solving the explainable AI conundrum by bridging clinicians’ needs and developers’ goals. npj Digit Med. 2023;6(1):94. 10.1038/s41746-023-00837-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deasy J, Liò P, Ercole A. Dynamic survival prediction in intensive care units from heterogeneous time series without the need for variable selection or curation. Sci Rep. 2020;10(1):22129. 10.1038/s41598-020-79142-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson A, Pollard T, Mark R. MIMIC-III Clinical Database [Internet]. PhysioNet; 2015 [cited 2023 Nov 28]. Available from: https://physionet.org/content/mimiciii/1.4/
- 22.DeGrave AJ, Janizek JD, Lee SI. AI for radiographic COVID-19 detection selects shortcuts over signal. Nat Mach Intell. 2021;3(7):610–9. 10.1038/s42256-021-00338-7 [DOI] [Google Scholar]
- 23.Eneanya ND, Boulware LE, Tsai J, Bruce MA, Ford CL, Harris C, et al. Health inequities and the inappropriate use of race in nephrology. Nat Rev Nephrol. 2022;18(2):84–94. 10.1038/s41581-021-00501-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garin SP, Parekh VS, Sulam J, Yi PH. Medical imaging data science competitions should report dataset demographics and evaluate for bias. Nat Med. 2023;29(5):1038–9. 10.1038/s41591-023-02264-0 [DOI] [PubMed] [Google Scholar]
- 25.Biden, JR. The White House. 2023 [cited 2024 Mar 19]. Executive Order on the Safe, Secure, and Trustworthy Development and Use of Artificial Intelligence. Available from: https://www.whitehouse.gov/briefing-room/presidential-actions/2023/10/30/executive-order-on-the-safe-secure-and-trustworthy-development-and-use-of-artificial-intelligence/
- 26.Hamon R, Junklewitz H, Sanchez I, Malgieri G, De Hert P. Bridging the gap between AI and explainability in the GDPR: towards trustworthiness-by-design in automated decision-making. IEEE Comput Intell Mag. 2022;17(1):72–85. 10.1109/MCI.2021.3129960 [DOI] [Google Scholar]
- 27.Chan B. Black-box assisted medical decisions: AI power vs .ethical physician care. Med Health Care Philos. 2023;26(3):285–92. 10.1007/s11019-023-10153-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghassemi M, Oakden-Rayner L, Beam AL. The false hope of current approaches to explainable artificial intelligence in health care. Lancet Digital Health. 2021;3(11):e745–50. 10.1016/S2589-7500(21)00208-9 [DOI] [PubMed] [Google Scholar]
- 29.Van Der Veer SN, Riste L, Cheraghi-Sohi S, Phipps DL, Tully MP, Bozentko K, et al. Trading off accuracy and explainability in AI decision-making: findings from 2 citizens’ juries. J Am Med Inform Assoc. 2021;28(10):2128–38. 10.1093/jamia/ocab127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatib F, Jian Z, Buddi S, Lee C, Settels J, Sibert K, et al. Machine-learning algorithm to predict hypotension based on high-fidelity arterial pressure waveform analysis. Anesthesiology. 2018;129(4):663–74. 10.1097/ALN.0000000000002300 [DOI] [PubMed] [Google Scholar]
- 31.Šribar A, Jurinjak IS, Almahariq H, Bandić I, Matošević J, Pejić J, et al. Hypotension prediction index guided versus conventional goal directed therapy to reduce intraoperative hypotension during thoracic surgery: a randomized trial. BMC Anesthesiol. 2023;23(1):101. 10.1186/s12871-023-02069-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips PJ, Hahn CA, Fontana PC, Yates AN, Greene K, Broniatowski DA, et al. Four Principles of Explainable Artificial Intelligence [Internet]. National Institute of Standards and Technology; 2021 Sep [cited 2023 May 29]. Available from: https://nvlpubs.nist.gov/nistpubs/ir/2021/NIST.IR.8312.pdf
- 33.Zednik C. Solving the black box problem: a normative framework for explainable artificial intelligence. Philos Technol. 2021;34(2):265–88. 10.1007/s13347-019-00382-7 [DOI] [Google Scholar]
- 34.Rudin C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat Mach Intell. 2019;1(5):206–15. 10.1038/s42256-019-0048-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasey B, Nagendran M, Campbell B, Clifton DA, Collins GS, Denaxas S, et al. Reporting guideline for the early-stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI. Nat Med. 2022;28(5):924–33. 10.1038/s41591-022-01772-9 [DOI] [PubMed] [Google Scholar]
- 36.Schneider J. Explainable Generative AI (GenXAI): A Survey, Conceptualization, and Research Agenda [Internet]. arXiv; 2024 [cited 2024 May 23]. Available from: http://arxiv.org/abs/2404.09554
- 37.Sendak M, Elish MC, Gao M, Futoma J, Ratliff W, Nichols M, et al. ‘The human body is a black box’: supporting clinical decision-making with deep learning. In: Proceedings of the 2020 Conference on Fairness, Accountability, and Transparency [Internet]. Barcelona Spain: ACM; 2020 [cited 2023 May 24]. p. 99–109. Available from: https://dl.acm.org/doi/10.1145/3351095.3372827
- 38.Zerilli J, Knott A, Maclaurin J, Gavaghan C. Transparency in algorithmic and human decision-making: Is there a double standard? Philos Technol. 2019;32(4):661–83. 10.1007/s13347-018-0330-6 [DOI] [Google Scholar]
- 39.Gopalan PD, Pershad S. Decision-making in ICU – A systematic review of factors considered important by ICU clinician decision makers with regard to ICU triage decisions. J Crit Care. 2019;50:99–110. 10.1016/j.jcrc.2018.11.027 [DOI] [PubMed] [Google Scholar]
- 40.Kempt H, Heilinger JC, Nagel SK. Relative explainability and double standards in medical decision-making: Should medical AI be subjected to higher standards in medical decision-making than doctors? Ethics Inf Technol. 2022;24(2):20. 10.1007/s10676-022-09646-x [DOI] [Google Scholar]
- 41.Poursabzi-Sangdeh F, Goldstein DG, Hofman JM, Wortman Vaughan JW, Wallach H. Manipulating and Measuring Model Interpretability. In: Proceedings of the 2021 CHI Conference on Human Factors in Computing Systems [Internet]. Yokohama Japan: ACM; 2021 [cited 2023 May 24]. p. 1–52. Available from: https://dl.acm.org/doi/10.1145/3411764.3445315
- 42.Selbst AD, Barocas S. The Intuitive Appeal of Explainable Machines. SSRN Journal [Internet]. 2018 [cited 2023 Oct 25]; Available from: https://www.ssrn.com/abstract=3126971
- 43.Cheng HF, Wang R, Zhang Z, O’Connell F, Gray T, Harper FM, et al. Explaining Decision-Making Algorithms through UI: Strategies to Help Non-Expert Stakeholders. In: Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems [Internet]. Glasgow Scotland Uk: ACM; 2019 [cited 2023 May 24]. p. 1–12. Available from: https://dl.acm.org/doi/10.1145/3290605.3300789
- 44.European Commission. Directorate General for Communications Networks, Content and Technology., High Level Expert Group on Artificial Intelligence. Ethics guidelines for trustworthy AI. [Internet]. LU: Publications Office; 2019 [cited 2023 Oct 26]. Available from: https://data.europa.eu/doi/10.2759/346720
- 45.Pinsky MR, Bedoya A, Bihorac A, Celi L, Churpek M, Economou-Zavlanos NJ, et al. Use of artificial intelligence in critical care: opportunities and obstacles. Crit Care. 2024;28(1):113. 10.1186/s13054-024-04860-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shick AA, Webber CM, Kiarashi N, Weinberg JP, Deoras A, Petrick N, et al. Transparency of artificial intelligence/machine learning-enabled medical devices. npj Digit Med. 2024;7(1):21. 10.1038/s41746-023-00992-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panigutti C, Hamon R, Hupont I, Fernandez Llorca D, Fano Yela D, Junklewitz H, et al. The role of explainable AI in the context of the AI Act. In: 2023 ACM Conference on Fairness, Accountability, and Transparency [Internet]. Chicago IL USA: ACM; 2023 [cited 2023 Oct 26]. p. 1139–50. Available from: https://dl.acm.org/doi/10.1145/3593013.3594069
- 48.Meskó B, Görög M. A short guide for medical professionals in the era of artificial intelligence. npj Digit Med. 2020;3(1):126. 10.1038/s41746-020-00333-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savage N. Breaking into the black box of artificial intelligence. Nature. 2022 Mar 29;d41586–022–00858–1. [DOI] [PubMed]
- 50.Jacovi A. Trends in Explainable AI (XAI) Literature [Internet]. arXiv; 2023 [cited 2024 May 23]. Available from: https://arxiv.org/abs/2301.05433
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. This document provides a comprehensive overview of explainable artificial intelligence (XAI), detailing definitions, the difference between explainability and interpretability, and the classification of explanations as global or local. It includes a taxonomy of XAI methods. Fig. S2. It also addresses the balance between model complexity and the necessity for explainability in healthcare.
Data Availability Statement
Not applicable.