Abstract
Adenovirus (Ad) efficiently delivers its DNA genome into a variety of cells and tissues, provided that these cells express appropriate receptors, including the coxsackie-adenovirus receptor (CAR), which binds to the terminal knob domain of the viral capsid protein fiber. To render CAR-negative cells susceptible to Ad infection, we have produced a bispecific hybrid adapter protein consisting of the amino-terminal extracellular domain of the human CAR protein (CARex) and the Fc region of the human immunoglobulin G1 protein, comprising the hinge and the CH2 and CH3 regions. CARex-Fc was purified from COS7 cell supernatants and mixed with Ad particles, thus blocking Ad infection of CAR-positive but Fc receptor-negative cells. The functionality of the CARex domain was further confirmed by successful immunization of mice with CARex-Fc followed by selection of a monoclonal anti-human CAR antibody (E1-1), which blocked Ad infection of CAR-positive cells. When mixed with Ad expressing eGFP, CARex-Fc mediated an up to 250-fold increase of transgene expression in CAR-negative human monocytic cell lines expressing the high-affinity Fcγ receptor I (CD64) but not in cells expressing the low-affinity Fcγ receptor II (CD32) or III (CD16). These results open new perspectives for Ad-mediated cancer cell vaccination, including the treatment of acute myeloid leukemia.
Adenoviruses (Ad) carrying therapeutic genes are currently among the leading candidate vectors for gene therapy and have been used to transduce numerous types of tissues and cell lines, although with varying efficiency. The efficacy of Ad entry is a major determinant of transgene expression. Entry of subgroup C Ad, including the currently used Ad2 and Ad5, depends on a primary Ad receptor, the coxsackie-adenovirus receptor (CAR) protein (5, 53). Secondary Ad receptors have earlier been identified as αvβ3- and αvβ5-type integrins (63). While the primary receptor is responsible for virus attachment by binding to the distal portion of the Ad fiber protein, the integrins bind to the RGD motif of the capsid protein penton base, thus mediating virus uptake into the cells by receptor-mediated endocytosis (56, 59). Binding assays using radiolabeled virions or purified fiber protein demonstrated that different cell cultures express variable amounts of primary Ad type C receptor(s) (12, 48, 63, 65). In polarized epithelial cells, the availability of CAR seems to be a limiting step to successful gene expression (58). CAR expression levels also seem to be limiting in brain tissue (10), skeletal muscle (1, 39), endothelial and smooth muscle cells (65), and cells of hematopoietic origin including human leukocytes (3, 27, 48). Furthermore, the expression levels of the primary receptor were found to vary considerably among tumors of different origins, including hematopoietic malignancies (9, 11, 18, 61), human melanoma cell cultures (26), bladder cancer cells (35), and ovarian tumors (30).
Even cells lacking CAR and/or αv integrin expression can be infected with high doses of vectors (reference 26 and unpublished data). However, a more economical and reliable procedure is to broaden the tropism of Ad vectors. The host range of Ad can be modified by three means: genetic or biochemical alterations of the Ad fiber protein or the use of bifunctional reagents. Genetic alterations include modifications of capsid fiber proteins, introducing new peptide sequences into the HI loop of the fiber knob (13, 31, 42) and insertion of a 10-amino-acid peptide linker sequence followed by the integrin-binding RGD motif at the carboxy terminus of the Ad5 fiber protein (66). Alternatively, the coding sequence for a polylysine stretch can be inserted at the C-terminal end of the fiber gene to allow binding to cells expressing heparin-binding motifs (64). A different approach includes the exchange of fiber of the commonly used Ad2/5 serotype with fiber of alternative serotypes such as Ad11 and Ad35 (20, 32, 41, 50), facilitating expression in hematopoietic cell lines (46) and with moderate success also in CD34+ cells (47). Biochemical alterations include the coupling of asialoglycoprotein-polylysine conjugates to wild-type Ad5 (67) or formulation of Ad complexes with polycationic polymers and cationic lipids (17). In addition, bispecific antibodies with specificities to αv integrin (65) or CD3 (62) and a second specificity to a FLAG epitope inserted in the penton base have been reported. Finally, bispecific proteins containing an Ad5 fiber-specific blocking antibody either fused or chemically coupled with receptor target-specific molecules like folate (15), epidermal growth factor (EGF) (60), fibroblast growth factor (22), CD40 (51), and the pancarcinoma antigen EpCAM (23), have been introduced.
In this study, we have produced a bispecific protein, CARex-Fc, with defined specificities and high affinities to both Ad capsid and cell surface Fcγ receptor I. The CARex-Fc fusion protein consists of the ectodomain of CAR fused to the immunoglobulin Fc domain. The protein was produced in COS7 cells and purified by affinity chromatography. CARex-Fc efficiently blocked transgene expression of a recombinant Ad expressing enhanced green fluorescent protein (eGFP) in A549 human lung carcinoma cells. The CARex-Fc protein was tested for its ability to redirect AdCMV-eGFP-mediated expression to cells lacking CAR expression but expressing one of the Fcγ receptors. In cells expressing high levels of the high-affinity Fcγ receptor I, CARex-Fc led to an up to 250-fold increase of eGFP expression. Moderate eGFP expression was obtained in cells with low levels of cell surface CD64 expression. No eGFP expression was obtained in cells expressing the low-affinity Fcγ receptors II and III, demonstrating the selectivity of CARex-Fc. In addition, the CARex-Fc fusion protein was used for immunization of mice and selection of an Ad infection-blocking monoclonal antibody specific for human CAR.
MATERIALS AND METHODS
Cell culture.
Cells were grown in RPMI 1640 plus 10% fetal calf serum (FCS) unless indicated differently. Jurkat (acute T-cell leukemia), U937 (D) (monocytic lymphoma), Daudi (Burkitt's B-lymphoma), and K562 (chronic myelogenous leukemia) cells were provided by F. Nestle, Department of Dermatology, University of Zürich, Zürich, Switzerland. HL-60 (promyelocytic leukemia) cells were obtained from A. Ziogas, Medical Virology, University of Zürich. Raji (Burkitt's B-lymphoma) cells were obtained from O. Georgiev, Institute of Molecular Biology, University of Zürich. U937 (H) (monocytic lymphoma), THP-1 (acute monocytic leukemia), Kasumi-1 (acute myeloid leukemia [AML]), and SigM5 (AML) cells, as well as primary AML cells were kindly provided by G. Schoedon and Peghini, Department of Medicine, University of Zürich. THP-1 cells were cultured in Iscove modified Dulbecco medium (IMDM) plus 10% FCS, whereas SigM5 cells were propagated in IMDM plus 20% human serum. All cell lines were originally purchased from the American Type Culture Collection, with exception of Kasumi-1 (Department of Human and Animal Cell Cultures, Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany) and SigM5 (57). Primary patient AML cells were isolated using the standard therapeutic leukapheresis procedure after obtaining informed consent, typed, and frozen in IMDM plus 50% pooled human serum. Short cultures of 2 days were performed in IMDM plus 10% FCS. Natural killer cells were isolated as described elsewhere (45) from the blood of healthy volunteers and were propagated in AIM-V medium containing 10% human serum, 10% leukocyte-conditioned medium (LCM), 2% HEPES, 1% sodium pyruvate, 1% glutamine, 1% nonessential amino acids, 1% penicillin-streptomycin and 100 U of interleukin-2 per ml. Human 911 cells were received from Fallaux et al. (16) and grown in Dulbecco's modified Eagle's medium (DMEM) plus 10% FCS. COS7 cells were kindly provided by S. Nagata, Osaka University, Osaka, Japan, and were grown in low-FCS medium (TurboDoma containing 1% FCS; Cell Culture Technologies, Gravesano, Switzerland). DO4 cells producing CTLA4-immunoglobulin G1 (IgG1) fusion protein specific for B7 (14) were obtained from R. Dummer, Department of Dermatology, University of Zürich, and were grown in DMEM plus 10% FCS. The A549 human lung carcinoma cell line was received from P. Sonderegger, Biochemisches Institut, University of Zürich, and was grown in DMEM plus 10% FCS. All cell lines were routinely screened for the absence of mycoplasma contamination.
Construction of pCMV1-CARex-Fc.
The pCMV1-Fc expression plasmid was originally constructed and kindly provided by X. He, Children's Hospital, Harvard Medical School, Boston, Mass. (unpublished results). The plasmid contains a 699-bp Fc fragment derived from the human IgG1 cDNA, encoding the hinge, CH2, and CH3 regions, which was amplified by the use of sequence-specific primers and PCR. In addition to the polylinker, the plasmid contains the human cytomegalovirus (CMV) enhancer/promoter, a polyadenylation sequence of the human growth hormone, and a simian virus 40 origin of replication.
The sequence encoding the first 236 amino acids of the extracellular domain of human CAR (including the endogenous Kozak motif and the N-terminal signal sequence) was amplified from cDNA synthesized from total RNA of 911 cells by using synthetic oligonucleotides corresponding to the published sequence (5). The PCR product was digested with HindIII and Xbal to allow in-frame fusion to the human IgG1-Fc sequence. The fusion junctions were verified by sequencing.
PAGE and Western blotting.
For analysis of the CARex-Fc protein, COS7 cells were transiently transfected as described elsewhere (24). To reduce the levels of unrelated proteins, the medium from transfected COS7 cells was replaced 1 day after transfection with TurboDoma medium lacking FCS. Supernatants of transfected cells and purified CARex-Fc protein were analyzed by polyacrylamide gel electrophoresis (PAGE) (33) followed by Coomassie blue staining or by Western blotting of electrotransferred protein to Immobilon-P membranes. Membranes were saturated in Tris-buffered saline plus 0.1% Tween 20 (TBS-T) containing 5% dry milk and incubated with fluorescein isothiocyanate (FITC)-conjugated rabbit anti-human IgG (Dako, Copenhagen, Denmark) for 1 h. Immunoreactivity was scored using a Fluoroimager 595 (Molecular Dynamics, Amersham, Berkhamsted, United Kingdom).
For analysis of CAR protein, cells were lysed in NETN (10 mM Tris [pH 8.0], 200 mM NaCl, 1 mM EDTA, 0.5% NP-40) containing 1 mM phenylmethylsulfonyl fluoride. Protein concentrations were determined using the bicinchoninic acid assay (BCA; Pierce, Rockford, Ill.), and protein separation was performed by sodium dodecyl sulfate (SDS)-PAGE (10% polyacrylamide) under nonreducing conditions. After transfer to Immobilon-P membranes, the blots were blocked in 5% dry milk in TBS-T, and incubated with mouse monoclonal antibody E1-1 (1:20 dilution of hybridoma supernatant) for 1 h and then with horseradish peroxidase-conjugated goat anti-mouse IgG (Boehringer Mannheim Biochemicals, Indianapolis, Ind.) for 1 h. Immunoreactivity was determined using the enhanced chemiluminescence Western blotting detection system (Amersham).
CARex-Fc protein purification.
Low-FCS supernatant containing CARex-Fc protein was adsorbed to protein G-Sepharose (Amersham Pharmacia). Following washing with phosphate-buffered saline (PBS), bound protein was eluted using 0.15 M glycine-HCl (pH 2.8) and immediately neutralized with 1 M Tris (pH 9.5). Eluted material was concentrated using centrifugal filter devices (Millipore, Bedford, Mass.), dialyzed against PBS, and kept frozen in aliquots. The protein concentration of purified CARex-Fc was determined by the BCA assay.
Immunization and hybridoma production.
BALB/c mice were injected twice intramuscularly with 30 μg of the pCMV1-CARex-Fc expression plasmid at 2-week intervals followed by a single 100-μg injection after 2 weeks. Sera from tail veins were tested by cytofluorometric analysis for the presence of anti-CAR antibodies. The mice were further boosted with one injection of a protein-adjuvant mix (270 μg of CARex-Fc mixed with 270 μg of polyadenylic-polyuridylic acid adjuvant [Sigma, St. Louis, Mo.]) after an additional 2 weeks and then 2 weeks later with an injection of 100 μg of protein without adjuvant 3 days before cell fusion. Spleen cell preparation and fusion with P3-X63Ag myeloma cells (a kind gift from H. Hengartner, Experimental Immunology, University of Zürich, Zürich, Switzerland) were performed as described previously (25). Supernatants of HAT (Gibco-BRL)-resistant colonies were screened by a cytofluorometric assay using 911 cells. The isotype of the E1-1 antibody was determined to be mouse IgG1, using a hemagglutination test (Serotec, Oxford, United Kingdom). For purification of E1-1 antibody, the hybridoma cells were cultured in TurboDoma medium and antibody was isolated by protein G-Sepharose chromatography as described above.
Immunoreagents and flow cytometric analysis.
CTLA4-IgG1 fusion protein specific for B7 (14) was produced and purified as described for E1-1 antibody. The mouse monoclonal antibody RmcB specific for human CAR (5) was provided by R. Finberg, Dana-Farber Cancer Institute, Boston, Mass. FITC-labeled anti-human CD16 (30624X), CD32 (30934X), and CD64 (31844X) and appropriate isotype controls were purchased from Pharmingen, San Diego, Calif., and secondary fluorochrome conjugates were purchased from Serotec.
For cytofluorometric analysis of adherent 911 cells, subconfluent cells were washed with PBS and detached by treatment with PBS–20 mM EDTA. Approximately 106 cells were incubated with either 50 μl of hybridoma supernatant or 1 μg of various antigen-specific antibodies in 250 μl of balanced salt solution (BSS)–5% FCS (BSS is 0.14 M NaCl, 1 mM CaCl2, 5.4 mM KCl, 0.8 mM MgSO4, 0.3 mM NaH2PO4, and 0.4 mM KH2PO4 [pH 6.9]) for 30 min on ice. If unlabeled primary antibodies were used, the cells were washed by pelleting in BSS–2% FCS, incubated with 1 μg of phycoerythrin-labeled secondary conjugates, and washed again before being subjected to cytofluorometric analysis (Epics XL; Coulter, Miami, Fla.). Fluorescence-activated cell sorter (FACS) measurements were performed with 10,000 viable cells per sample. For eGFP expression analysis, A549 cells were transduced with AdCMV-eGFP and 2 days later the cells were detached and washed as described above. To analyze whether Ad binding competed with E1-1 binding to 911 cells, cells were detached, incubated with increasing amounts of AdCMV–muB7-1 for 60 min on ice, washed, and stained with E1-1 as described above.
Recombinant Ad vectors and inhibition of Ad-mediated cell transduction.
Recombinant E1- and E3-deleted Ad vectors were constructed and CsCl purified as described previously (26). Viral titers were determined by incubating 2 ml of medium on 911 cell layers in six-well plates (e.g., 6 × 109 PFU/ml for AdCMV-eGFP [kindly provided by C. Kuhl, Institute of Molecular Biology, University of Zürich, Zürich, Switzerland] and 1.2 × 1010 PFU/ml for AdCMV-muB7-1, expressing the mouse B7-1 transgene). The virion concentration of AdCMV-eGFP was determined by the method of Maizel et al. (37) and found to be 3.4 × 1011 particles/ml.
For transduction inhibition assays using E1-1 or CARex-Fc, A549 monolayer cultures were grown to 30% confluence in six-well plates. For E1-1 blocking assays, the medium was replaced with 0.5 ml of E1-1 hybridoma supernatant or with binding medium (PBS plus DMEM–10% FCS, 1:1 mix) containing purified E1-1 and the cells were incubated on a rocking platform for 2.5 h on ice. Reporter AdCMV-eGFP was added at a multiplicity of infection (MOI) of 100, and the cells were incubated for an additional 2.5 h on ice. They were then washed twice with cold binding medium, and normal cell culture medium including E1-1 antibody or control antibody was supplied, followed by incubation for 2 days at 37°C in a CO2 incubator. To assay CARex-Fc-mediated blocking, purified CARex-Fc protein was mixed with reporter Ad for 60 min on ice and then added to A549 cells; this was followed by the washing procedure and analysis of transgene expression as described above.
CARex-Fc-mediated enhancement of cell transduction.
Hematopoietic cells were seeded at a concentration of 4 × 105 in 0.5 ml of medium in 12-well plates. Human serum was replaced with FCS as indicated. AdCMV-eGFP was mixed with CARex-Fc protein in a volume of 20 μl of medium and added to the cells. After overnight incubation, the volume was increased to 1 ml, and eGFP expression was analyzed 48 h postinfection. For inhibition of Fcγ receptor-dependent infections, THP-1 cells were preincubated on ice with either 2μg of CTLA4-lg or 1 μg of a mouse anti-human CD64 antibody (31841A; Pharmingen) for 30 min, washed, and processed as described above.
RESULTS
Expression and purification of the CARex-Fc fusion protein.
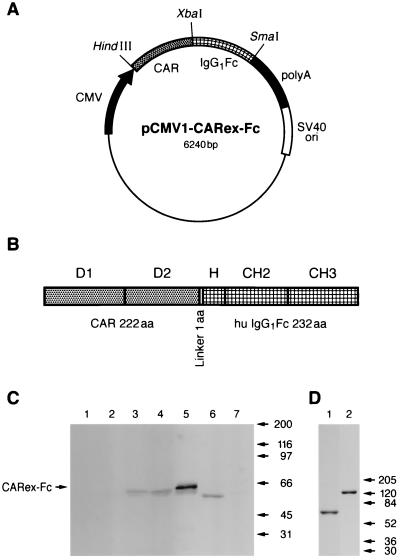
An expression plasmid encoding the ectodomain of the human Ad receptor CAR fused to the human IgG1 Fc domain was constructed for two reasons. First, we wanted to use such a protein for the production of new and eventually neutralizing CAR-specific monoclonal antibodies. Existing CAR-specific antibodies such as the RmcB produced by Hsu et al. (28) apparently do not block Ad infection (R. Finberg, personal communication). Second, we reasoned that such a bispecific protein might allow targeting of Ad to cells that express Fcγ receptors, but lack CAR expression. A PCR-amplified cDNA sequence corresponding to amino acids 1 to 236 of the human CAR sequence (including the endogenous Kozak motive and the N-terminal signal sequence of 14 amino acids which is cleaved off in the endoplasmic reticulum) was cloned in frame into the pCMV1-IgFc expression plasmid containing the human IgG1 sequence (Fig. 1A). The extracellular sequence of CAR contains the two Ig-like domains D1 and D2 (Fig. 1B), responsible for binding to Ad and group B coxsackievirus (5, 19). The human IgG1 Fc sequence encodes 232 amino acids comprising the hinge, CH2, and CH3 regions. The mature fusion protein is expected to consist of 455 amino acids with a calculated molecular mass of 50.7 kDa. Expression of the fusion protein was controlled by the human CMV promoter and an SV40 origin of replication (21) mediating efficient production of the CARex-Fc fusion protein from transiently transfected simian COS7 cells. Supernatants of transfected COS7 cells and purified fusion protein were analyzed by SDS-PAGE under reducing conditions and by Western blotting using an FITC-labeled rabbit anti-human Ig. Specific staining of a diffuse band migrating as a ∼60-kDa species was found in crude supernatants of COS7 cells transfected with the pCMV1-CARex-Fc expression plasmid (Fig. 1C, lanes 3 and 4) but not in supernatants of mock transfected COS7 cells (lane 7) or cell culture medium alone (lane 1). The difference between the apparent and calculated molecular masses and also the presence of a faster-running species may be due to N-linked glycosylation described previously for full-length CAR (53). As a control, the fusion protein CTLA4-Ig (14, 36), of ∼50 kDa, is shown in lane 6 (Fig. 1C). The amount of CARex-Fc protein in the supernatant increased over 5 days of cell growth, allowing the isolation of 4.5 mg of pure CARex-Fc protein from 1 liter of culture supernatant (Fig. 1D). Under nonreducing electrophoresis conditions, CARex-Fc migrated with molecular mass of ∼120 kDa, probably disulfide bonded by cysteines of the hinge region (Fig. 1D, lane 2). In addition, CARex-Fc behaved as a ∼120-kDa species when analyzed on a gel filtration column at physiological salt concentrations (S. Hemmi, unpublished results).
FIG. 1.
Production of CARex-Fc fusion protein. (A) The expression plasmid pCMV1-CARex-Fc was generated by fusing the cDNA encoding the 236 N-terminal amino acids, including the signal peptide of the human CAR protein, via a linker of 1 amino acid with the cDNA encoding 232 amino acids of the human IgG1 Fc polypeptide. (B) The mature, signal sequence-processed protein contains the extracellular two Ig-like domains D1 and D2 of CAR fused to the hinge, CH2, and CH3 regions of IgG1 resulting in a presumably secreted protein of 455 amino acids (aa). (C) Western blot analysis of CARex-Fc protein. COS7 cells were transiently transfected with pCMV1-CARex-Fc for 1 day (lane 2), 3 days (lane 3), or 5 days (lane 4) or were mock transfected (lane 7). Cell supernatants (30 μl) were subjected to SDS-PAGE. In addition, 1.5 μg of purified CARex-Fc (lane 5), 30 μl of DMEM plus 10% FCS (lane 1), and supernatant of stably transfected myeloma cells containing CTLA4-IgG1 (lane 6) were analyzed as controls. Human Igs were detected with FITC-labeled rabbit anti-human Ig antibody. (D) Analysis of purified recombinant CARex-Fc by PAGE and Coomassie blue staining. Purified CARex-Fc protein (3 μg) was loaded under reducing (lane 1) or nonreducing (lane 2) conditions.
Production of the human CAR-specific monoclonal antibody E1-1 and inhibition of Ad-mediated cell transduction by E1-1 and CARex-Fc protein.
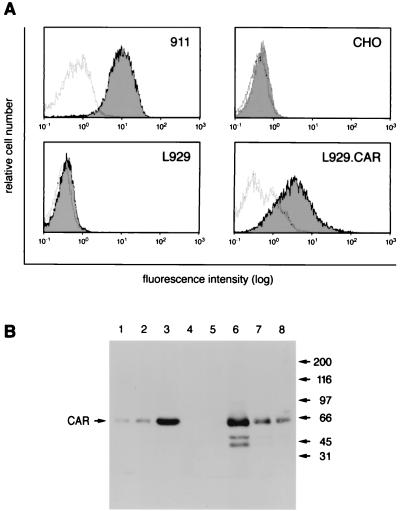
To produce monoclonal antibodies with specificity for human CAR, BALB/c mice were immunized and boosted first with the CAR expression plasmid, and then with purified CARex-Fc protein as described in Materials and Methods. Spleen cells of immunized mice were fused with P3-X63Ag myeloma cells, and hybridoma supernatants were first screened by cytofluorometric analysis for positive staining of 911 cells, which have high levels of CAR and then tested for Ad-blocking activity. The monoclonal antibody E1-1 turned out to have both features. When tested for cell binding, the E1-1 antibody stained 911 cells and L929.CAR mouse fibroblasts stably expressing the human CAR protein but not parental L929 cells or CAR-negative CHO cells (Fig. 2A). The staining pattern was comparable to that of RmcB (references 5 and 28 and results not shown). In addition, when performing Western blotting using protein extracts from different cells, the monoclonal E1-1 antibody recognized a single protein of ∼50 kDa (Fig. 2B), similar to results described for RmcB under nonreducing conditions (52). Even at the lowest protein amount of 911 or L929. CAR cell extracts tested (0.3 μg), we obtained a clear signal of the 50-kDa protein band (Fig. 2B, lanes 1 and 8, respectively). The additional two faster-running bands seen with the higher concentrations of extracts from L929.CAR cells may represent degradation products or proteins derived from additional RNA splicing variants (lane 6). Accordingly, even the largest amounts of protein extracts from CHO or L929 cells (30 μg) gave no signal, suggesting that the 50-kDa band was CAR (lanes 4 and 5, respectively).
FIG. 2.
Specificity of the E1-1 anti-human CAR monoclonal antibody. (A) Cytofluorometric analysis of CAR expression levels in different cell lines, using the E1-1 antibody. White histograms show background staining obtained using isotype control antibodies, and shaded histograms show CAR-specific staining. (B) Western blot analysis using E1-1 antibody and 0.3, 3, and 30 μg of protein lysate of 911 cells (lanes 1 to 3), 30 μg of protein lysate of CHO cells (lane 4), 30 μg of protein lysate of L929 cells (lane 5), and 30, 3, and 0.3 μg of protein lysate of L929.CAR cells (lanes 6 to 8, respectively).
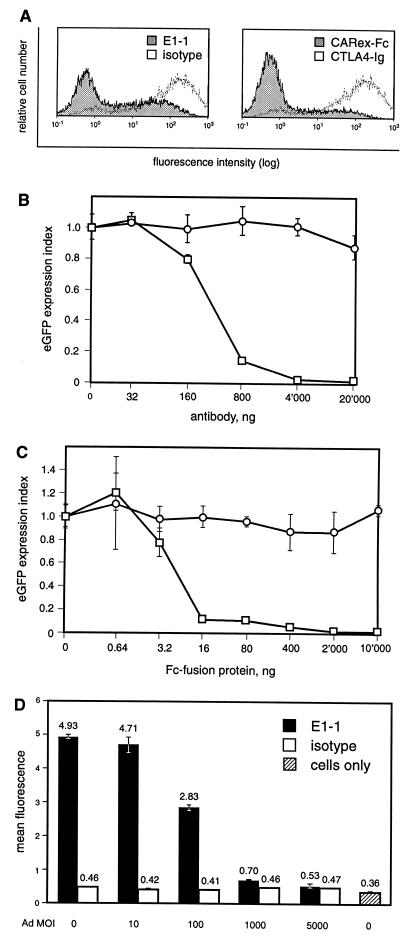
We then tested if E1-1 and CARex-Fc inhibited Ad-mediated transgene expression of a recombinant Ad expressing eGFP in A549 lung carcinoma cells (which are negative for the high-affinity Fcγ receptor CD64). Blocking or competition experiments were performed on ice to prevent possible internalization of ligands. As shown in Fig. 3A, both E1-1 and CARex-Fc reduced eGFP expression when the cells were treated with supernatant containing the E1-1 antibody (left panel) or when virus was preincubated with 1 μg of purified CARex-Fc protein (right panel). To quantitate this inhibition, purified E1-1 antibody and CARex-Fc protein were titrated and eGFP expression was determined (Fig. 3B and C, respectively). We found that 440 ng of E1-1 antibody and 11 ng of CARex-Fc protein resulted in a 50% inhibition of Ad-mediated eGFP expression, with the latter corresponding to a 200-fold molar excess of protein over virus particles. A maximal input of 20 μg of E1-1 antibody and 10 μg of CARex-Fc protein resulted in inhibition of 98% for both proteins.
FIG. 3.
Functional properties of E1-1 and CARex-Fc. (A) Inhibition of Ad-mediated eGFP expression by E1-1 anti-human CAR monoclonal antibody and CARex-Fc. A549 cells were either preincubated with 250 μl of E1-1 antibody supernatant or control antibody (left panel) and then incubated with virus or mixed with virus previously incubated on ice with 1 μg of CARex-Fc or CTLA4-Ig (right panel) protein. All incubations were performed for 2.5 h on ice, and the virus was used at an MOI of 100. The cells were then washed twice, and culture medium including E1-1, CARex-Fc, or control proteins was supplied. The cells were incubated for 2 days and analyzed for eGFP expression. (B and C) Quantification of the inhibitory effects of E1-1 (B) and CARex-Fc (C). Fivefold dilutions of purified E1-1 antibody or CARex-Fc protein (□) or control control proteins (○) were used as described for panel A. The eGFP expression index was calculated from the ratios of the mean eGFP expression measured in cells incubated either with E1-1 plus AdCMV-eGFP or CARex-Fc plus AdCMV-eGFP to the value in those treated with AdCMV-eGFP alone. Expression values represent the mean of triplicates including standard deviations. (D) Inhibition of E1-1 anti-human CAR monoclonal antibody binding to 911 cells by Ad. Cells were stained with E1-1 antibody following preincubation in the cold with the indicated amounts of AdCMV-muB7-1. Fluorescence intensities of stainings with isotype and specific antibody are given as the mean of triplicates including standard deviations of the mean.
Since the E1-1 antibody was capable of blocking Ad binding, we asked whether Ad binding would block E1-1 staining. 911 cells were incubated with increasing amounts of AdCMV-muB7-1, washed, and stained with E1-1 antibody. At the highest MOI (5,000) the mean fluorescence of E1-1 was reduced to background levels, confirming that CAR is a major determinant of Ad binding to these cells (Fig. 3D). Together, these results indicated that both the E1-1 antibody and the CARex-Fc fusion protein were functional CAR-specific reagents.
Binding of CARex-Fc to cells expressing CD64 high-affinity Fcγ receptor I.
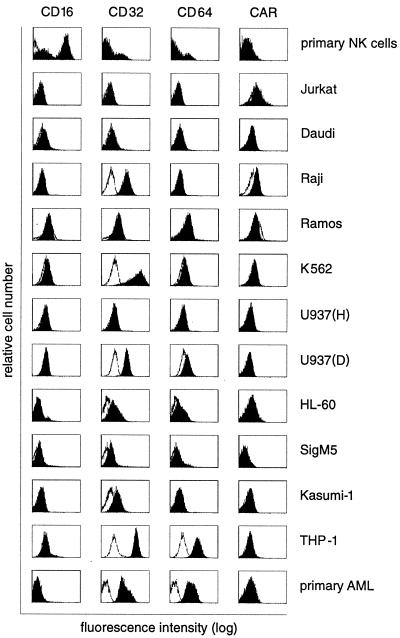
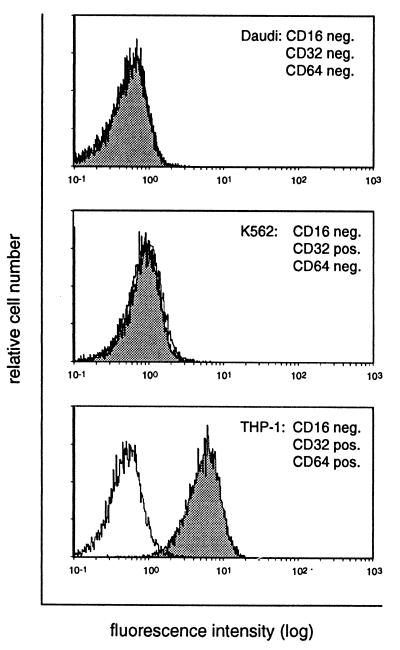
Using the E1-1 antibody, we next analyzed whether CARex-Fc fusion protein bound to cells expressing either the high-affinity Fcγ receptor I (CD64) or the low-affinity Fcγ receptors II and III (CD32 and CD16, respectively). Using specific anti-Fcγ receptor antibodies, expression levels of CD16, CD32, and CD64 were determined by cytofluorometric analysis on primary NK cells, primary AML cells, and a panel of hematopoietic cell lines listed in Table 1. CAR expression was measured using the E1-1 anti-CAR antibody both for cytofluorometric and Western blot (data not shown) analyses. Two cell lines, the monocytic lymphoma U937 (D) and the monocytic leukemia THP-1, and also patient-derived primary AML cells showed relatively high expression levels of both CD32 and CD64 but undetectable levels of CAR (Fig. 4). All cell lines were then tested for binding of CARex-Fc in a triple-sandwich staining assay. The first incubation was with CARex-Fc, and the second incubation was with the mouse E1-1 antibody followed by a phycoerythrin-labeled rabbit anti-mouse Ig conjugate. The FACS analyses in Fig. 5 shows three representative examples of cells with negative endogenous CAR staining. Daudi cells (negative for all three types of Fcγ receptor) and K562 cells (positive for CD32) showed no CARex-Fc binding. In contrast, THP-1 cells, which were CD32 and CD64 positive, also bound CARex-Fc but gave no signal in the absence of CARex-Fc. Taken together, these results demonstrated that CD64-positive cells but not CD16- and CD32-positive cells bound soluble CARex-Fc.
TABLE 1.
Expression of low and high-affinity Fcγ receptors and CAR and effects of CARex-Fc on Ad-mediated transduction
| Cell line | Expression of:
|
Transduction with or without CARex-Fc | ||||
|---|---|---|---|---|---|---|
| CD16 (FcγRIII) | CD32 (FcγRII) | CD64 (FcγRI) | CAR (FACS) | CAR (Western) | ||
| Primary NK cells | ++ | − | − | − | − | No change |
| Jurkat (acute T-cell leukemia) | − | − | − | ±a | ±a | 40% inhibition at highest CARex-Fc |
| Daudi (Burkitt's B lymphoma) | − | − | − | − | − | No change |
| Raji (Burkitt's B lymphoma) | − | ++ | − | + | + | No change |
| Ramos (Burkitt's B lymphoma) | − | − | − | − | + | No change |
| K562 (chronic myelogenous leukemia) | − | ++ | − | − | + | 20% inhibition at highest CARex-Fc |
| U937 (H) (monocytic lymphoma) | − | − | − | − | − | 20% inhibition at highest CARex-Fc |
| U937 (D) (monocytic lymphoma, alternative subline) | − | ++ | + | − | − | Up to 250-fold increase |
| HL-60 (promyelocytic leukemia) | − | + | ± | − | − | 5-fold- increase |
| SigM5 (AML) | − | + | ± | − | − | 2–3-fold increase |
| Kasumi-1 (AML) | − | + | ± | − | − | 2–3-fold increase |
| THP-1 (acute monocytic leukemia) | − | ++ | ++ | − | − | Up to 250-fold increase |
| Primary AML cells | − | + | ++ | − | − | Up to 200-fold increase |
Weak signal.
FIG. 4.
Cytofluorometric analysis of low-affinity (CD16 and CD32) and high-affinity (CD64) Fcγ receptor expression and CAR expression of primary NK and AML cultures and hematopoietic cell lines. Cells were stained using either isotype control (white histograms) or specific FITC-labeled antibodies (CD16, CD32, and CD64) or the E1-1 antibody in combination with phycoerythrin-conjugated rabbit anti-mouse IgG (black histograms).
FIG. 5.
CARex-Fc binds directly to CD64-positive cells. Daudi, K562, and THP-1 cells were incubated first with or without CARex-Fc (shaded and white histograms, respectively) and then with E1-1 and phycoerythrin-conjugated rabbit anti-mouse IgG.
CD64- and CARex-Fc-mediated increase of Ad transgene expression.
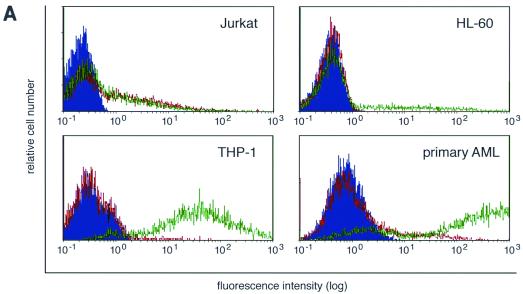
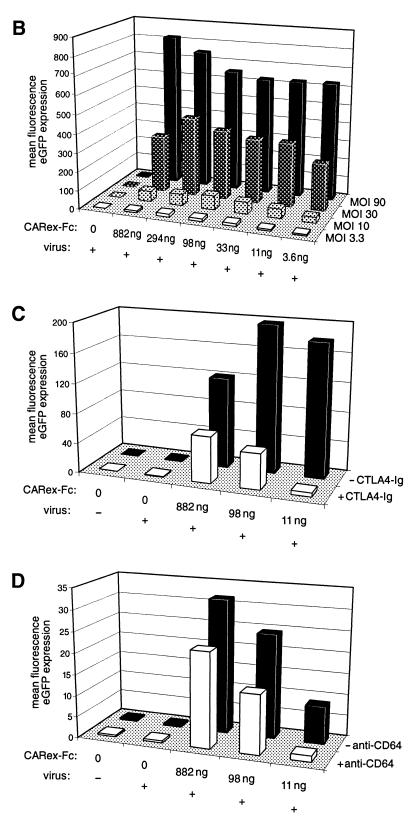
To test whether CARex-Fc protein allowed improved Ad-mediated eGFP expression in cells containing one of the Fcγ receptors, the cells listed in Table 1 were incubated with AdCMV-eGFP at an MOI of 10, either alone or premixed with 1.0 μg of CARex-Fc protein. eGFP staining was analyzed 48 h postinfection. A representative collection of histograms using four different types of cells (Jurkat, HL-60, THP-1, and primary AML) is shown in Fig. 6A. For HL-60, THP-1, and primary AML cells, virus alone gave rise to a small number of cells expressing eGFP at low mean fluorescence values. In contrast, premixing of Ad with CARex-Fc resulted in a strong increase of eGFP expression with 90% (THP-1) or 75% (AML) of the cells shifted to eGFP expression at high mean fluorescence values. When CARex-Fc was replaced by comparable amounts of the control CTLA4-Ig protein, which binds to the Fcγ receptor but not to Ad, no effect on eGFP expression was observed (data not shown), suggesting that ligation of the high-affinity Fcγ receptor alone does not induce uptake of Ad. For HL-60 cells, only about 20% of the cells showed a similar increase of expression, most probably reflecting the cell population expressing CD64. Jurkat cells demonstrated no change of eGFP expression on addition of CARex-Fc protein. A systematic analysis of eGFP expression levels of THP-1 cells as a function of CARex-Fc levels and Ad MOIs is depicted in Fig. 6B. MOIs of 3.3, 10, 30, and 90 were combined with different amounts of CARex-Fc, ranging from 882 to 3.6 ng, and the relative eGFP expression on day 2 postinfection was determined. For all MOIs used, a strong increase in eGFP expression was found, ranging from 30-fold at an MOI of 3.3 to 100-fold at MOIs of 30 and 90. For MOIs of 3.3, 10, and 30, peak induction was obtained with 294 or 98 ng of purified CARex-Fc protein, which corresponded to a protein/virus particle ratio of 6,400 to 2,100. Maximal (250-fold) induction was obtained when the analysis was performed on day 1 with an MOI of 90, and the minimal amount of CARex-Fc protein which still increased the eGFP expression level was 130 pg (data not shown). At all MOIs used except 90, the largest amount of CARex-Fc protein (882 ng) gave a weaker stimulation than did optimal amounts of CARex-Fc, presumably due to binding competition of soluble CARex-Fc protein with virus-bound CARex-Fc for the high-affinity Fcγ receptor. Likewise, high concentrations of CARex-Fc also had an inhibitory effect on Jurkat, U937 (H) and K562 cells (summarized in Table 1). Weak signals for endogenous CAR were seen for Jurkat and K562 cells in Western blots (data not shown) but not in FACS experiments. Surprisingly, Raji cells, which yielded a robust CAR signal in both assays, did not show any inhibitory effect. Ramos cells were completely resistant to Ad-mediated eGFP expression, possibly because they were shown to stain negative for several surface integrins (44).
FIG. 6.
CARex-Fc-mediated increase of Ad transgene expression in hematopoietic cells. (A) Jurkat, HL-60, THP-1, and primary AML cells were transduced with AdCMV-eGFP using an MOI of 10 in the absence (red) or presence (green) of 1 μg of CARex-Fc protein. Cells alone are shown as solid blue histograms. (B) MOI and CARex-Fc dose effects on the transduction efficiency of THP-1 cells. Results are given as means of duplicate determinations of eGFP mean values. The results of one of three comparable experiments are shown. (C) Inhibition of CARex-Fc-mediated transgene expression by preincubation of THP-1 cells with 2 μg of CTLA4-Ig for 30 min on ice. Experimental settings were comparable to those in panel B when using AdCMV-eGFP at an MOI of 10 and various amounts of CARex-Fc protein. (D) Inhibition of transgene expression by preincubation of THP-1 cells with 1 μg of an monoclonal mouse anti-human CD64 antibody.
Enhanced eGFP expression was obtained by two different protocols. Either CARex-Fc protein was premixed briefly with Ad and then added to cells and left on the cells for 48 h or cells were first incubated on ice with CARex-Fc only, washed, and incubated with virus (data not shown). The results of the two methods were comparable, suggesting that the CARex-Fc fusion protein was functionally independent of whether it was first bound to Ad or to the target cells.
Of the 11 cell lines and 2 primary cell cultures of myeloid or lymphatic origin, 2 cell lines, THP-1 and U937 (D), and one primary patient-derived AML cell culture showed a high increase of Ad transduction efficiency due to CARex-Fc (Table 1). The CARex-Fc-responsive cells expressed the high-affinity Fcγ receptor CD64 at relatively high levels (Fig. 4). Low levels of CD64 expression on HL-60, Kasumi-1, and SigM5 cells correlated with a low but significant increase of CARex-Fc-mediated expression.
To supply further evidence that the high-affinity Fcγ receptor is responsible for mediating the increased transduction efficiency, premixed virus and CARex-Fc was added to cells pretreated with either the CTLA4-Ig fusion protein binding to Fcγ receptor or a monoclonal antibody against human CD64 (Fig. 6C and D, respectively). In both cases, a reproducible 33- and 7-fold reduction of the eGFP expression levels were observed at small amounts of CARex-Fc. Taken together, these results indicate that the bispecific fusion protein CARex-Fc is capable of bridging the CD64 surface protein and the Ad fiber protein and thereby increases Ad infection in these cells.
DISCUSSION
Several approaches have been used to improve the Ad transduction efficiency of cells normally resistant to Ad. In the best cases, genetically modified Ad vectors containing heterologous peptides, such as RGD in the HI loop of the fiber (13, 31, 42) or at the C-terminal end (64, 66), were reported to increase the transduction efficiency up to 500-fold across a broad range of cell types. However, cells of hematopoietic origin with low levels of integrin expression (29) are not expected to be good targets of RGD-modified Ad. An additional limitation of such a genetic approach is the small size of the targeting peptide tolerated by the virus capsid structure (8, 66). Further extension of productive Ad-cell interactions by selective strategies is thus important to broaden Ad tropism for medical purposes. Toward this goal, we have designed bispecific and simple-to-produce fusion proteins. We have demonstrated that a bispecific fusion protein consisting of the ectodomain of CAR fused to the immunoglobulin Fc domain improves the transduction efficiency of hematopoietic cells devoid of the primary Ad receptor CAR by up to 250-fold. This transduction increase is highly significant and fully dependent on the presence of high-affinity Fcγ receptor I (CD64). The transduction increase is significantly reduced by CTLA4-Ig protein or an anti-human CD64 monoclonal antibody, which bind to the high-affinity Fcγ receptor I. The inclusion of the CAR ectodomain in our fusion construct is useful since this domain confers high-affinity binding to the fiber protein of Ad. If all the fibers are decorated with CARex-Fc, we expect that such modified virus prefers to bind to Fcγ receptors rather than to the receptor of native Ad, CAR.
Accordingly, we have demonstrated that coincubation of virus with CARex-Fc strongly reduced Ad-mediated expression in CAR-positive, Fcγ receptor-negative A549 cells, arguing for the correct folding of at least the D1 domain of CARex-Fc (19). In addition, the successful production of a virus-neutralizing monoclonal anti-human CAR-specific antibody using the CARex-Fc protein to boost the immune response, together with the demonstration of direct binding of the CARex-Fc protein to CD64-positive cells, adds further evidence for the functionality of our hybrid adapter protein.
Other strategies to alter Ad host range have involved conjugates containing blocking anti-Ad fiber knob Fab (15) or an anti-FLAG epitope antibody (65). Most closely related to our approach is the use of a bispecific protein consisting of the neutralizing scFv antibody fragment recognizing the fiber knob fused to the EGF ligand, termed the adenobody (60). In this system, viral gene delivery to cells expressing the EGF receptor was enhanced 16-fold. Since all the cells tested in these experiments also expressed CAR, the contribution of the additional EGF domain to retargeting Ad was difficult to define. Besides the EGF receptor, additional receptors like the folate receptor (15) and CD3 on T lymphocytes (62) have been used as targets for bispecific conjugates to extend Ad tropism. A similar approach was described for retroviruses by using a bispecific soluble virus receptor fused to EGF (6, 49). In this system, improved transduction was obtained by preincubating cells with the hybrid protein and then incubating them with virus. Alternatively, virus was incubated with the fusion protein and purified and then the complex was added to target cells.
In contrast to several of the previous reports, our approach was effective for cells lacking the natural virus receptor. The presence of the high-affinity Fcγ receptor I on the target cells was sufficient for a robust Ad-mediated gene expression. It is not known at present if CARex-Fc-modified Ad entered the CAR-less cells by a clathrin-dependent pathway, which was suggested to operate for Ad infection of fibroblast-type cells (59). Possibly, our CARex-Fc-modified Ad is taken up by a clathrin-independent route similar to Fc receptor-mediated endocytosis in phagocytic cells (2). This pathway operates in an actin-dependent manner, typically with particles larger than 0.5 μm. Since the CARex-Fc bridging protein seems to be dimeric, multiple Ads could become cross-linked and thus might induce phagocytosis. However, the low-affinity Fcγ receptors II and III, which primarily recognize immune complexes (55), were inefficient for CARex-Fc-Ad infection, suggesting that the CARex-Fc-Ad complexes were poorly recognized by the low-affinity Fcγ receptor uptake system. Further experiments are necessary to clarify the mechanisms of Ad-mediated transgene delivery into Fcγ receptor I-positive cells.
Nonetheless, CARex-Fc-modified Ad may have clinical potential. CD64 is expressed on monocytes, macrophages, and subtypes of AML cells but not on maturating dendritic cells (7, 43, 55) (Fig. 4). Since global survival rates for patients with AML are poor, the further development of immunotherapy and immune vaccine approaches is a valuable goal to pursue (4). The concept of immune gene therapy for cancer is based on the presumption that the host immune system is capable of recognizing tumor-associated antigens. For AML, little information is available about potential immunostimulatory antigens. Recently, isolation of AML-specific T-cell clones was reported (38, 40), but no corresponding peptide sequences have so far been published. High levels of transiently expressed cytokines and immune modulators might be sufficient to activate the immune system in vivo and thereby achieve the therapeutic goal (34, 54). Cells of the hematopoietic system are particularly suitable for gene therapy, since the techniques for bone marrow and blood cell transplantations are well established and, importantly, the transductions can be performed ex vivo. Primary AML cells can be obtained from patient bone marrow aspirates or peripheral blood mononuclear cells and can be kept in culture in the presence of interleukin-3, stem cell factor, or kit ligand (for a review, see reference 4). However, none of the currently used gene therapy vectors allow efficient gene transfer to malignant cells of hematopoietic origin. Therefore, the development of CARex-Fc for Ad-mediated gene transfer provides a promising alternative to specifically transfer therapeutic genes into CD64-positive AML malignancies. For other types of malignancies, alternative cell surface markers may serve as targets. Replacement of the Fc portion of CARex-Fc with other polypeptide sequences that bind to surface markers, such as receptor ligands or single-chain antibodies, may allow the production of a whole range of bifunctional, soluble Ad receptor-ligand fusion proteins.
ACKNOWLEDGMENTS
This work has been supported by the Kanton Zürich and by a grant of the Krebsliga of the Kanton Zürich (to S.H.).
We thank P. Forte (University Hospital, Zürich, Switzerland) for providing the cultivated NK cells, G. Schoedon (Department of Medicine, University of Zürich, Zürich, Switzerland) for several cell lines and patient AML cells, R. Fischer (Laboratory of Biochemistry, Swiss Federal Institute of Technology, Zürich, Switzerland) for advice relating to hybridoma production, L. Hangartner (Experimental Immunology, University of Zürich, Zürich, Switzerland) for isotype determination of the E1-1 antibody, and F. Ochsenbein for graphic designs.
ADDENDUM
After submission of this article, another group reported the use of a bispecific fusion protein consisting of the soluble truncated form of CAR fused with epidermal growth factor mediating Ad targeting to epidermal growth factor receptor-positive cells (13a).
REFERENCES
- 1.Acsadi G, Jani A, Massie B, Simoneau M, Holland P, Blaschuk K, Karpati G. A differential efficiency of adenovirus-mediated in vivo gene transfer into skeletal muscle cells of different maturity. Hum Mol Genet. 1994;3:579–584. doi: 10.1093/hmg/3.4.579. [DOI] [PubMed] [Google Scholar]
- 2.Aderem A, Underhill D M. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 3.Andiman W A, Miller G. Persistent infection with adenovirus types 5 and 6 in lymphoid cells from humans and woolly monkeys. J Infect Dis. 1982;145:83–88. doi: 10.1093/infdis/145.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Arceci R J. The potential for antitumor vaccination in acute myelogenous leukemia. J Mol Med. 1998;76:80–93. doi: 10.1007/s001090050195. [DOI] [PubMed] [Google Scholar]
- 5.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 6.Boerger A L, Snitkovsky S, Young J A. Retroviral vectors preloaded with a viral receptor-ligand bridge protein are targeted to specific cell types. Proc Natl Acad Sci USA. 1999;96:9867–9872. doi: 10.1073/pnas.96.17.9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyer A, Andreu G, Romet-Lemonne J L, Fridman W H, Teillaud J L. Generation of phagocytic MAK and MAC-DC for therapeutic use: characterization and in vitro functional properties. Exp Hematol. 1999;27:751–761. doi: 10.1016/s0301-472x(98)00070-8. [DOI] [PubMed] [Google Scholar]
- 8.Caillet-Boudin M L, Lemay P, Boulanger P. Functional and structural effects of an Ala to Val mutation in the adenovirus serotype 2 fibre. J Mol Biol. 1991;217:477–486. doi: 10.1016/0022-2836(91)90751-q. [DOI] [PubMed] [Google Scholar]
- 9.Cantwell M J, Sharma S, Friedmann T, Kipps T J. Adenovirus vector infection of chronic lymphocytic leukemia B cells. Blood. 1996;88:4676–4683. [PubMed] [Google Scholar]
- 10.Chillon M, Bosch A, Zabner J, Law L, Armentano D, Welsh M J, Davidson B L. Group D adenoviruses infect primary central nervous system cells more efficiently than those from group C. J Virol. 1999;73:2537–2540. doi: 10.1128/jvi.73.3.2537-2540.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu Y, Sperber K, Mayer L, Hsu M T. Persistent infection of human adenovirus type 5 in human monocyte cell lines. Virology. 1992;188:793–800. doi: 10.1016/0042-6822(92)90534-v. [DOI] [PubMed] [Google Scholar]
- 12.Defer C, Belin M T, Caillet-Boudin M L, Boulanger P. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J Virol. 1990;64:3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dmitriev I, Krasnykh V, Miller C R, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel D T. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Dmitriev I, Kashantseva E, Rogers B E, Krasnykh V, Curiel D T. Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J Virol. 2000;74:6875–6884. doi: 10.1128/jvi.74.15.6875-6884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dohring C, Angman L, Spagnoli G, Lanzavecchia A. T-helper-and accessory-cell-independent cytotoxic responses to human tumor cells transfected with a B7 retroviral vector. Int J Cancer. 1994;57:754–759. doi: 10.1002/ijc.2910570524. [DOI] [PubMed] [Google Scholar]
- 15.Douglas J T, Rogers B E, Rosenfeld M E, Michael S I, Feng M, Curiel D T. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–1578. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- 16.Fallaux F J, Kranenburg O, Cramer S J, Houweling A, Van Ormondt H, Hoeben R C, Van Der Eb A J. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther. 1996;7:215–222. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- 17.Fasbender A, Zabner J, Chillon M, Moninger T O, Puga A P, Davidson B L, Welsh M J. Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivo. J Biol Chem. 1997;272:6479–6489. doi: 10.1074/jbc.272.10.6479. [DOI] [PubMed] [Google Scholar]
- 18.Faucon N, Ogier G, Chardonnet Y. Changes in human adenovirus 5 propagated in Burkitt's lymphoma cells. JNCI. 1982;69:1215–1220. [PubMed] [Google Scholar]
- 19.Freimuth P, Springer K, Berard C, Hainfeld J, Bewley M, Flanagan J. Coxsackievirus and adenovirus receptor amino-terminal immunoglobulin V-related domain binds adenovirus type 2 and fiber knob from adenovirus type 12. J Virol. 1999;73:1392–1398. doi: 10.1128/jvi.73.2.1392-1398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gall J, Kass-Eisler A, Leinwand L, Falck-Pedersen E. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J Virol. 1996;70:2116–2123. doi: 10.1128/jvi.70.4.2116-2123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 22.Goldman C K, Rogers B E, Douglas J T, Sosnowski B A, Ying W, Siegal G P, Baird A, Campain J A, Curiel D T. Targeted gene delivery to Kaposi's sarcoma cells via the fibroblast growth factor receptor. Cancer Res. 1997;57:1447–1451. [PubMed] [Google Scholar]
- 23.Haisma H J, Pinedo H M, Rijswijk A, der Meulen-Muileman I, Sosnowski B A, Ying W, Beusechem V W, Tillman B W, Gerritsen W R, Curiel D T. Tumor-specific gene transfer via an adenoviral vector targeted to the pan-carcinoma antigen EpCAM. Gene Ther. 1999;6:1469–1474. doi: 10.1038/sj.gt.3300969. [DOI] [PubMed] [Google Scholar]
- 24.Hemmi S, Bohni R, Stark G, Di Marco F, Aguet M. A novel member of the interferon receptor family complements functionality of the murine interferon gamma receptor in human cells. Cell. 1994;76:803–810. doi: 10.1016/0092-8674(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 25.Hemmi S, Fenner M, Binz H, Winterhalter K, Wigzell H. Studies of monoclonal antibodies specific for major histocompatibility complex products of the rat. I. Production and characterization of monoclonal antibodies. Scand J Immunol. 1985;21:549–563. doi: 10.1111/j.1365-3083.1985.tb01845.x. [DOI] [PubMed] [Google Scholar]
- 26.Hemmi S, Geertsen R, Mezzacasa A, Peter I, Dummer R. The presence of HCAR (human coxsackievirus and adenovirus receptor) is associated with efficient adenovirus-mediated transgene expression in human melanoma cell cultures. Hum Gene Ther. 1998;9:2363–2373. doi: 10.1089/hum.1998.9.16-2363. [DOI] [PubMed] [Google Scholar]
- 27.Horvath J, Weber J M. Nonpermissivity of human peripheral blood lymphocytes to adenovirus type 2 infection. J Virol. 1988;62:341–345. doi: 10.1128/jvi.62.1.341-345.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu K H, Lonberg-Holm K, Alstein B, Crowell R L. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J Virol. 1988;62:1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang S, Endo R I, Nemerow G R. Upregulation of integrins alpha v beta 3 and alpha v beta 5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khuu H, Conner M, Vanderwaak T, Shultz J, Gomez-Navarro J, Alvarez R D, Curiel D T, Siegal G P. Detection of Coxsackie-Adenovirus Receptor (CAR) immunoreactivity in ovarian tumors of epithelial derivation. Appl Immunhistochem Mol Morphol. 1999;7:266–270. [Google Scholar]
- 31.Krasnykh V, Dmitriev I, Mikheeva G, Miller C R, Belousova N, Curiel D T. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72:1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krasnykh V N, Mikheeva G V, Douglas J T, Curiel D T. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70:6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Leimig T, Brenner M, Ramsey J, Vanin E, Blaese M, Dilloo D. High-efficiency transduction of freshly isolated human tumor cells using adenoviral interleukin-2 vectors. Hum Gene Ther. 1996;7:1233–1239. doi: 10.1089/hum.1996.7.10-1233. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Pong R C, Bergelson J M, Hall M C, Sagalowsky A I, Tseng C P, Wang Z, Hsieh J T. Loss of adenoviral receptor expression in human bladder cancer cells: a potential impact on the efficacy of gene therapy. Cancer Res. 1999;59:325–330. [PubMed] [Google Scholar]
- 36.Linsley P S, Brady W, Urnes M, Grosmaire L S, Damle N K, Ledbetter J A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maizel J V, Jr, White D O, Scharff M D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968;36:115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- 38.Mutis T, Schrama E, Melief C J, Goulmy E. CD80-transfected acute myeloid leukemia cells induce primary allogeneic T-cell responses directed at patient specific minor histocompatibility antigens and leukemia-associated antigens. Blood. 1998;92:1677–1684. [PubMed] [Google Scholar]
- 39.Nalbantoglu J, Pari G, Karpati G, Holland P C. Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells. Hum Gene Ther. 1999;10:1009–1019. doi: 10.1089/10430349950018409. [DOI] [PubMed] [Google Scholar]
- 40.Ostankovitch M, Buzyn A, Bonhomme D, Connan F, Bouscary D, Heshmati F, Dreyfus F, Choppin J, Guillet J G. Antileukemic HLA-restricted T-cell clones generated with naturally processed peptides eluted from acute myeloblastic leukemia blasts. Blood. 1998;92:19–24. [PubMed] [Google Scholar]
- 41.Roelvink P W, Kovesdi I, Wickham T J. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J Virol. 1996;70:7614–7621. doi: 10.1128/jvi.70.11.7614-7621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roelvink P W, Mi Lee G, Einfeld D A, Kovesdi I, Wickham T J. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286:1568–1571. doi: 10.1126/science.286.5444.1568. [DOI] [PubMed] [Google Scholar]
- 43.Rothe G, Schmitz G. Consensus protocol for the flow cytometric immunophenotyping of hematopoietic malignancies. Working Group on Flow Cytometry and Image Analysis. Leukemia. 1996;10:877–895. [PubMed] [Google Scholar]
- 44.Sanchez-Aparicio P, Dominguez-Jimenez C, Garcia-Pardo A. Activation of the alpha 4 beta 1 integrin through the beta 1 subunit induces recognition of the RGDS sequence in fibronectin. J Cell Biol. 1994;126:271–279. doi: 10.1083/jcb.126.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seebach J D, Comrack C, Germana S, LeGuern C, Sachs D H, DerSimonian H. HLA-Cw3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J Immunol. 1997;159:3655–3661. [PubMed] [Google Scholar]
- 46.Segerman A, Mei Y, Wadell G. Adenovirus types 11p and 35p show high binding efficiencies for committed hematopoietic cell lines and are infective to these cell lines. J Virol. 2000;74:1457–1467. doi: 10.1128/jvi.74.3.1457-1467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shayakhmetov D M, Papayannopoulou T, Stamatoyannopoulos G, Lieber A. Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector. J Virol. 2000;74:2567–2583. doi: 10.1128/jvi.74.6.2567-2583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silver L, Anderson C W. Interaction of human adenovirus serotype 2 with human lymphoid cells. Virology. 1988;165:377–387. doi: 10.1016/0042-6822(88)90582-x. [DOI] [PubMed] [Google Scholar]
- 49.Snitkovsky S, Young J A. Cell-specific viral targeting mediated by a soluble retroviral receptor-ligand fusion protein. Proc Natl Acad Sci USA. 1998;95:7063–7068. doi: 10.1073/pnas.95.12.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevenson S C, Rollence M, Marshall-Neff J, McClelland A. Selective targeting of human cells by a chimeric adenovirus vector containing a modified fiber protein. J Virol. 1997;71:4782–4790. doi: 10.1128/jvi.71.6.4782-4790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tillman B W, de Gruijl T D, Luykx-de Bakker S A, Scheper R J, Pinedo H M, Curiel T J, Gerritsen W R, Curiel D T. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. J Immunol. 1999;162:6378–6383. [PubMed] [Google Scholar]
- 52.Tomko R P, Johansson C B, Totrov M, Abagyan R, Frisen J, Philipson L. Expression of the adenovirus receptor and its interaction with the fiber knob. Exp Cell Res. 2000;255:47–55. doi: 10.1006/excr.1999.4761. [DOI] [PubMed] [Google Scholar]
- 53.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tung C, Federoff H J, Brownlee M, Karpoff H, Weigel T, Brennan M F, Fong Y. Rapid production of interleukin-2-secreting tumor cells by herpes simplex virus-mediated gene transfer: implications for autologous vaccine production. Hum Gene Ther. 1996;7:2217–2224. doi: 10.1089/hum.1996.7.18-2217. [DOI] [PubMed] [Google Scholar]
- 55.Van de Winkel J G, Capel P J. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14:215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 56.Varga M J, Weibull C, Everitt E. Infectious entry pathway of adenovirus type 2. J Virol. 1991;65:6061–6070. doi: 10.1128/jvi.65.11.6061-6070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walter R, Schoedon G, Baechli E, Betts D R, Hossie J P, Calandra T, Joller-Jemelka H, Fehr J, Schaffner A. Characterization of an arsenic-sensitive monoblast leukemia cell line SigM5. Br J Haematol. 2000;109:396–404. doi: 10.1046/j.1365-2141.2000.02013.x. [DOI] [PubMed] [Google Scholar]
- 58.Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 59.Wang K, Huang S, Kapoor-Munshi A, Nemerow G. Adenovirus internalization and infection require dynamin. J Virol. 1998;72:3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watkins S J, Mesyanzhinov V V, Kurochkina L P, Hawkins R E. The ‘adenobody’ approach to viral targeting: specific and enhanced adenoviral gene delivery. Gene Ther. 1997;4:1004–1012. doi: 10.1038/sj.gt.3300511. [DOI] [PubMed] [Google Scholar]
- 61.Wattel E, Vanrumbeke M, Abina M A, Cambier N, Preudhomme C, Haddada H, Fenaux P. Differential efficacy of adenoviral mediated gene transfer into cells from hematological cell lines and fresh hematological malignancies. Leukemia. 1996;10:171–174. [PubMed] [Google Scholar]
- 62.Wickham T J, Lee G M, Titus J A, Sconocchia G, Bakacs T, Kovesdi I, Segal D M. Targeted adenovirus-mediated gene delivery to T cells via CD3. J Virol. 1997;71:7663–7669. doi: 10.1128/jvi.71.10.7663-7669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 64.Wickham T J, Roelvink P W, Brough D E, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat Biotechnol. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 65.Wickham T J, Segal D M, Roelvink P W, Carrion M E, Lizonova A, Lee G M, Kovesdi I. Targeted adenovirus gene transfer to endothelial and smooth muscle cells by using bispecific antibodies. J Virol. 1996;70:6831–6838. doi: 10.1128/jvi.70.10.6831-6838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wickham T J, Tzeng E, Shears L L, 2nd, Roelvink P W, Li Y, Lee G M, Brough D E, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu G Y, Zhan P, Sze L L, Rosenberg A R, Wu C H. Incorporation of adenovirus into a ligand-based DNA carrier system results in retention of original receptor specificity and enhances targeted gene expression. J Biol Chem. 1994;269:11542–11546. [PubMed] [Google Scholar]