Abstract
During environmental risk assessments of chemicals, higher-tier biodegradation tests in soil, sediment, and surface-water systems are required using OECD standards 307, 308, and 309 guidelines, respectively. These guidelines are not suitable for testing highly volatile chemicals, and a biometer closed-incubation setup is recommended for testing slightly volatile chemicals. In this setup, the degradation kinetics of highly volatile chemicals can largely be influenced by volatilization. Additionally, guidelines lack sufficient information on test-system geometry and guidance on how to measure and maintain aerobic conditions during the test. Our objectives were (1) to design a closed test setup for biodegradation tests in soil in which the maintaining and measuring of aerobic conditions was possible without the loss of volatile test chemicals and (2) to suggest data-treatment measures for evaluating the degradation kinetics of volatile test chemicals. With the new setup, full-scale OECD 307 tests were performed using the volatile 14C-labeled chemicals decane and tetralin. For both test chemicals, reproducible complete mass balances were observed, and the new setup ensured that the volatilization losses were kept below the mineralized fraction. Based on the obtained data, an extended model was developed that enabled consideration of the volatilization in the modeling of degradation kinetics.
1. Introduction
During prospective environmental risk assessment of chemicals, biodegradation kinetics data are a key parameter in different regulatory frameworks like REACH,1 pesticide regulation,2 biocide regulation,3 etc. These degradation kinetics data are generated using a set of tiered laboratory biodegradation tests with increasing levels of complexity,4,5 which are performed using standard Organisation for Economic Co-operation and Development (OECD) guidelines.6 The highest tiers are formed by the environmental simulation biodegradation tests and were designed to simulate biodegradation in various environmental matrices such as soil (OECD 307),7 water and sediment (OECD 308),8 or surface water (OECD 309).9 The OECD guidelines were originally developed for pesticide regulation, for which they work well with most of the relevant chemicals. However, when those guidelines are applied to other regulatory frameworks, it should be considered whether the chemicals regulated under those frameworks possess different physical-chemical properties that might make them more challenging to test or render the existing test guidelines unsuitable. In particular, the previously mentioned guidelines face serious drawbacks when it comes to the testing of volatile chemicals. Some of these issues are listed below:
Although the scope of OECD 307, 308, and 309 guidelines clearly state that the tests are not suitable for volatile chemicals,6,7 with the exception of OECD 309, they do not define a threshold. In OECD 309, a threshold for the Henry’s law constant (KH) of 100 Pa m3/mol is stated.9
During the testing of volatile chemicals in a flow-through setup, incomplete mass balances is a major problem, and it is often impossible to meet the validity criteria of 90%–110% recovery for radiolabeled chemicals and 70%–110% for non-labeled chemicals.7−9 Prior work has suggested that incomplete mass balances mainly result due to inability of the traps to capture the volatile chemicals, and hence, they escape from the test setup. In addition, our own experience and other studies indicate that volatile test chemicals are also being sorbed to the plastic or silicone materials present in the fittings of the test setup.10
As an alternative, OECD 307, 308, and 309 allow us to apply static biometer-type incubation setups.7,8 However, these guidelines give only limited information on how to handle the headspace in the biometer type test setup for testing volatile chemicals. Several studies suggest that the headspace volume is quite important to keep the system aerobic in a static test setup. However, too much headspace might lead to higher mass distribution into the headspace volume, leading to a lowered fraction in the water, sediment, or soil available for degradation and a corresponding effect on degradation kinetics.10,11
The guideline does not stipulate any data requirements to prove whether the test system remains aerobic. This is a general problem with static test setups, especially if a co-solvent is used for the application of test chemicals. In a normal case, the co-solvent should be evaporated after test substance application, but during the application of volatile chemicals, the evaporation step has to be avoided as loss of test chemicals may occur. However, our own studies with closed biometer-type test setups have shown rapid oxygen depletion after solvent application. This can have a major impact on the degradation kinetics, but to monitor the oxygen content in the system containing volatile chemicals is a challenging task.
As suggested by Birch et al.,11 reductions in bioavailability as a result of partitioning to headspace can largely influence the degradation kinetics. Therefore, new data-treatment methods are needed for generating meaningful degradation kinetics for tests in which volatile chemicals are used.
To address these drawbacks, our objectives were (1) to develop an incubation test for testing degradation of volatile chemicals in soil, (2) to demonstrate the usefulness of the developed test setup in a full scale OECD 307, and (3) to suggest methods on how to treat the data obtained from such tests for evaluating degradation kinetics. The first objective was obtained during a series of pretests with different test setups. Maintainance of aerobic conditions inside the flask was also taken into consideration and was done by external oxygen measurements thereby minimizing test chemical losses due to opening of the vessel. Second, with the new test setup, a full-scale OECD 307 test was performed using two chemicals with different volatilization and degradation potential and four different soils, varying in terms of texture, organic carbon (OC) content, and microbial activity. The data obtained were used to demonstrate the reproducibility and reliability of the test setup in terms of mass balance. Third, the data from the tests were used to improve our understanding of different processes such as volatilization and sorption processes taking place during the test and its influence on the final biodegradation data obtained. Furthermore, data treatment methods were demonstrated for generating meaningful degradation kinetics for the volatile chemicals in the new test setup. The first hypothesis of the study was that improved methodology was necessary to determine the biodegradation kinetics of hydrophobic volatile chemicals in soils. The second hypothesis was that careful experimental testing and data analysis can facilitate the separation of degradation and evaporative losses.
2. Materials and Methods
2.1. Test Chemicals
A pair of 14C labeled chemicals were used for the degradation tests covered within this project: (1) tetralin (1,2,3,4-tetrahydronaphthalene, C10H12, CAS no. 119-64-2, item no. 17445740, >98% purity, Hartmann Analytics) and (2) decane (n-decane, C10H22, CAS no. 124-18-5, item no. 17539210, >98% purity Hartmann Analytics). The test chemicals were selected on the basis of their differences in air–water partitioning and sorption properties, with tetralin being slightly volatile [KH of 138 Pa m3 mol–1, vapor pressure (VP) of 0.37 mmHg at 25 °C, and organic carbon-water partition coefficient (Koc) of 1068 L/kg]13 and with decane being highly volatile but theoretically more sorbing than tetralin (KH of 522 000 Pa m3 mol–1, VP of 1.43 mmHg at 25 °C, and Koc of 22 270 L/kg).12 For the optimization of specific chemical analytics and also as a quality control, cold standards from Sigma-Aldrich with purities of >99% were used.
2.2. Soils
In total, four soils were used to cover a broad range of soil texture, organic carbon (OC) content, and microbial biomass, as required by the OECD guideline no. 307. A pair of soils with low OC content [01A (loamy sand, OC 0.80%) and 02A (silty loam, OC 0.98%)] and two with higher OC content [03G (silty loam, OC 3.05%) and 04A (loamy sand, OC 2.79%)] were used. The detailed soil properties are listed in Table S1. The soil texture was determined using the standard DIN ISO 11277,13 and OC content was determined using DIN EN 15936.14 Per the guideline requirements, the soil was sampled from the upper 20 cm layer and was sieved using a 2 mm sieve. The soils were maintained at 50% water-holding capacity (WHC) for pre-incubation under test conditions (at 20 °C in the dark) for a period of 2–3 weeks before the test chemical application.
2.3. Test-Chemical Application and Sample Incubation Conditions
The 14C-labeled test chemical application solution was prepared using the co-solvent acetone for tetralin and methanol for decane. The application solution was then spiked directly on the soil, and the sample bottle was immediately closed using an insert cap. The starting test substance concentration was 1 mg/kg (dry weight of soil) for all tests conducted within this project. The amount of applied radioactivity per soil sample of 50 g was 203 kBq in 219 μL of acetone for tetralin and 195 kBq in 139 μL of methanol for decane. After the application of the test chemical, the soil samples were incubated at 20 ± 2 °C in the dark.
2.4. Pretests
To develop a biodegradation test setup for volatile chemicals, a series of preliminary tests were conducted using 14C labeled tetralin. The first tests were composed of a soil degradation study using a flow-through setup as suggested by OECD 307 with slight modifications. The primary objective of this experiment was to check for a complete mass balance of tetralin during the incubation period. These tests were followed by other tests using closed-flask test setups in which maintaining and measuring aerobic conditions in the test setup was considered along with the complete mass balance of the test chemical.
2.4.1. Flow-Through Setup (OECD 307) with Volatile Combustion
A total of 50 g of dry weight (dw) of preincubated soil was weighed in a 100 mL sample flask and applied with 14C labeled tetralin (see section 2.3). A flow-through setup consisted of a sample flask connected with absorption traps containing 100 mL of 2N NaOH and ethylene glycol flasks to trap mineralized 14CO2 and volatile parent and intermediate, respectively. A schematic diagram of the flow-through setup used is given in the Figure S1. Behind the traps, a tube furnace operating at 850 °C was connected and filled with a CuO catalyst to combust any substance in the flow-through, which could not be captured in the prior traps. To trap the resulting 14CO2, one further sample flask with 100 mL of 2N NaOH was connected behind the oven. At specific sampling points (0, 3, 7, and 14 days after application), duplicate soil samples along with their traps were sampled, processed, and taken for chemical analysis (see sections 2.6 and 2.7).
2.4.2. Closed-Flask Test Setup 1
A biometer type closed test setup was designed consisting of a 500 mL bottle with 50 g of soil (dW), 0.5 g of tenax (for absorbing the volatiles), and 4 g of soda lime (for absorbing 14CO2). The flask was closed using a stainless steel, plastic-free lock system (Swagelok connections, Hamlet valves and fitting) (see Figure S2). The test was conducted exemplarily with just one soil (02-A) in duplicates. The test chemical application and sample incubation were done as described in section 2.3, and the incubation period was 14 days. At each sampling date, the air in the headspace was stripped out of the sample bottles through the tenax and was passed through 2N NaOH and ethylene glycol traps using a vacuum pump. The soil sample, traps, and the tenax were taken for further analysis (see sections 2.6 and 2.7).
2.4.3. Closed-Flask Test Setup 2
Unlike test setup 1, this new test setup composed of a smaller test-vessel 100 mL flask instead of 500 mL to lower the headspace volume, and the soda lime was replaced with 6 mL of 2N NaOH (see Figure 1). Instead of self-prepared tenax inserts, a tenax-adsorption tube (catalog no. 226-35, SKC Inc.) was permanently connected to the flask to passively trap the volatilized fraction. The same stainless-steel, plastic-free lock systems used in setup 1 were used to completely close the test setup. The test chemical application and sample incubation were done as described in section 2.3, and samples were taken in duplicates at 0, 7, and 14 days.
Figure 1.
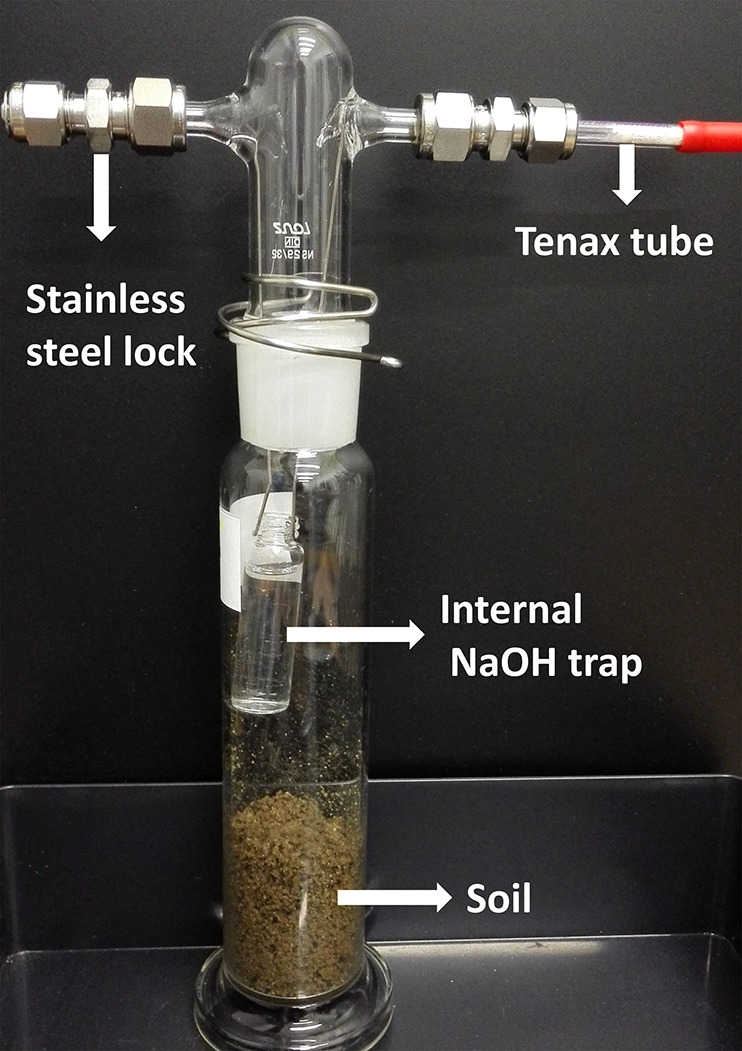
Closed-flask incubation test setup 2 used for testing the biodegradation of 14C labeled tetralin and decane in soil using guideline OECD 307. The test design consisted of a 100 mL glass flask with 50 g (dry matter) of soil with an internal CO2 absorbing flask and a permanently hanging tenax tube for passively trapping the volatile fraction. The entire test design was closed using a plastic-free stainless-steel lock system.
Additionally, two reference samples in duplicates (see section S5) were prepared according to test setup 2 without the tenax and NaOH flask but instead including an oxygen sensor spot (“Redflash technology”, Pyroscience) attached to the inner wall of the test vessel in the headspace. One of these references samples were applied with the same amount of cosolvent without test chemical as in the test samples applied with 14C tetralin, whereas the other reference sample was incubated under test conditions without solvent application. Every 2–3 days, the oxygen saturation in the headspace of these reference samples was measured with a fiber-optic oxygen meter (Firesting O2, Pyroscience) without the need to open the vessel (for details, see section S7). In the case that the oxygen concentration in the headspace was found to be <15%, the reference sample along with the 14C substance applied samples were aerated with oxygen-rich air for 20 s through a tube until 18–20% oxygen saturation in the headspace was reached. All of the air leaving the sample flask during the aeration procedure was passed through the tenax tube. The aeration procedure was tested on the reference sample and then applied to the test samples. On the sampling date, the air in the headspace was stripped out of the sample bottles through the tenax tube using a pump (see section S8). The soil sample, NaOH trap and the tenax tube were taken for further analysis (see section 2.6).
2.5. Main Test
To check the reproducibility and reliability of the test setup 2 in terms of obtaining complete mass balances for a longer incubation period, full-scale OECD 307 tests with two 14C-labeled chemicals (tetralin and decane) were performed across four different soils (see section 2.2). The sample preparation, chemical application, and sample treatment were done as described in section 2.4.3. The total sample incubation period in the tetralin study was 120 days for soils 04-A and 01-A and 28 and 60 days for soil 03-G and 02-A, respectively. For the decane study, the total sample incubation period was 7 days for soil 03-G and 12 days for the other soils. A total of 8–10 sampling points were scheduled within the incubation periods to obtain enough data points for a reliable modeling of the degradation kinetics. To check for the influence of abiotic degradation processes sterile samples were prepared, and separate samples were organized for the measurement of microbial biomass of the soil during the test (for details, see sections S5 and S6).
2.6. Sample Processing and Analysis
2.6.1. Soil Samples
At specific sampling dates, the soil was removed from the sample bottle and taken for extraction using acetonitrile (see section S9). The walls of the sample bottle along with other test materials, e.g., stainless steel caps and outer wall of CO2-absorbing flasks, were thoroughly rinsed with acetonitrile. The rinsing solution was combined with the soil extracts afterward. An aliquot of this extract in duplicates was mixed with an appropriate scintillation cocktail, taken for radioactive analysis (Hidex 600 SL, Hidey, Finland and Tricarb 2910, PerkinElmer), and then taken for specific chemical analysis (see section 2.7). After soil extraction, the remaining soil residue was air-dried, homogenized using a mortar mill (RM 2000, Retsch), and taken for combustion analysis using a Zinsser OX 700 oxidizer to determine the non-extractable residues (NER).
2.6.2. Absorption Traps
An aliquot of the trapping solution was analyzed by liquid scintillation counting (LSC). For the confirmation of CO2 trapped in the 2N NaOH flask, BaCl2 tests (see section S10) were performed for selected samples. The pH of the NaOH trap was monitored using a pH indicator (pH 0–14, SZBF 1390 V, Fluka Analytical). The soda lime used as a CO2 trap for the test setup 1 was ground using a mortar mill, and an aliquot was taken for combustion analysis.
2.6.3. Tenax Tubes
After the headspace air from the sample bottle was stripped through the tenax tubes at the sampling date, the tenax tubes were removed and eluted with 3 samples of 3 mL of acetonitrile. The extracts were taken for radioactive and specific chemical analysis.
2.7. Specific Chemical Analysis
Soil extracts with total recovery of >5% of the applied radioactivity (aR) were taken for specific chemical analysis. The extracts were centrifuged at 12300g for 5 min in a 2 mL vial (Eppendorf Germany) using a mini centrifuge (Microstar 12, VWR). The supernatant was taken for specific chemical analysis using radio-high-performance liquid chromatography (radio-HPLC). The tenax extracts were analyzed without any cleanup using radio-HPLC.
2.8. Radio-HPLC
For radio-HPLC analysis, a Dionex Ultimate 3000 was used coupled with a UV detector (PDA-100 photodiode Array detector) and a 14C detector (Ramona Raytest). The chromatographic separation was achieved by a Luna C18 (2) 3 μm 150 × 2 mm (MZ) column (Phenomenex, Germany), with the column temperature maintained at 30 °C. (For details on the radio-HPLC method used, see section S11)
2.9. Data Treatment
The starting point for fitting the degradation data was the degradation kinetics recommended by FOrum for the Coordination of pesticide fate models and their USe (FOCUS).15 FOCUS has developed various guidance documents originally to be used in pesticide registration. However, many documents were later adopted by other regulations, e.g., for biocides or veterinary compounds. FOCUS recommended a single first order (SFO) together with three different biphasic kinetic models (double first order in parallel, DFOP; hockey stick, HS; and first-order multi-compartment, FOMC). The fitting of the experimental residues was done with using the software CAKE version 3.2 running on R version 3.0.0. CAKE is a freely available software tool that completely follows the recommendations of FOCUS (2006/2014).
The recommendation for this type of data treatment is given for studies in which volatilization is not a significant process. Thus, to address the processes in these studies adequately, the volatilization losses were considered as an additional product that neither decline nor repartition into the soil. The volatilization is thus treated as a separate sink for the parent compound rather than as a partitioning process that can bring the parent compound back into the soil.11 The volatilization loss was considered to occur in parallel to the biodegradation. Therefore, in this extended model, the degradation and the volatilization of the compound were considered as two processes and separated so that individual rate constants could be calculated for the volatilization process as well as the degradation process. The calculation of DegT50 and DegT90 values was based on the fraction of radioactivity in extracts, which could be identified as parent substance. In general, the model assumes first order kinetics with k as an overall dissipation rate and c the concentration of test chemical according to following equation:
| 1 |
However, for the extended model, it is assumed that k consists of two rate constants volatilization rate (kV) and transformation rate (kT):
| 2 |
To describe the ratio of the two parallel processes, the model internally uses “fractions” FV (volatilization fraction) and FT (transformation fraction), which can be calculated based on the individual rates for volatilization and transformation together with the overall decline rate as follows:
| 3a |
| 3b |
For the structure of the standard and the extended model, see Figures S12 and 13.
3. Results and Discussion
3.1. Pretests
The results from the biodegradation tests carried out with 14C tetralin using different test setups after a 14 day incubation period in 02-A soil is shown in Figure S3. An incomplete mass balance was obtained for the soil degradation test carried out in a flow-through setup despite using a combustion oven to trap the volatile fraction in the form of completely oxidized 14CO2. This suggested that the volatilized fraction was either escaping or being adsorbed to the fittings used to connect the flow-through setup. Therefore, the flow-through setup even with this modification could not be utilized to attain a complete mass balance, which is a validation criteria for the test. In contrast, a complete mass balance was obtained using test setup 1. Nevertheless, the test setup was still considered inappropriate as 98% of the applied radioactivity was volatilized and trapped in the tenax and thus unavailable for microorganism in the soil. This made biodegradation measurements impossible, which is the primary goal of the test. Additionally, the soda lime used as internal 14CO2 trap in the test setup 1 was also considered impractical for direct analysis of mineralization as it had to be ground and taken for combustion for radioactive analysis. The high amount of radioactivity in the tenax was attributed to a higher headspace volume of the sample bottle, a higher amount of tenax filled in the inserts, and its dimensions and position within the sample bottle. A higher headspace volume will result in a greater mass fraction of the test substance partitioning into the headspace,10,11 which could subsequently be trapped in the tenax, thus making it unavailable for degradation. Considering these factors, a test using setup 2 with smaller headspace, a different type and positioning of the tenax adsorber, NaOH as CO2 absorber and with regular oxygen monitoring was performed The results observed using test setup 2 displayed a complete mass balance with only 20% of the applied radioactivity trapped on the tenax. Setup 2 was therefore considered operationally superior to setup 1 because it would permit measurement of biodegradation with less volatile losses. These results reveal the relevance of headspace and amount, dimensions, and position of tenax in a closed test setup for the distribution and the fate of a volatile chemical in the test system.
3.2. Main Test
3.2.1. Oxygen Monitoring in Reference Samples
Figure 2 shows the oxygen saturation in the headspace of the reference samples of test setup 2 during the initial 28 days of the tetralin main study. The results showed almost no oxygen depletion in the samples applied without co-solvent during the incubation period. In contrast, for soil samples applied with co-solvent, different oxygen depletion trends were observed, with the organic carbon rich silty loam (03-G) showing the most rapid oxygen depletion relative to the other soils. In general, rapid depletion of oxygen saturation took place between day 0 and 14 in all soil samples applied with co-solvent. If the oxygen saturation measured was below 15%, the samples were reoxygenated with oxygen-rich air to 18–20% saturation. Despite oxygenation occurring every 2–3 days, continuous depletion of oxygen saturation was observed between day 0 and 14 for all soils. This oxygen depletion could be attributed to the oxygen consumption due to the degradation of the co-solvent.
Figure 2.
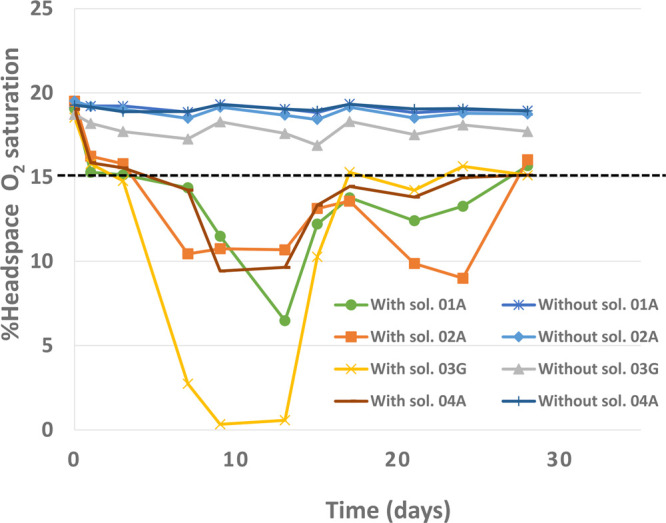
Oxygen saturation in the headspace of the reference samples for all four soils during the tetralin main study using test setup 2. A pair of reference samples were used, one being applied with and the other without solvent. The samples applied with solvent were oxygenated when oxygen saturation was <15%, and continuous depletion in the oxygen saturation was observed between 0 and 14 days. The oxygen measurements were done without the need to open the test vessel using an optical oxygen meter (Firesting, Pyroscience).
These results suggest that the conditions in a closed biometer test setup can rapidly turn anaerobic without regular oxygen supply if a cosolvent is not evaporated after the application of the test chemical. In all cases in which the co-solvent can be evaporated without extensive losses of test chemical, this is, therefore, preferable. In cases in which test-chemical losses are too high and co-solvents cannot be avoided, such as when testing highly volatile chemicals, changes from aerobic to anaerobic test conditions may influence the degradation kinetics due to effects on the microbial communities. Therefore, we strongly recommend monitoring the O2 saturation during tests conducted in a closed biometer test setup if no evaporation of cosolvent is performed. If the O2 saturation drops, a supplement of additional oxygen is needed.
3.2.2. Mass Balance
In line with the results observed in the preliminary tests, the average overall observed recovery was 99.9% ± SD of 10.6 (N = 90) for decane and 104.8% ± SD of 5.5 (N = 90) for tetralin in these studies. A mass balance of 100 ± 15% was observed in 88.9% (N = 90), and 100% (N = 90) of the test samples for decane and tetralin, respectively. Thus, results from the main study confirm that the test setup and approach that was developed to test volatile chemicals was highly effective and reproducible in terms of obtaining a complete mass balance. The complete degradation time series for all soil types for both test chemicals from the main study are shown in Figures S8–S11.
3.2.3. Competing Processes (Sorption, Volatilization, and Degradation)
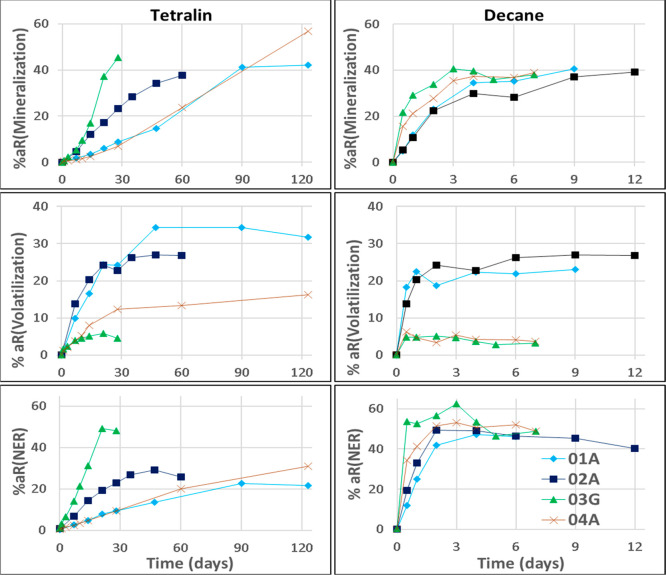
Figure 3 shows the mineralization of tetralin and decane, measured in the CO2 traps, the volatilization, measured in the tenax tubes, and the NER formation, measured as residues in the soil after extraction. The 03-G and 02-A soils showed faster mineralization of tetralin than the other two soils. In particular, very rapid mineralization of tetralin was observed for the 03-G soil in spite of the higher OC content in this soil compared with soil 01-A and 02-A. Slightly faster mineralization of decane was also observed in the 03-G soil. The higher mineralization rates in the 03-G soil can be explained by a combination of two factors. First, the 03-G soil had a higher amount of microbial biomass (see Tables S6 and S7) and a higher microbial activity, as revealed by the higher oxygen depletion16−18 in the reference samples of this soil (Figure 2). Second, the volatilization was reduced due to the higher OC content, relative to the other soils, leading to a higher fraction of test substance remaining in the soil available for degradation.
Figure 3.
Volatilization, mineralization, and NER formation of tetralin (left) and decane (right) in four different soils (see section 2.2). Volatilization, mineralization, and NER were calculated as a percentage of the initial applied radioactivity (%aR). The figure shows that volatilization is higher for low OC content soils (01-A and 02-A) in comparison with the high OC soils (03-G and 04-A). In contrast, fastest mineralization and NER formation was observed in 03-G soil with the highest OC content.
The radioactivity trapped in the tenax in both sterile and non-sterile samples was identified as 100% parent compound from the specific chemicals analysis data. As can be observed in Figure 3, a higher amount of test chemical was observed in the tenax for the soils with lower OC relative to the soils with a higher OC, suggesting greater volatilization from soils with lower OC content. In the sterile samples containing tetralin and decane, the amount of test chemical trapped in the tenax was also higher in the soils with lower OC carbon content (see Table S4). These results illustrate how sorption of the test chemical to organic matter in the soil reduces its volatilization19−23 and, with the extended residence time, the extent of biodegradation improves in a closed flask test setup.
The results show that the volatilized fraction of the test chemical gradually or rapidly increased and reached a plateau (see Figure 3). No considerable drop in the volatilized fraction was observed along the incubation period. Slight drops observed at some sampling points might have been due to the effect of overall lowered radioactivity recovery. This confirmed that in our closed incubation test setup, the volatilized fraction was not remobilized, and the tenax traps acted as intended as a contaminant sink24,10 rather than a partitioning reservoir.25,11
Although the KH value of decane is much higher than that of tetralin, the final fraction of volatilized decane at the test end was slightly lower than for tetralin. However, for the sterile samples, the distribution into the tenax was in line with the KH values (Table S4). These differences in mass distribution can be explained by the faster degradation rate of decane in the nonsterile samples relative to its volatilization rate. Furthermore, decane has a higher Koc value, which improves its sorption to the organic fraction of the soil.
A considerable portion of the applied radioactivity was recovered as NER in both the tetralin and decane studies. The rate of NER formation was faster and higher for decane compared to tetralin. Similar to the mineralization, highest and rapid NER formation was observed in the 03-G soil for tetralin, whereas in the decane study, the NER formation and mineralization at the end of study was more or less the same in all of the soils. Studies on NER26−28 suggest that the formation of bio-NER is linked to mineralization. This is also apparent in our study. Recently a microbial to biomass turnover (MTB) method was developed for predicting the fraction of bio-NER.29,30 This MTB method was applied using required inputs from our test and literature data to predict the bio-NER fraction (see Table S8). The short-term bio-NER prediction was done exemplarily until 28 days for tetralin and until the end of the test (12 days for soils 01A, 02A, and 04A and 7 days for soil 03G) for decane. The results showed that the predicted bio-NER was higher than the total NER observed in the test by a factor of 1.19–1.77 for decane, whereas for tetralin, it was slightly lower by a factor 0.65–0.77 (for details, see Table S9). Thus, MTB method also indicates high bio-NER formation for these test chemicals. This argument was also supported by the results observed in the sterile sample, for which almost no NER formation was observed (<2.5%) in any of the studies.
3.3. Data Analysis
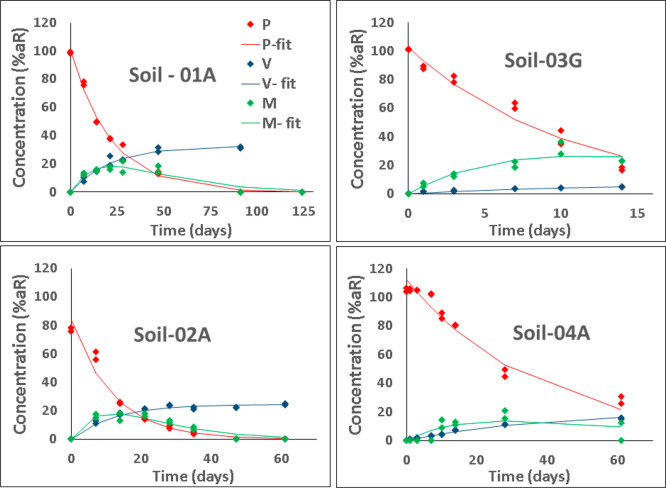
Due to rapid mineralization, NER formation and volatilization of decane, less than 9% of the applied radioactivity was observed in the extractable fraction after 1–2 days in all soils. Therefore, due to lack of enough data points, it was not possible to evaluate the parent dissipation kinetics for decane. For tetralin, the decline of the parent compound was first analyzed and the key information about the fitting of four different kinetic models was calculated using CAKE (see Table S5). Based on the results it was concluded that the SFO kinetic model generally showed the best performance as indicated by the small χ2 errors. The SFO rate constants are consistent with prior work on aerobic surface water.31−33 In the second step, the formation and transformation of the metabolites and the volatilization rate was evaluated based on SFO kinetics for the parent compound. As recommended by FOCUS (2006/2014), the transformation rate of the metabolites was also optimized considering SFO kinetics. The results are presented in Table 1. Apart from the metabolite residues in 04-A the χ2 values are all in all following the recommendations of FOCUS (2006/2014), which states that a χ2 value of about 15% for the parent compound is usually acceptable for these kinds of experiments. For metabolites, even higher percentages may be acceptable. However, that does not hold true for the high χ2 value for the metabolite in 04-A (44.2%). This is probably caused by the latency phase at the beginning of the experiment that cannot be handled by the fitting model. The results of the overall DT50 for the parent compound in the two models (see Tables 1 and S5) vary slightly because additional residues were included in the more-complex fitting model.
Table 1. CAKE Results for Tetralin When Using the Extended Model Including the Metabolite and Tenax Residues (Only SFO Kinetics)a.
| residue | χ2 (%) | overall DT50 (d) | FT | FV | DegT50 (days) | DT50, vol (days) |
|---|---|---|---|---|---|---|
| soil 01-A | ||||||
| parent | 4.45 | 15.2 | 0.48 | 0.33 | 22.6 | 46.4 |
| metabolite | 14.40 | 16 | – | – | – | – |
| tenax | 3.91 | – | – | – | – | – |
| soil 02-A | ||||||
| parent | 16.9 | 8.25 | 0.60 | 0.30 | 11.7 | 28.0 |
| metabolite | 16.4 | 8.01 | – | – | – | – |
| tenax | 4.25 | – | – | – | – | – |
| soil 03-G | ||||||
| parent | 7.19 | 7.13 | 0.62 | 0.06 | 7.6 | 111.1 |
| metabolite | 14.5 | 9.08 | – | – | – | – |
| tenax | 11.5 | – | – | – | – | – |
| soil 04-A | ||||||
| parent | 5.37 | 25.7 | 0.43 | 0.18 | 31.1 | 143.1 |
| metabolite | 44.2 | 15.9 | – | – | – | – |
| tenax | 6.41 | – | – | – | – | – |
FT is the transformed fraction, Fv is the volatilized fraction, DegT50 is the degradation half-time, and DT50, vol is the removal half-time due to volatilization.
However, in addition to the overall DT50 for the parent compound the extended model can separate between the decline due to transformation and due to volatilization. The two fractions (FT and FV) in Table 1 describe how the optimization tool evaluated the importance of the respective processes, transformation and volatilization, in the experiment. An FT of 0.48 (see Table 1) means that the fit is best when 48% of the original molecules are transformed to metabolites, whereas an Fv of 0.33 means that in the optimum fit 33% of the parent compound is collected in the tenax. Based on the fractions for volatilization, respective half-lives can be calculated using the following equation (Fv, fraction due to volatilization; DT50,vol, half-life due the volatilization of the compound in the soil; DT50, overall disappearance of the compound):
| 4 |
The half-lives for volatilization are presented in Table 1. The DegT50 values represent all (primary) transformation processes. It was calculated according to following equation:
| 5 |
Note that this DegT50 includes not only the formation of metabolites but also other processes (e.g., formation of NER). If the DegT50 were to represent the formation of metabolites, the term 1 – Fv should be replaced by the FT shown in Table 1. In Figure 4, visual representations of the fitting are presented. They confirm the previous conclusions on the basis of the χ2 errors (fits are acceptable) because the curves follow the experimental data closely, and the deviations are randomly distributed around the curves. There is only one exception for 04-A, in which the beginning of the experiment could not be fitted correctly because the SFO model cannot consider latency periods.
Figure 4.
Graphs shows the CAKE results for tetralin when using the extended model including the metabolite and tenax residues (only SFO kinetics). Time-dependent observed (markers) and fitted (lines) residues are shown. P, parent; V, volatilized; and M, metabolite.
In summary, it can be concluded that, based on the extended model, it is possible to evaluate the experimental findings and to calculate meaningful half-lives for compounds even in situations where losses due to volatilization are an important process.
4. Implication and Suggestions
It was demonstrated that the closed flask test setup was suitable for obtaining complete mass balances for a range of volatile chemicals in a standard soil-degradation test. However, it was observed that the rapid oxygen depletion might be an issue in closed-flask test setups applied with co-solvent without an evaporation step. This might considerably change the test conditions during the incubation period and also the degradation of the test chemical. Therefore, we recommend monitoring the oxygen saturation during such special tests and supply oxygen by either manual (as demonstrated here) or automatic oxygen-delivery methods (for, e.g., a plastic-free sapromat system).
In our tests, using 14C-labeled test chemical was a major advantage for obtaining the complete mass balance because mineralization, NER formation, and volatilization comprised considerable fractions of the applied radioactivity. Without 14C labeling, it would not have been possible to trace NER formation and mineralization and, thus, make a clear distinction between volatilization and related processes. Hence, 14C labeling was not just an advantage in obtaining the complete mass balance but also important for understanding underlying processes in the new test setup.
The closed setup used in the present study provided a basis for evaluating the degradation of volatile chemicals while accounting for its sorption and volatilization. For an improved biodegradation test setup, it is crucial to minimize the escape of test chemicals from the soil by volatilization and sorption to test system materials to reduce the impact of these processes on the biodegradation test results. It was here demonstrated that it is feasible to design such systems and keep the major fraction of the chemicals in the soil during the test. Nonetheless, obtaining degradation kinetics using the same test setup for slowly degrading and highly volatile chemicals could be challenging as volatilization might then be a dominant process of removal from the soil making it unavailable for degradation and hence masking the possibility of degradation of the chemical. For such chemicals, an even-further reduction of the headspace may be necessary, combined with application strategies that minimizes or removes the need for co-solvents in the test. As seen in our tests, the choice of soil type can also play a crucial role in volatilization and degradation of test chemicals.
Within our study, we have primarily focused on the degradation of volatile chemicals in soil, but there are other simulation tests with aqueous medium (such as OECD 308 and 309) in which volatilization can be more significant. Thus, it should be studied further whether this improved test setup with a tenax trap can be adapted for such aqueous tests or whether closed-headspace test setups without a tenax trap are more suitable. In the absence of a trap, the headspace will act as a reservoir for the test substance instead of a sink and thus require a different modeling approach for generating biodegradation kinetics.11
Acknowledgments
We acknowledge Concawe for funding the project and especially Concawe ecology group for helpful comments and feedbacks during the manuscript preparation process. We also thank Fraunhofer IME-AE staff members Christoph Eggenstein Deimel, Claudia Knoche, and Joana Bräutigam for their valuable input and work within the project.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.8b05079.
Additional details, including figures and tables, on soil parameters, flow-through setup, test setup 1, mass balance, the preparation of samples, oxygen measurement in the headspace, headspace air-stripping procedure, soil extraction, the BaCl2 test, the radio-HPLC method, degradation time series, variability in mass balance, %aR in tenax in sterile samples, degradation kinetics using standard and extended modeling, microbial biomass measurements results, and predicted bio-NER vs total NER from the tests (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Commission regulation (EU) No 253/2011 of 15 March 2011 amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards Annex XIII. Off. J. of European Union 2011, L69/7–12. [Google Scholar]
- Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. of European Union 2009, 309, 1–50. [Google Scholar]
- Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products. Off. J. of European Union 2012, 167, 1–123. [Google Scholar]
- OECD . Revised introduction to OECD Guidelines for the Testing of Chemicals, Section 3, Part 1, Principles and strategies related to the testing of degradation of organic chemicals; OECD Publishing: Paris, France, 2002.
- Kowalczyk A.; Martin T. J.; Price O. R.; Snape J. R.; van Egmond R. A.; Finnegan C. J.; Schäfer H.; Davenport R. J.; Bending G. D. Refinement of biodegradation tests methodologies and the proposed utility of new microbial ecology techniques. Ecotoxicol. Environ. Saf. 2015, 111, 9–22. 10.1016/j.ecoenv.2014.09.021. [DOI] [PubMed] [Google Scholar]
- OECD . Guidelines for the Testing of Chemicals, Section 3 Environmental fate and behavior; OECD: Paris, France, 2017; 10.1787/2074577x. [DOI]
- OECD . Test No. 307: Aerobic and Anaerobic Transformation in Soil, OECD Guidelines for the Testing of Chemicals, Section 3, No. 307; OECD Publishing: Paris, France, 2002. DOI 10.1787/9789264070509-en. [DOI]
- OECD . Test No. 308: Aerobic and Anaerobic Transformation in Aquatic Sediment Systems, OECD Guidelines for the Testing of Chemicals, Section 3, No. 308; OECD Publishing: Paris, France, 2002; 10.1787/9789264070523-en. [DOI]
- OECD . Test No. 309: Aerobic Mineralisation in Surface Water – Simulation Biodegradation Test, OECD Guidelines for the Testing of Chemicals, Section 3, No. 309, OECD Publishing: Paris, France, 2002; 10.1787/9789264070547-en. [DOI]
- Brown D. M.; Hughes C. B.; Spence M.; Bonte M.; Whale G. Assessing the suitability of a manometric test system for determining the biodegradability of volatile hydrocarbons. Chemosphere 2018, 195, 381–389. 10.1016/j.chemosphere.2017.11.169. [DOI] [PubMed] [Google Scholar]
- Birch H.; Andersen H. R.; Comber M.; Mayer P. Biodegradation testing of chemicals with high Henry’s constants–Separating mass and effective concentration reveals higher rate constants. Chemosphere 2017, 174, 716–721. 10.1016/j.chemosphere.2017.02.003. [DOI] [PubMed] [Google Scholar]
- National Food Institute DTU . Danish (Q)SAR Database. http://qsardb.food.dtu.dk/database/index.html (accessed November 23, 2017).
- Soil quality - Determination of particle size distribution in mineral soil material - Method by sieving and sedimentation; International Organization for Standardization: Switzerland, 2009.
- DIN EN 15936:2012–11. Sludge, treated biowaste, soil and waste - Determination of total organic carbon (TOC) by dry combustion; German version EN 15936:2012.
- FOCUS (2006). Guidance Document in Estimating Persistence and Degradation Kinetics from Environmental Fate Studies in Pesticides in EU Registration. EC Document Reference Sanco/10058/2005 version 2.0, Report of the FOCUS work group on degradation kinetics: 434.
- Shrestha P.; Junker T.; Fenner K.; Hahn S.; Bakkour R.; Diaz C.; Hennecke D.; Honti M. Simulation Studies to Explore Biodegradation in Water–Sediment Systems: From OECD 308 to OECD 309. Environ. Sci. Technol. 2016, 50 (13), 6856–6864. 10.1021/acs.est.6b01095. [DOI] [PubMed] [Google Scholar]
- Leahy J. G.; Olsen R. H. Kinetics of toluene degradation by toluene-oxidizing bacteria as a function of oxygen concentration, and the effect of nitrate. FEMS Microbiol. Ecol. 1997, 23 (1), 23–30. 10.1111/j.1574-6941.1997.tb00387.x. [DOI] [Google Scholar]
- Michaelsen M.; Hulsch R.; Hopner T.; Berthe-Corti L. Hexadecane mineralization in oxygen-controlled sediment-seawater cultivations with autochthonous microorganisms. Appl. Environ. Microbiol. 1992, 58, 3072–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhard N.; Guth J. A. Rate of volatilisation of pesticides from soil surfaces; Comparison of calculated results with those determined in a laboratory model. Pestic. Sci. 1981, 12, 37–44. 10.1002/ps.2780120106. [DOI] [Google Scholar]
- Chiou T. C.; Shoup D. T. Soil sorption of organic vapors and effects of humidity on sorption mechanism and capacity. Environ. Sci. Technol. 1985, 19, 1196–1200. 10.1021/es00142a010. [DOI] [PubMed] [Google Scholar]
- Basile M.; Senesi N.; Lamberti F.A. Study of some factors affecting volatilization losses of 1,3-dichloropropene (1,3-D) from soil. Agric., Ecosyst. Environ. 1986, 17, 269–279. 10.1016/0167-8809(86)90047-2. [DOI] [Google Scholar]
- Alvarez-Benedi J.; Tabemero M. T.; Atienza J.; Bolado S. A coupled model representing volatilisation and sorption of soil incorporated herbicides. Chemosphere 1999, 38 (7), 1583–1593. 10.1016/S0045-6535(98)00385-3. [DOI] [Google Scholar]
- Spencer W. F.; Farmer W. J.; Cliath M. M. Pesticide volatilization. Residue Reviews 1973, 49, 1–40. 10.1007/978-1-4613-9377-1_1. [DOI] [Google Scholar]
- Mayer P.; Olsen J. L.; Gouliarmou V.; Hasinger M.; Kendler R.; Loibner A. P. A Contaminant Trap as a Tool for Isolating and Measuring the Desorption Resistant Fraction of Soil Pollutants. Environ. Sci. Technol. 2011, 45 (7), 2932–2937. 10.1021/es1033124. [DOI] [PubMed] [Google Scholar]
- Birch H.; Hammershøj R.; Comber M.; Mayer P. Biodegradation of hydrocarbon mixtures in surface waters at environmentally relevant levels-effect of inoculum origin on kinetics and sequence of degradation. Chemosphere 2017, 184, 400–407. 10.1016/j.chemosphere.2017.05.169. [DOI] [PubMed] [Google Scholar]
- Nowak K. M.; Miltner A.; Gehre M.; Schäffer A.; Kästner M. Formation and fate of bound residues from microbial biomass during 2,4-D degradation in soil. Environ. Sci. Technol. 2011, 45, 999–1006. 10.1021/es103097f. [DOI] [PubMed] [Google Scholar]
- Nowak K.; Girardi C.; Miltner A.; Gehre M.; Schäffer A.; Kästner M. Contribution of microorganisms to non-extractable residue formation during biodegradation of ibuprofen in soil. Sci. Total Environ. 2013, 445–446, 377–384. 10.1016/j.scitotenv.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Poßberg C.; Schmidt B.; Nowak K.; Telscher M.; Lagojda A.; Schaeffer A. Quantitative identification of biogenic non-extractable pesticide residues in soil by 14C-analysis. Environ. Sci. Technol. 2016, 50 (12), 6415–6422. 10.1021/acs.est.6b00689. [DOI] [PubMed] [Google Scholar]
- Brock A. L.; Kästner M.; Trapp S. Microbial growth yield estimates from thermodynamics and its importance for degradation of pesticides and formation of biogenic non-extractable residues. SAR QSAR Environ. Res. 2017, 28 (8), 629–650. 10.1080/1062936X.2017.1365762. [DOI] [PubMed] [Google Scholar]
- Trapp S.; Brock A. L.; Nowak K.; Kästner M. Prediction of the Formation of Biogenic Nonextractable Residues during Degradation of Environmental Chemicals from Biomass Yields. Environ. Sci. Technol. 2018, 52 (2), 663–672. 10.1021/acs.est.7b04275. [DOI] [PubMed] [Google Scholar]
- Prosser C. M.; Redman A. D.; Prince R. C.; Paumen M. L.; Letinski D. J.; Butler J. D. Evaluating persistence of petroleum hydrocarbons in aerobic aqueous media. Chemosphere 2016, 155, 542–549. 10.1016/j.chemosphere.2016.04.089. [DOI] [PubMed] [Google Scholar]
- Birch H.; Hammershøj R.; Mayer P. Determining Biodegradation Kinetics of Hydrocarbons at Low Concentrations: Covering 5 and 9 Orders of Magnitude of Kow and Kaw. Environ. Sci. Technol. 2018, 52 (4), 2143–2151. 10.1021/acs.est.7b05624. [DOI] [PubMed] [Google Scholar]
- Howard P.; Meylan W.; Aronson D.; Stiteler W.; Tunkel J.; Comber M.; Parkerton T. F. A new biodegradation prediction model specific to petroleum hydrocarbons. Environ. Toxicol. Chem. 2005, 24 (8), 1847–1860. 10.1897/04-453R.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.