Abstract
Obesity has emerged as a worldwide health concern due to its increasing prevalence. Adipocytes have the ability to express angiotensin-converting enzyme 2 receptors (ACE2) and several adipocytokines. These expressions could lead to the activation of a cytokine storm, which in turn promotes the development of cardiovascular diseases. The aim of this study was to investigate the impact of perindopril and losartan exposure on the ACE2 and interleukin 6 (IL-6) levels in adipocyte cells. This study used an in vivo true experimental design utilizing a post-test-only control group. A total of 24 adult male albino rats were divided into four groups, one group served as the non-obese (negative control), while the other three groups were obese: (1) the positive control (untreated obese rats); (2) perindopril group (2 mg/kg BW/day orally for 4 weeks); and (3) losartan group (20 mg/kg BW/day for 4 weeks). Afterwards, the rats were euthanized, and the visceral fat tissue were obtained during dissection. The levels of ACE2 and IL-6 were measured using the enzyme-linked immunosorbent assay (ELISA). Losartan administration in obese rats resulted in a notable elevation in ACE2 levels compared to both the perindopril group (losartan vs perindopril, p=0.011) and the positive control (p=0.004). In addition, the treatment of perindopril and losartan in obese rats resulted in a significant reduction in IL-6 levels when compared to the positive control (perindopril vs positive control, p=0.020; losartan vs positive control, p=0.002, respectively). This study provides insight into the administration of perindopril and losartan, which could suppress the pro-inflammatory (IL-6) but increase the ACE2 levels in adipose tissue.
Keywords: Obesity, adipocyte, pro-inflammatory, ACE2, IL-6
Introduction
Obesity has emerged as a worldwide health concern with a growing incidence [1]. According to the World Health Organization (WHO), 43% of adults aged 18 or older globally are categorized as overweight [2], while 16% are categorized as obese [3]. Obesity is recognized as a major risk factor for cardiovascular disease, and a link between obesity and various cardiovascular conditions has been identified, such as acute myocardial infarction, stable coronary disease, cardiac arrhythmias, heart failure, and sudden cardiac death [4].
Angiotensin-converting enzyme 2 receptor (ACE2) is an essential component of the renin-angiotensin system (RAS) and plays a critical role in regulating overall blood pressure. This role is accomplished by enzymatically breaking down angiotensin I (Ang I), resulting in the production of inactive Ang 1–9 peptides, and directly converting angiotensin II (Ang II), which leads to the formation of restricted Ang 1–7 [5]. These processes have an impact on vasoconstriction and fibrosis [6]. Obese individuals may have adipocytes that contribute to the expression of ACE2, with higher levels of ACE2 expression in adipose tissue compared to lung tissue [7,8]. Obesity also stimulates the production of many adipocytokines such as interleukin 6 (IL-6), interleukin 10 (IL-10), tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), leptin, and resistin [9], which in turn could lead to a cytokine storm [10]. This process promotes the progression of cardiovascular diseases, including acute myocardial infarction and heart failure [4].
Cardiovascular drug classes such as ACE inhibitors (ACEI) and angiotensin II receptor blockers (ARB) can increase ACE2 expression and prevent cytokine storms that provide benefits for the cardiovascular system [11]. One type of ACEI and ARB that has been shown to affect ACE2 expression is perindopril [12] and losartan [13]. The aim of this study was to assess the effects of perindopril and losartan on ACE2 expression in obese rat models as the basis for developing therapy using perindopril and losartan in an effort to prevent cardiovascular disease in obese patients.
Methods
Study design and setting
A true in vivo experiment with a post-test-only control group design was conducted at Airlangga University’s Experimental Animal Laboratory, Anatomical Pathology Laboratory, and Molecular Genetics Laboratory in Surabaya, Indonesia. The sample for this study consisted of 24 adult male albino Wistar rats, aged two months, with a body weight of approximately 80–120 grams. The Wistar strain was selected due to the rats exhibiting quicker weight gain and signs of obesity compared to the Sprague Dawley strain [14].
The inclusion criteria for this study were (1) Wistar strain male Rattus norvegicus rats aged 2 months (8 weeks); (2) rats were healthy and had not been used in previous studies (verified by a test animal certificate); and (3) rats were successfully induced to become obese. All rats that became sick or died during obesity induction or intervention were excluded. All rats were randomized and divided into four groups: (a) negative control (non-obese group); (b) positive control (untreated obese rats); (c) obese rat group treated with perindopril; and (d) obese rat group treated with losartan.
The independent variables in this study were the administration of perindopril and losartan to rats induced by obesity. The dependent variables were ACE2 and IL-6 levels in adipose tissue.
Obesity induction
Before obesity was induced, the rats were acclimatized in the cage for one week. Obesity was induced by providing a high-fat diet by administering 1 mL peroral pork oil daily. The study initially included 28 adult male albino rats from a 10% expected attrition or death of animal’s formula [15], to ensure that at least 24 rats remained after the obesity induction process. The high-fat feed was given for eight weeks (two months). At the beginning of the fifth week, the intervention (perindopril and losartan) was started along with a high-fat diet until the eighth week [16].
Obese rats were identified by assessing the increase in body weight (>10% compared to the standard group or >30% of the initial weight) [17]. The obesity was also confirmed by measuring the body length and body mass index (BMI) weekly to determine adipocyte levels and signs of obesity [18].
Administration of perindopril and losartan
Obese rats were subsequently administered perindopril at a dose of 2 mg/kg BW/day, dissolved in 3 cc of distilled water as adapted from a previous study [12]. Losartan was given at a dose of 20 mg/kg BW/day, dissolved in 3 cc of distilled water [19]. Both perindopril and losartan were administered orally on an empty stomach through a gastric tube daily for four weeks. After all treatments, the rats were sacrificed and dissected to collect visceral fat (adipose) tissues.
Measurement of the levels of ACE2 and IL-6
ACE2 and IL-6 levels were measured using the enzyme-linked immunosorbent assay (ELISA) method with the ELISA kits (Thermofisher EH489RB and Elabscience E-EL-R0015, respectively). Adipose tissue samples were transformed into a suspension solution of 100 μL, which was subsequently placed into wells treated with primary antibodies and incubated. Following this, secondary antibodies were added and incubated once again. The procedure continued with the addition of horseradish peroxidase (HRP) conjugate solution and substrate. The results were read at a wavelength of 450 nm using an ELISA reader.
Data analysis
The data obtained were analyzed using one-way ANOVA followed by Tukey HSD post-hoc analysis. If p<0.05, the outcome is considered statistically significant. Data analysis was performed using SPSS software version 25.0 [20].
Results
Losartan significantly elevated ACE2 levels compared to both perindopril and the control group
We evaluated the impact of ACEI/ARB treatment along with a high-fat diet on in vivo ACE2 levels. Our data revealed that the positive control group had a relatively higher level of ACE2 (697.25 ng/mL) compared to the negative control group (606.91 ng/mL), although this difference was not statistically significant (p=0.637) (Table 1). Perindopril and losartan were used in our study to determine the effects of ACEI and ARB treatment. The losartan group had the highest ACE2 levels, followed by the perindopril group and positive control (Table 1).
Table 1.
Result of angiotensin-converting enzyme 2 (ACE2) and IL-6 levels between group
| Groups | n | ACE2 (ng/mL) | IL-6 (ng/mL) | ||
|---|---|---|---|---|---|
| Mean±SD | Min-max | Mean±SD | Min-max | ||
| Negative control (non-obese group) | 6 | 606.91±239.20 | 179.00–903.50 | 11.21±1.34 | 9.65–13.15 |
| Positive control (obese group) | 6 | 697.25±273.04 | 423.50–1031.00 | 15.35±2.58 | 10.78–17.85 |
| Perindopril | 6 | 782.33±477.47 | 179.00–1548.50 | 12.11±1.52 | 10.24–14.65 |
| Losartan | 6 | 1310±259.17 | 968.50–1681.00 | 10.65±3.00 | 6.22–17.85 |
The mean level of ACE2 in rats that received perindopril (782.33 ng/mL) was not significantly different compared to the positive control group (697.25 ng/mL). However, there was a statistically significant difference in the average level of ACE2 between the losartan group and the positive control group (p=0.004) and between the losartan group and the perindopril group (p=0.011) (Figure 1).
Figure 1.
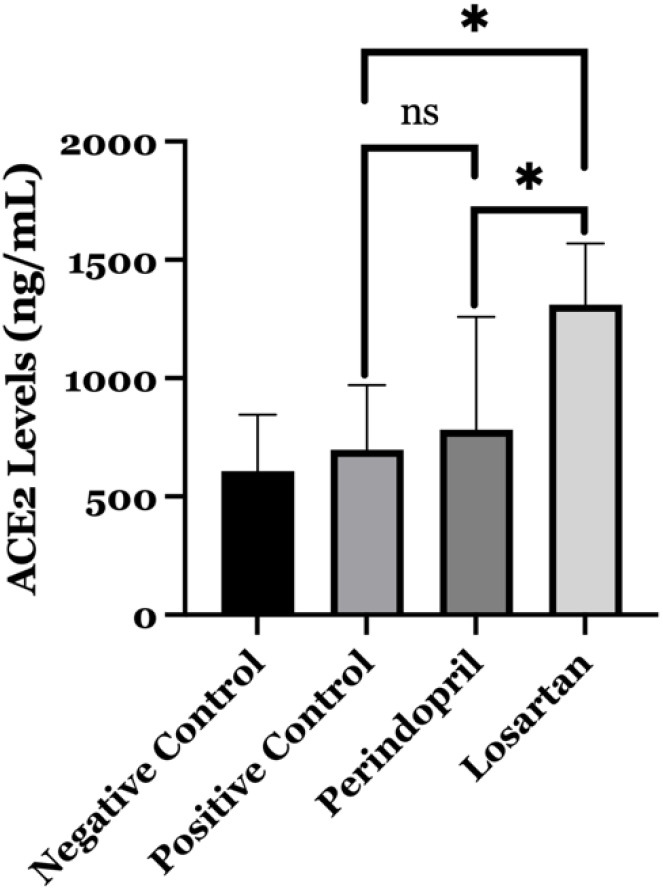
Comparison level of angiotensin-converting enzyme 2 receptors (ACE2) between groups. The level of ACE2 is significantly higher in the losartan group compared to the perindopril group (p=0.011) and positive control group (untreated obese rats) (p=0.004). ns: not significant; * Significant at p=0.05.
A high-fat diet increased IL-6 levels but ACEI/ARB decreased IL-6 level
The level of IL-6, as an indicator of inflammation, increased significantly in the positive control group compared to the negative control (15.35 vs 11.21 ng/mL, p=0.004). We also evaluated the impact of losartan and perindopril administration on the inflammatory marker (IL-6) in our study (Table 1). The findings demonstrated a statistically significant difference in IL-6 levels between the obese rat treated with perindopril and the positive control (12.11 vs 15.35 ng/mL, p=0.020) (Figure 2). Furthermore, the administration of losartan to obese rats resulted in a statistically significant (p=0.002) reduction in IL-6 levels (10.65 ng/mL) as compared to the positive control group (Figure 2). In contrast, perindopril and losartan did not differ significantly in their ability to decrease IL-6 levels (p=0.272).
Figure 2.
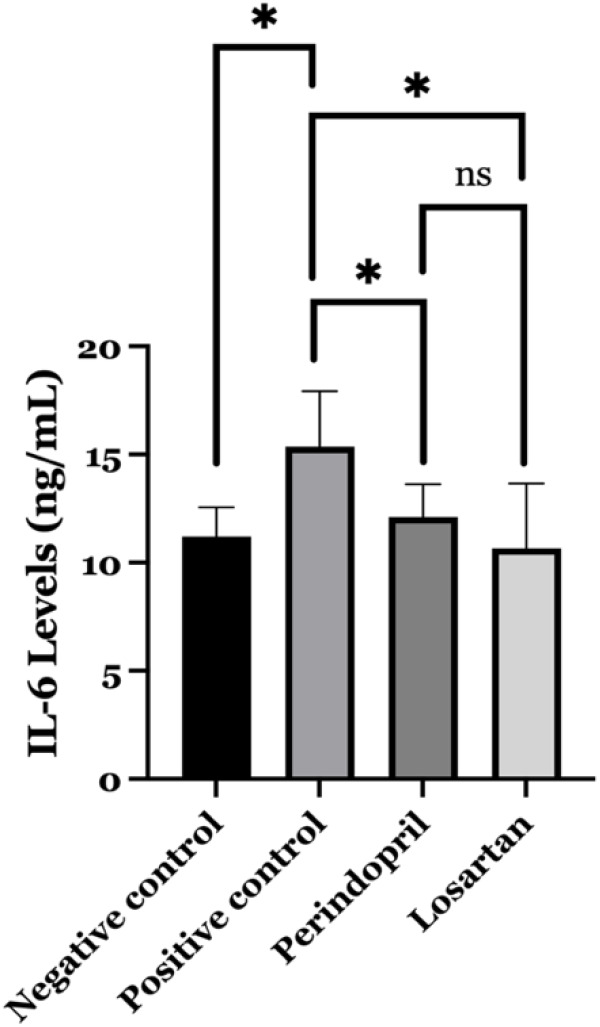
Comparison level of interleukin 6 (IL-6) between study groups. The level of IL-6 is significantly lower in the perindopril group (p=0.020) and the losartan group (p=0.002) compared to the positive control group (untreated obese rats). ns: not significant; * Significant at p=0.05.
Discussion
Losartan increases ACE2 levels
Obesity is a contributing factor to the development of cardiovascular disease [4]. Obese individuals may have elevated levels of ACE2 expressed in their adipocytes compared to other tissues [7,8]. The presence of ACE2 in adipose tissue is critical for maintaining the local adipose RAS homeostasis. Further systemic RAS effects may result from any disruption to this equilibrium [21]. The aim of our study was to investigate the influence of obesity on adipocyte ACE2 levels through in vivo experiments.
We found that ACE2 levels in obese rats that were fed a high-fat diet for eight weeks did not differ significantly from those in non-obese rats. Adipocytes are known to express ACE2, and its expression is upregulated in response to high-fat diet-induced obesity [22]. Adipocyte ACE2 deficiency is also associated with an increased risk of chronic inflammation, a condition that can lead to insulin resistance and cardiac failure, among others [23]. The complex mechanism of ACE2 has led to the conclusion that the ACE2/Ang 1–7 axis is responsible for avoiding inflammatory responses, lipotoxicity, and oxidative stress [24]. A previous in vivo investigation demonstrated variations in ACE2 levels following short-term (1 week) and long-term (4 months) administration of a high-fat diet. The ACE2 mRNA expression in adipose tissue is known to be elevated by short-term administration of a high-fat diet, resulting in enhanced protein expression and enzymatic activity [22,25]. Despite prolonged administration of a high-fat diet leading to an increase in ACE2 mRNA expression, there was no corresponding significant increase in ACE2 levels or enzymatic activity compared to short-term administration [25]. The levels of mRNA disintegrin and metalloproteinase domain 17 (Adam17) in adipose tissue have shown a significant increase in comparison to rats that were fed a low-fat diet (LFD) [25]. Adam17 plays a role in the process of shedding ACE2, leading to a reduction in ACE2 levels and contributing to the inflammatory response [24,26,27].
The aim of our study was to evaluate and compare the impacts of perindopril and losartan on obese rat models. After four weeks of intervention, our data revealed that the administration of losartan resulted in a significant rise in ACE2 levels compared to the other groups. A previous study demonstrated that only ARBs tend to elevate ACE2 levels within the body due to ACE2’s suppression of angiotensin 2 release [28,29]. It has been demonstrated that the Ang II type 1 receptor (AT1R) blocker, losartan, inhibited the internalization and degradation of ACE2 [28,30]. These findings support the hypothesis that the ACE/Ang axis II/AT1R is responsible for lowering ACE2 levels and triggering the inflammatory response [28,31]. A previous animal study indicated that ACEI/ARB therapy could lead to an increase in ACE2 expression and provide advantages for the cardiovascular system [32]. It is postulated that the variations in the effects of angiotensin II on ACEI and ARB might explain the observed disparities in the effects between the perindopril and losartan groups in this study [33]. ACEIs function by decreasing the levels of circulating Ang II, whereas ARBs elevate the levels of free Ang II in the bloodstream [33].
Losartan and perindopril decrease IL-6 levels
Our study investigated the effect of a high-fat diet and medicines that activate the RAS system on IL-6 levels in obese rats. The results of the study showed a substantial difference between the high-fat diet group and the negative control group. It was expected that these effects would happen since adipose tissue releases about a third of all IL-6 levels in the blood [34]. The activation of the AT1R results in increased levels of Ang II, which in turn activates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway and starts the synthesis of IL-6 [35,36]. The pro-inflammatory impact of Ang II is counteracted homeostatically by another RAS peptide, Ang 1–7, which is created by the alternate metabolism of Ang I or Ang II by ACE2 [37].
A result similar to the previous study showed that high ACE2 levels correlated with low IL-6 values. This finding suggests that increased ACE2 expression may have an anti-inflammatory effect, as evidenced by the reduced levels of the inflammatory marker IL-6. Our study is also in line with the results of a previous study where serum IL-6 levels were significantly higher in overweight and obese subjects compared to healthy controls [38]. The consistent results illustrate a robust correlation between metabolic pathways and inflammation. Macrophages and adipocytes play a significant role in producing different adipocytokines, including IL-1, IL-6, IL-8, IL-12, interferon (IFN), TNF-α, transforming growth factor beta (TGF-β), leukemia inhibitory factor (LIF), MCP-1, macrophage inflammatory protein (MIP-1), leptin, and resistin [39]. When inflammation occurs, IL-6 is among the first cytokines to become active. Excessive release can lead to hyperinflammation, which in turn causes cytokine storms [40]. Down-regulation of ACE2 after the virus entry into the cell causes the accumulation of Ang II levels, which activates the AT1R receptor [41]. Activation of the AT1R-dependent induction of nicotinamide adenine dinucleotide phosphate oxidase (NOX), mostly Nox2 and Nox4, will increase ROS production [42] and activate NF-κB and AP-1 pathways to increase inflammatory cytokines, especially the IL-6 [42-44]. The transcription regulation of AT1R gene expression by IL-6 leads to a vicious cycle [45]. Therefore, the combination of these factors could worsen cardiovascular diseases such as hypertension, arrhythmia, and coronary artery disease [4,46].
The role of ACEIs and ARBs in the pro-inflammatory marker IL-6 is also well-studied. Our study attempted to prove the beneficial effects of ACEI and ARB on reducing the inflammatory response that can be applied to obese patients. Our study showed that the ACEI and ARB groups had significantly lower IL-6 levels than the untreated obese group. These findings support the claim that targeting the ACE2/Ang-(1–7)/Mas Receptor (MasR) axis can be an effective therapeutic strategy to counteract the harmful effects of Ang II, which promotes vasoconstriction and inflammatory responses, and prevent its dominance over the ACE/Ang II/AT1R axis [28]. Coincidentally, a recent study documented that individuals with obesity exhibited elevated levels of Ang II in comparison to those who were in good health [47]. Suppression of Ang II is expected to significantly decrease IL-6 expression by reducing the activity of the transcription factor NF-kB [48], which aligns with the findings of our study. Our findings confirm previous research indicating that ACE2 plays a role in promoting viral-induced inflammation [49]. This result suggests a possible treatment strategy involving the activation of ACE2, which could help suppress the occurrence of cytokine storms without compromising the immune system’s capacity [50], such as the use of anti-IL6 (tocilizumab) [51], or corticosteroids [52].
Despite the study’s valuable insights, it lacks comprehensiveness in evaluating the clinical effects of perindopril and losartan. It would have been beneficial to include assessments of clinical parameters such as blood pressure, heart rate, and other components of the renin-angiotensin system (RAS), including angiotensin II and angiotensin-(1–7). These parameters could potentially exhibit significant changes due to the administration of perindopril and losartan.
Conclusion
ACE2 levels in the positive control (obese control group) were not significantly different compared to the negative control (non-obese control group). ACE2 levels in the losartan group were significantly higher compared to all other groups (perindopril, untreated obese group and non-obese group). In addition, losartan and perindopril treatment decrease the level of IL-6 significantly compared to untreated obese animals. These suggest that losartan and perindopril may decrease pro-inflammatory cytokine IL-6 in adipose tissues, and in addition, losartan also increases the level of ACE2 in adipose tissues.
Acknowledgments
The authors would like to thank Mr. Pardi and the head of the Experimental Animal Laboratory, Anatomical Pathology Laboratory and Molecular Genetics Laboratory, Universitas Airlangga, Surabaya, Indonesia.
Ethics approval
This study was approved with ethical approval statement 2.KEH.077.07.2022 issued by the Animal Care and Utilization Committee (ACUC), Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia.
Competing interests
All the authors declared that there is no conflict of interest.
Funding
This study was funded by the Medical Faculty of Universitas Airlangga, Surabaya, Indonesia, with grant number 1315/UN3.1.1/HK/202.
Underlying data
Derived data supporting the findings of this study are available from the corresponding author on request.
How to cite
Andrianto A, Hermawan HO, Harsoyo PM, et al. Perindopril and losartan affect ACE-2 and IL-6 expression in obese rat model. Narra J 2024; 4 2: e681 - http://doi.org/10.52225/narra.v4i2.681.
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet 2014;384(9945):766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Prevalence of overweight among adults. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed: 11 December 2023.
- 3.World Health Organization. Prevalence of obesity among adults. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-obesity-among-adults-bmi-=-30-(age-standardized-estimate)-(-). Accessed: 11 December 2023.
- 4.Csige I, Ujvárosy D, Szabó Z, et al. The impact of obesity on the cardiovascular system. J Diabetes Res 2018;2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad H, Khan H, Haque S, et al. Angiotensin-converting enzyme and hypertension: A systemic analysis of various ACE inhibitors, their side effects, and bioactive peptides as a putative therapy for hypertension. J Renin Angiotensin Aldosterone Syst 2023;2023:7890188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Heialy S, Hachim M, Senok A, et al. Regulation of angiotensin converting enzyme 2 (ACE2) in obesity: Implications for COVID-19. BioRxiv 2020:2020.04.17.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia X, Yin C, Lu S, et al. Two things about COVID-19 might need attention. Preprints (Basel) 2020:2020020315. [Google Scholar]
- 8.Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes Med 2020;19:100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab 2008;93(11 Suppl 1):S64–S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nigro E, D’Agnano V, Quarcio G, et al. Exploring the network between adipocytokines and inflammatory response in SARS-CoV-2 infection: A scoping review. Nutrients 2023;15(17):3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020;126(12):1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang M, Li X, Meng Y, et al. Upregulation of angiotensin-converting enzyme (ACE) 2 in hepatic fibrosis by ACE inhibitors. Clin Exp Pharmacol Physiol 2010;37(1):e1–e6. [DOI] [PubMed] [Google Scholar]
- 13.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005;111(20):2605–2610. [DOI] [PubMed] [Google Scholar]
- 14.Marques C, Meireles M, Norberto S, et al. High-fat diet-induced obesity rat model: A comparison between Wistar and Sprague-Dawley rat. Adipocyte 2016;5(1):11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charan J, Kantharia N.. How to calculate sample size in animal studies? J Pharmacol Pharmacother 2013;4(4):303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurnijasanti R, Winarti D, Wahjuni RS, et al. Hispathology of coronary artery of male rat (Ratus Norvegicus) with high fat diet after being given ethanol extract of Indian acalypha (Acalipha indica. L). KnE Life Sciences 2017;3(6):241. [Google Scholar]
- 17.Leopoldo AS, Lima-Leopoldo AP, Nascimento AF, et al. Classification of different degrees of adiposity in sedentary rats. Braz J Med Biol Res 2016;49(4):e5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Licholai JA, Nguyen KP, Fobbs WC, et al. Why do mice overeat high-fat diets? How high-fat diet alters the regulation of daily caloric intake in mice. Obesity 2018;26(6):1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klimas J, Olvedy M, Ochodnicka-Mackovicova K, et al. Perinatally administered losartan augments renal ACE2 expression but not cardiac or renal Mas receptor in spontaneously hypertensive rats. J Cell Mol Med 2015;19(8):1965–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IBM Corp. IBM SPSS Statistics version 25.0. New York: IBM Corp; 2017. [Google Scholar]
- 21.El-Sayed MJS, Jackson AU, Brotman SM, et al. ACE2 expression in adipose tissue is associated with cardio-metabolic risk factors and cell type composition—implications for COVID-19. Int J Obes 2022;46(8):1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez-Zorita S, Milton-Laskibar I, García-Arellano L, et al. An Overview of adipose tissue ACE2 modulation by diet and obesity. Potential implications in COVID-19 infection and severity. Int J Mol Sci 2021;22(15):7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loot AE, Roks AJM, Henning RH, et al. Angiotensin-(1–7) attenuates the development of heart failure after myocardial infarction in rats. Circulation 2002;105(13):1548–1550. [DOI] [PubMed] [Google Scholar]
- 24.Patel VB, Basu R, Oudit GY. ACE2/Ang 1-7 axis: A critical regulator of epicardial adipose tissue inflammation and cardiac dysfunction in obesity. Adipocyte 2016;5(3):306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupte M, Boustany-Kari CM, Bharadwaj K, et al. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol 2008;295(3):R781–R788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zipeto D, da F Palmeira J, Argañaraz GA, et al. ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID-19. Front Immunol 2020;11:576745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang F, Yang J, Zhang Y, et al. Angiotensin-converting enzyme 2 and angiotensin 1-7: Novel therapeutic targets. Nat Rev Cardiol 2014;11(7):413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deshotels MR, Xia H, Sriramula S, et al. Angiotensin II mediates angiotensin-converting enzyme type 2 internalization and degradation through an Angiotensin II type I receptor-dependent mechanism. Hypertension 2014;64(6):1368–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005;111(20):2605–2610. [DOI] [PubMed] [Google Scholar]
- 30.Ogunlade BO, Lazartigues E, Filipeanu CM. Angiotensin type 1 receptor-dependent internalization of SARS-CoV-2 by angiotensin-converting enzyme 2. Hypertension 2021;77(4):E42–E43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forrester SJ, Booz GW, Sigmund CD, et al. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol Rev 2018;98(3):1627–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kriszta G, Kriszta Z, Váncsa S, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on angiotensin-converting enzyme 2 levels: A comprehensive analysis based on animal studies. Front Pharmacol 2021;12:619524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaduganathan M, Vardeny O, Michel T, et al. Renin–angiotensin–aldosterone system inhibitors in patients with COVID-19. N Engl J Med 2020;382(17):1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruun JM, Verdich C, Toubro S, et al. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-α. Effect of weight loss in obese men. Eur J Endocrinol 2003;148(5):535–542. [DOI] [PubMed] [Google Scholar]
- 35.Hojyo S, Uchida M, Tanaka K, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen 2020;40(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nazerian Y, Vakili K, Ebrahimi A, et al. Developing cytokine storm-sensitive therapeutic strategy in COVID-19 using 8P9R chimeric peptide and soluble ACE2. Front Cell Dev Biol 2021;9:717587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simões E Silva AC, Teixeira MM. ACE inhibition, ACE2 and angiotensin-(1-7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res 2016;107:154–162. [DOI] [PubMed] [Google Scholar]
- 38.El-Mikkawy DME, EL-Sadek MA, EL-Badawy MA, et al. Circulating level of interleukin-6 in relation to body mass indices and lipid profile in Egyptian adults with overweight and obesity. Egypt Rheumatol Rehabil 2020;47(1):7. [Google Scholar]
- 39.Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007;56(4):901–911. [DOI] [PubMed] [Google Scholar]
- 40.Majidpoor J, Mortezaee K.. Interleukin-6 in SARS-CoV-2 induced disease: Interactions and therapeutic applications. Biomed Pharmacother 2022;145:112419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ Res 2020;126(10):1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nejat R, Sadr AS. Are losartan and imatinib effective against SARS-CoV2 pathogenesis? A pathophysiologic-based in silico study. In Silico Pharmacol 2021;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Arif G, Khazaal S, Farhat A, et al. Angiotensin II Type I Receptor (AT1R): The gate towards COVID-19-associated diseases. Molecules 2022;27(7):2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiese OJ, Allwood BW, Zemlin AE. COVID-19 and the renin-angiotensin system (RAS): A spark that sets the forest alight? Med Hypotheses 2020;144:110231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elton TS, Martin MM. Angiotensin II type 1 receptor gene regulation: Transcriptional and posttranscriptional mechanisms. Hypertension 2007;49(5):953–961. [DOI] [PubMed] [Google Scholar]
- 46.El-Sayed MJS, Jackson AU, Brotman SM, et al. ACE2 expression in adipose tissue is associated with cardio-metabolic risk factors and cell type composition—implications for COVID-19. Int J Obes 2022;46(8):1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al Heialy S, Hachim MY, Senok A, et al. Regulation of angiotensin-converting enzyme 2 in obesity: Implications for COVID-19. Front Physiol 2020;11:555039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sano M, Fukuda K, Sato T, et al. ERK and p38 MAPK, but not NF-κB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res 2001;89(8):661–669. [DOI] [PubMed] [Google Scholar]
- 49.Ardiana M, Suryawan IGR, Hermawan HO, et al. Effect of SARS-CoV-2 spike protein exposure on ACE2 and interleukin 6 productions in human adipocytes: An in-vitro study. Narra J 2023;3(3):e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pantazi I, Al-Qahtani AA, Alhamlan FS, et al. SARS-CoV-2/ACE2 Interaction Suppresses IRAK-M Expression and Promotes Pro-Inflammatory Cytokine Production in Macrophages. Front Immunol 2021;12:683800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo P, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 2020;92(7):814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russell B, Moss C, George G, et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. Ecancermedicalscience 2020;14:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]


