Abstract
Objective:
Nephrolithiasis is prevalent and burdensome worldwide. At present, evidence on the risk factors for nephrolithiasis is unconsolidated and the associations remain uncertain. The authors systematically evaluate the robustness of the meta-analytic evidence and aid more reliable interpretations of the epidemiological relationships.
Methods:
The authors conducted a comprehensive review of the meta-analyses, screened the included studies with the aid of the AMSTAR 2 evaluation tool, and then used R (4.1.1) software to perform data analysis to evaluate the association between candidate risk factors and kidney stones, and evaluated the credibility of the evidence of the association between risk factors and kidney stones according to the GRADE classification, and finally obtained the strength and effectiveness of the association.
Results:
The authors finally included 17 meta-analyses regarding 46 risk factors, 34 of which (73.9%) showed statistically significant association with nephrolithiasis. Among the significant associations, the authors found that waist circumference, BMI, dietary intake and fructose intake were positively correlated with the occurrence and development of nephrolithiasis. Caffeine, dietary fiber and DASH-diet showed a tendency to reduce kidney stones. Interestingly, calcium supplementation, dietary calcium, and vitamin D, which are widely believed to be responsible for stone formation, made no difference or even reduced the risk of nephrolithiasis.
Conclusions:
The authors’ study demonstrates the suggestive causal (central obesity, type 2 diabetes, gout, dietary sodium, fructose intake and higher temperatures) risk factors of nephrolithiasis. The authors also demonstrate the suggestive causal (coffee/alcohol/beer intake, dietary calcium and DASH-diet) protective factors of nephrolithiasis. To provide epidemiological basis for the treatment and prevention of nephrolithiasis.
Keywords: kidney stone disease, nephrolithiasis, risk factors, umbrella review, urology
Introduction
Highlights
The first paper presents a more systematic umbrella review of risk factors and prevention factors for kidney stone disease, and a re-review of published meta-analyses results in a more credible conclusion.
Central obesity, type 2 diabetes, gout, dietary sodium, fructose intake, and high temperature have been shown to be potential risk factors for kidney stones.
Coffee/alcohol/beer intake, dietary calcium and DASH-diet can be considered protective factors for kidney stone disease.
Nephrolithiasis, as a disease with a high incidence and recurrence rate, is always troubling people all over the world. In the United States, ~10.6% of men and 7.1% of women suffer deeply from nephrolithiasis, which is comparable to diabetes (9.7%)1–3. In China, the incidence in men and women is estimated to be 6.5% and 5.1%, respectively4, which is also non-negligible. The presence of nephrolithiasis can lead to more than short-term pain and infection. Progressive hypertension5, chronic kidney diseases, and even end-stage kidney failure6,7 will be the ultimate outcomes of nephrolithiasis without effective intervention and treatment. Unfortunately, despite surgery or medication, recurrence rates of 50% within 5 years and up to 75% within 20 years leave many patients suffering8. Meanwhile, the medical expenses incurred by patients due to nephrolithiasis crush their economic lives either. As early as 2005, the annual medical cost of nephrolithiasis in the United States had already exceeded $5 billion9. Taking into account the continued growth of people at risk of obesity and diabetes, it is estimated that the cost could increase by $1.24 billion per year until 203010,11.
In the face of such intractable disease, scientists have proposed a series of measures to prevent kidney stones based on epidemiological investigations and statistics, aiming at relevant risk factors, such as increasing fluid intake, supplementing citrate and limiting animal protein intake12. Nonetheless, the enhancing incidence of nephrolithiasis can still be observed around the world13, which reflects that the summary of the risk factors for the onset of nephrolithiasis is not comprehensive enough and needs to be refined and improved.
Umbrella review, also known as systematic review of systematic reviews, is a research method that systematically evaluates all systematic reviews and meta-analyses on a specific medical research topic to obtain more reliable conclusions14,15. Umbrella reviews not only help researchers save time and effort from starting from scratch but also provide a bird’s eye view and recommendations on how certain medical phenomena are associated with exposure to related risk factors16.
To date, there have been numerous systematic reviews and meta-analyses on the pathogenesis of nephrolithiasis, but their methodological quality and the quality of the evidence still need to be further validated and evaluated. Therefore, this article aimed to provide an umbrella review of published systematic reviews and meta-analyses to obtain the available evidence on risk factors associated with nephrolithiasis and to provide guidance for the prevention of nephrolithiasis.
Methods
The umbrella review was registered with PROSPERO. Detailed methods for umbrella review were demonstrated in the Supplementary materials 1, Supplemental Digital Content 1, http://links.lww.com/JS9/C677. Moreover, this research evaluated the effect of kinds of factors on nephrolithiasis formation and followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) Guideline17, see in Supplementary Fig 1, Supplemental Digital Content 2, http://links.lww.com/JS9/C678. Besides, according to the World Medical Association’s Declaration of Helsinki in 2013, this study has taken through the registration process at Research Registry (http://www.researchregistry.com).
Literature search strategy, inclusion and exclusion criteria, and data extraction
The PubMed, Embase and the Cochrane library databases were searched by two independent researchers from inception to 1 November 2021, with English language restrictions, to identify studies. The detailed search terms are provided as follows. We included meta-analyses that explored the association between any risk factor and nephrolithiasis in the observational study. We excluded systematic reviews without meta-analysis. The first author, publication year, number of cases and participants, risk factor of interest, estimate with its 95% CI from the largest primary study, metrics used for pooling analyses, and study design were the main information that was extracted. Other information based on the random-effects model, including I2, tau2, Z value, and the P value of Egger’s test, was also collected to verify the subsequent re-analyzed meta-analysis results.
Search strategy for umbrella review
PubMed
The specific search methods used to search the included literature can be found in the Supplementary Materials, Supplemental Digital Content 1, http://links.lww.com/JS9/C677 of this paper. The search strategies used for the other databases were almost identical or slightly modified depending on the circumstances of each database.
AMSTAR 2 for the umbrella review
AMSTAR 2 is a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions. When more than one meta-analysis was included, we extracted information from the meta-analysis with the highest AMSTAR 2 level18, the detail checklist could be saw in the Supplementary Fig 2, Supplemental Digital Content 3, http://links.lww.com/JS9/C679.
Statistical analysis for the umbrella review
We re-analyzed each eligible meta-analysis using a random-effects model. The following indexes were used to evaluate bias: a small study effect could be recognized if the P value of Egger’s test is small of 0.05 or P less than 0.1 with the pooling estimate larger than the estimate of its largest component study19. The I² statistic was calculated, and high heterogeneity was defined as I² greater than 50%20. The 95% prediction interval could predict that the probability of the effect size of each future individual study falling within the prediction interval is 95%21. The χ2 test was used to detect excess significance bias22.
We used a Bonferroni-corrected P value to account for multiple testing: P less than 0.0016 (0.05 divided by 32) was the significance level, and a P value between 0.0016 and 0.05 was considered to be a suggestive association. Statistical analyses and data visualization were achieved by the “meta” packages in R (4.1.1).
Credibility assessment and certainty of evidence evaluation
We divided the level of evidence with nominal statistical significance (P < 0.05) into four categories that mainly referred to a study that previously proposed a credibility assessment23: convincing (class I), highly suggestive (class II), suggestive (class III), and weak (class IV). The evaluation criteria can be found as follows. We assessed the certainty of evidence for associations in the umbrella review under the guidance of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach24.
Credibility assessment for meta-analysis in umbrella review
We mainly referred to the previously proposed study for credibility assessment23. Specific evaluation criteria can be found in the Supplementary Materials 2, Supplemental Digital Content 1, http://links.lww.com/JS9/C677.
Results
Twenty-four articles were included to further assessment after applying the inclusion or exclusion criteria (Fig. 1). Finally, 17 studies on 46 associations were further analyzed by using the AMSTAR 2.0 tool to evaluate all meta-analyses (Supplementary Table S1 and S2, Supplemental Digital Content 1, http://links.lww.com/JS9/C677). All metrics calculated in this umbrella review were the same as those that extracted form original study. In total, 73.9% (34/46) of the associations were statistically significant according to the random-effects model results, of which 10 achieved the P less than 1E-06 level. 22 (47.8%) associations had significant heterogeneity (I2 > 50%). Obvious flaws (heterogeneity, small study effect, excess significance bias, P less than 0.05 of the largest study in meta-analysis) were not detected for 18 (39.1%) associations.
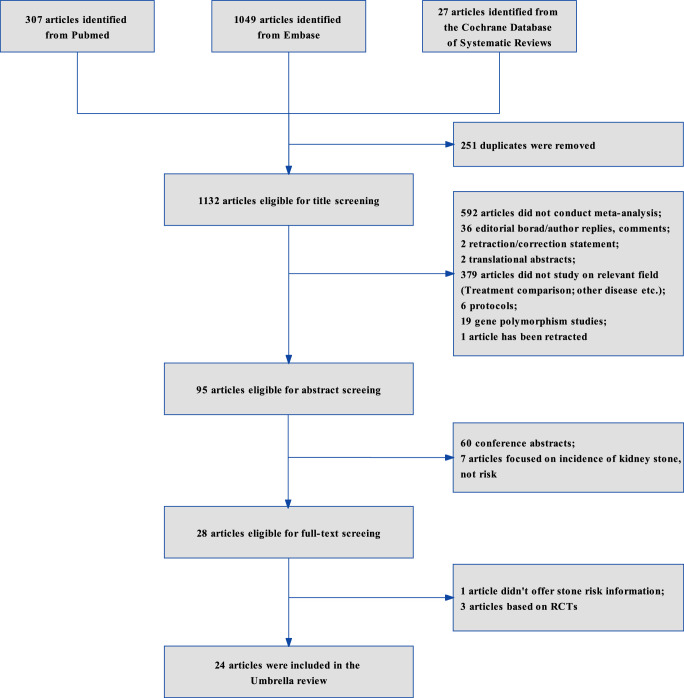
Figure 1.
Flow diagram to demonstrate the search and selection process in umbrella review.
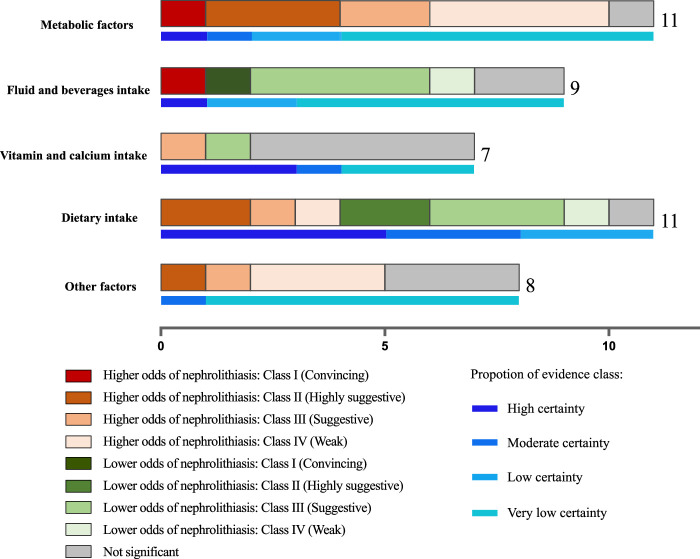
The summary results for number of meta-analyses included and its corresponding class of umbrella review and certain of evidence were demonstrated in Fig. 2. The details of associations between exposures and nephrolithiasis, and evidence class reported in meta-analyses were collected in Table 1.
Figure 2.
Histogram demonstrating the summary results for number of meta-analyses included and its corresponding class of umbrella review and certain of evidence.
Table 1.
Associations between exposures and nephrolithiasis, and evidence class reported in meta-analyses.
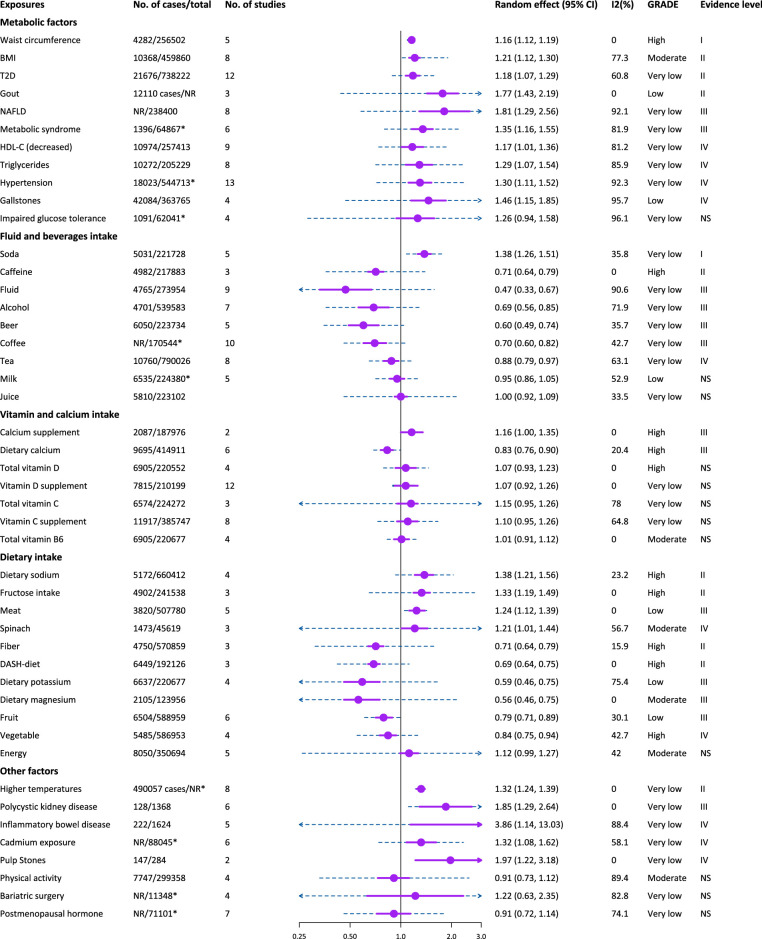
| Exposures | No. cases/total population | No. of study estimates | Study design | Effect metrics | Effect of largest study in meta-analysis | Random effect summary estimate (95% CI) | Random-effects P value | I2 (%) | 95% prediction interval | P value for Egger test | Excess significance bias P value | Large heterogeneity, small study effect, excess significance bias, P>0·05 of the largest study in meta-analysis | Evidence level |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolic factors | |||||||||||||
| Waist circumference | 4282/256 502 | 3 studies, 5 cohort | cohort | RR | 1.18 (1.12, 1.25) | 1.16 (1.12, 1.19) | 1.63E-19 | 0 | (1.10, 1.20) | 0.136 | 0.731 | None | I |
| BMI | 10 368/459 860 | 5 studies, 8 cohorts | Cohorts/case-control | RR | 1.39 (1.26, 1.53) | 1.21 (1.12, 1.30) | 1.06E-06 | 77.3 | (1.02, 1.90) | 0.240 | 0.141 | Large heterogeneity | II |
| T2D | 21 676/738 222 | 12 studies, 12 cohorts | Cohort/case-control | RR | 1.11 (0.91, 1.34) | 1.18 (1.07, 1.29) | 1.84E-13 | 60.8 | (0.89, 1.57) | 0.705 | 0.401 | Large heterogeneity | II |
| Gout | At least 12 110 cases/NR | 3 studies, 3 cohorts | Cross-sectional | OR | 1.49 (1.04, 2.14) | 1.77 (1.43, 2.19) | 1.52E-07 | 0 | (0.44, 7.05) | 0.97 | 0.156 | None | II |
| NAFLD | NR/238 400 | 8 studies, 8 cohorts | Cohort/Cross-section | OR | 1.08 (0.90, 1.30) | 1.81 (1.29, 2.56) | 6.99E-04 | 92.1 | (0.58, 5.61) | 0.737 | 0.256 | Large heterogeneity, P>0·05 of the largest study in meta-analysis | III |
| Metabolic syndrome | At least 1396/64 867a | 6 studies, 6 cohorts | Cross-sectional | OR | 1.11 (1.03, 1.19) | 1.35 (1.16, 1.55) | 1.77E-04 | 81.9 | (0.80, 2.28) | 0.147 | 0.122 | Large heterogeneity | III |
| HDL-C (decreased) | 10 974/257 413 | 9 studies, 9cohorts | Cohort/Cross-section | OR | 0.97 (0.89, 1.06) | 1.17 (1.01, 1.36) | 0.031 | 81.2 | (0.74, 1.87) | 0.319 | 0.709 | Large heterogeneity, P>0·05 of the largest study in meta-analysis | IV |
| Triglycerides | 10 272/205 229 | 8 studies, 8 cohorts | Cohort/Cross-section | OR | 1.03 (0.97, 1.10) | 1.29 (1.07, 1.54) | 0.007 | 85.9 | (0.71, 2.33) | 0.098 | 0.949 | Large heterogeneity, small study effect. | IV |
| Hypertension | At least 18 023/544 713a | 11 studies, 13 cohorts | Cohort | RR | 0.95 (0.91, 1.01) | 1.30 (1.11, 1.52) | 0.002 | 92.3 | (0.72, 2.36) | 0.035 | 0.435 | Large heterogeneity, small study effect, P>0·05 of the largest study in meta-analysis | IV |
| Gallstones | 42 084/363 765 | 2 studies, 4 cohorts | Cohort | RR | 1.97 (1.81, 2.15) | 1.46 (1.15, 1.85) | 0.002 | 95.7 | (0.47, 4.57) | 0.424 | 0.255 | Large heterogeneity | IV |
| Impaired glucose tolerance | At least 1091/62041a | 4 studies, 4 cohorts | Cross-sectional | OR | 1.03 (0.96, 1.10) | 1.26 (0.94, 1.58) | 0.196 | 96.1 | (0.28, 5.41) | 0.971 | 0.919 | Large heterogeneity, P>0·05 of the largest study in meta-analysis | NS |
| Fluid and beverages intake | |||||||||||||
| Soda | 5031/221 728 | 3 studies, 5 cohorts | Cohorts/case-control | RR | 1.51 (1.37, 1.66) | 1.38 (1.26, 1.51) | 8.49E-12 | 35.8 | (1.08, 1.76) | 0.275 | 0.811 | None | I |
| Caffeine | 4982/217 883 | 1 study, 3 cohorts | Cohorts | HR | 0.69 (0.58, 0.82) | 0.71 (0.64, 0.79) | 8.03E-11 | 0 | (0.36, 1.39) | 0.874 | 0.362 | None | II |
| Fluid | 4765/273 954 | 9 studies, 9 cohorts | RCTs/Cohort/case-control | RR | 0.68 (0.56, 0.83) | 0.47 (0.33, 0.67) | 2.79E-05 | 90.6 | (0.14, 1.58) | 0.172 | 0.37 | Large heterogeneity | III |
| Alcohol | 4701/539 583 | 6 studies, 7cohorts | Cohort/Case-control | RR | 0.54 (0.47, 0.62) | 0.69 (0.56, 0.85) | 2.16E-04 | 71.9 | (0.36, 1.32) | 0.296 | 0.785 | Large heterogeneity | III |
| Beer | 6050/223 734 | 4 studies, 5 cohorts | Cohort/case-control | RR | 0.59 (0.46, 0.76) | 0.60 (0.49, 0.74) | 1.67E-06 | 35.7 | (0.35, 1.04) | 0.766 | 0.547 | None | III |
| Coffee | NR/170 544a | 6 studies, 10cohorts | Cohort/case-control | OR | 0.51 (0.36, 0.75) | 0.70 (0.60, 0.82) | 1.63E-05 | 42.7 | (0.46, 1.07) | 0.439 | 0.648 | None | III |
| Tea | 10 760/790 026 | 6 studies, 8 cohorts | Cohort/case-control | RR | 0.85 (0.78, 0.92) | 0.88 (0.79, 0.97) | 0.013 | 63.1 | (0.65, 1.19) | 0.722 | 0.697 | Large heterogeneity | IV |
| Milk | At least 6535/224 380a | 5 studies, 5 cohorts | Cohort/Case-control | RR | 1.00 (0.94, 1.07) | 0.95 (0.86, 1.05) | 0.325 | 52.9 | (0.71, 1.27) | 0.652 | 0.579 | Large heterogeneity, P>0·05 of the largest study in meta-analysis | NS |
| Juice | 5810/223 102 | 3 studies | Cohort/Case-control | RR | 0.95 (0.88, 1.03) | 1.00 (0.92, 1.09) | 0.951 | 33.5 | (0.46, 2.17) | 0.369 | 0.748 | P>0·05 of the largest study in meta-analysis | NS |
| Vitamin and calcium intake | |||||||||||||
| Calcium supplement | 2087/187 976 | 2 studies, 2 cohorts | Cohorts | RR | 1.13 (0.92, 1.36) | 1.16 (1.00, 1.35) | 0.047 | 0 | / (study number not enough) | / (study number not enough) | 0.811 | P>0·05 of the largest study in meta-analysis | III |
| Dietary calcium | 9695/41 4911 | 4 studies, 6 cohorts | Cohort | RR | 0.79 (0.69, 0.89) | 0.83 (0.76, 0.90) | 2.43E-05 | 20.4 | (0.69, 0.99) | 0.206 | 0.1425 | None | III |
| Total vitamin D | 6905/220 552 | 2 studies, 4 cohorts | Cohorts | RR | 1.18 (0.94, 1.48) | 1.07 (0.93, 1.23) | 0.358 | 0 | (0.79, 1.46) | 0.101 | 0.715 | P>0·05 of the largest study in meta-analysis | NS |
| Vitamin D supplement | 7815/210 199 | 10 studies, 12 cohorts | RCTs/Cohorts/case-control | RR | 1.38 (1.03, 1.85) | 1.07 (0.92, 1.26) | 0.381 | 0 | (0.90, 1.28) | 0.359 | 0.348 | None | NS |
| Total vitamin C | 6574/224 272 | 2 studies, 3 cohorts | Cohorts | RR | 0.99 (0.90, 1.09) | 1.15 (0.95, 1.26) | 0.268 | 78.0 | (0.06, 21.46) | 0.491 | 0.678 | Large heterogeneity, P>0·05 of the largest study in meta-analysis | NS |
| Vitamin C supplement | 11 917/385 747 | 5 studies, 8 cohorts | Cohorts/case-control | RR | 0.90 (0.79, 1.04) | 1.10 (0.95, 1.26) | 0.204 | 64.8 | (0.73, 1.66) | 0.208 | 0.583 | Large heterogeneity, P>0·05 of the largest study in meta-analysis | NS |
| Total vitamin B6 | 6905/220 677 | 2 studies, 4 cohorts | Cohorts | RR | 1.06 (0.91, 1.24) | 1.01 (0.91, 1.12) | 0.881 | 0 | (0.83, 1.27) | 0.092 | 0.681 | None | NS |
| Dietary intake | |||||||||||||
| Dietary sodium | 5172/660 412 | 4 studies, 4 cohorts | cohort | RR | 1.33 (1.12, 1.58) | 1.38 (1.21, 1.56) | 7.42E-07 | 23.2 | (0.93, 2.05) | 0.513 | 0.449 | None | II |
| Fructose intake | 4902/241 538 | 1 study, 3 cohorts | Cohort | RR | 1.27 (1.04, 1.54) | 1.33 (1.19, 1.49) | 8.65E-07 | 0 | (0.65, 2.74) | 0.795 | 0.194 | None | II |
| Meat | 3820/507 780 | 4 studies, 5 cohorts | Cohorts/case-control | RR | 1.21 (1.05, 1.39) | 1.24 (1.12, 1.39) | 8.49E-05 | 0 | (1.05, 1.46) | 0.256 | 0.131 | None | III |
| Spinach | 1473/45 619 | 1 study, 3 cohorts | Cohort | RR | 1.34 (1.10, 1.64) | 1.21 (1.01, 1.44) | 0.035 | 56.7 | (0.18, 8.07) | 0.390 | 0.266 | Large heterogeneity | IV |
| Fiber | 4750/570 859 | 3 studies, 3 cohorts | Cohort | RR | 0.67 (0.60, 0.75) | 0.71 (0.64, 0.79) | 4.11E-11 | 15.9 | (0.31, 1.63) | 0.727 | 0.223 | None | II |
| DASH-diet | 6449/192 126 | 1 study, 3 cohort | Cohort | RR | 0.65 (0.56, 0.76) | 0.69 (0.64, 0.75) | 6.83E-17 | 0 | (0.42, 1.12) | 0.403 | 0.367 | None | II |
| Dietary potassium | 6637/220 677 | 2 studies, 4 cohorts | Cohort | RR | 0.67 (0.57, 0.78) | 0.59 (0.46, 0.75) | 8.59E-06 | 75.4 | (0.21, 1.65) | 0.990 | 0.725 | Large heterogeneity | III |
| Dietary magnesium | 2105/123 956 | 3 cohorts | Cohort | RR | 0.62 (0.43, 0.89) | 0.56 (0.46, 0.75) | 5.90E-06 | 0 | (0.20, 2.15) | 0.06 | 0.102 | None | III |
| Fruit | 6504/588 959 | 5 studies, 6 cohorts | Cohorts/case-control | RR | 0.79 (0.71, 0.89) | 0.79 (0.71, 0.89) | 1.09E-04 | 30.1 | (0.61, 1.03) | 0.527 | 0.135 | None | III |
| Vegetable | 5485/586 953 | 4 studies, 4 cohorts | Cohorts | RR | 0.90 (0.81, 1.00) | 0.84 (0.75, 0.94) | 0.002 | 42.7 | (0.55, 1.26) | 0.695 | 0.164 | None | IV |
| Energy | 8050/350 694 | 3 studies, 5 cohorts | Cohort | RR | 1.01 (0.85, 1.18) | 1.12 (0.99, 1.27) | 0.075 | 42.0 | (0.26, 4.65) | 0.022 | 0.234 | Large heterogeneity, P>0·05 of the largest study in meta-analysis, small study effect. | NS |
| Other factors | |||||||||||||
| Higher temperatures | At least 490 057 cases/NRa | 4 studies, 8 cohorts | Cohort/case-control | OR | 1.30 (1.20, 1.41) | 1.32 (1.24, 1.39) | 2.54E-21 | 0 | (1.22, 1.43) | 0.175 | 0.044 | Excess significance bias | II |
| Polycystic kidney disease | 128/1368 | 6 studies, 6 cohorts | Case-controls | RR | 1.75 (0.98, 2.64) | 1.85 (1.29, 2.64) | 6.99E-04 | 0 | (1.11, 3.08) | 0.177 | 0.724 | P>0·05 of the largest study in meta-analysis | III |
| Inflammatory bowel disease | 222/1624 | 5 studies, 5 cohorts | Cohort/case-control | RR | 5.62 (4.37, 7.24) | 3.86 (1.14, 13.03) | 0.030 | 88.4 | (0.08, 197.67) | 0.835 | 0.629 | Large heterogeneity | IV |
| Cadmium exposure | NR/88 045a | 6 studies, 6 cohorts | Cohort/ Cross-sectional study | OR | 1.29 (1.01, 1.61) | 1.32 (1.08, 1.62) | 0.007 | 58.1 | (0.74, 2.35) | 0.001 | 0.114 | Large heterogeneity, small study effect. | IV |
| Pulp Stones | 147/284 | 2 studies, 2 cohorts | Case-control | OR | 1.71 (0.90, 3.5) | 1.97 (1.22, 3.18) | 0.006 | 0 | / (study number not enough) | / (study number not enough) | 0.495 | None | IV |
| Physical activity | 7747/299 358 | 2 studies, 4 cohorts | Cohort | RR | 1.02 (0.85, 1.21) | 0.91 (0.73, 1.12) | 0.379 | 89.4 | (0.33, 2.54) | 0.204 | 0.884 | Large heterogeneity, P>0·05 of the largest study in meta-analysis | NS |
| Bariatric surgery | NR/11 348a | 4 studies, 4 cohorts. | RCTs/cohorts | RR | 1.71 (1.44, 2.04) | 1.22 (0.63, 2.35) | 0.551 | 82.8 | (0.07, 20.20) | 0.443 | 0.077 | Large heterogeneity | NS |
| Postmenopausal hormone | NR/71101a | 3 studies, 7 cohorts | RCTs/cohorts/case-control | RR | 1.08 (0.87, 1.34) | 0.91 (0.72, 1.14) | 0.397 | 74.1 | (0.46, 1.81) | 0.007 | 0.274 | Large heterogeneity, P>0·05 of the largest study in meta-analysis, small study effect | NS |
DASH-diet, dietary approaches to stop hypertension-diet; HDL-C, high-density lipoprotein cholesterol; NAFLD, non-alcoholic fatty liver disease; OR, odds ratio; RR, risk ratio; T2D, type 2 diabetes.
Some original studies did not offer detailed number of participants.
Metabolic factors
Of the 10 significant associations, 4 (40%) associations achieved highly suggestive or convincing evidence including waist circumference/BMI, type 2 diabetes (T2D) and gout. The same proportion occurred in associations with weak suggestive evidence, which contained high-density lipoprotein cholesterol (HDL-C) on decreased, triglycerides, hypertension and gallstones. The nephrolithiasis associations with non-alcoholic fatty liver disease (NAFLD) and metabolic syndrome were identified as suggestive evidence. No significant evidence was found for the association, impaired glucose tolerance, by evaluation.
Fluid and beverages intake
Of the 7 significant associations, highly suggestive, convincing, weak evidences were achieved by only 1 (14.3%) association each, which were soda, caffeine and tea, respectively. 4 (57.1%) associations, covering fluid, alcohol, beer and coffee, got suggestive evidence. Milk and juice were recorded as associations covered no significant evidence.
Vitamin and calcium intake
Only two associations among all associations of vitamin and calcium intake, calcium supplement and dietary calcium, were significant and achieved suggestive evidence. Other five associations like total vitamin D, vitamin D supplement, total vitamin C, vitamin C supplement and total vitamin B6 were deemed to have no significant evidence.
Dietary intake
Of the 10 significant associations, 4 (40%) associations achieved Class II credibility level including dietary sodium, fructose intake, fiber and dietary approaches to stop hypertension-diet (DASH-diet). Meat, dietary potassium, dietary magnesium and fruit, these 4 (40%) significant associations got Class III credibility level. For significant associations with weak evidence, spinach and vegetables were classified as them. Of all over associations, only one association without significance and no convincing one were observed.
Other factors
Two associations, higher temperatures and polycystic kidney disease, owned highly suggestive or suggestive evidence among the five significant associations. Other three significant associations embodying inflammatory bowel disease, cadmium exposure and pulp Stones only achieved Class IV credibility level. By analyzing and evaluating data, physical activity, bariatric surgery and postmenopausal hormone were recorded as associations covered no significance.
Evaluation of associations by GRADE approach
Of the 46 associations in the umbrella review, we found that only 9 (19.6%) and 7 (15.2%) were supported by high and moderate evidence certainty based on the GRADE approach, respectively (Fig. 3 and Supplementary Table S3, Supplemental Digital Content 1, http://links.lww.com/JS9/C677). This indicated that meta-analyses based on extensive prospective cohort studies for most risk factors are urgently needed in the future.
Figure 3.
Forest plot to demonstrate the main results of umbrella review. Purple box and solid line are the effect size with its 95% CIs of meta using random-effect model, and blue dotted line represent 95% prediction intervals. 2hGlu, 2-h glucose after an oral glucose challenge; 25(OH)D, 25-Hydroxyvitamin D; HDL-C, high-density lipoprotein cholesterol; NAFLD, non-alcoholic fatty liver disease; T2D, type 2 diabetes.
Discussion
Our study provided a panoramic display of 46 reported associations from meta-analyses of observational studies between exposures and nephrolithiasis. Of the 34 significant associations, only 11 (32.4%) associations achieved highly suggestive or convincing evidence. Besides, Among the 46 associations, we found that only nine (19.6%) and seven (15.2%) were supported by high and moderate evidence certainty, respectively. Integrating the evidence from the observational studies enables a more reliable interpretation of epidemiological relationships. We found that increased BMI, T2D, higher levels of circulating calcium, urinary calcium, circulating 25(OH)D, and urinary sodium causally increased the risk of nephrolithiasis. Increased waist circumference and waist-hip ratio were suggestively associated with a higher nephrolithiasis risk. In addition, we demonstrated that caffeine and tea intake were closely associated with a lower nephrolithiasis risk. Higher coffee, alcohol, and beer intake, and higher levels of urinary magnesium were suggestively associated with a decreased nephrolithiasis risk.
Metabolic factors
Metabolic syndrome and its components have been established to be associated with a higher nephrolithiasis risk in observational studies. Besides, obesity/central obesity was supported by high and moderate evidence certainty. Therefore, it would be advisable to conclude an influential relationship between obesity and nephrolithiasis risk. And future similar research is unlikely to change this evidence. In addition, T2D is likely a causal risk factor for the development of nephrolithiasis. A meta-analysis that simultaneously enrolled prospective and retrospective studies simultaneously with high heterogeneity decreased the evidence level (very low certainty). Thus, a prospective updated meta-analysis incorporating a larger number of prospective cohort studies is warranted to increase the level of evidence. The main underlying mechanisms by which obesity and T2D affect kidney stone formation involve urinary derangements, especially a lower urinary pH25 and inflammation26.
For HDL-C and NAFLD, there is weak evidence that a lower HDL-C level and NAFLD increases the risk of nephrolithiasis. However, neither the primary study with the largest sample nor the only prospective cohort study was not significant. For triglycerides, the results of the largest sample primary study and the only prospective cohort study were inconsistent. Therefore, a meta-analysis based on extensive prospective cohort studies may be required to resolve the disparity between the results of observational studies. Similar to lipid traits, there is weak evidence that hypertension increases the risk of nephrolithiasis, which indirectly suggests the close relationship between abnormal blood lipids and hypertension27. On the other hand, gallstone, one of the outcomes of abnormal lipid metabolism28, and nephrolithiasis belong to pathological component deposition in the body, but the evidence level for the relevance between the two is very limited. This discrepancy may be partially attributable to the high heterogeneity and small study effect in the meta-analysis of observational studies. Thus, an updated meta-analysis is necessary.
Although gout was found to be associated with nephrolithiasis in observational studies (highly suggestive), we found no formal meta-analysis on serum urate levels and urolithiasis. A cohort study of 239 331 Korean adults reported that increased serum urate levels increased the risk of nephrolithiasis in a dose-dependent manner29. In contrast, a recent observational study of the UK Biobank data demonstrated none causal effect of serum urate levels on nephrolithiasis30. Therefore, it would be inadvisable to conclude a causal relationship based on the present evidence. Given that nephrolithiasis is a heterogeneous disease that includes, but is not limited to uric acid calculus, the causal effect of serum urate levels could be diluted by other types of urolithiasis, such as calcium oxalate stones. Associations between serum urate levels and gout with urolithiasis should consider the components of stone.
Fluid and beverages intake
Results of umbrella review demonstrated that caffeine, coffee and tea intake were associated with a lower risk of nephrolithiasis. However, only caffeine reached the highly suggestive class with a high level of evidence certainty. Regarding alcohol and beer intake, the observational studies indicate a suggestive association between them and kidney stone development. However, this association should be treated with caution because the results of partial sensitivity analyses were not significant. The diuretic effects of alcohol, tea, and coffee might be the common mechanism that lowers the risk of nephrolithiasis31,32. In addition, tea and coffee might exert many other protective effects to against stone formation, such as caffeine intake, additional fluid volume intake and the effects of antioxidant components32. Although taking more coffee, tea, alcohol and beer may help prevent nephrolithiasis, people should balance their potential harms on other organs, especially for alcohol consumption. The long-term impact of over intaking of alcohol on the liver may ultimately involve the formation of nephrolithiasis. Interestingly, the result of Soda in the umbrella review seems to be ambivalent. The attitude that Soda could be the risk factor for nephrolithiasis became whirling due to convincing evidence but poor GRADE-score, which may hint that the key for preventing nephrolithiasis can be caught by clarifying the boundary range of soda intake rather than focusing on the identity definition of Soda. Over the range, soda intake promotes the excretion of calcium, uric acid, and oxalic in the urine33,34.
In our review, the effect of juice on the formation of nephrolithiasis was not significant, which may be related to the composition and its influence. In addition to the additional liquid replenishment of juice, orange, lemon, and grapefruit juices are rich in citrate, which plays a role in alkalizing urine in the body35, at the same time, the citrate in urine also plays a preventive role in preventing stones36,37. On the other hand, the role of orange juice and grapefruit juice in enhancing urine oxalate cannot be ignored38,39, and the high carbohydrates and sugars in juice are also risk factors for the formation of kidney stones40,41. The role of juice needs to consider not only the influence of multiple components but also the variety of juice, which reflects the need for more specific research. For milk, it is important to note that, contrary to popular beliefs about the relationship between milk and nephrolithiasis, milk does not promote the formation of nephrolithiasis according to our result. Milk, which contains whey protein and albumin, did not affect the average urinary calcium, uric acid, citrate, oxalate, pH, and urinary saturation index in urine42.
Vitamin and calcium intake
Although once reviews demonstrated that higher levels of circulating calcium and urinary calcium were causally associated with kidney stone formation43,44, it is crucial to separate genetically predicted higher serum calcium levels and external calcium intake because calcium in the intestine acts as a chelator for oxalate. A low-calcium diet will increase oxalate absorption in the intestine, thereby leading to oxaluria and increasing the risk of calcium oxalate crystal formation. This was supported in the umbrella review that calcium supplement increases, but dietary calcium intake decreases the risk of nephrolithiasis. Neither total vitamin D intake nor vitamin D supplementation was associated nephrolithiasis in the meta-analysis of observational studies. However, our previously published mendelian randomization study45 suggested that higher 25(OH)D levels are causally associated with kidney stones. But the effect in the real world depends on the cumulative exposure to vitamin D intervention over time. In theory, long-term extensive supplementation with exogenous vitamin D alone, calcium alone, or a combination of the two to elevate circulating 25(OH)D and calcium levels may increase the risk of nephrolithiasis due to the cumulative effects over time.
In addition to vitamin D, the results of vitamin C and vitamin B6 in our article suggested that there was no significant association with the formation of nephrolithiasis. However, total vitamin C and vitamin C supplementation were observed to be risk factors for kidney stones in men but not in women46. This difference may involve hormonal differences between men and women, but the exact mechanism is still not well understood. In the case of vitamin B6, as a cofactor of alanine-glyoxylate aminotransferase, deficiency of vitamin B6 may eventually lead to increased oxalate production and excretion via raising the amount of glyoxylate converted to oxalate through lactate dehydrogenase. Some studies have shown that vitamin B6 intake can reduce oxalate excretion in the urine47–49, but other studies have reached contradictory conclusions50,51. Reviewing the results we obtained, the impact of vitamin B6 on kidney stones may depend on the degree of intake, high intake may achieve the purpose of stone suppression, but the daily intake of vitamin B6 usually was not paid attention by residents, at the same time, the efficacy of vitamin B6 may also be related to its metabolism in the body.
Dietary intake
Evidence from epidemiological studies and randomized controlled trials (RCTs) has shown a significant association of sodium intake and urinary sodium levels with urinary calcium excretion52. Our study supported a link between urinary sodium levels and nephrolithiasis. The level of urinary sodium reflects the complex interplay between dietary sodium intake and homeostatic mechanisms. Kidney function, potential genetic influence, and other pathways might all contribute to the control of sodium excretion53. For patients with high sodium levels in spot urine samples, decreasing dietary salt intake to lower urinary sodium levels would be an effective intervention to reduce nephrolithiasis risk. This view corresponds to DASH-diet in our study, which advocated low in salt, fat and sugar.
Rich in non-dairy animal protein, meat consumption was considered a risk factor for kidney stones because the renal acid load in this diet tends to be inversely proportional to the excretion of citrate in the urine54,55, resulting in a negative calcium balance, low citrate, low potassium and low magnesium in the urine, which reduces greatly the ability to inhibit the crystallization of oxalates in the urine56. As shown in our article, meat promotes the formation of nephrolithiasis, while dietary potassium and dietary magnesium act as inhibitors. In addition, through animal experiments, meat intake has been verified to affect the changes of gut microbiota. Several studies have found that high-protein diets led to pro-inflammatory changes in the intestines of mice, an increase in disease-causing microorganisms, and a decrease in oxalate-degrading bacteria57,58. Other studies have manifested that protein intake, particularly chicken-derived protein, may be associated with positive changes in the gut microbiota of mice, and a significant increase in oxalic-degrading lactobacillus was observed in the gut of mice59,60. Differences in these effects may depend on the absolute amount of protein intake, with beneficial flora at an advantage when intake was moderate; When intake was at both extremes, the representation of beneficial bacteria tended to be lowest61.
According to our results, dietary fiber can be regarded as an inhibitor of kidney stones, and not surprisingly, fruits and vegetables rich in dietary fiber also exert the same effect. Notably, the effect of fructose intake and spinach contradict the above conclusion. Li and colleagues reported that negatively charged protein biomolecules in spinach leaching solution and calcium ions could biosynthesize calcium oxalate crystals by mutual reaction, which may pave the way for the explanation of the special outcome of spinach.
Other factors
The increased risk of kidney stones after bariatric surgery has been confirmed by several studies62–64, and although this contradicted the results obtained in our study, it also suggests that the results should be understood from multiple perspectives and multi-factorial directions. There are various types of bariatric surgery, including sleeve gastrectomy (SG), biliopancreatic diversion with duodenal switch (BPD-DS) and Roux-en-Y gastric bypass (RYGB); however, only the postoperative status of RYGB was esteemed as a risk factor for kidney stones64. After RYGB, decreased urine output, decreased urine citrate, and increased urine oxalate are all visible pathogenetic factors for nephrolithiasis, meanwhile, through animal experiments, it was observed that Oxalobacter formigines, a non-pathogenic gut commensal that could consume oxalate, was colonized in mice to be able to reduce the increase of urinary oxalate after RYGB65, which suggests that the effect of bariatric surgery on intestinal flora may be a factor in the pathogenesis of kidney stones.
Based on epidemiological statistics, studies have indicated that men do have a higher incidence of kidney stones than women, by a ratio of about 2-3: 111,66. Gender differences in nephrolithiasis incidence were usually attributed to hormonal differences between men and women, despite the exact mechanisms are still unclear. The existing literatures have reported that the promoting effect of androgen and the inhibiting effect of estrogen in urinary oxalate excretion66, but combined with our results, we could conclude that the promoting effect of androgen on kidney stones was more dominant than the inhibiting effect of estrogen. In addition, several studies have updated the role of androgen receptor (AR) signaling in nephrolithiasis formation, for example, upregulation of liver glycolate oxidase and renal epithelial nicotinamide adenine dinucleotide phosphate oxidase (NAPDH) to increase oxalate biosynthesis67,68, and inhibition of macrophage recruitment and its ability to phagocytose crystals69. Therefore, targeted therapy for AR can theoretically be used as a viable therapeutic intervention for kidney stones. In fact, dimethyl curcumin (ASC‑J9), a kind of AR degradation enhancer, has been reported to restrain oxalate crystal formation by controlling the kidney tubular epithelial cell injury and oxalate biosynthesis of rats69. Since the current progress is still in the research stage, the task of developing therapeutic drugs in this direction is still onerous.
From our results, higher temperatures, polycystic kidney disease, inflammatory bowel disease, cadmium exposure and pulp stones were believed to increase the risk of kidney stones, but according to the GREAD score, the evaluation results for these risk factors are not yet robust, indicating that further exploration and research are needed to help identify targeted treatment measures for possible factors that may lead to nephrolithiasis.
To sum up, the extensive and complex pathogenesis of kidney stones were observed by us, which did not only affect kidney singularly to result in the formation of stones. According to our article, various factors tended to affect the circulatory metabolism of the whole body, especially the metabolic association between liver, intestine and kidney, which suggests that kidney stones should be regarded as the consequence of systemic metabolic diseases, rather than ordinary kidney diseases.
The major strength of our study is that we systematically and thoroughly summarized and presented evidence of the associations between exposures and nephrolithiasis and then applied well-defined criteria to assess the credibility of the included studies. However, our study also had several limitations. Firstly, important exposures that were not reported in meta-analyses may be overlooked because umbrella reviews focus on existing meta-analyses. Secondly, outdated meta-analyses might provide incomplete conclusions with less power if a high-quality original study existed. Thirdly, we were unable to investigate the nonlinear causal associations between exposures and nephrolithiasis even if they did exist. More researches are needed to assess the causality in the future.
Conclusion
In summary, our study demonstrates the suggestive causal (central obesity, T2D, gout, dietary sodium, fructose intake and higher temperatures) risk factors of nephrolithiasis. We also demonstrate the suggestive causal (coffee/alcohol/beer intake, dietary calcium and DASH-diet) protective factors of nephrolithiasis. It will be helpful to judge the relative priority of exposures associated with nephrolithiasis for future study and prevention of this disease. However, updated meta-analyses based on extensive prospective cohort studies for most risk factors are still urgently needed in the future.
Ethics committee approval
No ethics committee approval was conducted because this study did not involve an animal and human experiments or data containing individual information.
Consent
No ethics committee approval was conducted because this study did not involve an animal and human experiments or data containing individual information.
Source of funding
This study was supported by the Foundation of Science & Technology Department of Sichuan Province [2022YFS0304, 2023YFS0029], the Post-Doctor Research Project, West China Hospital of Sichuan University[ 2020HXBH016], the National Natural Science Foundation of China [82200851] and the Natural Science Foundation of Sichuan Province of China [2023NSFSC1533].
Author contribution
The authors’ responsibilities were as follows: C.C., M.Y., J.Z., W.J., L.X., L.H., W.K., J.X. for concept and design; C.C., M.Y. for the data acquisition; J.Z., M.Y., X.L. for statistical analysis and interpretation of data; C.C. for drafting of the manuscript; W.J., L.H., W.K., J.X. for critical revision of the manuscript. All authors interpreted data, revised the document, and read and approved the final manuscript.
Conflicts of interest disclosure
None.
Research registration unique identifying number (UIN)
The umbrella review was registered with Research Registry (researchregistry10240).
Guarantor
Xi Jin.
Data availability statement
All data generated or analyzed during this study are included in this article. The data are available from the corresponding author upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Footnotes
C.C., Y.M. and Z.J. contribute equally.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 29 May 2024
Contributor Information
Yucheng Ma, Email: yuchengma88@163.com.
Chao Cheng, Email: Doc_cheng1999@163.com.
Zhongyu Jian, Email: jzyhx@scu.edu.cn.
Jun Wen, Email: 18280033326@163.com.
Liyuan Xiang, Email: 280907594@qq.com.
Hong Li, Email: lihonghxhx@scu.edu.cn.
Kunjie Wang, Email: wangkj@scu.edu.cn.
Xi Jin, Email: jinxi@wchscu.cn.
References
- 1. Sorokin I, Mamoulakis C, Miyazawa K, et al. Epidemiology of stone disease across the world. World J Urol 2017;35:1301–1320. [DOI] [PubMed] [Google Scholar]
- 2. Scales CD, Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol 2012;62:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu G, Liu B, Sun Y, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ (Clin Res Ed) 2018;362:k1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeng G, Mai Z, Xia S, et al. Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. BJU Int 2017;120:109–116. [DOI] [PubMed] [Google Scholar]
- 5. Strazzullo P, Barba G, Vuotto P, et al. Past history of nephrolithiasis and incidence of hypertension in men: a reappraisal based on the results of the Olivetti Prospective Heart Study. Nephrol Dial Transplant 2001;16:2232–2235. [DOI] [PubMed] [Google Scholar]
- 6. Shoag J, Halpern J, Goldfarb DS, et al. Risk of chronic and end stage kidney disease in patients with nephrolithiasis. J Urol 2014;192:1440–1445. [DOI] [PubMed] [Google Scholar]
- 7. Keddis MT, Rule AD. Nephrolithiasis and loss of kidney function. Curr Opin Nephrol Hypertens 2013;22:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eisner BH, Goldfarb DS. A nomogram for the prediction of kidney stone recurrence. J Am Soc Nephrol 2014;25:2685–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hyams ES, Matlaga BR. Economic impact of urinary stones. Transl Androl Urol 2014;3:278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Antonelli JA, Maalouf NM, Pearle MS, et al. Use of the National Health and Nutrition Examination Survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur Urol 2014;66:724–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ziemba JB, Matlaga BR. Epidemiology and economics of nephrolithiasis. Investig Clin Urol 2017;58:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peerapen P, Thongboonkerd V. Kidney stone prevention. Adv Nutr (Bethesda, Md) 2023;14:555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stamatelou K, Goldfarb DS. Epidemiology of kidney stones. Healthcare (Basel, Switzerland) 2023;11:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aromataris E, Fernandez RS, Godfrey C, et al. Methodology for jbi umbrella reviews. In: 2014. Faculty of Science, Medicine and Health - Papers: part A. 3344.
- 15. Smith V, Devane D, Begley CM, et al. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol 2011;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belbasis L, Bellou V, Ioannidis JPA. Conducting umbrella reviews. BMJ Med 2022;1:e000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 18. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clin Res Ed) 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009;172:137–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials 2007;4:245–253. [DOI] [PubMed] [Google Scholar]
- 23. Kim JY, Son MJ, Son CY, et al. Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. Lancet Psychiatry 2019;6:590–600. [DOI] [PubMed] [Google Scholar]
- 24. Foroutan F, Guyatt G, Zuk V, et al. GRADE Guidelines 28: Use of GRADE for the assessment of evidence about prognostic factors: rating certainty in identification of groups of patients with different absolute risks. J Clin Epidemiol 2020;121:62–70. [DOI] [PubMed] [Google Scholar]
- 25. Bamberger JN, Rosen DC, Khusid JA, Kaplan-Marans E, Gallante B, Kapoor A, Paranjpe I, Atashsokhan DJ, Atallah WM, Gupta M. The impact of metabolic syndrome components on urinary parameters and risk of stone formation. World J Urol 2021;39:4483–4490. [DOI] [PubMed] [Google Scholar]
- 26. Sakhaee K. Unraveling the mechanisms of obesity-induced hyperoxaluria. Kidney Int 2018;93:1038–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yi Q, Hu H, Zeng Q. Association of triglycerides to high density lipoprotein cholesterol ratio with hypertension in Chinese adults: a cross-sectional study. Clin Exp Hypertens (New York, NY : 1993) 2023;45:2195996. [DOI] [PubMed] [Google Scholar]
- 28. Zeng D, Wu H, Huang Q, et al. High levels of serum triglyceride, low-density lipoprotein cholesterol, total bile acid, and total bilirubin are risk factors for gallstones. Clin Lab 2021;67. doi: 10.7754/Clin.Lab.2021.201228 [DOI] [PubMed] [Google Scholar]
- 29. Kim S, Chang Y, Yun KE, et al. Development of nephrolithiasis in asymptomatic hyperuricemia: a cohort study. Am J Kidney Dis 2017;70:173–181. [DOI] [PubMed] [Google Scholar]
- 30. Narang RK, Gamble GG, Topless R, et al. Assessing the relationship between serum urate and urolithiasis using Mendelian randomization: an analysis of the UK Biobank. Am J Kidney Dis 2021;78:210–218. [DOI] [PubMed] [Google Scholar]
- 31. Jones P, Karim Sulaiman S, Gamage KN, et al. Do lifestyle factors including smoking, alcohol, and exercise impact your risk of developing kidney stone disease? Outcomes of a systematic review. J Endourol 2021;35:1–7. [DOI] [PubMed] [Google Scholar]
- 32. Barghouthy Y, Corrales M, Doizi S, et al. Tea and coffee consumption and pathophysiology related to kidney stone formation: a systematic review. World J Urol 2021;39:2417–2426. [DOI] [PubMed] [Google Scholar]
- 33. Nguyen NU, Dumoulin G, Henriet MT, et al. Increase in urinary calcium and oxalate after fructose infusion. Hormone Metab Res 1995;27:155–158. [DOI] [PubMed] [Google Scholar]
- 34. Fox IH, Kelley WN. Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism 1972;21:713–721. [DOI] [PubMed] [Google Scholar]
- 35. Baia Lda C, Baxmann AC, Moreira SR, et al. Noncitrus alkaline fruit: a dietary alternative for the treatment of hypocitraturic stone formers. J Endourol 2012;26:1221–1226. [DOI] [PubMed] [Google Scholar]
- 36. Pak CY. Citrate and renal calculi: an update. Miner Electrolyte Metab 1994;20:371–377. [PubMed] [Google Scholar]
- 37. Pak CY. Citrate and renal calculi: new insights and future directions. Am J Kidney Dis 1991;17:420–425. [DOI] [PubMed] [Google Scholar]
- 38. Wabner CL, Pak CY. Effect of orange juice consumption on urinary stone risk factors. J Urol 1993;149:1405–1408. [DOI] [PubMed] [Google Scholar]
- 39. Goldfarb DS, Asplin JR. Effect of grapefruit juice on urinary lithogenicity. J Urol 2001;166:263–267. [PubMed] [Google Scholar]
- 40. Gamage KN, Jamnadass E, Sulaiman SK, et al. The role of fluid intake in the prevention of kidney stone disease: a systematic review over the last two decades. Turk J Urol 2020;46:S92–s103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lemann J, Jr, Piering WF, Lennon EJ. Possible role of carbohydrate-induced calciuria in calcium oxalate kidney-stone formation. N Eng J Med 1969;280:232–237. [DOI] [PubMed] [Google Scholar]
- 42. Hattori CM, Tiselius HG, Heilberg IP. Whey protein and albumin effects upon urinary risk factors for stone formation. Urolithiasis 2017;45:421–428. [DOI] [PubMed] [Google Scholar]
- 43. Bargagli M, Ferraro PM, Vittori M, et al. Calcium and vitamin D supplementation and their association with kidney stone disease: a narrative review. Nutrients 2021;13:4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alexander RT. Kidney stones, hypercalciuria, and recent insights into proximal tubule calcium reabsorption. Curr Opin Nephrol Hypertens 2023;32:359–365. [DOI] [PubMed] [Google Scholar]
- 45. Jian Z, Huang Y, He Y, et al. Genetically predicted lifelong circulating 25(OH)D levels are associated with serum calcium levels and kidney stone risk. J Clin Endocrinol Metab 2021;107:e1159–e1166. [DOI] [PubMed] [Google Scholar]
- 46. Ferraro PM, Curhan GC, Gambaro G, et al. Total, Dietary, and Supplemental Vitamin C Intake and Risk of Incident Kidney Stones. Am J Kidney Dis 2016;67:400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Balcke P, Schmidt P, Zazgornik J, et al. Pyridoxine therapy in patients with renal calcium oxalate calculi. Pro Eur Dial Transplant Assoc 1983;20:417–421. [PubMed] [Google Scholar]
- 48. Yendt ER, Cohanim M. Response to a physiologic dose of pyridoxine in type I primary hyperoxaluria. N Eng J Med 1985;312:953–957. [DOI] [PubMed] [Google Scholar]
- 49. Mitwalli A, Ayiomamitis A, Grass L, et al. Control of hyperoxaluria with large doses of pyridoxine in patients with kidney stones. Int Urol Nephrol 1988;20:353–359. [DOI] [PubMed] [Google Scholar]
- 50. Edwards P, Nemat S, Rose GA. Effects of oral pyridoxine upon plasma and 24-hour urinary oxalate levels in normal subjects and stone formers with idiopathic hypercalciuria. Urol Res 1990;18:393–396. [DOI] [PubMed] [Google Scholar]
- 51. Kaelin A, Casez JP, Jaeger P. Vitamin B6 metabolites in idiopathic calcium stone formers: no evidence for a link to hyperoxaluria. Urol Res 2004;32:61–68. [DOI] [PubMed] [Google Scholar]
- 52. Afsar B, Kiremit MC, Sag AA, et al. The role of sodium intake in nephrolithiasis: epidemiology, pathogenesis, and future directions. Eur J Intern Med 2016;35:16–19. [DOI] [PubMed] [Google Scholar]
- 53. Pazoki R, Evangelou E, Mosen-Ansorena D, et al. GWAS for urinary sodium and potassium excretion highlights pathways shared with cardiovascular traits. Nat Commun 2019;10:3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Trinchieri A. Diet and renal stone formation. Minerva Med 2013;104:41–54. [PubMed] [Google Scholar]
- 55. Vezzoli G, Dogliotti E, Terranegra A, et al. Dietary style and acid load in an Italian population of calcium kidney stone formers. Nutr Metab Cardiovasc Dis 2015;25:588–593. [DOI] [PubMed] [Google Scholar]
- 56. Kok DJ, Iestra JA, Doorenbos CJ, et al. The effects of dietary excesses in animal protein and in sodium on the composition and the crystallization kinetics of calcium oxalate monohydrate in urines of healthy men. J Clin Endocrinol Metab 1990;71:861–867. [DOI] [PubMed] [Google Scholar]
- 57. Mu C, Yang Y, Luo Z, et al. The colonic microbiome and epithelial transcriptome are altered in rats fed a high-protein diet compared with a normal-protein diet. J Nutr 2016;146:474–483. [DOI] [PubMed] [Google Scholar]
- 58. Kostovcikova K, Coufal S, Galanova N, et al. Diet rich in animal protein promotes pro-inflammatory macrophage response and exacerbates colitis in mice. Front Immunol 2019;10:919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhu Y, Shi X, Lin X, et al. Beef, chicken, and soy proteins in diets induce different gut microbiota and metabolites in rats. Front Microbiol 2017;8:1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mehra Y, Rajesh NG, Viswanathan P. Analysis and characterization of Lactobacillus paragasseri and Lacticaseibacillus paracasei: two probiotic bacteria that can degrade intestinal oxalate in hyperoxaluric rats. Probiotics Antimicrob Proteins 2022;14:854–872. [DOI] [PubMed] [Google Scholar]
- 61. Qi X, Xu W, Guo M, et al. Rice- or pork-based diets with similar calorie and content result in different rat gut microbiota. Int J Food Sci Nutr 2017;68:829–839. [DOI] [PubMed] [Google Scholar]
- 62. Laurenius A, Sundbom M, Ottosson J, et al. Incidence of kidney stones after metabolic and bariatric surgery-data from the scandinavian obesity surgery registry. Obes Surg 2023;33:1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gonzalez RD, Canales BK. Kidney stone risk following modern bariatric surgery. Curr Urol Rep 2014;15:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Uy M, Di Lena R, Hoogenes J, et al. Bariatric surgery in patients with a history of nephrolithiasis: 24-h urine profiles and radiographic changes after Roux-en-Y gastric bypass versus sleeve gastrectomy. Obes Surg 2021;31:1673–1679. [DOI] [PubMed] [Google Scholar]
- 65. Canales BK, Hatch M. Kidney stone incidence and metabolic urinary changes after modern bariatric surgery: review of clinical studies, experimental models, and prevention strategies. Surg Obes Relat Dis 2014;10:734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fan J, Chandhoke PS, Grampsas SA. Role of sex hormones in experimental calcium oxalate nephrolithiasis. J Am Soc Nephrol 1999;10(suppl 14):S376–380. [PubMed] [Google Scholar]
- 67. Yoshihara H, Yamaguchi S, Yachiku S. Effect of sex hormones on oxalate-synthesizing enzymes in male and female rat livers. J Urol 1999;161:668–673. [PubMed] [Google Scholar]
- 68. Liang L, Li L, Tian J, et al. Androgen receptor enhances kidney stone-CaOx crystal formation via modulation of oxalate biosynthesis & oxidative stress. Mol Endocrinol (Baltimore, Md) 2014;28:1291–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhu W, Zhao Z, Chou F, et al. Loss of the androgen receptor suppresses intrarenal calcium oxalate crystals deposition via altering macrophage recruitment/M2 polarization with change of the miR-185-5p/CSF-1 signals. Cell Death Dis 2019;10:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. The data are available from the corresponding author upon reasonable request.