Abstract
Background
Treatment decisions for persons with relapsing–remitting multiple sclerosis (RRMS) rely on clinical and radiological disease activity, the benefit-harm profile of drug therapy, and preferences of patients and physicians. However, there is limited evidence to support evidence-based personalized decision-making on how to adapt disease-modifying therapy treatments targeting no evidence of disease activity, while achieving better patient-relevant outcomes, fewer adverse events, and improved care. Serum neurofilament light chain (sNfL) is a sensitive measure of disease activity that captures and prognosticates disease worsening in RRMS. sNfL might therefore be instrumental for a patient-tailored treatment adaptation. We aim to assess whether 6-monthly sNfL monitoring in addition to usual care improves patient-relevant outcomes compared to usual care alone.
Methods
Pragmatic multicenter, 1:1 randomized, platform trial embedded in the Swiss Multiple Sclerosis Cohort (SMSC). All patients with RRMS in the SMSC for ≥ 1 year are eligible. We plan to include 915 patients with RRMS, randomly allocated to two groups with different care strategies, one of them new (group A) and one of them usual care (group B). In group A, 6-monthly monitoring of sNfL will together with information on relapses, disability, and magnetic resonance imaging (MRI) inform personalized treatment decisions (e.g., escalation or de-escalation) supported by pre-specified algorithms. In group B, patients will receive usual care with their usual 6- or 12-monthly visits. Two primary outcomes will be used: (1) evidence of disease activity (EDA3: occurrence of relapses, disability worsening, or MRI activity) and (2) quality of life (MQoL-54) using 24-month follow-up. The new treatment strategy with sNfL will be considered superior to usual care if either more patients have no EDA3, or their health-related quality of life increases. Data collection will be embedded within the SMSC using established trial-level quality procedures.
Discussion
MultiSCRIPT aims to be a platform where research and care are optimally combined to generate evidence to inform personalized decision-making in usual care. This approach aims to foster better personalized treatment and care strategies, at low cost and with rapid translation to clinical practice.
Trial registration
ClinicalTrials.gov NCT06095271. Registered on October 23, 2023
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08454-6.
Introduction
Multiple sclerosis (MS) is an inflammatory, demyelinating, and neurodegenerative disease of the central nervous system, typically affecting persons in early adulthood and is a leading cause of non-traumatic disability in young adults [1]. MS presents heterogeneous courses of the disease, the most common form being relapsing–remitting MS (RRMS), with high variability in symptoms and treatment responses. There are currently over 20 disease-modifying therapies (DMTs) that have been approved but all have diverse benefits, harms, and burdens [2]. High-efficacy DMTs such as for example natalizumab [3], alemtuzumab [4], and ocrelizumab [5] which are given as infusions, ranging from every 4 weeks to yearly, lead to an almost complete suppression of acute disease activity. However, such DMTs inevitably inhibit the natural immune response putting patients at risk for harm due to, for example, viral or bacterial infections [6].
As more potent DMTs are being developed [7] one should also explore the optimization of currently available DMTs [8]. Personalized treatment strategies for persons with MS are urgently needed [8, 9] to treat patients as little as possible but as much as necessary and at the right time [10]. Practically, this means ensuring no evidence of disease activity, while achieving better patient-relevant outcomes such as, for example, improved quality of life, fewer adverse events, and improved care. Such an approach requires detailed information on disease activity and treatment response. Currently, in usual care, this includes information on relapses, new/enlarging T2 weighted (w) MRI lesions or T1w contrast-enhancing lesions, and confirmed disability worsening. MRIs are time- and resources-consuming, and the occurrence of disease worsening despite stable standard MRI features is well documented [11]. There is an urgent need for a body fluid biomarker [9] allowing for reliable and rapid detection of disease activity leading to prompt treatment escalation or as important additional surveillance to ensure disease stability during treatment de-escalation.
Serum neurofilament light chain (sNfL) has emerged as a promising fluid biomarker reflecting neuro-axonal damage in MS and correlating with disease activity and severity [12–15]. Recent advancements in immunoassay technology allow for sensitive detection of subtle sNfL level increases in serum samples [16]. sNfL levels are associated with future MS disease activity, disability worsening, MRI activity, and treatment response [17–22]. When added to clinical assessments (i.e., relapses and disability worsening assessment) and conventional MRI, sNfL increases sensitivity in detecting disease activity and worsening in disability score [14].
However, while sNfL shows potential for personalized treatment decisions, its routine use in clinical care is not widespread or recommended in major clinical guidelines. To the best of our knowledge, there are currently no planned or ongoing randomized controlled trials assessing the clinical usefulness of sNfL in MS therapy monitoring in clinical practice.
Objectives
The primary objective of this trial is to assess whether a treatment strategy including sNfL monitoring improves patient-relevant outcomes and care of patients with RRMS by either increasing the proportion of patients with no evidence of disease activity or by improving patients’ health-related quality of life. We assume that introducing a 6-monthly monitoring of sNfL within SMSC usual care will inform more personalized treatment decisions and result in either:
Better quality of life for patients with MS through biomarker (i.e., sNfL) guided de-escalation by reducing treatment burden and risk for side effects associated with highly effective DMT, or
Lower disease activity by early and/or more sensitive biomarker-guided escalation of DMT.
Secondary objectives include assessing if a treatment strategy including 6-monthly monitoring is associated with a decrease in the proportion of patients with relapses, disability worsening (assessed using Expanded Disability Status Scale (EDSS) scores), and/or MRI activity (i.e., new/enlarging T2w lesions or T1w contrast-enhancing lesions). We also aim to assess if specific patient subgroups benefit from 6-monthly sNfL monitoring, i.e., patients at higher risk in comparison to patients at lower risk for future disease activity. We further aim to evaluate the economic implications of monitoring sNfL in terms of direct and indirect RRMS-related costs, quality-adjusted life-time, and incremental cost-effectiveness.
Methods
MultiSCRIPT-Cycle 1 is a pragmatic multicenter, 1:1 randomized, trial embedded in the SMSC to compare a new treatment strategy including sNfL monitoring in addition to SMSC usual care compared with SMSC usual care alone. Patients are randomized in a 1:1 ratio to two groups with these different treatment strategies, one of them new (group A) and one of them usual care (group B).
MultiSCRIPT-Cycle 1 is a pragmatic trial that aims to provide evidence closely reflecting what happens in routine care (PRagmatic Explanatory Continuum Indicator Summary (PRECIS-2) [23] available in Appendix 1). It is patient-centered and clearly focused on real-world decision-making. It is embedded in the existing SMSC data structure and is based on routinely collected data from well-established and field-tested processes with central quality controls.
Trial Setting
MultiSCRIPT-Cycle 1 is fully embedded in the SMSC, leveraging the existing research infrastructure and processes. The SMSC (NCT02433028—BASEC ID: 2023–02367) is a prospective multicenter cohort study performed across eight Swiss academic medical centers (the University Hospitals of Basel, Berne, Geneva, Lausanne, and Zurich, and the Cantonal Hospitals of Aarau, Lugano, and St. Gallen).
Usual care within the SMSC consists of 6 or 12-monthly clinical visits, including routine assessment of relapses and disability status (measured using the EDSS), a blood draw (mandatory within SMSC usual care), quality of life questionnaires at least once a year and may include MRI at the discretion of the treating physician and patient’s preferences (facultative but it is routine for persons with MS to get yearly MRI to assess new/enlarging T2w lesions and/or T1w contrast-enhancing lesions on cranial and/or spinal MRIs) [24]. MRI protocols are standardized and aligned across centers. The centers scan the patients by default always at the same scanner with the same scanning parameters (e.g. head position in the scanner) to ensure maximum of comparability between the scans.
The SMSC 6 or 12-monthly schedule is at the discretion of the physician and patient’s preferences and may vary over time. The SMSC collates routinely collected data (i.e., data not collected for the purpose of research) into a standardized and unique database and all blood samples collected as part of the SMSC usual care are biobanked.
Eligibility criteria
We include all patients who have been diagnosed with RRMS according to the most recent McDonald criteria (2017) [25] for at least a year and have already consented to take part in the SMSC. Patients who are included (or planned to be included) in another DMT trial are excluded as they are (or will) most likely not follow the SMSC usual care.
The eligibility criterion that participants must have been diagnosed with RRMS for at least a year is used because it frequently takes up to a minimum of a year of treatment before a DMT adaptation may be considered. We are explicitly not excluding pregnant women from MultiSCRIPT-Cycle 1, or patients with specific conditions or concomitant diseases. We are aiming to generate evidence for all patients, including vulnerable populations, for which the tested intervention may be used in real-world care settings.
Patients who are not participating in MultiSCRIPT Cycle 1 but are in the SMSC will serve as external control subjects.
Primary outcomes
The two independent primary outcomes are (1) EDA3 (evidence of disease activity) and (2) quality of life using the Multiple Sclerosis Quality of Life (MSQoL)-54 instrument. Both primary outcomes will be assessed using 24 months follow-up data (i.e., 24 months since randomization).
EDA3 is defined as the occurrence of a relapse as defined in the McDonald criteria [25], confirmed disability worsening defined as an EDSS increase of ≥ 1.5 steps if baseline EDSS was 0, ≥ 1.0 step if baseline EDSS 1.0 to 5.5 and 0.5 steps if baseline EDSS > 5.5 [11], or new/enlarging T2w lesions compared to the last MRI or T1w contrast-enhancing lesions based on local MRI readings. EDA3 has a better predictive value of disease worsening compared to taking its components individually [26] and no (N)EDA3 is regarded as the most adequate indicator of treatment response [27].
The MSQoL-54 Instrument is an extension of the well-established Short Form-36 (SF-36) specifically for MS patients. It is a validated instrument with an adequate test–retest reliability, construct validity, and internal consistency [28, 29]. MSQoL-54 is a structured self-reported questionnaire including 54 items generating 12 subscales with two summary scores, the physical health composite summary and the mental health composite summary [28]. For the primary outcome, we will use the sum of both composite summaries as a total score [30].
Secondary outcomes
The secondary outcomes include assessments of EDA3 and MSQoL-54 at 12 months. The individual components of EDA3 will also be assessed separately including relapses, disability worsening measured by EDSS, and new/enlarging T2w lesions and T1w contrast-enhancing lesions based on local MRI reading and on centralized MRI readings. Similarly, the individual summary scores of MSQoL-54 will also be assessed separately. The amount of immunosuppressive/ immunomodulatory drug treatment (or DMTs) will be monitored. Quality of life will be further assessed using EQ-5D-5 and SF-36. All secondary outcomes will be assessed using 12- and 24-month follow-up data (i.e., 12 and 24 months since randomization).
For the health economical evaluation, we will assess health-related quality of life measured with the European Quality of Life 5 Dimension 5 Severity (EQ-5D-5L), quality-adjusted life years, professional activity status and change, indirect costs, and direct medical costs based on healthcare utilization (e.g., hospitalizations).
To better understand treatment pathways and clinical decision-making, we will collect information on treatment changes (e.g., how many patients were escalated or de-escalated) and the reason for treatment change.
Harm outcomes
Any serious adverse events (SAEs) related to the key intervention in the new strategy (i.e., blood draw for sNfL measurement) will be monitored and collected until the end of the study conduct at 42 months of follow-up.
In addition, the following harm indicators will be assessed during the safety interim analysis: mortality, harms related to immunosuppression (e.g., relevant infections), occurrence of relapses, and/or disability worsening in patients previously stable.
All follow-up data on SAEs and indicators of harms available at the database closure will be used for the safety analysis.
New care strategy (group A)
At randomization, patients are allocated to a new treatment strategy (group A) with 6-monthly sNfL monitoring in addition to SMSC usual care or SMSC usual care alone (group B).
Patients allocated to the new treatment strategy have 6-monthly sNfL monitoring including 6-monthly blood draws and communication of sNfL values to treating physicians in addition to their usual care within the SMSC.
The monitoring of sNfL requires a 6-monthly blood draw. In practice, for the duration of the trial, patients allocated to group A who came to the SMSC every 12 months are asked to now come in-between their yearly visits (i.e., at 6 months) for an additional blood draw to monitor sNfL. For patients allocated to group A who came to the SMSC every 6 months anyway, the blood draw is always performed as part of the SMSC usual care, and no additional blood for the purpose of the trial will be taken. One serum aliquot per blood draw is used to measure sNfL centrally at the Clinical Neuroimmunology Laboratory, Department of Biomedicine in Basel, Switzerland. Two sNfL assays are used: single molecule array (Simoa, Quanterix, USA) and Cobas (Roche, CH) technologies. Percentiles and z scores normalized sNfL values [14] are reported to the clinicians within 14 days from blood sampling.
The SMSC treating physicians receive 6-monthly sNfL values for all patients allocated to the intervention arm and at a maximum 10 days after the patient’s visit. If and how the physician and patient act upon the sNfL value is beyond the scope of this trial. To facilitate the implementation of sNfL in treatment decision-making, we have established treatment decision algorithms that integrated sNfL in addition to usual care assessments for the most common clinical scenarios (Appendix 2). Those algorithms were decided upon by consensus among experts in the field and patient consultants using a modified Delphi approach [31, 32].
Usual care comparator (group B)
Patients allocated to the usual care comparator (group B) continue with their usual care in the SMSC, including their usual 6 to 12 monthly visits. As the goal is to improve this usual care by a more personalized approach to MS treatment and to demonstrate the superiority of such approach, this is the most appropriate and pragmatic control.
Patients who do not consent to be randomly allocated and participate in the MultiSCRIPT-Cycle 1 trial will continue their SMSC usual care and will not be exposed to any influence of the study. This group of patients will allow to explore the external validity of the randomized trial results using only the SMSC routinely collected data (for example by exploring differences of characteristics of patients in MultiSCRIPT-Cycle 1 and the other cohort patients), but will not be used to determine intervention effects [33].
Participant timeline
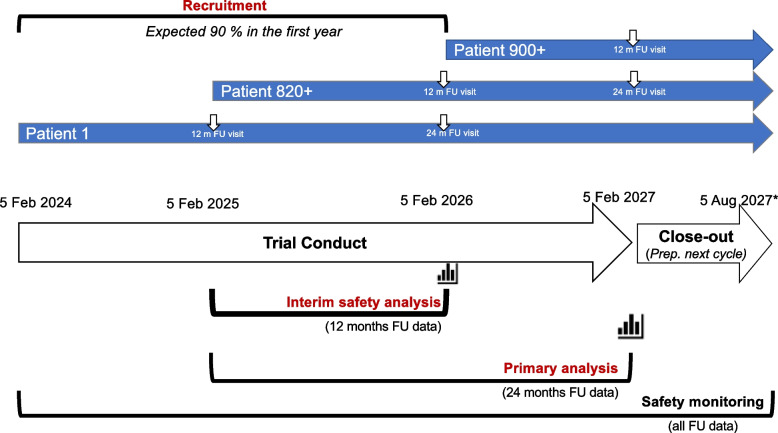
The recruitment period will last 2 years and the trial conduct until the primary analysis will last 3 years, but participants will be followed until the end of the close-out phase for an additional 6 months (Fig. 1). The expected duration of participants’ follow-up will range from 1.5 years to 3.5 years depending on their timepoint of recruitment. Table 1 illustrates the study assessment timeline for individual participants.
Fig. 1.
MultiSCRIPT Cycle 1 timeline. *Timelines may be adapted depending on recruitment rates
Table 1.
Study assessment timeline
| Pre-allocation | Allocation |
Post-allocation Follow-up visits |
Post-allocation Close-out |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Visit | - | V1 | V2 | V3 | V6 | V7 | V8 | V9 | V10 |
| Time | M0 (− 14d) | M0 | M6 (± 45d) | M12 (± 45d) | M18 (± 45d) | M 24 (± 45d) | M30 (± 45d) | M36 (± 45d) | M42 (± 45d) |
| Recruitment | |||||||||
| Eligibility screening | X | ||||||||
| Informed consent | X | ||||||||
| Randomization | X | ||||||||
| Baseline characteristicsa | X | X | |||||||
| Intervention | X | X | X | X | X | X | X | X | |
| Primary outcomes | |||||||||
| MSQoL-54a | X | X | |||||||
| EDA3a | X | X | |||||||
| Secondary outcomes | |||||||||
| MSQoL-54a | X | X | X | ||||||
| EDA3a | X | X | X | ||||||
| Relapsesa | X | X | X | ||||||
| EDSSa | X | X | X | ||||||
| Lesionsa | X | X | X | ||||||
| Amount of DMTab | X | X | |||||||
| SAE | X | X | X | X | X | X | X | X | |
|
Indicators of harma - Relapses - EDSS - Opportunistic infections |
X x x x |
(X) (x) (x) (x) |
X x x x |
(X) (x) (x) (x |
X x x x |
(X) (x) (x) (x |
X x x x |
(X) (x) (x) (x |
|
| Other outcomes | |||||||||
| EQ-5D-5La | X | X | X | ||||||
|
Other PROs: - Professional activitya - Healthcare utilization—Hospitalizationa |
X X |
X X |
X X |
||||||
aRoutinely collected data within the SMSC; (X) only when it coincides with a SMSC visi
bDefined as the daily dose following WHO recommendations
Sample size
We plan to recruit all eligible patients from the SMSC and aim to recruit 915 patients. This estimation was based on a number of eligible persons with RRMS in the SMSC across all 8 centers and assuming an 80% acceptance rate. We further estimate that the primary analysis will include 824 patients with 24-month follow-up data available. This was based on the assumption that we will recruit 90% of our target sample size in the first year of recruitment.
Based on the data of the SMSC (date of last analysis: 15 October 2021), we assume 52% patients under usual care without sNfL biomarker monitoring will have an EDA3 during 24-month follow-up. We assume a relative risk reduction of 25% of EDA3 with sNfL biomarker monitoring compared to usual care to be a minimal important difference (MID). A sample size of 824 patients would have 93% power to detect the MID.
We are not aware of an established MID for MSQoL-54. We assume a difference of 0.2 (Hedges’ g) as a MID for the MSQoL-54, considering current guidelines for health-technology assessment and reimbursement decisions on quality-of-life assessments [34]. Using a systematic search for trials using quality of life outcomes, we identified two recent trials (SUNBEAM [35] and RADIANCE [36]) using the MSQoL-54 instrument in a population that had a higher disease activity compared to the SMSC population. We used a conservative approach assuming a SD = 20 based on the pooled standard deviation at baseline in the SUNBEAM [35] and RADIANCE [36] trials, an intra-patient correlation (baseline and 24 m follow-up) of 0.8 and a correlation of the composite scores of 0.5 (r = 0.66 reported by Vickrey et al. [28]), which shows that a sample size of 824 patients in MultiSCRIPT-Cycle 1 would have > 95% power to detect the MID of 0.2 or a difference in 4 points on the MSQoL-54 total score. The power for 3 points would be 91% in the primary analysis. Given the pragmatic approach of MultiSCRIPT-Cycle 1 with a probably more heterogeneous study population than the more explanatory SUNBEAM and RADIANCE patient populations, a larger variability of quality of life may be plausible. A recently published survey in MS patients from the Netherlands, France, the UK, Spain, Germany, and Italy, however, reported SD for the composites of 17.2 (physical) and 21.5 (mental) [29]. However, even in a scenario with a 25% larger variability (SD of 25), the statistical power for detecting a difference of 4 points would be large with a sample size of 824 (94%).
The power calculations have been adjusted for multiple testing of the two primary outcomes.
Recruitment and informed consent
We aim to recruit 915 participants which as of January 2024 represent 61% of the eligible SMSC participants with RRMS (n = 1503). Participants are recruited by their SMSC physician during their usual care visit to the SMSC.
Making use of the existing SMSC database, eligible patients are identified ahead of their SMSC visit. Depending on the study site, the information sheet and consent form for the trial may be sent ahead of the patient’s visit. On the day of their SMSC visit, patients are invited to take part in MultiSCRIPT Cycle 1 by their SMSC treating physician. If they accept to sign the inform consent, patients are then randomized and their allocated group is directly communicated to them because patients allocated to the new treatment strategy need to know if they have to come back in 6 months.
We adopted proactive mitigation measures to address recruitment risks [37], including (a) a dedicated ‘diagnosis’ of factors supporting and hindering recruitment informed by QuinteT Recruitment Intervention (QRI) methods, with a view to sharing good practice and training materials on how to recruit patients informed by QRI [38–40]; (b) a recruitment log to record reasons for declining participation, informed by the SEAR (Screened, Eligible, Approached, Randomised) process [41]; (c) a quarterly newsletter will be submitted to all recruiting centers, reporting the progress and expected recruitment in all centers; and (d) online conferences (one per quarter in the first year of recruitment) and annual investigator meetings will be used to evaluate processes and share experiences.
Assignment of treatment strategies
Allocation
Participants are randomized in a 1:1 ratio to the trial groups stratified by the study site. The randomization will be implemented in a centralized manner using the SMSC web-based electronic data capture implemented by RodanoTech, an electronic data capture (EDC) and data management services provider for the SMSC. Allocation concealment is ensured by centralized and instant web-based randomization.
Blinding procedures
MultiSCRIPT-Cycle 1 is a pragmatic trial that aims to provide evidence closely reflecting what happens in usual care. Therefore, being aware of the treatment and having a real-world assessment of treatment outcomes are part of the evaluated intervention within this pragmatic trial framework.
Data collection and management
Assessment and collection of outcomes
Most data related to outcome measures are routinely collected within the SMSC and at a minimum on a yearly basis. For MultiSCRIPT-Cycle 1, the primary outcomes will be assessed at 24 months follow-up and secondary outcomes at 12 and 24 months follow-up. The additional data collected for the purpose of MultiSCRIPT are the sNfL measurements and MRI-related data based on radiological reports from the local centers.
Retention and adherence
MultiSCRIPT-Cycle 1 uses a pragmatic approach, and therefore no specific steps are taken to maximize adherence for the purpose of the trial as this would create an artificial setting and deviate from usual care. Patients are followed within the SMSC and the SMSC usual care mimics routine care for patients with MS. Concomitant care and use of concomitant interventions are at the full discretion of the treating physician, as in usual care. No concomitant interventions are prohibited for the purpose of this trial.
Treatment changes will be recorded but no mitigation action will be taken to enforce implementation of the treatment decision algorithms. It remains the treating physician and the patient’s choice to implement a treatment change based on sNfL as it would occur in usual care.
Data management
In the SMSC, individual clinical data is extracted from the hospital patient files (source data) by dedicated study nurses/coordinators and reported in an SMSC-specific web-based EDC system developed and operated by RodanoTech (Geneva) in collaboration with the coordinating center (Basel). All quality-of-life questionnaires may either be entered by the study participant (with or without the aid of the study personnel) directly as a survey online in REDCap or done on pen and paper. Questionnaire data is regularly transferred to the RodanoTech EDC on completion of the questionnaire.
For MultiSCRIPT-Cycle 1, new study-specific electronic case report form (eCRFs) will be added to a dedicated area in the EDC for “nested projects” within the SMSC. Study-specific data (screening information and randomization) will be added to the eCRF by the local SMSC personnel. All other data used in MultiSCRIPT-Cycle 1 (i.e., outcomes and questionnaires) is collected in the course of the clinical routine within the SMSC and is already captured and entered in the SMSC eCRF.
Data entry and modifications are recorded in an audit trail. Password protection and user-right management ensure that only authorized study investigators, monitors, and data managers have access to the data. Each patient is identified using an SMSC participant number.
Project and participant data will be handled with uttermost discretion and are only accessible to authorized personnel who require the data to fulfill their duties within the scope of the study. Participant contact information is available at the centers for (e)mailing and follow-up reminders but only local research staff involved in this study have access to the participants’ contact data and those data are never shared between sites. This information will be stored safely by the sites. On the eCRFs and other study-specific documents, participants are only identified by their unique SMSC participant number.
For quality assurance the sponsor, the Ethics Committee, or an independent trial monitor may visit the research sites. Direct access to the source data and all study-related files is granted on such occasions. All involved parties keep the participant data strictly confidential.
Statistical methods
Statistical analysis for primary and secondary outcomes
Superiority of a treatment strategy will be assumed when there is an improvement in at least one of the two primary outcomes, i.e., the strategy either reduces the risk of EDA3 or increases the quality of life. Since there are two primary outcomes the significance level will be 2.5%.
Primary analyses
The primary analyses for the two primary outcomes will be conducted when 90% of the total number of participants (i.e., n = 824) have been recruited, randomized, and have at least 24 months of follow-up data (expected to occur after 3 years of trial conduct). The primary analysis will follow the intention to treat (ITT).
To test the between-group difference 24 months after randomization regarding the proportion of patients experiencing at least one EDA3 event, Pearson’s χ2-test will be applied. Odds ratio and 95% confidence intervals will be reported. To test the between-group difference in the MSQoL-54 scores 24 months after randomization, a linear regression model will be used. The model will be adjusted on the following covariate: baseline MSQoL-54 score. The score differences and 95% confidence intervals will be reported. To adjust for multiple testing of the two primary outcomes, the Bonferroni correction will be applied and the threshold for rejecting null hypotheses will be set at α = 2.5%.
Secondary analyses
The analysis of the primary outcomes will be repeated with 12-month follow-up data using all patients who were randomized and thus have a minimum of 12-month follow-up. In addition, each component of the EDA3 primary outcome (i.e., proportions of patients with at least one relapse, EDSS worsening, new/enlarging T2w lesion or T1w contrast-enhancing lesion, and proportions of patients with at least two criteria of EDA3) and of the MSQoL-54 score (i.e., physical health composite summary and the mental health composite summary) will be assessed using Pearson’s χ2-test and linear regression models, respectively. Secondary outcomes will also be assessed at 24-month follow-up.
The amount of immunosuppressive drug treatment will be summarized using the defined daily dose as defined by the World Health Organization (WHO) [42], i.e., the assumed average maintenance dose per day for a drug used for its main indication in adults.
Interim harm analyses
An interim analysis will be conducted when 90% of the total number of participants (i.e., n = 824) have been recruited, randomized, and have at least 12 months of follow-up data (expected to occur after 2 years of trial conduct). The rationale being that indicators of harms are collected at a minimum on a yearly basis (as some participants only come once a year to the SMSC) we therefore need a minimum of 1 year follow-up data. Using 12-month follow-up data, the interim analyses will focus of reviewing any SAEs related to blood draw and indicators of harms including mortality, harms related to immunosuppression (e.g., relevant infections), and any event related to the occurrence of relapses and/or disability worsening in patients previously stable. The analysis will be conducted and reviewed in a blinded fashion by the Data Safety Monitoring Board (DSMB) made of study-independent experts, including a statistician and experts in trial methodology, ethics, and/or care management in MS.
The DSMB will request unblinding of the data if any of the aforementioned outcomes exhibit statistically significant differences when comparing the two trial groups. We will consider that the causal pathway between the key intervention (i.e., monitoring and providing the sNfL information) and indicators of harms is complex and difficult to assess because sNfL is only one parameter among many that is considered in shared decision-making about treatment adaptation in usual care.
Interim analyses will be considered indicative and will not lead to an automatic early stopping of the trial. In case of major safety concerns, based on their expertise and review of the literature, the DSMB may recommend an early termination of the trial. Of note, “efficacy” will not be assessed in the interim analysis.
Harm analysis
Incidence rates of SAEs and multiple occurrences of SAEs within individual patients will be reported separately and summarized and compared between treatment arms using Pearson’s χ2-tests. Harm outcomes will be assessed until the end of the assessment cycle (i.e., up to 42-month follow-up).
Additional analyses
Subgroup effects on the primary outcomes will be analyzed by interaction tests. To this end, EDA3 will be assessed in a logistic regression model and MSQoL-54 in a linear regression model. All subgroup analyses will be pre-specified in the final statistical analyses plan (SAP) which will be published before unblinding the data.
The key subgroups of interest are patients with low or high baseline risk of further EDA (secondary objective; Sect. 5). In addition, we plan to analyze the following subgroups: age (< 40 years vs. > 40 years); sex (male or female); treatment-naive or previously treated for multiple sclerosis (with any DMT at any time before study enrolment); number of relapses in the year before the study (≤ 1 relapse or > 1 relapse); Number of relapses in the 2 years before the study (≤ 1, 2, or > 2 relapses); Baseline disability (EDSS score 0–3.5 or > 3.5); number of contrast-enhancing lesions at baseline (0 or ≥ 1); number of T2w lesions (< 3; 3 to 9; > 9); and T2w lesion volume (≤ 3300 µL or > 3300 µL).
We will consider the criteria as determined by the Instrument to assess the Credibility of Effect Modification in Analyses (ICEMAN) when interpreting subgroup effects and reporting the results [43].
In addition, to better understand the causal pathway that leads to the outcomes, we will also explore the outcomes in patients who have been treated as expected according to the treatment guidelines (e.g., patients with a relatively clear indication to escalation and subsequently escalated). In the same direction, we will explore the treatment decision (categorical: no (major) change, escalation, or de-escalation) as a mediator in the causal pathway from the intervention to the outcomes, as we do not expect the intervention to have a direct causal effect on the outcomes, but an indirect effect through this mediator (complete mediation). For this, we will record each treatment change. We will also explore the impact of lack of blinding using the centralized reading of MRI compared with local MRI reports.
Since such analyses rely on complex assumptions, are based on non-randomized comparisons, and are prone to time-dependent confounding bias, we will carefully prespecify such additional analyses and consider them entirely as exploratory [44].
Finally, health economic analyses will be detailed in a specific health economic analysis plan that will be finalized before the primary analysis starts.
Handling of missing data and drop-outs
Patients are already participating in the SMSC which include at a minimum yearly visit with an assessment of their EDA3 status (i.e., EDSS, relapses, and MRI data are routinely collected), thus the occurrence of missing data should be minimal for the EDA3 primary outcome. MSQoL-54 is also part of the SMSC usual care and in addition to careful planning and conduct of the trial should also minimize the occurrence of missing data on the MSQoL-54 primary outcome.
To test the robustness of our results to missing data, various imputation methods will be implemented, and a detailed description of imputation methods will be provided in the SAP. In the unlikely event that missing data exceeds 40%, we will only report the complete case analysis [45] and we will interpret it only as exploratory.
Dissemination plans
Statistical code will be made available on GitHub while the patient-level data set will be made available upon request to the primary investigator. Results will be published in an open-access peer-reviewed medical journal. Authorship to publications will be granted according to the rules of the International Committee of Medical Journal Editors (ICMJE) [46].
Discussion
MS is the most frequent non-traumatic cause of disability in young adults and MS patients have a lower health-related quality of life compared with the general population but also compared with other chronic diseases [47, 48]. An estimated 15,000 persons are living with MS in Switzerland [49] of whom > 1800 are included in the SMSC with a median follow-up of 6.3 years, as of January 2024. Patient care in the SMSC reflects the usual care of MS patients nationwide.
Within the SMSC, almost half of the patients with RRMS receive DMTs known for their high efficacy but with uncertainties on the long-term exposure to these treatments. A significant number (over 200) of patients in the SMSC also remain untreated and potentially at risk of disease activity. Improvement of patients’ quality of life by providing patients and physicians with information that may reduce the uncertainty and enable a biomarker-guided de-escalation when applicable would be highly relevant and a significant step towards a more personalized approach to MS treatment and care management. This may reduce the treatment burden and the risk for potential severe side effects associated with highly effective DMT. On the other hand, there may be a better basis for informed decision-making and better informed, earlier, and/or more sensitive biomarker-guided escalation that may lead to fewer patients with evidence of disease activity. Better and adequate monitoring of treatment response would also reduce the costs generated by over- or under-treating patients. Moreover, a continuous sNfL monitoring may improve patients’ quality of life by providing an additional sense of security in a disease which is largely unpredictable.
The pragmatic attitude of MultiSCRIPT being fully embedded in the SMSC usual care with minimal disruption to patient care, leveraging routinely collected data and aiming to generate evidence to inform decisions lays the foundation for creating a learning healthcare system. A learning system whereby accumulating data will enable the continuous generation of new hypotheses on how treatment and care strategies can be further personalized to treat patients as little as possible but as much as necessary at the right time, i.e., ensuring no evidence of disease activity (with more sensitive biomarkers such as sNfL), while achieving better patient-relevant outcomes, and improved care by better informing shared decision-making.
Trial status
The current manuscript reflects the Version 2.0 of the protocol dated 19 March 2024.
The recruitment started with the first patient included on 5 February 2024. At the submission of this manuscript on 9 April 2024, 125 patients have been included and randomized. Recruitment is ongoing and should be completed by February 2026.
Supplementary Information
Acknowledgements
We would like to thank all study coordinators and nurses involved in the SMSC as well as persons with MS participating in the SMSC. We would also like to thank the patient consultants who provided feedback on the protocol proposal.
Roles and responsibilities
This is an investigator-initiated trial conducted under the supervision of Professor Özgür Yaldizli and coordinated by the SMSC coordinating center in Basel. All SMSC centers and investigators are involved. The Department of Clinical Research (DKF) at the University Hospital Basel is responsible for operating REDCap, data monitoring, and for performing the statistical analysis. Professor Özgür Yaldizli will be involved in every step connected to this study including being responsible for the project development and implementation, the recruitment and enrolment of participants, conduct of the trial, and coordinating the dissemination of results.
Abbreviations
- DMTs
Disease-Modifying Therapies
- DSMB
Data Safety Monitoring Board
- eCRF
Electronic Case Report Form
- EDA
Evidence of Disease Activity
- EDC
Electronic Data Capture
- EDSS
Expanded Disability Status Scale
- EQ-5D-5L
European Quality of Life 5 Dimension 5 Severity
- ICEMAN
Instrument to assess the Credibility of Effect Modification in Analyses
- ICMJE
International Committee of Medical Journal Editors
- ITT
Intention-To-Treat
- MID
Minimal Important Difference
- MRI
Magnetic Resonance Imaging
- MS
Multiple Sclerosis
- MSQoL-54
Multiple Sclerosis Quality of Life (MSQoL)-54 instrument
- NEDA3
No Evidence of Disease Activity
- PRECIS-2
PRagmatic Explanatory Continuum Indicator Summary (PRECIS-2) tool
- QRI
QuinteT Recruitment Intervention
- RRMS
Relapsing-Remitting Multiple Sclerosis
- SAEs
Serious Adverse Events
- SAP
Statistical Analysis Plan
- SEAR
Screened, Eligible, Approached, Randomised
- SF-36
Short Form-36
- SMSC
Swiss Multiple Sclerosis Cohort
- sNfL
Serum Neurofilament Light chain
- w
Weighted
- WHO
World Health Organization
Authors’ contributions
Authors contributions according to the CRedIT author statement: Conceptualization: PJ, LK, JK, LGH, ÖY. Data curation: Formal analysis: PJ, PB, SS, LGH. Funding acquisition: CZ, AS, JK, LGH, ÖY. Investigation (trial-ongoing): CZ, AO, LD, AM, CG, JO, TD, LA, OF, PR, PL, SM, CP, RH, GD, CG, JK, ÖY. Methodology: PJ, SS, LR, MS, MJL, LGH. Project administration: PJ. Resources:./.Software:./. Supervision: JK, LGH, ÖY. Validation:./. Visualization:./. Writing—original draft: PJ, LGH. Writing—review and editing: all authors.
Funding and sponsor
Open access funding provided by University of Basel This project is supported by the Swiss National Science Foundation as part of the investigator-initiated clinical trial program (33IC30_205806/1) sponsored by the University Hospital Basel, Switzerland. The funder and sponsor had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Availability of data and materials
The datasets analyzed during the current study and statistical code will be available from the corresponding author on reasonable request, as is the full protocol.
Declarations
Ethics approval and consent to participate
The Ethikkommission Nordwest- und Zentralschweiz (EKNZ) reviewed and approved the protocol (BASEC 2023–01578). Written, informed consent to participate will be obtained from all participants. Protocol amendments will be submitted for review to the EKNZ.
Consent for publication
Not applicable—no identifying images or other personal or clinical details of participants are presented here or will be presented in reports of the trial results. The participant information materials and informed consent form are available from the corresponding author on request.
Competing interests
PJ, PB, SS, AO, LD, AM, DL, GD, and LR declares no conflict of interest; OF, MU, MJL, and MS declared none; Ente Ospedaliero Cantonale, employer of CZ, received grants and/or compensation for Zecca’s lecturer and consultant activities and/or grants from Abbvie, Almirall, Biogen Idec, Bristol Meyer Squibb, Genzyme, Lilly, Lundbeck, Merck, Mylan, Novartis, Pfizer, Teva Pharma, Roche, Sanofi; AS received speaker honoraria for activities with Bristol Myers Squibb, CSL Behring, Novartis, and Roche, and research support by the Baasch Medicus Foundation, the Medical Faculty of the University of Bern and the Swiss MS Society, all not related to this work; The University Hospital Basel (USB) and the Research Center for Clinical neuroimmunology and Neuroscience (RC2NB), as the employers of CG, have received the following fees which were used exclusively for ( research support from Siemens, GeNeuro, Genzyme-Sanofi, Biogen, Roche. They also have received advisory board and consultancy fees from Actelion, Genzyme-Sanofi, Novartis, GeNeuro, Merck, Biogen, and Roche; as well as speaker fees from Genzyme-Sanofi, Novartis, GeNeuro, Merck, biogen and Roche; JO received research support by the Swiss MS Society and served on advisory boards for Roche and Merck; TD has served on scientific advisory boards, steering committees, and data safety monitoring boards for Alexion, Actelion, Biogen, Celgene, Genzyme, GeNeuro, Merck, Mitsubishi Pharma, Novartis, Roche, Octapharma, and MedDay; has received travel and/or speaker honoraria from Biogen, Genzyme, Merck, Novartis, Roche, and Merck-Serono; has received research support from Alexion, Biogen, Novartis, Roche, the Swiss MS Society, the European Union, and the Swiss National Foundation. LA served on scientific advisory boards for Celgene, Novartis Pharmaceuticals, Merck, Biogen, Sanofi Genzyme, Roche, and Bayer; received funding for travel and/or speaker honoraria from Celgene, Biogen, Sanofi Genzyme, Novartis, Merck Serono, Roche, Teva, and the Swiss MS Society; and research support from Biogen, Sanofi, Genzyme, and Novartis; PR has received honoraria for lectures or advisory board participation from Alexion, Bristol-Myers Squibb, Boehringer Ingelheim, Debiopharm, Merck Sharp and Dohme, Laminar, Midatech Pharma, Novocure, QED, Roche, and Sanofi and research support from Merck Sharp and Dohme and Novocure; PR reports that the Geneva University Hospital received honoraria for speaking from Biogen, Merck, Roche; consulting fees from Biogen, Merck, Novartis, Roche; research grants from Biogen, Merck, Novartis; SM received honoraria for travel, honoraria for lectures/consulting and/or grants for studies from Almirall, Alexion, Bayer, Biogen, Bristol-Myers Squibb SA/Celgene, Genzyme, Merck-Serono, Teva, Novartis and Roche; CP reports that the Lausanne University Hospital received speaker honoraria, travel grants and consulting services for her activities with Novartis, Roche, Biogen, Merck, Sanofi-Aventis none related to this work; RH received speaker/advisor honorary from Merck, Novartis, Roche, Biogen, Alexion, Sanofi, Janssen, Bristol-Myers Squibb, Teva/Mepha and Almirall. He received research support within the last 5 years from Roche, Merck, Sanofi, Biogen, Chiesi, and Bristol-Myers Squibb. He also received research grants from the Swiss MS Society and the SITEM Insel Support Fund and is a member of the Advisory Board of the Swiss and International MS Society. He also serves as deputy editor-in-chief for the Journal of Central Nervous System Disease. All conflicts are not related to this work; Ente Ospedaliero Cantonale, an employer of CG, received grants and/or compensation for Gobbi’s lecturer and consultant activities and/or grants from Almirall, Biogen Idec, Bristol Meyer Squibb, Genzyme, Lilly, Lundbeck, Merck, Mylan, Novartis, Teva Pharma, Roche, Sanofi. Faculty of Biomedical Sciences, Università della Svizzera Italiana (USI), Lugano, Switzerland; LK has received no personal compensation. His institutions (University Hospital Basel/Stiftung Neuroimmunology and Neuroscience Basel) have received and used exclusively for research support payments for steering committee and advisory board participation, consultancy services, and participation in educational activities from: Actelion, Bayer, BMS, df-mp Molnia & Pohlmann, Celgene, Eli Lilly, EMD Serono, Genentech, Glaxo Smith Kline, Janssen, Japan Tobacco, Merck, MH Consulting, Minoryx, Novartis, F. Hoffmann-La Roche Ltd, Senda Biosciences Inc., Sanofi, Santhera, Shionogi BV, TG Therapeutics, and Wellmera, and license fees for Neurostatus-UHB products; grants from Novartis, Innosuisse, and Roche; ÖY received grants from ECTRIMS/MAGNIMS, University of Basel, Pro Patient Stiftung, University of Basel, Free Academy Basel, Swiss Multiple Sclerosis Society, Swiss National Science Foundation and advisory board/lecture and consultancy fees from Roche, Sanofi Genzyme, Allmirall, Biogen and Novartis; RC2NB is supported by Foundation Clinical Neuroimmunology and Neuroscience Basel. RC2NB has a contract with Roche for a steering committee participation of LGH.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jens Kuhle, Özgür Yaldizli, and Lars G. Hemkens shared the last authorship.
References
- 1.Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9:520–32. 10.1016/S1474-4422(10)70064-8 [DOI] [PubMed] [Google Scholar]
- 2.Jakimovski D, Bittner S, Zivadinov R, Morrow SA, Benedict RH, Zipp F, et al. Multiple sclerosis. Lancet. 2024;403:183–202. 10.1016/S0140-6736(23)01473-3 [DOI] [PubMed] [Google Scholar]
- 3.Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. 10.1056/NEJMoa044397 [DOI] [PubMed] [Google Scholar]
- 4.Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung H-P, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–28. 10.1016/S0140-6736(12)61769-3 [DOI] [PubMed] [Google Scholar]
- 5.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung H-P, Hemmer B, et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med. 2017;376:221–34. 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]
- 6.Giovannoni G. Disease-modifying treatments for early and advanced multiple sclerosis: a new treatment paradigm. Curr Opin Neurol. 2018;31:233–43. 10.1097/WCO.0000000000000561 [DOI] [PubMed] [Google Scholar]
- 7.Amin M, Hersh CM. Updates and advances in multiple sclerosis neurotherapeutics. Neurodegener Dis Manag. 2023;13:47–70. 10.2217/nmt-2021-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalincik T, Manouchehrinia A, Sobisek L, Jokubaitis V, Spelman T, Horakova D, et al. Towards personalized therapy for multiple sclerosis: prediction of individual treatment response. Brain. 2017;140:2426–43. 10.1093/brain/awx185 [DOI] [PubMed] [Google Scholar]
- 9.Rotstein D, Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol. 2019;15:287–300. 10.1038/s41582-019-0170-8 [DOI] [PubMed] [Google Scholar]
- 10.Cutter GR, Liu Y. Personalized medicine. Neurol Clin Pract. 2012;2:343–51. 10.1212/CPJ.0b013e318278c328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C, et al. Contribution of Relapse-Independent Progression vs Relapse-Associated Worsening to Overall Confirmed Disability Accumulation in Typical Relapsing Multiple Sclerosis in a Pooled Analysis of 2 Randomized Clinical Trials. JAMA Neurol. 2020;77:1132–40. 10.1001/jamaneurol.2020.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelhak A, Benkert P, Schaedelin S, Boscardin WJ, Cordano C, Oechtering J, et al. Neurofilament Light Chain Elevation and Disability Progression in Multiple Sclerosis. JAMA Neurol. 2023;80:1317–25. 10.1001/jamaneurol.2023.3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhle J, Kropshofer H, Haering DA, Kundu U, Meinert R, Barro C, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92:e1007–15. 10.1212/WNL.0000000000007032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benkert P, Meier S, Schaedelin S, Manouchehrinia A, Yaldizli Ö, Maceski A, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21:246–57. 10.1016/S1474-4422(22)00009-6 [DOI] [PubMed] [Google Scholar]
- 15.Bar-Or A, Thanei G-A, Harp C, Bernasconi C, Bonati U, Cross AH, et al. Blood neurofilament light levels predict non-relapsing progression following anti-CD20 therapy in relapsing and primary progressive multiple sclerosis: findings from the ocrelizumab randomised, double-blind phase 3 clinical trials. EBioMedicine. 2023;93:104662. 10.1016/j.ebiom.2023.104662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius Å, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016;54:1655–61. 10.1515/cclm-2015-1195 [DOI] [PubMed] [Google Scholar]
- 17.Kapoor R, Smith KE, Allegretta M, Arnold DL, Carroll W, Comabella M, et al. Serum neurofilament light as a biomarker in progressive multiple sclerosis. Neurology. 2020;95:436–44. 10.1212/WNL.0000000000010346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barro C, Benkert P, Disanto G, Tsagkas C, Amann M, Naegelin Y, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. 2018;141:2382–91. 10.1093/brain/awy154 [DOI] [PubMed] [Google Scholar]
- 19.Disanto G, Barro C, Benkert P, Naegelin Y, Schädelin S, Giardiello A, et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81:857–70. 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bittner S, Steffen F, Uphaus T, Muthuraman M, Fleischer V, Salmen A, et al. Clinical implications of serum neurofilament in newly diagnosed MS patients: A longitudinal multicentre cohort study. EBioMedicine. 2020;56:102807. 10.1016/j.ebiom.2020.102807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thebault S, Abdoli M, Fereshtehnejad S-M, Tessier D, Tabard-Cossa V, Freedman MS. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci Rep. 2020;10:10381. 10.1038/s41598-020-67504-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uphaus T, Steffen F, Muthuraman M, Ripfel N, Fleischer V, Groppa S, et al. NfL predicts relapse-free progression in a longitudinal multiple sclerosis cohort study: Serum NfL predicts relapse-free progression. EBioMedicine. 2021;72:103590. 10.1016/j.ebiom.2021.103590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. 10.1136/bmj.h2147 [DOI] [PubMed] [Google Scholar]
- 24.Disanto G, Benkert P, Lorscheider J, Mueller S, Vehoff J, Zecca C, et al. The Swiss Multiple Sclerosis Cohort-Study (SMSC): A Prospective Swiss Wide Investigation of Key Phases in Disease Evolution and New Treatment Options. PLoS ONE. 2016;11:e0152347. 10.1371/journal.pone.0152347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. The Lancet Neurology. 2018;17:162–73. 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 26.Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol. 2015;72:152–8. 10.1001/jamaneurol.2014.3537 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Salter A, Wallström E, Cutter G, Stüve O. Evolution of clinical trials in multiple sclerosis. Ther Adv Neurol Disord. 2019;12:1756286419826547. 10.1177/1756286419826547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995;4:187–206. 10.1007/BF02260859 [DOI] [PubMed] [Google Scholar]
- 29.Visser LA, Louapre C, Uyl-de Groot CA, Redekop WK. Health-related quality of life of multiple sclerosis patients: a European multi-country study. Archives of Public Health. 2021;79:39. 10.1186/s13690-021-00561-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giordano A, Testa S, Bassi M, Cilia S, Bertolotto A, Quartuccio ME, et al. Viability of a MSQOL-54 general health-related quality of life score using bifactor model. Health Qual Life Outcomes. 2021;19:224. 10.1186/s12955-021-01857-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67:401–9. 10.1016/j.jclinepi.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 32.McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm. 2016;38:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mc Cord KA, Al-Shahi Salman R, Treweek S, Gardner H, Strech D, Whiteley W, et al. Routinely collected data for randomized trials: promises, barriers, and implications. Trials. 2018;19:29. 10.1186/s13063-017-2394-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.IQWIG. General Methods (Version 6.0). 2020. https://www.iqwig.de/methoden/general-methods_version-6-0.pdf. Accessed 9 Oct 2021.
- 35.Comi G, Kappos L, Selmaj KW, Bar-Or A, Arnold DL, Steinman L, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. The Lancet Neurology. 2019;18:1009–20. 10.1016/S1474-4422(19)30239-X [DOI] [PubMed] [Google Scholar]
- 36.Cohen JA, Comi G, Selmaj KW, Bar-Or A, Arnold DL, Steinman L, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. The Lancet Neurology. 2019;18:1021–33. 10.1016/S1474-4422(19)30238-8 [DOI] [PubMed] [Google Scholar]
- 37.Kasenda B, von Elm E, You J, Blümle A, Tomonaga Y, Saccilotto R, et al. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA. 2014;311:1045–51. 10.1001/jama.2014.1361 [DOI] [PubMed] [Google Scholar]
- 38.Donovan JL, Rooshenas L, Jepson M, Elliott D, Wade J, Avery K, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials. 2016;17:283. 10.1186/s13063-016-1391-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donovan JL, Jepson M, Rooshenas L, Paramasivan S, Mills N, Elliott D, et al. Development of a new adapted QuinteT Recruitment Intervention (QRI-Two) for rapid application to RCTs underway with enrolment shortfalls—to identify previously hidden barriers and improve recruitment. Trials. 2022;23:258. 10.1186/s13063-022-06187-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rooshenas L, Scott LJ, Blazeby JM, Rogers CA, Tilling KM, Husbands S, et al. The QuinteT Recruitment Intervention supported five randomized trials to recruit to target: a mixed-methods evaluation. J Clin Epidemiol. 2019;106:108–20. 10.1016/j.jclinepi.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson C, Rooshenas L, Paramasivan S, Elliott D, Jepson M, Strong S, et al. Development of a framework to improve the process of recruitment to randomised controlled trials (RCTs): the SEAR (Screened, Eligible, Approached, Randomised) framework. Trials. 2018;19:50. 10.1186/s13063-017-2413-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. Defined Daily Dose (DDD). https://www.who.int/tools/atc-ddd-toolkit/about-ddd. Accessed 10 Oct 2021.
- 43.Schandelmaier S, Briel M, Varadhan R, Schmid CH, Devasenapathy N, Hayward RA, et al. Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ. 2020;192:E901–6. 10.1503/cmaj.200077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ewald H, Speich B, Ladanie A, Bucher HC, Ioannidis JPA, Hemkens LG. Marginal structural models and other analyses allow multiple estimates of treatment effects in randomized clinical trials: Meta-epidemiological analysis. J Clin Epidemiol. 2019;107:12–26. 10.1016/j.jclinepi.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 45.Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts. BMC Med Res Methodol. 2017;17:162. 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ICMJE Recommendations Defining the Role of Authors and Contributors. https://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html. Accessed 9 Apr 2024.
- 47.Hermann BP, Vickrey B, Hays RD, Cramer J, Devinsky O, Meador K, et al. A comparison of health-related quality of life in patients with epilepsy, diabetes and multiple sclerosis. Epilepsy Res. 1996;25:113–8. 10.1016/0920-1211(96)00024-1 [DOI] [PubMed] [Google Scholar]
- 48.Rudick RA, Miller D, Clough JD, Gragg LA, Farmer RG. Quality of Life in Multiple Sclerosis: Comparison With Inflammatory Bowel Disease and Rheumatoid Arthritis. Arch Neurol. 1992;49:1237–42. 10.1001/archneur.1992.00530360035014 [DOI] [PubMed] [Google Scholar]
- 49.Kaufmann M, Puhan MA, Kuhle J, Yaldizli Ö, Magnusson T, Kamm CP, et al. A Framework for Estimating the Burden of Chronic Diseases: Design and Application in the Context of Multiple Sclerosis. Front Neurol. 2019;10:953. 10.3389/fneur.2019.00953 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study and statistical code will be available from the corresponding author on reasonable request, as is the full protocol.