Abstract
A versatile and popular Cucurbitaceous vegetable, pumpkin has recently gained much attention because of its variety of phytochemicals and health advantages. Pumpkins are a type of winter squash, traditionally with large, spherical, orange fruits and a highly nutrient food. Pumpkin by-products comprise various parts, such as seeds, peels, and pulp residues, with their bioactive composition and many potential benefits poorly explored by the food industry. Pumpkin and their by-products contain a wide range of phytochemicals, including carotenoids, polyphenols, tocopherols, vitamins, minerals, and dietary fibers. These compounds in pumpkin by-products exhibit antioxidant, anticancer, anti-inflammatory, anti-diabetic, and antimicrobial properties and could reduce the risk of chronic diseases. This comprehensive review aims to provide a detailed overview of the phytochemicals found in pumpkin and its by-products, along with their extraction methods, health benefits, and diverse food and industrial applications. This information can offer valuable insights for food scientists seeking to reevaluate pumpkin’s potential as a functional ingredient. Reusing these by-products would support integrating a circular economy approach by boosting the market presence of valuable and sustainable products that improve health while lowering food waste.
Keywords: pumpkin, by-products, phytochemicals, food applications
1. Introduction
Pumpkins represent an extensive group of plants collectively known as pumpkins or squash in the family Cucurbitaceae and the genus Cucurbita. Cucumbers, melons, watermelons, pumpkins, squash, and gourds are the family’s valuable culinary plants. Their distinctive trait is that they are primarily herbaceous climbing vines with separate male and female blooms. In the tropics and warm temperate zones, the family is represented by more than 118 genera and 845 species. It belongs to the plant kingdom’s most morphologically diverse genera [1]. The pumpkin is thought to be the vegetable with the widest variety of traits, including size, shape, weight, and color. Below a central seed cavity, it contains a thick layer of edible pulp covered in a relatively hard rind. From July to September, pumpkins bloom, and from August to October, the seeds ripen [2]. Pumpkins are one of the greatest sources of bioactive compounds, and current studies on functional foods are increasingly focusing on them [3]. Pumpkin scientific classification refers to Kingdom—Plantae, Phylum—Tracheophyta, Class—Magnoliopsida, Order—Cucurbitales, Family—Cucurbitaceae, and Genus—Cucurbita L., and is classified as 75 species [4]. The pumpkin species have two distinct regions of origin: Mexico and Central and South America. Specifically, Cucurbita moschata and Cucurbita pepo are primarily associated with Mexico, while Cucurbita ficifolia and Cucurbita mixta are native to Central and South America [5]. However, Cucurbita is a small genus with about 27 species, the most cultivated and economically significant species being C. moschata, C. maxima, C. mixta, and C. pepo, which have been extensively investigated for their chemical makeup and biological activity. All around the world, there are numerous kinds and varieties of the cultivated edible fruit known as the pumpkin. Their fruit sizes are extremely diverse, ranging from several grams to hundreds of kilograms. One of the most varied domesticated species is the cultivated squash, Cucurbita maxima, which has at least five different varieties. The pulp, seeds, flowers, leaves, branches, and roots of the pumpkin plant are all utilized in daily diets around the world and in the pharmaceutical and cosmetics sectors [6].
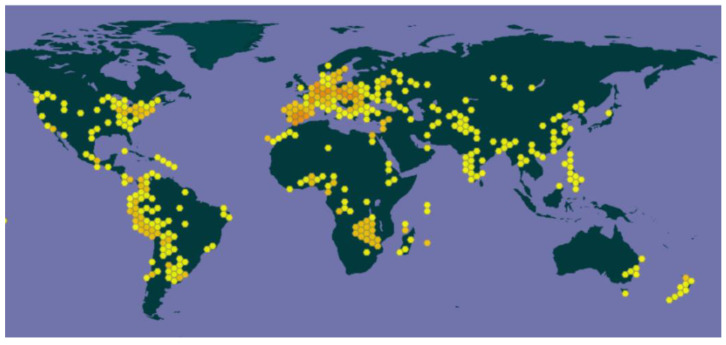
Pumpkins are a significant cash crop that is grown extensively and consumed all over the globe (Figure 1). Pumpkins and squash are cultivated on a worldwide scale of around 3 million hectares, resulting in a total production of 27.832 million tons. China produces roughly 58% of the world’s pumpkins, followed by India with 20%, Ukraine with 4%, and Russia with 4%. Moreover, according to FAO [7], in 2020, Asia was the top global producer of pumpkins, squash, and gourds, with an output of 16,746,673 tons, accounting for 59.89% of the total. Europe follows with a production of 4,886,280 tons, representing 17.47%. The statistics related to the cultivation of pumpkins, squash, and gourds at the level of the member states of the European Union (E.U.-27) show that, in the last five years, the total production has registered an increase; therefore, in 2016, a production of 1,847,838 tons, and in 2020, the production reported by member states (EU-27) was 2,413,440 tons. Pumpkin by-products represent a common agro-food waste; data on the production of pumpkins, squash, and gourds in Romania indicate a value of 23,701 tons in 2016 and 26,850 tons in 2020, which is 1.03% lower compared to the previous year. Romanian edible varieties of Cucurbita maxima: “Alb mare” (from 2009), “Tudor” (from 2012), “De Bihor” (from 2006), “Mădăraș” (2010), etc.
Figure 1.
Cucurbita maxima Duchesne around the world [4].
The pumpkin is one of the most underappreciated and underused food and medicinal plants. It is a remarkable vegetable that can be used both as a nourishing food and as medicine. Pumpkin (Cucurbita maxima) is an annual herbaceous plant cultivated for its fruit, flowers, and seeds.
The nutritional content of pumpkins and other squashes includes sources of carbohydrates, fatty acids, fiber, carotenoids, vitamins, and minerals. The content of nutritional compounds depends a lot on the nature of the pumpkin varieties and the influence of environmental conditions (type of soil for planting, humidity, temperature, light, and mineral nutrients) [8]. Generally, the significant variability within a single species, based either on their subsequent variety or environmental factors and ripening stage, makes it difficult to establish clear qualitative or quantitative characteristics between the species. They can be consumed in a variety of ways. They serve the same purpose as animal feed. Fresh pumpkins can be consumed, cooked, baked, or processed into marinades, smoothies, soups, and juices. In the food industry, it is utilized as an ingredient in pastries, baked goods, biscuits, bread, candies, and baby food [9,10,11].
The formulation of pumpkin pulp is a common practice in the food sector, and it produces large amounts of waste. Wastes generated by certain industries have the potential to be utilized as raw materials for the production of various innovative products that offer notable health advantages and increased value to the industry. This approach allows for the reuse of industrial waste and reduces the need to depend on alternative sources for the manufacturing of different goods. The industry has been interested in the pumpkin wastes produced by industrialization, particularly the seeds, peel, and pomace, because of their beneficial constituents and high levels of carotenoids, dietary fiber, and polyphenols [12,13]. Thus, these leftovers possess significant potential as a reservoir of biomolecules with established health-enhancing properties for utilization in food items.
When pumpkins are processed, a substantial quantity of waste is generated in the form of (3.1–4.4%) and peels (2.6–16%), and the pulp constitutes 72–76% after processing. Pumpkin is commercialized worldwide, and its processing generates tons of seeds and peels as byproducts. The main sources of pumpkin waste are household waste, agricultural waste, and the food industry. The development of functional/nutritional foods, energy production, and biogas are only a few uses for pumpkin waste in the processing sectors. Pumpkin peels have been extensively used in the food industry as a natural colorant and dietary fiber source.
Pumpkin seeds possess a significant amount of antioxidants and oil. The oil and protein content of pumpkin seed flour is around 51.0% and 36.5%, respectively [14,15].
Pumpkin seeds were shown to have a high protein content (48 ± 1%) when compared to other nutrients and other fruit seeds/kernels [16,17]. The research conducted by Łaba et al. [18] highlights the viability, efficiency, and cost-effectiveness of extracting and hydrolyzing pumpkin oilseed cake proteins using Bacillus subtilis. The technique not only improves protein recovery but also increases the value of agricultural waste. Pumpkin peels contain a lot of pectin [19] and Jun et al. [20] used them to extract an alcohol-insoluble polysaccharide and characterize the pectic polysaccharides they contained.
As part of a sustainable approach, the current trend aims to combine the concepts of agriculture, industrial production, and circular economy by employing residues from the production of pumpkins as matrices to extract bioactive compounds of interest to the industry. Reducing, alternatively reusing, recycling, and recovering are used in place of the term “end-of-life”, which should result in a direct decrease in the amount of waste produced. Thus, it is anticipated that this approach will promote sustainable development, improve environmental quality, and increase economic prosperity [21].
Pumpkin seeds are used in salads, baked goods, and snacks due to their nutritional value and pleasant taste. Scientists are competently aware of the oxidizing and modulating effects of zinc on enzyme activation in the human organism during the COVID-19 pandemic, with pumpkin seeds being abundant in zinc. Pumpkin pulp and peels contain a significant number of phytochemicals, including β-carotene, total flavonoids, and total phenolics. Those compounds possess the capacity to increase the immune system and combat the effects of aging. Pumpkin peels are widely utilized in the food industry for their natural coloring properties and as a source of dietary fiber. The presence of terpenoids and polyphenols in this plant species makes it a valuable food addition. Carotenoids, of which β-carotene, α-carotene, neoxanthin, violaxanthin, and lutein stand out, are the most significant terpenoids in pumpkins [22]. The food sector is continuously altering as a result of an expanding population and increasing health concerns. The innovative approach of utilizing inexpensive and organic resources, such as agricultural and food byproducts, to produce innovative food products, has the potential to satisfy the increasing demand from consumers for environmentally friendly goods and transparent labeling. Given the difficulties faced by the food processing business, efforts are undertaken to optimize processing technology in order to reduce waste production. Wastes from the food processing sector are biologically treated and used as co-products, raw materials for other industries, or as food or feed. Pumpkin paste can be prepared in advance and stored in the freezer for future use, as inexpensive powders derived from pumpkin components can be utilized in the food and pharmaceutical sectors to effectively incorporate useful additives and nutraceuticals [23].
Pumpkin bioactive components are thought to protect against a wide range of illnesses, including coronary heart disease, cancer, diabetes, and hypertension. Pumpkin waste, generated during manufacture, processing, preparation, and distribution, can be transformed into sustainable products to prevent the loss of biomass and valuable nutrients, such as bioactive components [24]. These by-products offer significant nutritional benefits and present new market opportunities for the food, pharmaceutical, and cosmetic industries. Moreover, their reuse aligns with the United Nations’ sustainable development goals, reducing industrial waste and promoting sustainable growth through effective stabilization and extraction methods [25].
The present paper explores the basic composition of pumpkin waste, the necessary extraction techniques, and the applications of this waste in developing high-value goods within the framework of a circular bioeconomy. This review aims to highlight the most recent and crucial findings in the identification of pumpkin by-products as valuable sources of phytochemical constituents, the associated health benefits, the extraction methods of pumpkin by-products’ bioactive compounds, and the investigation of their potential diverse applications in various industrial sectors. Overall, this review makes a significant and beneficial contribution to sustainable pumpkin processing and circular economy.
2. Characterization of Pumpkin By-Products
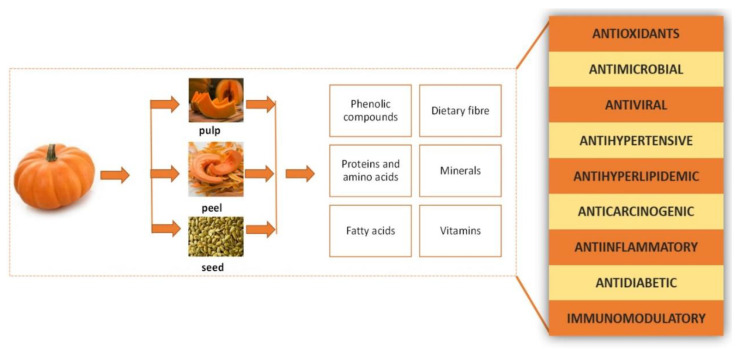
Because of the nutritional and health-protective properties of the proteins and oil from the seeds, as well as the polysaccharides from the fruit’s pulp, agriculture, food processing, the pharmaceutical industry, and the feed industry all have taken an increasing interest in pumpkin fruit and products derived from the pumpkin in recent years. Significant quantities of by-products in peels, seeds, and pomace are generated during the industrial processing of pumpkins into puree, juice, and pumpkin seed oil [26]. Figure 2 illustrates the types of pumpkin by-products and their corresponding biochemical elements.
Figure 2.
Biochemical components of pumpkin by-products and their role.
Pumpkin by-products are a valuable source of health-promoting compounds, including antioxidants, polyphenols, and carotenoids. Research indicates that diets abundant in antioxidants are associated with a reduced incidence of diabetes, cancer, and cardiovascular disease [2,8]. Additionally, studies have demonstrated that antioxidant compounds found in pumpkin seeds can help regulate blood sugar levels in animals with impaired glucose metabolism. Furthermore, the consumption of antioxidants has been linked to a lower risk of neurodegenerative diseases such as Alzheimer’s. Insufficient antioxidant levels in the body are also associated with increased oxidative stress, which has been implicated in the development of depression [5].
2.1. Pumpkin Pomace
The pomace produced as a by-product when pumpkins are used for food coloring agents is a heterogeneous matrix comprising various fractions of crushed skins, seeds, and mesocarp. This side stream, a low-value outlet for this by-product, is currently used as animal feed [27]. Although pumpkins are not typically used as a source of food fibers, several studies have examined the functionality of pumpkin polysaccharide fractions, concentrating on the characterization of cell wall-enriched powder from residual mesocarp [28] and pectic polysaccharides from peels and kernel cake [29]. The raw material displays a more complex chemical makeup and is also rich in proteins and lipids when made from industrially produced pumpkin pomace, which is derived from crushed seeds, skins, and mesocarp combined. In contrast to distinct isolated fractions in earlier studies, the inclusion of these distinct elements can offer different features and capabilities.
Pumpkin pomace is a good source of dietary fiber, vitamins, minerals, and carbs. The pumpkin pomace powder stands out due to its high carotenoid content of 35.55 mg/100 g dry weight (D.W.). In addition, it has high levels of minerals (calcium: 90 mg/100 g; phosphorus: 14 mg/100 g), cellulose (19.6%), hemicellulose (3.5%), and pectin (5.4%) [28].
Bakery and pastry goods can be fortified using pumpkin pomace powder. In the past, several scientists added pumpkin powder to various baked goods to examine the degree of suitability, modifications to the physico-chemical composition, impact on sensory parameters, and nutritional value of baked goods. When pumpkin peels and pulp were made into flour, their physicochemical examination showed that they are good sources of nutrients, and their use in making biscuits significantly impacted the biscuit industry [30].
2.2. Pumpkin Peels
Pumpkin peels are rich in bioactive components and include substantial quantities of fiber, protein, as well as minerals like calcium and magnesium. Nevertheless, the nutritional composition of pumpkin peels is lower in carbs, lipids, and potassium compared to pumpkin pulp [31]. Pumpkin peel contains a high concentration of carotenoids, which are pigment chemicals that can promote health [32].
Pumpkin peel includes the greatest amounts of total carotenoids (neoxanthin, β-carotene, violaxanthin, all-trans-lutein) and isomers (9-cis β-carotene, β-ionon, β-cyclocitral, 13-cis-β-carotene, α-ionon, dihydropseudoionon, tocopherol) among all fractions, but in different quantities in different varieties. According to Nuerbiya et al. [33], pumpkin peels include 10–40% of the overall carotenoid content present in pumpkins. Additionally, they serve as a valuable resource of provitamin A. Various factors, such as variations in the ripening stage, pedoclimatic circumstances, and harvest and postharvest treatment, can influence the content of carotenoids in pumpkin products and by-products [34]. Furthermore, the peels include fiber that is predominantly composed of cellulose [35], protein (9–17%), pectin, and amino acids [9]. Dietary fibers, such as pectin derived from pumpkin peels, have been shown to slow the digestion of starch, contributing to the management and prevention of diet-related conditions, including diabetes. Lutein, α-carotene, β-carotene, and zeaxanthin are some of the carotenoids found in pumpkin peels and have potential use in the food sector. Kurian and Kripan and [36] conducted a study to analyze the nutritional composition of pumpkin peel. They revealed that the peel contains 11.89 mg/100 g of β-carotene, 18.90 mg/100 g of ascorbic acid, 42.99 mg/100 g of iron, and 319.13 mg/100 g of phosphorus.
Badr et al. [37] determined the chemical makeup and biological value of pumpkin (C. pepo) peel powder [37]. The pumpkin peel powder contained 751.9 μg/100 g of β-carotene. Aspartic acid was discovered to be the most abundant amino acid in pumpkin peel powder (2.64%), followed by glutamic acid (2.53%) and leucine (1.2%). Comparatively, the peel section displayed the highest concentration of tryptophan compared to the pulp and seeds. The antibacterial properties of pumpkin peel extract were evaluated using the agar disc method. The results showed a 17 mm diameter of inhibition zone against the Streptomyces viridochromogenes and a 15 mm diameter of inhibition zone against the fungus Mucor meihi. These findings suggest that the extract has a wide range of antimicrobial activity.
Pectic polysaccharide is also present in significant amounts in pumpkin peels. Jun et al. [20] examined the chemical and physical properties of the pectic polysaccharides present in pumpkin skin. The researchers discovered that the pectic component derived from pumpkin peels has the ability to enhance the growth of gut bacteria and has implications for slowing down the absorption of glucose and bile acids. This suggests that pumpkin peels could be a valuable material for producing functional food.
2.3. Pumpkin Seeds
Although the edible portion of a pumpkin is its seeds, they are typically discarded as agro-industrial wastes. Pumpkin seeds are the most important component due to their high protein and low fat content. Flat, oval in shape, and pale green characterize the seeds. In addition to being utilized for both edible and medicinal uses, pumpkin seeds are utilized to produce oil and are eaten raw, boiled, and roasted in many countries. Pumpkin seeds are widely appreciated as a delicacy in several cultures and the seed oil has culinary and therapeutic uses due to its high concentration of bioactive compounds, including polyunsaturated fatty acids, essential amino acids, lutein, vitamins, phytosterols, γ-tocopherols, and β-carotene pigments, fibers, substantial amounts from micronutrients (P, Mg, Mn, K, and Ca) [38]. Karanja et al. [39] conducted research that discovered that pumpkin seeds contain high levels of crude protein, crude fiber, and crude oil. Pumpkin seeds exhibit a significant amount of polyunsaturated fatty acids, similar to the fatty acid composition observed in soybean and sunflower oils. According to a general examination of various pumpkin seed types, crude protein ranged from 14.05 to 33.29%, crude fiber from 11.69 to 24.85%, crude fat from 31.9 to 41.37%, and crude carbohydrates from 8.66 to 27.35%. The analysis of pumpkin seed extracts showed that they included substantial quantities of unsaturated fatty acids, particularly linoleic acid (26.18–81.21%), stearic acid (0.16–5.56%), oleic acid (15.56–30.79%), and palmitic acid (1.16–20.81%), which were the most abundant.
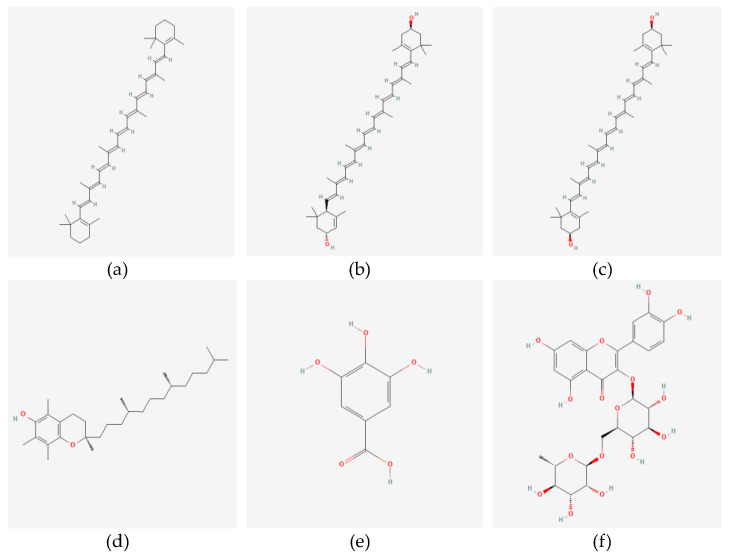
The potential of pumpkin seed oil as both a nutraceutical and an edible oil has attracted considerable attention. Andjelkovic et al. [40] characterize it as a dichromatic, viscous substance with powerful antioxidant properties. Research has demonstrated that pumpkin seed oil exerts beneficial benefits on urinary issues, as well as on the regulation of bladder and urethral pressure, preventing hypertension, and reducing inflammation in the prostate [41,42,43]. Pumpkin seeds and pumpkin seed oil also contain significant amounts of vitamin E, an antioxidant. Pumpkin seed oil, with its fat content of 41.59% and protein content of 25.4%, is a highly abundant source of both protein and edible oil. Upon a thorough analysis of pumpkin seeds, it was determined that the moisture content was 5.2%, the crude fiber content was 5.34%, the total ash content was 2.49%, and the carbohydrate content was 25.19% [44]. Figure 3 illustrates the chemical compositions of various bioactive compounds present in pumpkin by-products.
Figure 3.
Examples of structures of various important bioactive compounds in pumpkin by-products: (a) β-carotene, (b) Zeaxanthin, (c) Lutein, (d) α-tocopherol, (e) Gallic acid, (f) Rutin [45].
3. Biochemical Components of By-Products from Pumpkins
3.1. Phenolic Compounds
Phenolic substances, a secondary plant metabolite that is prevalent in fruits and their by-products, have antioxidant, anti-mutagenic, and anti-inflammatory characteristics, among others [46]. According to Saavedra et al. [2], in the best operational conditions assessed, peels had a higher phenolic content than seeds, with values of 11 and 6.1 mg GAE/g D.W., respectively.
Pumpkin seeds have a complex phenolic profile that includes flavonoids, phenolic acids, and lignans. The primary chemicals identified were vanillic and caffeic acids, with values of 0.6 and 0.41 mg/100 g oil, respectively [47,48].
Upon examination of various pumpkin types, it was shown that C. pepo and C. moschata seeds contained higher levels of tocopherols than C. maxima seeds. In addition, the peels of pumpkins had a higher concentration of carotenoids than the seeds, especially in the case of C. maxima. β-carotene was found to be abundant throughout all types and varieties of pumpkins [9]. Ascorbic acid, chlorophyll a, and chlorophyll b were also determined by Blanco-Daz et al. [12], who obtained values for the first one ranging from 0.42 to 1.2 mg/g D.W. The same scientists demonstrated that peels had chlorophyll concentrations that were around 21 times higher than those of pulp.
3.2. Proteins and Amino Acids
Proteins are abundant in pumpkin by-products, notably in the seeds. According to Banerjee et al. [49], these bioactive chemicals have a range of biological properties, including antihypertensive, antioxidant, antibacterial, and immunomodulatory actions. Pumpkin seeds have been evaluated for protein content, with results ranging from 21 to 44% [14], and for the pumpkin peel, the values are lower, between 1.8 to 25% [50]. Kim et al. [9] measured the protein and amino acid contents of C. maxima, C. pepo, and C. moschata [9]. They found that C. maxima has a peel with a much greater protein content (17 g/kg fresh weight) than the other species (9.3 and 11 g/kg fresh weight, respectively). However, the protein content of C. pepo seeds was higher than that of C. maxima seeds (309 g/kg fresh weight vs. 275 g/kg, respectively). With the exception of aspartic acid, C. maxima peels had the highest levels of amino acids, but C. pepo seeds had the highest levels of amino acids due to their higher levels of arginine (64 mg/kg fresh weight).
3.3. Dietary Fiber
Pumpkin by-products can be viewed as a significant dietary fiber source that is used in the food sector. Fiber is a valuable tool for enhancing food products’ nutritional and functional qualities and/or physical characteristics, aiding in the prevention of illnesses including costiveness, cholesterolemia, cancer, and diabetes [35]. Due to the considerable amounts of cellulose and hemicelluloses present in these basic materials, pumpkin by-products (peels) have high levels of total dietary fiber (approximately 80%), with insoluble fiber constituting the majority of them [14].
Pectic oligosaccharides were extracted from the peel of pumpkins and separated into three groups (water, EDTA, and alkali-soluble oligosaccharides). The alkali-soluble fraction exhibited the highest levels of total sugar and uronic acid, measuring 25 and 46 g/100 g, respectively [20]. Pectin from pumpkin peel was extracted as efficiently as possible, and the resulting pectin and protein from pumpkin seeds were combined to generate biodegradable films. Pumpkin seeds and peels contain pectin, which can be extracted using acid hydrolysis. This pectin has great potential for replacing commercial pectin as a gelling agent and thickener in the food industry [51]. Compared to other pectin sources, pumpkin-derived pectin extracted from Cucurbita maxima with high molecular weight and a high degree of methoxylation offers advantages such as cytoprotective and antioxidant effects on cell models, and can be considered as an ingredient able to enhance the nutritional profile of the final food product [29].
3.4. Fatty Acids
The diversity of the cultivar, the degree of maturity, the growth conditions, and the preparation technique all influence the significant difference in the composition of fatty acids in pumpkin seeds. Thus, pumpkin seeds are valuable sources of lipids (rich in polyunsaturated fatty acids). They contain essential and non-essential fatty acids, which can prevent various conditions, such as cancer and other cardiovascular diseases. Pumpkin seeds have a fatty oil concentration of 45 to 60%. Pumpkin oil exhibits a substantial proportion of polyunsaturated fatty acids, comprising over 50% of the overall fatty acid content, alongside approximately 19.4% saturated fatty acids. The primary unsaturated fatty acids observed were linoleic acid, accounting for 21–55.6% of the total, followed by oleic acid, which accounted for 20.4–60.8%. The predominant saturated fatty acids were palmitic acid, ranging from 9.5% to 14.5%, and stearic acid, ranging from 3.1% to 7.7% [52].
A study conducted by Amin et al. [14] found that the lipid content of pumpkin seed oil varied between 18% and 55%. In Bangladesh, two kinds of pumpkin (Cucurbita maxima) (indigenous and hybrid) were examined for their FA profiles using GC/MS, and Amin et al. [14] found that the hybrid variety was particularly high in saturated fatty acids (capric, myristic, and stearic acids). Conversely, the native diversity of seeds exhibited a significant presence of unsaturated fatty acids, specifically oleic, linoleic, and linolenic acids. In their study, Nawirska-Olszańska et al. [48] observed that the oil content of C. maxima seeds is usually higher than comparable to C. pepo seeds. Oleic and linoleic acids were the primary fatty acids in C. pepo, C. maxima, and C. moschata [14].
In their investigation of C. maxima L. (var. Berrettina) pumpkin seed oil, Montesano et al. [53] found that the most prevalent fractions were polyunsaturated fatty acids and monounsaturated fatty acids (37.2% and 41.7%, respectively). The fatty acids that were most abundant were oleic and linoleic acids. Türkmen et al. [54] discovered that the unsaturated fatty acid content of edible pumpkin seeds collected from different locations in Turkey varied, up to 73.3%, with 18.4% oleic acid, 52.6% linoleic acid, and 1.27% linolenic acid being the main components; 27.7% of the fatty acids were saturated, with 16.4% being palmitic acid, and 11.1% being stearic acid.
3.5. Minerals
The maintenance of a healthy diet depends on the consumption of minerals. In addition to helping to cure and prevent hypertension, calcium is crucial for the growth of bones and teeth. Other micronutrients, like magnesium and potassium, play a crucial role in controlling blood pressure and regulating heartbeat [55]. The chemical composition of pumpkin (C. pepo) peel powder was determined by Badr et al. [37]. The calcium, iron, zinc, and copper values (mg/100 g dry weight) for pumpkin peel powder were 5571, 247.33, 42.92, and 12.91 mg/100 g, respectively. According to Idouraine et al. [56], squash seeds were shown to have significantly higher amounts of potassium (2069–2838 mg/100 g D.W.) compared to magnesium and calcium (which varied from 735 to 856 and 24–279 mg/100 g D.W., respectively). A vital mineral called zinc is in charge of improving the immune system and metabolism. Pumpkin seeds include peptides that are naturally associated with zinc, which are excellent minerals for promoting a healthy immune system [57,58].
3.6. Vitamins
Pumpkin has high levels of pro-vitamin A carotenoids, including lutein (a bright yellow color) and β-carotene (an orange hue). β-Carotene has a pro-vitamin A activity level of 100%. Once converted into vitamin A inside the body, it plays a crucial function in the immune system, night vision, development, and the formation and repair of epithelial tissue [59].
Pumpkin pulp contains 2–10 mg of vitamin C, 0.426 mg of vitamin A, and 9–10 mg of vitamin E per 100 g [14]. Vitamins like vitamin A, vitamin C, thiamine, folate, pyridoxine, riboflavin, niacin, and pantothenic acid were found in pumpkin seeds, in addition to, tocopherol or vitamin E [60,61]. δ-tocopherol, which made up about 42.2% of all the tocopherols in the Bejaoui variety’s seed oil, was the primary component, followed by α-tocopherol, γ-tocopherol [62].
4. Innovative Applications of Pumpkin By-Products
Around the world, pumpkins are grown for their use as food and medicine, and they are used to make various food products, including jams, syrups, jellies, purees, etc. In the last few decades, utilizing pumpkin wastes as functional food has presented new challenges. Snacks, bread, muffins, crackers, cookies, cakes, cereal bars, and other baked goods are all made with pumpkin seeds. The nutritional value of the manufactured product is improved by using pumpkin seed powder as a taste enhancer in dressings, soups, and baked goods [63,64,65].
Pumpkins have significant quantities of protein, fat, fiber, and tocopherol, all of which are abundant in pumpkin seeds. Additionally, they serve as an excellent reservoir of phytochemicals. Past studies have explored the use of fiber as a substance that can help prevent or treat diabetes. Additionally, all of these components possess nutritional qualities when consumed as part of a diet. Pumpkin, an abundant source of dietary fiber, is frequently added to culinary dishes to enhance their nutritional value and texture. Food engineers are using it more frequently to create unique foods with high nutritious value because its seed flour has a nutty flavor and an appealing green hue [66].
4.1. Natural Food Additives
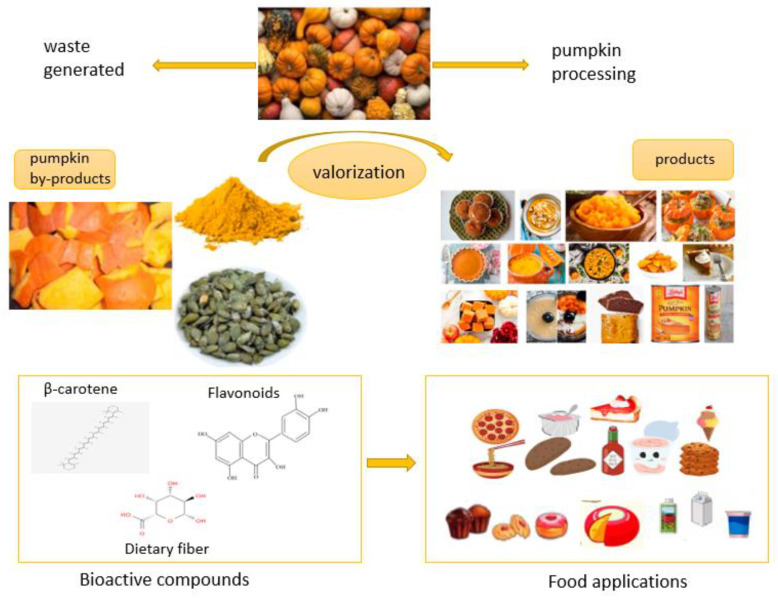
Pumpkin by-products are more widely used in food processing due to their high nutritional content and potential health advantages. Pumpkin powder is an excellent source of dietary fiber, minerals, and antioxidants such as β-carotene. However, bread mixes and baked items that incorporate pumpkin contain a higher caloric content and are enriched with vitamin A. Figure 4 illustrates the utilization of pumpkin by-product powder in diverse food applications. Industrialists and food technologists are now pursuing the development of functional foods.
Figure 4.
Valorization of pumpkin by-products and potential product applications.
Pumpkin flour, derived from both the seeds and peel of a pumpkin, can be utilized in a highly popular manner for utilizing pumpkin by-products. Apart from ascorbic acid and calcium, pumpkin peels are also rich in protein and fiber compared to the pulp. In the manufacture of bread, pumpkin peel flour was combined in part with wheat flour [11]. Bread made with 5% pumpkin peel flour has good qualities, including a high protein level, raw fiber, and a low carbohydrate content. By providing a brand-new category of nutritious and useful food, helping to decrease food waste, and creating a new added value for the utilization of discarded pumpkin peels, the usage of pumpkin peel flour stands out as a good market option. Burger Staichok et al. [11] used pumpkin hull flour to make bread as a technological application to partially replace wheat flour; 2.5% and 5% concentrations of pumpkin hull flour were used to make the bread. Although using pumpkin flour boosted the bread’s overall protein content, which is advantageous from a nutritional standpoint, there was a slight decline in cohesiveness, volume, and elasticity, which was to be expected given the low production of gluten.
Based on a nine-point hedonic sensory evaluation scale, Mishra and Sharma [67] made a pumpkin peel-based biscuit with 20% pumpkin peel flour that was the most appreciated. Based on its proximate evaluation (moisture, protein, fat, fiber, carbohydrate, energy, and ash value), the produced biscuit is rich in nutrients and can be utilized as a supplement in malnutrition and pregnant women. In order to make muffins, pumpkin powder was added to a wheat flour mixture. The nutritional, sensory, and physicochemical impacts of the addition were investigated [68]. When additional pumpkin powder was added to the baked muffins, the amount of β-carotene increased. Based on the sensory study, 20% pumpkin powder was shown to be the optimal proportion for acceptability; formulations above 20% had an adverse effect on the color and general acceptability of the product. In muffins, pumpkin flour was used in place of wheat flour to some extent, which resulted in a drop in saturated fatty acids and an increase in unsaturated fatty acids [69]. Children between the ages of 7 and 12 sensory-tested the product, and the results showed a 33% improvement in taste preference and general acceptability.
Many culinary products, including gluten-free noodles and pasta, have been made by partially substituting pumpkin flour (up to 50%) for corn flour. An analysis has been conducted on the sensory, physicochemical, and cooking yield aspects of the formulation products [70]. Pasta with 25% pumpkin flour had desirable qualities leading to improved color, texture properties, and sensory attributes of gluten-free pasta. The partial substitution of pumpkin flour with corn flour enhanced the moisture content, ash, cooking yield, and a* color parameter. Corn flour and vegetables processed with flour were used to make gluten-free spaghetti products [71]. The pumpkin combination tested highly in terms of its mechanical and sensory qualities, color, and general appeal.
The quality of the beef sausage that contained 30% dried pumpkin powder was enhanced [72]. Additionally, the formula was free of Salmonella. It enhanced protein and fat content while decreasing moisture content. Also, increasing the amount of dried pumpkin powder reduced pH and improved water holding capacity. Table 1 displays the industrial food applications for pumpkin by-products.
Table 1.
Potential food utilization of different forms of pumpkin by-products.
| Form of the Pumpkin By-Product | Food Product | Functional/ Technological Benefits |
References |
|---|---|---|---|
| Pumpkin peel flour | Bread | The bread with 5% pumpkin peel flour showed good characteristics with high protein content, raw fiber, and a lesser amount of carbohydrate. | [11] |
| Pumpkin seed flour | Muffins | Compared with other muffins, muffins containing 33% seed flour had a better sensory profile and improved nutritional value. | [69] |
| Pumpkin peel flour | Biscuits | Biscuits prepared with 20% pumpkin peel flour were most appreciated with good taste and appearance. | [67] |
| Pumpkin pulp flour | Gluten-free pasta | The gluten-free pasta with 25% (13.5 g/100 g) pumpkin flour had the most desirable overall acceptability and sensory attributes among all formulated pasta. The moisture content, ash, cooking yield, and a* were increased by the partial replacement of the corn flour with pumpkin flour. | [70] |
| Dried pumpkin powder | Beef sausage | The sample formulated with 30% dried pumpkin powder recorded the lowest moisture content (64.22%) compared with (72.43%) for the control sample. Significant increase (p < 0.05) in fat (9.30%) and protein (20.55%) content was also recorded. | [72] |
| Pumpkin seed | Chicken burgers | Improved stability during the period of storage. | [73] |
| Pumpkin seed kernel flour | Beef meat balls | Fat replacement. | [74] |
| Pumpkin seed and pulp | Beef patties | There was no change in the texture and a decrease in the moisture content. | [75] |
| Pumpkin pulp | Pumpkin-based juice blends | The results reveal that the pumpkin juice developed in this study had high levels of hydration, crude protein, fiber, ash, and carbohydrates, suggesting that it is a rich source of these essential nutrients. The sensory analysis indicated that consumer groups deemed the pumpkin-based juice blends acceptable. | [76] |
| Pumpkin pulp | Yogurt | Increase health benefits. | [77] |
| Pumpkin peel | Yogurt | Adding pumpkin peel powder to yogurt enhanced the nutritional profile, namely in terms of β-carotene and bioactive components. The use of powder also beneficially impacted the yogurt’s textural characteristics, demonstrating improved consistency and mouthfeel. | [78] |
| Pumpkin seed | Ice cream | Augmenting the protein content boosts the level of fullness and improves sensory attributes. | [79] |
| Pumpkin seed powder | Cereal milk | Incorporating pumpkin seed powder improved the sensory and physicochemical properties of cereal milk. The refrigerated storage extended the shelf life of cereal milk to 9 days. |
[80] |
| Pumpkin pomace powder | Cheese | The addition of PP powder to the cheeses led to an increase in both the carotenoid content and antioxidant activity, resulting in improved sensory evaluation scores. | [81] |
All ages and diverse cultures enjoy a variety of ground beef dishes, such as meatballs and burgers. According to a previous study, substituting flour and dry pumpkin seeds for red meat in beef patties resulted in a patty with a poorer texture and more water retention. [73]. To manufacture beef balls, powder is used in place of fat. Although the hamburger’s great qualities were allegedly lost, the cooking properties allegedly persisted. Moreover, opting for nutritious fat-free vegetable substitutes like soybean oil and pumpkin seed meal is a feasible choice [82]. In order to create a beef pellet with enhanced nutritional value and increased benefits, pumpkin seed meal was employed as a replacement for beef fat during the entire production procedure. Its nutritional value is assessed by its fatty acid content and health index, while consumer satisfaction is gauged by sensory evaluation. According to a study that looked at the impacts of including pumpkin seeds in chicken patties, these components might enhance the end product’s baking and lipid oxidation capabilities [73].
A study was conducted to examine the possibility of creating a novel ice cream recipe with a significant percentage of pumpkin pulp. The outcomes demonstrated that the ice cream developed had better texture and emulsification capabilities and a moderate level of fat [77]. Also, in the category of dairy products, Gavril (Rațu) et al. [78] incorporated pumpkin peel powder into yogurt and resulted in enhancements in the nutritional composition, namely in terms of β-carotene and bioactive substances. The sensory evaluation results indicated that the addition of pumpkin peel did not adversely affect the overall acceptance of the yogurt. In fact, some samples (YPP2) demonstrated preferred sensory characteristics over the control. Therefore, the study suggests that incorporating pumpkin by-products like pumpkin peel can contribute to sustainable food production and provide consumers with more diverse food choices that offer enhanced nutritional and sensory attributes.
Another study was also conducted to look into the effects of adding pumpkin seed powder to cereal milk that had already been fermented with Lactobacilli spp. and Bifidobacteria spp. cultures for the production of non-dairy probiotic goods [80]. The outcomes showed that adding pumpkin seed powder to cereal milk enhanced its physicochemical and sensory qualities.
Interest in researching and producing blended juices has grown since they reflect high-quality consistency and increased nutritional or phytochemical qualities. A study was conducted to develop a juice fortified with vitamins, antioxidants, and minerals derived from pumpkin pulp. The results of the study revealed that adding mango and strawberry juice to pumpkin juice improved the sensory quality [76].
Additional Applications of Pumpkin By-Products
Pumpkin seeds and pumpkin peels are very capable of being transformed and used to produce valuable goods with higher value, such as edible or biodegradable film. In order to create biodegradable films, defatted pumpkin seed meal, and peels were effectively used, and bio-based films made with co-products helped to lessen the environmental problem associated with composting. Such a film is suitable for baked goods, cakes, and desserts. These wastes have a high carbon content and could be desirable renewable substrates for producing added-value products. Cell viability was examined in wastes made from pumpkin peel and pulp (30:70 w/w), which had been treated with Lactobacillus casei at an initial inoculum size of 9 log CFU/mL and incubated at 37 °C. Increased cell viability after 24 h of fermentation showed that pumpkin was an effective carrier substrate for probiotics and an adequate carbon supply for their fermentation [83]. In their study, Lalnunthari et al. [51] investigated the production of an edible film by utilizing pectin and protein derived from pumpkin seeds and peels, respectively. Edible film, a type of coating material, is utilized as packaging or wrapping to protect products and culinary ingredients. They can be made by forming a film with proteins, polysaccharides, pectin, and lipids. The authors discovered that the procedure of various variables had an impact on the extraction of protein and pectin. Lalnunthari et al. [51] discovered a 69.89 ± 2.90% pectin production from pumpkin peels when the optimal conditions were an extraction temperature of 89.98 °C, an extraction time of 13 min, and a pH of 2.85. The films showed good elongation (9.74 ± 0.46%) and the greatest tensile strength values (1401 ± 5.4 kPa). Such edible film may be employed in the production of snack-friendly foods such as chopped fruit, baked goods, confectionery, etc. Based on minimally processed pumpkin residue extract (0 to 6%), glycerol, cassava starch, and oregano essential oil (0 to 2%), research by Dos Santos Caetano et al. [84] generated biodegradable films. The addition of pumpkin residue is essential since it gives the film opacity.
The manufacture of bioethanol from pumpkin peel wastes demonstrated the potential benefits of these materials. Artificial neural networks (ANNs) with estimation and prediction capabilities were employed in the study to optimize the bioethanol production process from waste pumpkin peel. This study utilized a multilayer feed-forward neural network that was developed using an error back-propagation learning approach. The mechanism involves hydrolyzing the reducing sugars present in the pumpkin peel using the enzyme amyloglucosidase, which breaks down the polysaccharides into fermentable sugars. Subsequently, these sugars undergo fermentation by the yeast Saccharomyces cerevisiae, resulting in the production of 50.60 g/L of bioethanol. This optimized process, guided by ANN predictions, effectively enhances the yield of bioethanol from pumpkin peel waste [85]. Furthermore, it was shown that the pumpkin peel contains a significant amount of carbohydrates, specifically 65.30%, along with fiber, water, protein, ash, and a tiny amount of fat. According to this information, pumpkin peel may be an efficient source of glucose. The ideal parameters for the fermentation process were anticipated based on time, temperature, substrate loading, α-amylase, and amyloglucosidase concentration. The results showed that the key factors affecting the levels of reducing sugar and bioethanol were the amount of substrate used and the temperature during fermentation.
H3PO4 was employed as a chemical agent to create activated carbon using pumpkin seed shells [86]. The structure of the pores of the activated carbon was dramatically impacted by carefully adjusting the impregnation proportion and activation temperature. So, pumpkin seed shells could serve as a different raw material for producing commercially viable activated carbon.
5. Various Techniques for the Extraction of Bioactives from Pumpkin By-Products
Extraction is the most important step, which is normally carried out with various solvents, acids, alkalis, and hydrodistillation methods depending on the type of extracted component.
5.1. Conventional Techniques
Conventional extraction (CE) is among the most popular techniques. The solubility and volatility of the compounds in the chosen solvent have an impact on extraction efficacy. While phenolic chemicals are easily soluble in polar protic media (methanol, ethanol, acetone), carotenoids are fat-soluble in polar aprotic solvents (acetone, ethanol, acid acetic), or nonpolar solvents (ethyl acetate and benzene). Natural pigment extraction traditionally involves employing Soxhlet extraction techniques using organic or inorganic solvents, maceration, or hydro-distillation. Water or diluted alcohol is typically used for the conventional extraction of pigments that are water soluble, while non-polar solvents are used for the extraction of pigments that are lipophilic (hexane, acetone, methanol). The majority of them are harmful in nature, but because they are volatile, they can effectively break down target pigments to make removal easier. Instead of being technically advantageous, these solvents can pollute the environment and are dangerous for human consumption [87].
Conventional extraction methods have poor extraction efficiency, high energy consumption, take a long time to extract, and are not economically profitable. Due to these issues, innovative ecologically friendly methods are required to enable efficient extraction using green solvents derived from renewable resources, such as plant-based edible oils.
The effects of various extraction methods for carotenoids in pumpkin pulp were examined in a study. Temperature (15, 30, and 45 °C), extraction period (8, 12, and 16 h), and the solid-to-solvent ratio (1:50, 1:100, and 1:150) were all optimized for carotenoid extraction. To purify nonpolar carotenoids from pumpkin pulp, nonpolar solvents like lycopene and β-carotene are necessary [88]. Moreover, it was demonstrated that enhancing the solid-to-solvent ratio to a maximum of 1:150 improved carotenoid production.
5.2. Modern Extraction Techniques
New techniques have been introduced as a result of the limitations of conventional procedures. Traditional extraction techniques are characterized by the difficulties of obtaining high purity, the use of costly solvents, longer extraction durations, the potential for heat-labile chemical degradation, and low extraction selectivity. There are currently various novel and developing methods being employed for the extraction process.
Due to many advantages, including the need for minimal solvents, speed, convenience, and the ability to boost extraction yield while protecting pigments from deterioration and improving the quality of natural colorants, these approaches are more popular than traditional extraction methods. An overview of the extraction procedures for the valorization of pumpkin by-products is shown in Table 2.
Table 2.
Overview of extraction processes for pumpkin by-products.
| Pumpkin By-Products | Process Conditions | Compounds | Yield | References |
|---|---|---|---|---|
| Pumpkin peels | CE Methanol 80% 1:33 w/v |
Phenolic compounds | 319–529 mg GAE/100 g D.W. | [12] |
| Pumpkin peels | CE 70 °C/0.5 h Water/70% ethanol/70% methanol 1:25 w/v |
Phenolic compounds | 200–1069 mg GAE/100 g D.W. | [2] |
| Pumpkin peels | CE, on ice until colorless THF:methanol (1:1 v/v) containing 0.1% BHT, 1:1.5 w/v |
Carotenoids | 40–130 mg/kg F.W. | [9] |
| Pumpkin peels | CE, until colorless acetone:hexane (1:1 v/v) |
Carotenoids | 12–1751 mg/kg D.W. | [89] |
| Pumpkin peels | UAE, hexane:acetone (3:1 v/v) Central composite design extraction temperature (6.25–98.75 °C), extraction duration (13.98–128.98 min), and solvent ratio (0.23–50.23 mL) |
Carotenoids and antioxidant activity | 0.53 to 1.06 mg/g D.W. and 0.34 to 7.28 µM TE/g D.W. | [78] |
| Pumpkin peels | UAE 20 °C/0.5–0.83 h, Ethanol:petroleum ether 1:30–1:40 w/v |
Carotenoids | 98–239 μg trans-lutein/g D.W. | [90] |
| Pumpkin peels | UAE 25 °C/30 min corn oil as an alternative solvent, amplitude-20%, |
Carotenoids and phenolic compounds | 38.03 ± 4.21 µg/g of Oil Extracts 588.68 ± 7.26 mg GAE/g of Extract |
[3] |
| Pumpkin peel extract | Subcritical water extraction (SWE) and SFE, SWE-Water, and SFE–SC–CO2 120 °C/3 h/5 MPa (SWE) 60 °C/3 h/25 MPa (SFE) |
Carotenoids | 15.22 mg/100 g extract (SWE) and 11.48 mg/100 g extract (SFE) | [91] |
| Pumpkin peels | SFE 59 °C/0.5 h CO2, 15.5% Ethanol |
Carotenoids | 85% total carotenoid recovery | [92] |
| Pumpkin peels | CE, on ice until colorless Hexane containing 0.1% BHT, 1:1.5 w/v |
Tocopherols | 5–13 mg/kg F.W. | [9] |
| Pumpkin seeds | CE 70 °C/0.5 h Water/70% ethanol/70% methanol, 1:25 w/v |
Phenolic compounds | 95–343 mg GAE/100 g D.W. | [2] |
| Pumpkin seed | CE 20 °C/16 h 70% acetone/100% dichloromethane, 1:25 w/v |
Phenolic compounds | 132–612 mg GAE/g D.W. | [2] |
| Pumpkin seeds | CE Ethanol–hexane 80 °C, 4 h |
Phenolic compounds | 3.5–42.4 mg GAE/g D.W. | [93] |
| Pumpkin seeds | MAE, hexane, 5–30 min/200 W, 1:10 (g/mL), 20 kHz |
Oil | 51–62.5% | [94] |
| Pumpkin seeds | MAE Ethanol–water 100–150 °C, 20 min, 2.45 GHz |
Phenolic compounds | 35–84 mg GAE/g D.W. | [95] |
| Pumpkin seeds | UAE Ethanol–water 40 °C/20 min, 1:10 (g/mL), 100 W, 20 kHz |
Phenolic compounds | 34.2 mg GAE/g D.W. | [93] |
| Pumpkin seeds | CE 0.05 h 0.1% HCOOH in methanol 70% 1:10 w/v |
Phenolic compounds | 379 mg GAE/100 g D.W. | [47] |
| Pumpkin seeds | UAE, 0.25 h Ethanol 50%/Methanol 80%/Acetone 1:5 w/v/1:15 w/v |
Phenolic compounds | 34–113 mg GAE/ 100 g F.W. |
[48] |
| Pumpkin seeds | CE on ice until colorless THF:methanol (1:1 v/v) containing 0.1% BHT, 1:1.5 w/v |
Carotenoids | 7–32 mg/kg F.W. | [9] |
| Pumpkin seeds | CE on ice, until colorless Hexane containing 0.1% BHT, 1:1.5 w/v |
Tocopherols | 49–93 mg/kg F.W. | [9] |
| Pumpkin seeds | UAE 0.17 h Dichloromethane:Methanol (1:4 v/v) 1:50 w/v |
Tocopherols | 29–71 mg/100 g D.W. | [14] |
| Pumpkin seeds | CE Chloroform:methanol (2:1 v/v) 1:20 w/v |
Fatty acids/oil | 440–524 g/kg F.W. | [9] |
| Pumpkin Peels | MAE 45 °C/130 W/30 min corn oil, 1:10 ratio |
Carotenoids and phenolic compounds | 34.94 ± 3.60 µg/g of Oil Extracts 554.54 ± 10.25 mg GAE/g |
[3] |
| Pumpkin by-products | UAE, Caprylic acid:Capric acid (3:1) 50 °C,/52.5 W/cm3 ultrasonic power/7 mL/g ratio/10 min |
β-carotene | 15,141 mg/100 g | [95] |
GAE, gallic acid equivalents; D.W., dry weight; BHT, 2,6-di-tert-butyl-4-methylphenol; PVP, polyvinyl-pyrolidone; RME, reverse micelle extraction; CTAB, cetyltrimethylammoniumbromide; ATPS, aqueous two-phase system; FPU, filter paper unit; SFE, supercritical fluid extraction; UAE, ultrasound-assisted extraction; CAE, chlorogenic acid equivalents; EAE, enzyme assisted extraction; F.W., fresh weight; MAE, microwave-assisted extraction; CE, conventional extraction; r.m. raw material; THF, tetrahydrofuran; PLE, pressurized liquid extraction.
5.2.1. Ultrasound-Assisted Extraction (UAE)
UAE employs mechanical vibrations with an ultrasonic frequency greater than 20 kHz. This causes a cavitation effect, which speeds up the mass and heat transmission in the particles, disrupting cells, and enabling chemical release. Using ultrasound in the extraction process enables improved preservation of the bioactives’ properties. This technique significantly enhances extraction efficiency, reduces the necessary temperature, and shortens the extraction time [96].
Song et al. [90] evaluated the efficacy of ultrasound-assisted extraction (UAE) technology compared to conventional solvent extraction. Due to the UAE technique’s great efficiency, quick extraction time, and straightforward and uncomplicated operation, it is generally known that it can be employed to help extract carotenoids from natural sources. The study investigated the impact of ultrasonic power and frequency, solvent type, time, and solvent-to-material ratio on trans-lutein production and total trans-carotenoids. The utilization of ultrasound-assisted extraction (UAE) resulted in a substantial quantity of trans-lutein and trans-carotenoids, exceeding the conventional solvent extraction method. This is due to the UAE’s ability to prevent degradation and isomerization of the compounds. Also, in the study using pumpkin peel, Song et al. [90] enhanced the UAE by using traditional methods to determine the optimal solvent for extracting carotenoids. They discovered that a blend of ethanol and petroleum ether was the most effective solvent. In order to achieve this purpose, researchers assessed the impact of the key parameters that affect the yield of carotenoids, including ultrasonic power, extraction duration, and liquid–solid ratio. UAE achieved a carotenoid content of 363 µg/g under optimal conditions (203 W, 30 min, and a solvent-to-material ratio of 31 mL/g), resulting in a 92% yield increase compared to conventional extraction methods.
Sharma and Bhat [97] used maize oil as an eco-friendly solvent and three distinct extraction techniques (ultrasound-assisted extraction, microwave-assisted extraction, and conventional extraction) to isolate carotenoids from the pulp and peel of two pumpkin cultivars. The carotenoid concentration in the samples obtained by UAE was higher compared to the samples obtained through MAE and CE using organic solvents (hexane and isopropyl alcohol). Additionally, it was discovered that the amount of carotenoids varied based on the plant portion, with the peel powder having a higher amount than the pulp powder. In comparison to traditional extraction (19.21 ± 4.39; 16.21 ± 2.52 µg/g), the results showed that total carotenoids were almost two times higher when using ultrasound (38.03 ± 4.21; 33.78 ± 1.76 µg/g).
Gavril et al. [78] study aims to determine the optimal extraction parameters for an ultrasonic-assisted extraction technique to extract whole carotenoids and evaluate the antioxidant activity of pumpkin peel. The most effective parameters for extracting carotenoids from pumpkin peel were a temperature of 80 °C, a solvent ratio of 10 mL, and an extraction period of 100 min. This resulted in a carotenoid concentration of 0.97 mg/g D.W. and an antioxidant activity of 7.25 µM TE/g D.W.
5.2.2. Microwave-Assisted Extraction (MAE)
MAE is a method that combines conventional solvent extraction with nonionizing electromagnetic waves at microwave wavelengths between 0.3 and 300 GHz. By heating the entire sample simultaneously, the compounds are subsequently extracted because of the effects of dipole rotation and ionic conduction. The technique makes use of the particle-by-particle absorption of radiation by the material. The sample is heated and transfers the heat to the extractant as a result of employing a solvent that is not polar and does not absorb microwave radiation. The method is cost-effective since it shortens the extraction process and uses less solvent. Dipole particles move during microwave heating and generate heat energy via friction. The factors that can affect MAE efficiency include power, frequency, extraction duration, temperature, matrix moisture, solvent type, and the ratio of solid to liquid [98,99].
Hexane and ethanol were used as the extraction solvents in Ferreira et al.’s [93] comparison of UAE, MAE, and the traditional method for recovering phenolic components from pumpkin seeds (Curcubita sp.); the highest phenolic content and antioxidant activity were acquired using MAE under subcritical solvents (ethanol and water, 8.12 and 8.71 mg GAE/g, respectively) with good extraction yields [93]. However, the extract produced with subcritical ethanol had a higher concentration of phenols, yielding 83.95 mg GAE/g of extract as opposed to 55.52 mg from subcritical water. These results, 3.93 mg GAE/g matrix and 42.42 mg GAE/g extract, were better than those from traditional extraction. The authors explained that this is caused by the decrease in solvent polarity that occurs in subcritical conditions, which encourages the solubilization of a variety of phenolic compounds.
5.2.3. Supercritical Fluid Extraction (SFE)
SFE operates at higher pressures and temperatures than the critical points of fluids such as CO2, water, methanol, ethanol, n-butane, and ethylene. The most often utilized supercritical fluid is CO2, which is readily available in high purity, affordable, flammable, chemically inert, and recyclable. Low temperature and low pressure, crucial for extracting natural compounds, especially those with thermally unstable components, are the key benefits of employing supercritical CO2. The critical point for CO2 is at 31.1 °C (304.2 K) and 7.3 MPa (72.8 bar), allowing for work at ambient temperature and low pressure, which is suitable for the extraction of thermolabile substances [100].
By extracting pumpkin seed oil and the fruit’s peel, a high-quality extract that is acceptable for use in food, medicine, and cosmetics has been produced [100]. By including the pumpkin peel during the oil extraction process, bioactive components from the peel, including carotenoids, phytosterols, tocopherols, and antioxidant activity, were added to the extract. The solvent solution for the simultaneous extraction was sub- and supercritical CO2. The utilization of optimal pressured extraction conditions, including temperature and pressure, using CO2 resulted in the production of a higher quality extract compared to those obtained through conventional and ultrasonic methods, as evidenced by the concentration of β-carotene, phytosterols, tocopherols, phenolics, antioxidant activity, and thermal stability.
Mitra et al. [101] examined the Soxhlet method of extracting oil from pumpkin seeds using hexane and CO2 supercritical extraction, achieving comparable yields with both methods. SFE also made it possible to shorten extraction times and use less organic solvent in both studies. Consequently, the oil yield obtained at the ideal conditions (68.1 °C, 94.6 min, 32,140 kPa) was 31%. Supercritical CO2 extraction was also used by Wang et al. [61] to extract β-carotene (18.50 mg/100 g sample) from pumpkin seeds.
5.2.4. Deep Eutectic Solvents Extraction (DESE)
Deep eutectic solvents are new green solvents that recently have been discovered that are nontoxic, affordable, biodegradable, and safe for the environment; they are a mixture of two or three components generating hydrogen bonds. An organic salt (quaternary ammonium or phosphonium salt) serves as the hydrogen bond acceptor, whereas alcohol, amino acids, sugar, organic acids, etc., serve as the hydrogen bond donors [102]. Because they have the potential for recycling and make it simple to purify the extracted bioactive components, these solvents are seen as intriguing alternatives. To extract β-carotene from refuse pumpkin materials, Stupar et al. [95] utilized hydrophobic natural deep eutectic solvents derived from fatty acids. One of the most effective natural deep eutectic solvents was selected as caprylic acid:capric acid (3:1), yielding 151.41 µg/mL of β-carotene at the ideal conditions of 50 °C, 60% (52.5 W/cm3) ultrasonic power, and a solvent-to-solid ratio of 7 mL/g over the course of 10 min of extraction. In order to develop a sustainable and effective extraction method of pigment from pumpkin peels, Huang et al. [103] conducted an experiment using different combinations of deep eutectic solvent alcohol and two aqueous phase systems. Subsequently, they implemented a Box–Behnken design and ultrasound-assisted cellulase to optimize the parameters. The results showed that the extraction solvents utilized were DES (choline chloride:triethylene glycol, 1:3) and ethanol (6:4). At a solid–liquid ratio of 1:40 g/mL, a cellulase dose of 2.10%, and an ultrasonic power of 300 W for 40 min; the pigment yield was 2.460 ± 0.037%.
6. Potential Health Benefits of Pumpkin By-Products
The therapeutic potential of pumpkin and its by-products against a variety of diseases is due to bioactives that are able to produce a distinct physiological effect in the human body. Therefore, bioactive substances are of great relevance for human health after evaluating studies on the chemical, biological, and pharmacological features of pumpkin and taking its by-products, taking peels, seeds, and fruit into consideration.
6.1. Antioxidant Activity
Antioxidants are useful in managing and preventing various diseases by preventing the accumulation of free radicals. Pumpkin by-products are abundant sources of various antioxidant substances such as flavanoids, tocopherols, phenolic acids, and carotenoids that preserve proper oxidative stability [99]. By using acidified methanol to extract pumpkin seeds, Yasir et al. [104] found that the extraction yield increased by 1.4 to ten times, the phenolic content increased (149.5–396.4 mg GAE/g), the DPPH radical scavenging activity increased, and the geno-protective activity was enhanced using the pBR322 plasmid assay.
In order to assess the antioxidant properties of pumpkin seeds and shells, Saavedra et al. [2] implemented numerous extraction solvents and dehydration techniques. Applying a 70% ethanol solution to both the seeds and shell samples showed that the presence of 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals was reduced by 18.92–70.96%. The way in which various dehydration techniques suppress DPPH radicals is significantly different. The freeze-dried samples exhibited inhibition ranging from 1.47% to 52.41%, whereas the oven-dried samples at 65 °C showed inhibition ranging from 2.65% to 72.36%. The scavenging activity of pumpkin peel and pulp extract is around 80% at a concentration of 50 mg/mL [36].
6.2. Antihypertensive and Cardioprotective Activity
Blood pressure in the arteries is constantly high due to the long-term health condition known as hypertension, which is also a risk factor for cardiovascular illnesses. Several medicinal components, including mono- and polyunsaturated fatty acids like omega 3, omega 6, oleic acid, and linoleic acid, are abundant in pumpkin seed oil and play a significant role in lowering the risk of hypertension and cardiovascular illness [105]. In mice with high cholesterol, a supplement of pumpkin seeds showed anti-atherogenic and hepatoprotective effects [106].
Zuhair et al. [107] reported that pumpkin seeds exhibit hypotensive effects. Pumpkin seed oil demonstrated a favorable drug interaction outcome when administered in animal models with commonly used hypotensive medicines such as felodipine.
6.3. Anticancer Activity
The phytoestrogenic chemicals found in pumpkin seeds make them a promising applicant for avoiding hormone-dependent cancer. The anticancer activity of the seeds extract, for instance, was tested on MCF7, Jeg3, and BeWo (chorionic carcinoma), and the results showed good cytotoxic activity (10 or 50 µg/mL) and significant estradiol production. These findings indicated that, like many phytoestrogenic substances, pumpkin seeds, which are high in lignans and flavones, exhibit a biphasic effect through a variety of pathways with both estrogenic and antiestrogenic activities [108]. The seed oil of C. pepo effectively inhibited the MCF7 (breast cancer) cell line with an IC50 of 0.4 mg [109].
In a study comparing the cytotoxic effects of various pumpkin portions on the HEPG2 (liver carcinoma) and MCF7 cell lines, all extracts (pulp, peel, seed, and defatted seed extracts) showed strong cytotoxic effects with IC50 values ranging between 0.60 and 5.03 g/mL and 0.40–1.01 µg/mL, respectively [38]. Pectin has been identified as the most favorable polysaccharide found in pumpkin peels or pulp. Numerous papers have described pectin as a bioactive substance that effectively prevents cancer [110]. The ethanol-based extracts of C. maxima seed demonstrated complete suppression of esophageal adenoid cystic carcinoma cell lines at a concentration of 100 µg/mL, indicating strong anticancer activity [111].
Numerous carotenoid pigments, which have been demonstrated to lower the risk of cancer, are abundant in pumpkin seed oil. It has been claimed that pumpkin seed consumption has an adverse relationship with the development of several cancers, including lung, rectal, and breast cancer [66].
It is claimed that consuming 20–40 mg/kg of pumpkin seed oil orally for 20 days can be beneficial in reducing the enlargement of the prostate gland caused by testosterone [112]. Studies conducted in both living animals (in vivo) and laboratory settings (in vitro) have shown that pumpkin inhibits prostate cancer. Furthermore, a study on Sprague Dawley rats showed that applying pumpkin seed oil successfully decreased the progression of testosterone-induced hyperplasia. These findings indicate that pumpkin seed oil may have substantial advantages in the management of benign prostatic hyperplasia [113].
6.4. Anti-Hyperlipidemic Effect
In their study, Majid et al. [114] examined the effects of pumpkin seed oil on human subjects under controlled conditions. The findings demonstrated a significant decrease in endpoint LDL (low-density lipoprotein) cholesterol and a substantial increase in HDL (high-density lipoprotein) cholesterol. The experimental findings suggest that pumpkin seed oil exhibits hypolipidemic and antihypertensive effects since it reduces LDL levels and elevates HDL levels.
Pumpkin seed powder contains arginine, a precursor of nitric oxide, at a concentration of 2.6%. Arginine is associated with apoptosis, blood pressure regulation, cardiac function, and inflammatory response [55]. A study examined the impact of pumpkin seed extract, which consists mainly of arginine, on rats with hyperlipidemia. The study discovered that administering pumpkin seed extract to these rats resulted in a rise in nitric oxide synthesis, most likely attributed to arginine in the extract. In addition, the production of nitric oxide leads to a decrease in the expression of the vascular cell adhesion molecule, hence reducing the oxidation of LDL [115]. Thus, it can be concluded that altering lifestyle and incorporating regular consumption of pumpkins into a diet can be a helpful dietary strategy to treat hypercholesterolemia.
6.5. Anti-Diabetic Effect
Studies have indicated that dietary fibers, like pectin found in pumpkin skins, can inhibit the digestion of starch. This can be beneficial in managing diet-related conditions such as diabetes [116]. Adams et al. [117] identified several bioactive components in pumpkin that contribute to the reduction in blood glucose levels. Specifically, pectin and non-pectin polysaccharides derived from the epidermis and pulp, as well as hypoglycemic proteins and seed oils from pumpkin seeds, were found to be effective in lowering blood glucose. In studies involving alloxan-induced diabetic rats, protein-bound polysaccharides were reported to significantly reduce blood glucose levels, increase plasma insulin concentrations, and enhance glucose tolerance. In rats fed a diet containing 2% cholesterol, a combination of pumpkin seeds with flax seeds or purslane seeds was shown to have an anti-atherogenic impact by lowering total cholesterol, triglycerides, and total lipids in both the liver and serum [118]. By-products from pumpkins are a plentiful source of several nutraceuticals that can treat hypercholesterolemia and diabetes. For instance, the high dietary fiber content of pumpkin peels may help avoid hypercholesteremia and diabetes [115]. The anti-diabetic properties of C. maxima seed are attributed to the inhibitory impact of its aqueous extract on α-amylase and α-glucosidase enzymes. The anti-diabetic effectiveness of a newly discovered protein-bound polysaccharide from pumpkin seeds was assessed based on its ability to inhibit α-amylase. When administered at a concentration of 5.0 mg/mL, it was found to exhibit α-amylase inhibition with a percentage of 41.3 ± 2.1%. Pumpkin-based polysaccharide is a useful treatment for diabetic individuals [119].
6.6. Anti-Inflammatory Effect
The management of many diseases can be aided by preventing inflammation, which is a risk factor for many pathological disorders like diabetes, cancer, and other concerns. The anti-inflammatory effects of pumpkin by-products are attributed to their rich content of polyphenols and other antioxidant compounds, which help in reducing inflammation by neutralizing free radicals and modulating inflammatory pathways. In several disorders, it has been claimed that pumpkin seed oil can reduce and avoid inflammation. In rats subjected to pyloric ligation, water immersion stress, and indomethacin-induced ulcer procedures, a newly obtained tetracyclic triterpenoid from pumpkin seeds oil at a dose of 300 µg/mL showed optimal antiulcer action, with the percentage of inhibition being 55.7%, 67.1%, and 59.1%, respectively [120]. Through a decrease in DNA fragmentation, Bardaa et al. [121] demonstrated that oral therapy of azathioprine-induced cutaneous lesions in rats with pumpkin seed oil (4 mL/kg b.wt) improved macroscopic, morphometric, and histological results compared to the untreated group. The isolation of 3-hydroxycholest-7-en-24-one from C. pepo seeds activated macrophages by inhibiting the generation of nitric oxide in RAW 264 cells [122].
6.7. Antimicrobial Effect
The linoleic and oleic acids in the seed oil may also have strong antibacterial properties against S. aureus, with a zone of inhibition of 15 mm, according to Adnan et al. [109]. In tests against Bacillus subtilis, S. aureus, and E. coli, pumpkin seed extracts in petroleum ether and methanol at concentrations of 10, 50, 100, 200, 500, and 1000 g/mL were successful [109].
The disc diffusion method reveals that the methanolic and ethanolic extracts derived from pumpkin peel exhibit antibacterial properties, resulting in an inhibitory zone ranging from 6 to 10 mm [123]. According to Ahmad and Khan [124], research has shown that pumpkin seed oil has strong antibacterial action against Pseudomonas aeruginosa, Candida albicans, Acinetobacter baumanii, Enterococcus faecalis, Klebsiella pneumonia, E. coli, and Staphylococcus aureus.
Asif et al. [125] found that pumpkin peel extract showed significant antibacterial properties against four bacterial strains. This was determined using the disc diffusion method, and the inhibition zone values ranged from 10 to 15 mm. The high antioxidant and antibacterial capacity of pumpkin peel suggests that this section of the fruit could be used to develop antioxidant-rich functional foods.
A polysaccharide derived from pumpkin seeds was shown to have antibacterial properties. At a dosage of 0.5 mg/mL, significant suppression was seen against the bacteria E. coli (12.8 ± 1.0 mm) and B. subtilis (7.2 ± 0.3 mm), whereas a modest zone of inhibition was seen against Pichia fermentans (2.3± 0.2 mm) and Staphylococcus aureus (2.3 ± 0.2 mm) [3,61].
6.8. Other Health Benefits
Rezig et al. [126] showed that pumpkin seed oil decreases the pressure inside the urethra and bladder, by lowering crystal growth, which lowers the chance of bladder stones, among other disorders. According to Baek et al. [127], the greater iron concentration in pumpkin seeds aids in alleviating the anemia brought on by iron scarcity. Consuming raw and processed pumpkin seeds may be beneficial for treating depression. Trypsin might be inhibited and the Hageman factors could be activated by protein isolates from pumpkin seeds. Additionally, pumpkin seeds are used in a variety of cosmetic products, including dry facial masks, body scrubbers, body butter, and scrubs for the body [13]. A study was conducted to see how aspartame and pumpkin seed oil interacted. Reports suggest that administering a dosage of 4 mL/kg of pumpkin seed oil mixed with water for a duration of four weeks can mitigate the adverse effects of aspartame and provide protection to the liver. This treatment with pumpkin seed oil effectively preserved the globulin levels, liver enzymes (aspartate transaminase, alkaline phosphatase), albumin, and bilirubin [128].
By preserving the gastric mucosa in a dose-dependent manner, pumpkin seeds have additionally demonstrated anti-ulcerative capabilities [120]. Vitamin C-rich C. pepo pulp is used as a remedy for enteritis, dyspepsia, and stomach problems. Sarkar et al. [129] found that albino rats given pumpkin fruit pulp extract showed higher alkaline phosphate activity, thinner mucosal lining, and lower ulcer index. In contrast, albino rats given aspirin had reduced alkaline phosphate activity, thicker mucosal lining, and higher ulcer index. This indicates that the pulp of C. pepo has properties that protect the stomach and duodenum from damage and can be used to treat ulcers.
Researchers’ attention is always on developing more efficient and affordable methods of wound healing since they believe that current medical treatments are insufficient [130]. Pumpkin seed oil’s high concentrations of plant sterols, tocopherols, and polyunsaturated fatty acids have a beneficial impact on wound healing [121]. It has also been noted that similar findings regarding pumpkin peel exist [22].
7. A Biorefinery Approach for the Valorization of Industrial Pumpkin By-Products
Several processing options for their integrated valorization might be addressed, taking into consideration the composition of the pumpkin by-products and the large range of high-added-value bio-products available. To do this, biorefineries should be developed that concentrate on the selective separation of their primary constituents and their afterward transformation into building block chemicals and their derivatives. In order to extract fatty acids and/or oils from the pumpkin by-products, a traditional extraction using organic solvents could be chosen as the initial step. Based on the Alonso et al. [131] and Gullón et al. [132] studies, the solid produced after this initial processing might be submitted to an autohydrolysis procedure to extract the key components. Two distinct fractions would result from this processing: a liquid fraction rich in oligosaccharides and phenolic chemicals, and a solid fraction high in cellulose and lignin. To recover oligosaccharides and other molecules with antioxidant activity individually, further ethyl acetate extraction could be performed on the resulting liquid phase. A delignification process (using alkali or deep eutectic solvents) on the autohydrolyzed solid enables the solubilization of lignin, producing a solid residue primarily made of cellulose. The dark fluid would then be acidified to precipitate lignin. The depolymerization of lignin for the generation of aromatic building blocks with significant application potential might also be discussed as a means to develop a practical biorefinery. Several techniques, including base-catalyzed hydrolysis, pyrolysis, hydrogenolysis, or oxidation, could be used to carry it out. Following the delignification process, the resulting solid can be utilized for either the enzymatic hydrolysis of cellulose to produce saccharification or the acid hydrolysis to generate nanocrystalline cellulose. The upgrading of the resultant glucose to various platform chemicals (such as bio-ethanol and succinic acids) through the fermentative process or its catalytic oxidation to furan derivatives are two intriguing ways that could be taken into consideration. Applying the principles of the circular economy, the biorefinery concept would enable the pumpkin by-products’ re-incorporation into productive processes as abundant and affordable raw materials, satisfying the needs of regulations addressing the prevention and management of waste [132,133,134,135].
8. Conclusions and Future Perspectives
Pumpkin and its by-products have drawn attention for its nutrient profile, pharmacological properties, and industrial usage, which may be related to its enormous number of phytochemicals and bioactive compounds.
Bioactives found in various pumpkin parts have been shown to lower the risk of certain chronic diseases. Due to its phytochemical profile, health benefits, and industrial uses, it is imperative to investigate the significance of pumpkins for consumers. A circular and sustainable economy centered on the pumpkin industry would be supported by the reuse of these biomolecules, which have applications in the food, pharmaceutical, and cosmetic industries. With the aim of eliminating food waste and lowering losses in manufacturing and supply chains, this strategy is pertinent to achieving sustainable development goals. In addition to having a higher cost–benefit ratio, eliminating food loss, increasing energy efficiency, and lowering environmental pollution, the bioactive compounds recovered using green extraction processes have considerable economic potential [136].
More research is required to determine the nutritional profile and potential industrial uses of pumpkins.
Pumpkin seeds are valued for their oil, rich in essential fatty acids, and as a strong source of proteins with essential amino acids. Extracted compounds need further clinical testing. Promoting pumpkin consumption in developing nations can reduce costs and offer therapeutic benefits.
Different foods may prevent oxidation using the phytochemical-rich bioactive substances in pumpkin and its by-products. Current processing techniques could be applied to demonstrate the functional influence that pumpkin by-products have on various food products. In addition, research can be carried out on the household consumption of pumpkin by-products.
The previously mentioned investigations indicate that pumpkin wastes, including peel and seeds, possess substantial potential to be employed as raw materials for various purposes. However, in order to maximize the production of the desired chemical, such as cellulose, pectin, bioethanol, etc., they require an appropriate pretreatment procedure. Future research must focus on improving the pretreatment process to extract the important lignocellulosic material from pumpkin wastes in its entirety.
Researchers, food industry specialists, and consumers can encourage healthy and sustainable nutrition by acknowledging and utilizing the potential of these by-products, while also presenting new and nutritious food options.
Consequently, every part of the pumpkin plant can be utilized in extracting phytochemicals that provide beneficial biological impacts on human health. By adopting this approach, the byproduct generated from pumpkin cultivation and processing might be effectively repurposed. These items could potentially be repurposed and altered for use in new commercial goods, thus supporting a circular economy framework that is recognized for its economic and ecological benefits.
Future research should focus on optimizing extraction methods, the economic feasibility of using pumpkin waste, exploring new applications for these by-products, and integrating them into food products to enhance nutritional value and sustainability in the food industry. Integrating pumpkin by-products into food industry products and processes can offer several benefits: nutritional value, innovation in product development, cost efficiency, support for circular economy, and sustainability.
Author Contributions
Conceptualization: R.N.G., F.S., G.R., N.S., I.A., and O.E.C.; writing—original draft: R.N.G., F.S., G.R., O.E.C., and F.D.L.; writing—review and editing: R.N.G., F.S., O.E.C., and G.R.; supervision, G.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Aruah C.B., Uguru M.I., Oyiga B.C. Variations among Some Nigerian Cucurbita Landraces. Afr. J. Plant Sci. 2010;4:374–386. doi: 10.5897/AJPS.9000257. [DOI] [Google Scholar]
- 2.Saavedra M.J., Aires A., Dias C., Almeida J.A., De Vasconcelos M.C.B.M., Santos P., Rosa E.A. Evaluation of the Potential of Squash Pumpkin By-Products (Seeds and Shell) as Sources of Antioxidant and Bioactive Compounds. J. Food Sci. Technol. 2015;52:1008–1015. doi: 10.1007/s13197-013-1089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P., Kaur G., Kehinde B.A., Chhikara N., Panghal A., Kaur H. Pharmacological and Biomedical Uses of Extracts of Pumpkin and Its Relatives and Applications in the Food Industry: A Review. Int. J. Veg. Sci. 2020;26:79–95. doi: 10.1080/19315260.2019.1606130. [DOI] [Google Scholar]
- 4.Global Biodiversity Information Facility (GBIF) Global Biodiversity Information Facility (GBIF) [(accessed on 9 May 2024)]. Available online: https://www.gbif.org/species/2874515.
- 5.Men X., Choi S.-I., Han X., Kwon H.-Y., Jang G.-W., Choi Y.-E., Park S.-M., Lee O.-H. Physicochemical, Nutritional and Functional Properties of Cucurbita Moschata. Food Sci. Biotechnol. 2021;30:171–183. doi: 10.1007/s10068-020-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulczyński B., Gramza-Michałowska A. The Profile of Carotenoids and Other Bioactive Molecules in Various Pumpkin Fruits (Cucurbita Maxima Duchesne) Cultivars. Molecules. 2019;24:3212. doi: 10.3390/molecules24183212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FAOSTAT Statistic Database FAOSTAT. [(accessed on 11 July 2023)]. Available online: https://www.fao.org/faostat/en/#data/qcl.
- 8.Bemfeito C.M., Carneiro J.d.D.S., Carvalho E.E.N., Coli P.C., Pereira R.C., Vilas Boas E.V.D.B. Nutritional and Functional Potential of Pumpkin (Cucurbita Moschata) Pulp and Pequi (Caryocar Brasiliense Camb.) Peel Flours. J. Food Sci. Technol. 2020;57:3920–3925. doi: 10.1007/s13197-020-04590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim M.Y., Kim E.J., Kim Y.-N., Choi C., Lee B.-H. Comparison of the Chemical Compositions and Nutritive Values of Various Pumpkin (Cucurbitaceae) Species and Parts. Nutr. Res. Pract. 2012;6:21–27. doi: 10.4162/nrp.2012.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Białek A., Jelińska M., Tokarz A. Influence of Maternal Diet Enrichment with Conjugated Linoleic Acids on Lipoxygenase Metabolites of Polyunsaturated Fatty Acids in Serum of Their Offspring with 7,12-Dimethylbenz[a]Anthracene Induced Mammary Tumors. Prostaglandins Other Lipid Mediat. 2015;116–117:10–18. doi: 10.1016/j.prostaglandins.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Burger Staichok A.C., Mendonça K.R.B., Santos P.G.A.d., Garcia L.G.C., Damiani C. Pumpkin Peel Flour (Cucurbita Máxima L.)—Characterization and Technological Applicability. J. Food. Nutr. Res. 2016;4:327–333. doi: 10.12691/jfnr-4-5-9. [DOI] [Google Scholar]
- 12.Blanco-Díaz M.T., Font R., Martínez-Valdivieso D., Del Río-Celestino M. Diversity of Natural Pigments and Phytochemical Compounds from Exocarp and Mesocarp of 27 Cucurbita Pepo Accessions. Sci. Hortic. 2015;197:357–365. doi: 10.1016/j.scienta.2015.09.064. [DOI] [Google Scholar]
- 13.Ratnam N., Vandana, Najibullah M., Ibrahim M. A Review on Cucurbita Pepo. Int. J. Pharmacogn. Phytochem. Res. 2017;9:1190–1194. [Google Scholar]
- 14.Amin M.Z., Islam T., Mostofa F., Uddin M.J., Rahman M.M., Satter M.A. Comparative Assessment of the Physicochemical and Biochemical Properties of Native and Hybrid Varieties of Pumpkin Seed and Seed Oil (Cucurbita Maxima Linn.) Heliyon. 2019;5:e02994. doi: 10.1016/j.heliyon.2019.e02994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacheco A.F.C., Pacheco F.C., Cunha J.S., dos Santos F.R., Pacheco J.C.C., Correa K.d.P., Junior W.d.A.O., Paiva P.H.C., Junior B.R.d.C.L. Bibliometric analysis of pumpkin seed proteins: A review of the multifunctional properties of their hydrolysates and future perspectives. Food Bioscie. 2024;59:104269. doi: 10.1016/j.fbio.2024.104269. [DOI] [Google Scholar]
- 16.Lolli V., Viscusi P., Bonzanini F., Conte A., Fuso A., Larocca S., Leni G., Caligiani A. Oil and protein extraction from fruit seed and kernel by-products using a one pot enzymatic-assisted mild extraction. Food Chem. 2024;19:100819. doi: 10.1016/j.fochx.2023.100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallqui L.A., Canchumanya M.B., Papa H.R., Rodríguez G., Aguirre E., Hidalgo A. Physico-chemical characteristics of flours obtained from raw, roasted and autoclaved Cucurbita, Passiflora and Annona seeds. Int. J. Food Sci. Technol. 2024;59:2079–2822. doi: 10.1111/ijfs.16959. [DOI] [Google Scholar]
- 18.Łaba W., Wilk M., Cwynar M., Ciurko D., Piegza M. Simultaneous Extraction and Hydrolysis of Pumpkin Oilseed Cake Proteins Using Active Culture of Proteolytic Bacillus subtilis. Waste Biomass Valori. 2024;15:5013–5023. doi: 10.1007/s12649-024-02515-2. [DOI] [Google Scholar]
- 19.Chen X., Chen L., Li J., Xu Y., Wu J., Peng J., Cheng L., Fu M., Yu Y., Li L. Pectins from food waste: Characterization and functional properties of pectic polysaccharide extracted from pumpkin (Cucurbita moschata Duch.) peels. Eur. Food Res. Technol. 2024;250:1803–1814. doi: 10.1007/s00217-024-04506-y. [DOI] [Google Scholar]
- 20.Jun H.-I., Lee C.-H., Song G.-S., Kim Y.-S. Characterization of the Pectic Polysaccharides from Pumpkin Peel. LWT—Food Sci. Technol. 2006;5:554–561. doi: 10.1016/j.lwt.2005.03.004. [DOI] [Google Scholar]
- 21.Kirchherr J., Reike D., Hekkert M. Conceptualizing the Circular Economy: An Analysis of 114 Definitions. Resour. Conserv. Recycl. 2017;127:221–232. doi: 10.1016/j.resconrec.2017.09.005. [DOI] [Google Scholar]
- 22.Bahramsoltani R., Farzaei M.H., Abdolghaffari A.H., Rahimi R., Samadi N., Heidari M., Esfandyari M., Baeeri M., Hassanzadeh G., Abdollahi M., et al. Evaluation of Phytochemicals, Antioxidant and Burn Wound Healing Activities of Cucurbita Moschata Duchesne Fruit Peel. Iran. J. Basic. Med. Sci. 2017;20:798–805. doi: 10.22038/IJBMS.2017.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussain A., Kausar T., Din A., Murtaza M.A., Jamil M.A., Noreen S., ur Rehman H., Shabbir H., Ramzan M.A. Determination of Total Phenolic, Flavonoid, Carotenoid, and Mineral Contents in Peel, Flesh, and Seeds of Pumpkin (Cucurbita Maxima) J. Food Process. Preserv. 2021;45:e15542. doi: 10.1111/jfpp.15542. [DOI] [Google Scholar]
- 24.Habtemariam S. The Chemical and Pharmacological Basis of Pumpkins (Cucurbita Species) as Potential Therapy for Type-2 Diabetes. In: Habtemariam S., editor. Medicinal Foods as Potential Therapies for Type-2 Diabetes and Associated Diseases. Academic Press; Cambridge, MA, USA: 2019. pp. 473–502. [Google Scholar]
- 25.Ahmed Z., Chen J., Tufail T., Latif A., Arif M., Ullah R., Alqahtani A.S., Xu B. Fundamental opportunities and challenges of nutraceutical noodles enriched with agri-food by-products. Trends Food Sci. Technol. 2024;143:104299. doi: 10.1016/j.tifs.2023.104299. [DOI] [Google Scholar]
- 26.Villamill R.-A., Escobar N., Romero L.N., Huesa R., Plazas A.V., Gutiérrez C., Robelto G.E. Perspectives of pumpkin pulp and pumpkin shell and seeds uses as ingredients in food formulation. Nutr. Food Sci. 2023;53:459–473. doi: 10.1108/NFS-04-2022-0126. [DOI] [Google Scholar]
- 27.Valdez-Arjona L.P., Ramírez-Mella M. Pumpkin Waste as Livestock Feed: Impact on Nutrition and Animal Health and on Quality of Meat, Milk, and Egg. Animals. 2019;9:769. doi: 10.3390/ani9100769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derkanosova N.M., Vasilenko O.A., Peregonchaya O.V., Sokolova S.A., Zajceva I.I., Ponomareva I.N., Shelamova S.A. Research of Composition and Properties of Pumpkin Pomace as Functional Food Ingredient. Atlantis Press; Paris, France: 2018. pp. 771–775. [Google Scholar]
- 29.Torkova A.A., Lisitskaya K.V., Filimonov I.S., Glazunova O.A., Kachalova G.S., Golubev V.N., Fedorova T.V. Physicochemical and Functional Properties of Cucurbita Maxima Pumpkin Pectin and Commercial Citrus and Apple Pectins: A Comparative Evaluation. PLoS ONE. 2018;13:e0204261. doi: 10.1371/journal.pone.0204261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turksoy S., Özkaya B. Pumpkin and Carrot Pomace Powders as a Source of Dietary Fiber and Their Effects on the Mixing Properties of Wheat Flour Dough and Cookie Quality. Food Sci. Technol. Res. 2011;17:545–553. doi: 10.3136/fstr.17.545. [DOI] [Google Scholar]
- 31.Dhiman A., Sharma K., Attri S. Functional Constituents and Processing of Pumpkin: A Review. J. Food Sci. Technol. 2009;46:411–417. [Google Scholar]
- 32.Song J., Wang X., Li D., Meng L., Liu C. Degradation of Carotenoids in Pumpkin (Cucurbita Maxima L.) Slices as Influenced by Microwave Vacuum Drying. Int. J. Food Prop. 2017;20:1479–1487. doi: 10.1080/10942912.2016.1212875. [DOI] [Google Scholar]
- 33.Nuerbiya Y., Agbaje R., Abdulla A. Optimization of Extraction Pigment from Pumpkin Skin Product’s Stability. Food Ferment. Ind. 2014;40:216–222. [Google Scholar]
- 34.Ninčević Grassino A., Rimac Brnčić S., Badanjak Sabolović M., Šic Žlabur J., Marović R., Brnčić M. Carotenoid Content and Profiles of Pumpkin Products and By-Products. Molecules. 2023;28:858. doi: 10.3390/molecules28020858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyam K.L., Lau M., Tan C.P. Fibre from Pumpkin (Cucurbita Pepo L.) Seeds and Rinds: Physico-Chemical Properties, Antioxidant Capacity and Application as Bakery Product Ingredients. Malays. J. Nutr. 2013;19:99–109. [PubMed] [Google Scholar]
- 36.Kurian A., Kripanand S. Nutritional Composition and Antioxidant Activity of Pumpkin Wastes. RJPBCS. 2016;6:336–344. [Google Scholar]
- 37.Badr S.E.A., Shaaban M., Elkholy Y.M., Helal M.H., Hamza A.S., Masoud M.S., El Safty M.M. Chemical Composition and Biological Activity of Ripe Pumpkin Fruits (Cucurbita Pepo L.) Cultivated in Egyptian Habitats. Nat. Prod. Res. 2011;25:1524–1539. doi: 10.1080/14786410903312991. [DOI] [PubMed] [Google Scholar]
- 38.Hernández-Santos B., Rodríguez-Miranda J., Herman-Lara E., Torruco-Uco J.G., Carmona-García R., Juárez-Barrientos J.M., Chávez-Zamudio R., Martínez-Sánchez C.E. Effect of Oil Extraction Assisted by Ultrasound on the Physicochemical Properties and Fatty Acid Profile of Pumpkin Seed Oil (Cucurbita Pepo) Ultrason. Sonochemistry. 2016;31:429–436. doi: 10.1016/j.ultsonch.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 39.Karanja J., Mugendi B.J., Khamis F., Muchugi A. Nutritional Composition of the Pumpkin (Cucurbita Spp.) Seed Cultivated from Selected Regions in Kenya. J. Hortic. Lett. 2013;3:17–22. [Google Scholar]
- 40.Andjelkovic M., Van Camp J., Trawka A., Verhé R. Phenolic Compounds and Some Quality Parameters of Pumpkin Seed Oil. Eur. J. Lipid Sci. Technol. 2010;112:208–217. doi: 10.1002/ejlt.200900021. [DOI] [Google Scholar]
- 41.Al-Okbi S.Y., Mohamed D.A., Kandil E., Abo-Zeid M.A., Mohammed S.E., Ahmed E.K. Anti-Inflammatory Activity of Two Varieties of Pumpkin Seed Oil in an Adjuvant Arthritis Model in Rats. Grasas Y Aceites. 2017;68:e180. doi: 10.3989/gya.0796161. [DOI] [Google Scholar]
- 42.Abdel-Salam O.M.E., El-Sayed El-Shamarka M., Salem N.A., El-Mosallamy A.E.M.K., Sleem A.A. Amelioration of the Haloperidol-Induced Memory Impairment and Brain Oxidative Stress by Cinnarizine. EXCLI J. 2012;11:517–530. [PMC free article] [PubMed] [Google Scholar]
- 43.Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., Guyton R.A., O’Gara P.T., Ruiz C.E., Skubas N.J., Sorajja P., et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–2492. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 44.Gohari Ardabili A., Farhoosh R., Haddad Khodaparast M.H. Chemical Composition and Physicochemical Properties of Pumpkin Seeds (Cucurbita Pepo Subsp. Pepo Var. Styriaka) Grown in Iran. J. Agric. Sci. Technol. 2011;13:1053–1063. [Google Scholar]
- 45.PubChem PubChem. [(accessed on 22 July 2024)]; Available online: https://pubchem.ncbi.nlm.nih.gov/
- 46.Brglez Mojzer E., Knez Hrnčič M., Škerget M., Knez Ž., Bren U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules. 2016;21:901. doi: 10.3390/molecules21070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghisoni S., Chiodelli G., Rocchetti G., Kane D., Lucini L. UHPLC-ESI-QTOF-MS Screening of Lignans and Other Phenolics in Dry Seeds for Human Consumption. J. Funct. Foods. 2017;34:229–236. doi: 10.1016/j.jff.2017.04.037. [DOI] [Google Scholar]
- 48.Nawirska-Olszańska A., Kita A., Biesiada A., Sokół-Łętowska A., Kucharska A.Z. Characteristics of Antioxidant Activity and Composition of Pumpkin Seed Oils in 12 Cultivars. Food Chem. 2013;139:155–161. doi: 10.1016/j.foodchem.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 49.Banerjee J., Singh R., Vijayaraghavan R., MacFarlane D., Patti A.F., Arora A. Bioactives from Fruit Processing Wastes: Green Approaches to Valuable Chemicals. Food Chem. 2017;225:10–22. doi: 10.1016/j.foodchem.2016.12.093. [DOI] [PubMed] [Google Scholar]
- 50.Quintana S.E., Marsiglia R.M., Machacon D., Torregroza E., García-Zapateiro L.A. Chemical Composition and Physicochemical Properties of Squash (Cucurbita Moschata) Cultivated in Bolivar Department (Colombia) Contemp. Eng. Sci. 2018;11:1003–1012. doi: 10.12988/ces.2018.8384. [DOI] [Google Scholar]
- 51.Lalnunthari C., Devi L.M., Amami E., Badwaik L.S. Valorisation of Pumpkin Seeds and Peels into Biodegradable Packaging Films. FBP. 2019;118:58–66. doi: 10.1016/j.fbp.2019.08.015. [DOI] [Google Scholar]
- 52.Andrikopoulos N.K., Chiou A., Mylona A. Triacylglycerol Species of Less Common Edible Vegetable Oils. Food Rev. Int. 2004;20:389–405. doi: 10.1081/FRI-200033470. [DOI] [Google Scholar]
- 53.Montesano D., Blasi F., Simonetti M.S., Santini A., Cossignani L. Chemical and Nutritional Characterization of Seed Oil from Cucurbita Maxima L. (Var. Berrettina) Pumpkin. Foods. 2018;7:30. doi: 10.3390/foods7030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Türkmen Ö., Özcan M.M., Seymen M., Paksoy M., Uslu N., Fidan S.M. Physico-Chemical Properties and Fatty Acid Compositions of Some Edible Pumpkin Seed Genotypes and Oils. J. Agroaliment. Process. Technol. 2017;23:229–235. [Google Scholar]
- 55.Glew R.H., Glew R.S., Chuang L.-T., Huang Y.-S., Millson M., Constans D., Vanderjagt D.J. Amino Acid, Mineral and Fatty Acid Content of Pumpkin Seeds (Cucurbita Spp.) and Cyperus Esculentus Nuts in the Republic of Niger. Plant Foods Hum. Nutr. 2006;61:49–54. doi: 10.1007/s11130-006-0010-z. [DOI] [PubMed] [Google Scholar]
- 56.Idouraine A., Kohlhepp E.A., Weber C.W., Warid, Martinez-Tellez J.J. Nutrient Constituents from Eight Lines of Naked Seed Squash (Cucurbita Pepo L.) J. Agric. Food Chem. 1996;44:721–724. doi: 10.1021/jf950630y. [DOI] [Google Scholar]
- 57.Hussain A., Kausar T., Sehar S., Sarwar A., Quddoos M.Y., Aslam J., Liaqat A., Siddique T., An Q.U., Kauser S., et al. A Review on Biochemical Constituents of Pumpkin and Their Role as Pharma Foods; a Key Strategy to Improve Health in Post COVID 19 Period. Food Prod. Process. Nutr. 2023;5:22. doi: 10.1186/s43014-023-00138-z. [DOI] [Google Scholar]
- 58.Lin C.-T., Tejano L.A., Panjaitan F.C.A., Permata V.N.S., Sevi T., Chang Y.-W. Protein identification and potential bioactive peptides from pumpkin (Cucurbita maxima) seeds. Food Sci. Nutr. 2024;12:5388–5402. doi: 10.1002/fsn3.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murkovic M., Mülleder U., Neunteufl H. Carotenoid Content in Different Varieties of Pumpkins. J. Food Comp. Anal. 2002;15:633–638. doi: 10.1006/jfca.2002.1052. [DOI] [Google Scholar]
- 60.Tokudome Y., Imaeda N., Ikeda M., Kitagawa I., Fujiwara N., Tokudome S. Foods Contributing to Absolute Intake and Variance in Intake of Fat, Fatty Acids and Cholesterol in Middle-Aged Japanese. J. Epidemiol. 1999;9:78–90. doi: 10.2188/jea.9.78. [DOI] [PubMed] [Google Scholar]
- 61.Wang X., Wang C., Zha X., Mei Y., Xia J., Jiao Z. Supercritical Carbon Dioxide Extraction of β-Carotene and α-Tocopherol from Pumpkin: A Box–Behnken Design for Extraction Variables. Anal. Methods. 2017;9:294–303. doi: 10.1039/C6AY02862D. [DOI] [Google Scholar]
- 62.Rezig L., Martine L., Nury T., Msaada K., Mahfoudhi N., Ghzaiel I., Prost-Camus E., Durand P., Midaoui A.E., Acar N., et al. Profiles of Fatty Acids, Polyphenols, Sterols, and Tocopherols and Scavenging Property of Mediterranean Oils: New Sources of Dietary Nutrients for the Prevention of Age-Related Diseases. J. Oleo Sci. 2022;71:1117–1133. doi: 10.5650/jos.ess22110. [DOI] [PubMed] [Google Scholar]
- 63.Noh N.A.N.M., Karim L., Omar S.R. Value-Added Products from Pumpkin Wastes: A Review. Malays. J. Sci. Health Technol. 2022;8:77–84. doi: 10.33102/mjosht.v8i1.231. [DOI] [Google Scholar]
- 64.Sadeghi A., Ebrahimi M., Raeisi M., Ghods Mofidi S.M. Improving the Antioxidant Capacity of Bread Rolls by Controlled Fermentation of Rice Bran and Addition of Pumpkin (Cucurbita Pepo) Puree. Food Meas. 2019;13:2837–2845. doi: 10.1007/s11694-019-00204-6. [DOI] [Google Scholar]
- 65.Çiftçi S., Suna G. Functional Components of Peanuts (Arachis Hypogaea L.) and Health Benefits: A Review. Future Foods. 2022;5:100140. doi: 10.1016/j.fufo.2022.100140. [DOI] [Google Scholar]
- 66.Syed Q.A., Akram M., Shukat R. Nutritional and Therapeutic Importance of the Pumpkin Seeds. Biomed J Sci Tech Res. 2019;21:3586. doi: 10.26717/BJSTR.2019.21.003586. [DOI] [Google Scholar]
- 67.Mishra S., Sharma K. Development of Pumpkin Peel Cookies and Its Nutritional Composition. J. Pharmacogn. Phytochem. 2019;8:370–372. [Google Scholar]
- 68.Kripanand S., Aathira P., E Kurian A., Srinivasulu K., Guruguntla S. Effect of Pumpkin Powder Incorporation on the Physico-Chemical, Sensory and Nutritional Characteristics of Wheat Flour Muffins. IFRJ. 2018;25:1081–1087. [Google Scholar]
- 69.Białek M., Rutkowska J., Adamska A., Bajdalow E. Partial Replacement of Wheat Flour with Pumpkin Seed Flour in Muffins Offered to Children. CyTA—J. Food. 2016;14:391–398. doi: 10.1080/19476337.2015.1114529. [DOI] [Google Scholar]
- 70.Mirhosseini H., Abdul Rashid N.F., Tabatabaee Amid B., Cheong K.W., Kazemi M., Zulkurnain M. Effect of Partial Replacement of Corn Flour with Durian Seed Flour and Pumpkin Flour on Cooking Yield, Texture Properties, and Sensory Attributes of Gluten Free Pasta. LWT—Food Sci. Techno. 2015;63:184–190. doi: 10.1016/j.lwt.2015.03.078. [DOI] [Google Scholar]
- 71.Padalino L., Mastromatteo M., Lecce L., Cozzolino F., Del Nobile M.A. Manufacture and Characterization of Gluten-Free Spaghetti Enriched with Vegetable Flour. J. Cereal Sci. 2013;57:333–342. doi: 10.1016/j.jcs.2012.12.010. [DOI] [Google Scholar]
- 72.Ahmed W.M.M., Alsiddig S.A., Abdelgadir M.O., Ismail A.E., Basheer E.O., Elhassan I.H. Quality Evaluation of Beef Sausage Formulated with Different Levels of Dried Pumpkin Powder. Int. J. Multidiscip. Curr. Res. 2020;8:150–154. [Google Scholar]
- 73.Öztürk T., Turhan S. Physicochemical Properties of Pumpkin (Cucurbita Pepo L.) Seed Kernel Flour and Its Utilization in Beef Meatballs as a Fat Replacer and Functional Ingredient. J. Food Process. Preserv. 2020;44:e14695. doi: 10.1111/jfpp.14695. [DOI] [Google Scholar]
- 74.Longato E., Lucas-González R., Peiretti P.G., Meineri G., Pérez-Alvarez J.A., Viuda-Martos M., Fernández-López J. The Effect of Natural Ingredients (Amaranth and Pumpkin Seeds) on the Quality Properties of Chicken Burgers. Food Bioprocess. Technol. 2017;10:2060–2068. doi: 10.1007/s11947-017-1978-0. [DOI] [Google Scholar]
- 75.Serdaroğlu M., Kavuşan H.S., İpek G., Öztürk B. Evaluation of the Quality of Beef Patties Formulated with Dried Pumpkin Pulp and Seed. Korean J. Food Sci. Anim. Resour. 2018;38:1–13. doi: 10.5851/kosfa.2018.38.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.AlJahani A., Cheikhousman R. Nutritional and Sensory Evaluation of Pumpkin-Based (Cucurbita Maxima) Functional Juice. Nutr. Food Sci. 2017;47:346–356. doi: 10.1108/NFS-07-2016-0109. [DOI] [Google Scholar]
- 77.Barakat H., Hassan M.F.Y. Chemical, Nutritional, Rheological, and Organoleptical Characterizations of Stirred Pumpkin-Yoghurt. Food Nutr. Sci. 2017;8:746–759. doi: 10.4236/fns.2017.87053. [DOI] [Google Scholar]
- 78.Gavril (Rațu) R.N., Cârlescu P.M., Veleșcu I.D., Arsenoaia V.N., Stoica F., Stănciuc N., Aprodu I., Constantin O.E., Râpeanu G. The Development of Value-Added Yogurt Based on Pumpkin Peel Powder as a Bioactive Powder. Agric. Food Res. 2024;16:101098. doi: 10.1016/j.jafr.2024.101098. [DOI] [Google Scholar]
- 79.Soleimanian Y., Sanou I., Turgeon S.L., Canizares D., Khalloufi S. Natural Plant Fibers Obtained from Agricultural Residue Used as an Ingredient in Food Matrixes or Packaging Materials: A Review. Compr. Rev. Food Sci. Food Saf. 2022;21:371–415. doi: 10.1111/1541-4337.12875. [DOI] [PubMed] [Google Scholar]
- 80.Shendge S., Patharkar S. Standardize the Processing Technology for Preparation of Cereal Milk Fortification with Garden Cress (Lepidium Sativum) Seed and Pumpkin (Cucurbita) Seed Powder. Pharma Innov. 2020;9:423–426. [Google Scholar]
- 81.Rațu R.N., Stoica F., Lipșa F.D., Petrescu C.A., Stănciuc N., Aprodu I., Constantin O.E., Râpeanu G. Preliminary Studies on Obtaining a Cheese Made Exclusively from Whey Enriched with Pumpkin Pomace Powder. Sci. Papers. Ser. D Anim. Scie. 2024;LXVII:548–557. [Google Scholar]
- 82.Ferrer-González B.M., García-Martínez I., Totosaus A. Textural Properties, Sensory Acceptance and Fatty Acid Profile of Cooked Meat Batters Employing Pumpkin Seed Paste or Soybean Oil Oleogel as Fat Replacers. Grasas Y Aceites. 2019;70:e320. doi: 10.3989/gya.1055182. [DOI] [Google Scholar]
- 83.Genevois C., Flores S., de Escalada Pla M. Byproduct from Pumpkin (Cucurbita Moschata Duchesne Ex Poiret) as a Substrate and Vegetable Matrix to Contain Lactobacillus Casei. J. Funct. Foods. 2016;23:210–219. doi: 10.1016/j.jff.2016.02.030. [DOI] [Google Scholar]
- 84.Dos Santos Caetano K., Almeida Lopes N., Haas Costa T.M., Brandelli A., Rodrigues E., Hickmann Flôres S., Cladera-Olivera F. Characterization of Active Biodegradable Films Based on Cassava Starch and Natural Compounds. Food Packag. Shelf Life. 2018;16:138–147. doi: 10.1016/j.fpsl.2018.03.006. [DOI] [Google Scholar]
- 85.Chouaibi M., Daoued K.B., Riguane K., Rouissi T., Ferrari G. Production of bioethanol from pumpkin peel wastes: Comparison between response surface methodology (RSM) and artificial neural networks (ANN) Ind. Crops Prod. 2020;155:112822. doi: 10.1016/j.indcrop.2020.112822. [DOI] [Google Scholar]
- 86.Demiral İ., Şamdan C. Preparation and Characterisation of Activated Carbon From Pumpkin Seed Shell Using H3PO4. Anadolu Univ. J. Sci. Technol.-A Appl. Sci. Eng. 2016;17:125–138. doi: 10.18038/btda.64281. [DOI] [Google Scholar]
- 87.Ngamwonglumlert L., Devahastin S., Chiewchan N. Natural Colorants: Pigment Stability and Extraction Yield Enhancement via Utilization of Appropriate Pretreatment and Extraction Methods. Crit. Rev. Food Sci. Nutr. 2017;57:3243–3259. doi: 10.1080/10408398.2015.1109498. [DOI] [PubMed] [Google Scholar]
- 88.Norshazila S., KOY C.N., Rashidi O., Hoon H.L., AZRINA I., NURUL R.A., Zarinah Z. The Effect of Time, Temperature and Solid to Solvent Ratio on Pumpkin Carotenoids Extracted Using Food Grade Solvents. Sains Malays. 2017;46:231–237. doi: 10.17576/jsm-2017-4602-07. [DOI] [Google Scholar]
- 89.Kreck M., Kürbel P., Ludwig M., Paschold P.J., Dietrich H. Identification and Quantification of Carotenoids in Pumpkin Cultivars (Cucurbita Maxima L.) and Their Juices by Liquid Chromatography with Ultraviolet-Diode Array Detection. J. Appl. Bot Food Qual. 2006;80:93–99. [Google Scholar]
- 90.Song J., Yang Q., Huang W., Xiao Y., Li D., Liu C. Optimization of Trans Lutein from Pumpkin (Cucurbita Moschata) Peel by Ultrasound-Assisted Extraction. Food Bioprod. Process. 2018;107:104–112. doi: 10.1016/j.fbp.2017.10.008. [DOI] [Google Scholar]
- 91.Salami A., Asefi N., Kenari R.E., Gharekhani M. Extraction of Pumpkin Peel Extract Using Supercritical CO2 and Subcritical Water Technology: Enhancing Oxidative Stability of Canola Oil. J. Food Sci. Technol. 2021;58:1101–1109. doi: 10.1007/s13197-020-04624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Andrade Lima M., Kestekoglou I., Charalampopoulos D., Chatzifragkou A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules. 2019;24:466. doi: 10.3390/molecules24030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferreira D.F., Barin J.S., Binello A., Veselov V.V., Cravotto G. Highly Efficient Pumpkin-Seed Extraction with the Simultaneous Recovery of Lipophilic and Hydrophilic Compounds. Food Bioprod. Process. 2019;117:224–230. doi: 10.1016/j.fbp.2019.07.014. [DOI] [Google Scholar]
- 94.Hernández-Santos B., Martínez-Sánchez C.E., Torruco-Uco J.G., Rodríguez-Miranda J., Ruiz-López I.I., Vajando-Anaya E.S., Carmona-García R., Herman-Lara E. Evaluation of Physical and Chemical Properties of Carrots Dried by Refractance Window Drying. Dry. Technol. 2016;34:1414–1422. doi: 10.1080/07373937.2015.1118705. [DOI] [Google Scholar]
- 95.Stupar A., Šeregelj V., Ribeiro B.D., Pezo L., Cvetanović A., Mišan A., Marrucho I. Recovery of β-Carotene from Pumpkin Using Switchable Natural Deep Eutectic Solvents. Ultrason. Sonochemistry. 2021;76:105638. doi: 10.1016/j.ultsonch.2021.105638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marić M., Grassino A.N., Zhu Z., Barba F.J., Brnčić M., Rimac Brnčić S. An Overview of the Traditional and Innovative Approaches for Pectin Extraction from Plant Food Wastes and By-Products: Ultrasound-, Microwaves-, and Enzyme-Assisted Extraction. Trends Food Sci. Technol. 2018;76:28–37. doi: 10.1016/j.tifs.2018.03.022. [DOI] [Google Scholar]
- 97.Sharma M., Bhat R. Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil) Foods. 2021;10:787. doi: 10.3390/foods10040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sarfarazi M., Jafari S.M., Rajabzadeh G., Galanakis C.M. Evaluation of Microwave-Assisted Extraction Technology for Separation of Bioactive Components of Saffron (Crocus Sativus L.) Ind. Crops Prod. 2020;145:111978. doi: 10.1016/j.indcrop.2019.111978. [DOI] [Google Scholar]
- 99.Sánchez-Camargo A., Mendiola J., Ibáñez E. Supercritical Fluid Extraction. In: Dalcanale E., Krebs B., Marquardt R., Morbidelli M., Nakai H., Panza L., Poole C., Quack M., Wandelt K.R., editors. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. Elsevier; Amsterdam, The Netherlands: 2013. [Google Scholar]
- 100.Cuco R.P., Cardozo-Filho L., da Silva C. Simultaneous Extraction of Seed Oil and Active Compounds from Peel of Pumpkin (Cucurbita Maxima) Using Pressurized Carbon Dioxide as Solvent. J. Supercrit. Fluids. 2019;143:8–15. doi: 10.1016/j.supflu.2018.08.002. [DOI] [Google Scholar]
- 101.Mitra P., Ramaswamy H.S., Chang K.S. Pumpkin (Cucurbita Maxima) Seed Oil Extraction Using Supercritical Carbon Dioxide and Physicochemical Properties of the Oil. J. Food Eng. 2009;95:208–213. doi: 10.1016/j.jfoodeng.2009.04.033. [DOI] [Google Scholar]
- 102.Zhuang B., Dou L.-L., Li P., Liu E.-H. Deep Eutectic Solvents as Green Media for Extraction of Flavonoid Glycosides and Aglycones from Platycladi Cacumen. J. Pharm. Biomed. Anal. 2017;134:214–219. doi: 10.1016/j.jpba.2016.11.049. [DOI] [PubMed] [Google Scholar]
- 103.Huang L., Zuo S., Gao X., Li Z., Wang S., Chen B., Li X., Zhu L., Zhang Y. Extraction and Functional Properties of Pigment from Pumpkin Peels by a Novel Green Deep Eutectic Alcohol Two-Phase System. Sustain. Chem. Pharm. 2023;33:101067. doi: 10.1016/j.scp.2023.101067. [DOI] [Google Scholar]
- 104.Yasir M., Sultana B., Nigam P.S., Owusu-Apenten R. Antioxidant and Genoprotective Activity of Selected Cucurbitaceae Seed Extracts and LC–ESIMS/MS Identification of Phenolic Components. Food Chem. 2016;199:307–313. doi: 10.1016/j.foodchem.2015.11.138. [DOI] [PubMed] [Google Scholar]
- 105.Das U.N. Essential Fatty Acids: Biochemistry, Physiology and Pathology. Biotechnol. J. 2006;1:420–439. doi: 10.1002/biot.200600012. [DOI] [PubMed] [Google Scholar]
- 106.Makni M., Sefi M., Fetoui H., Garoui E.M., Gargouri N.K., Boudawara T., Zeghal N. Flax and Pumpkin Seeds Mixture Ameliorates Diabetic Nephropathy in Rats. Food Chem. Toxicol. 2010;48:2407–2412. doi: 10.1016/j.fct.2010.05.079. [DOI] [PubMed] [Google Scholar]
- 107.Zuhair H.A., Abd El-Fattah A.A., El-Sayed M.I. Pumpkin-Seed Oil Modulates the Effect of Felodipine and Captopril in Spontaneously Hypertensive Rats. Pharmacol. Res. 2000;41:555–563. doi: 10.1006/phrs.1999.0622. [DOI] [PubMed] [Google Scholar]
- 108.Richter D., Abarzua S., Chrobak M., Vrekoussis T., Weissenbacher T., Kuhn C., Schulze S., Kupka M.S., Friese K., Briese V., et al. Effects of Phytoestrogen Extracts Isolated from Pumpkin Seeds on Estradiol Production and ER/PR Expression in Breast Cancer and Trophoblast Tumor Cells. Nutr. Cancer. 2013;65:739–745. doi: 10.1080/01635581.2013.797000. [DOI] [PubMed] [Google Scholar]
- 109.Adnan M., Gul S., Batool S., Bibi F., Rehman A., Yaqoob S., Shabir H., Yousaf T., Mussarat S., Ali N., et al. A Review on the Ethnobotany, Phytochemistry, Pharmacology and Nutritional Composition of Cucurbita Pepo L. J. Phytopharmacol. 2017;6:133–139. doi: 10.31254/phyto.2017.6211. [DOI] [Google Scholar]
- 110.Rico X., Gullón B., Alonso J.L., Yáñez R. Recovery of High Value-Added Compounds from Pineapple, Melon, Watermelon and Pumpkin Processing by-Products: An Overview. Food Res. Inter. 2020;132:109086. doi: 10.1016/j.foodres.2020.109086. [DOI] [PubMed] [Google Scholar]
- 111.Abou-Elella F., Mourad R. Anticancer and Anti-Oxidant Potentials of Ethanolic Extracts of Phoenix Dactylifera, Musa Acuminata and Cucurbita Maxima. Res. J. Pharm. Biol. Chem. Sci. 2015;6:710–720. [Google Scholar]
- 112.Gossell-Williams M., Davis A., O’Connor N. Inhibition of Testosterone-Induced Hyperplasia of the Prostate of Sprague-Dawley Rats by Pumpkin Seed Oil. J. Med. Food. 2006;9:284–286. doi: 10.1089/jmf.2006.9.284. [DOI] [PubMed] [Google Scholar]
- 113.Gossell-Williams M., Lyttle K., Clarke T., Gardner M., Simon O. Supplementation with Pumpkin Seed Oil Improves Plasma Lipid Profile and Cardiovascular Outcomes of Female Non-Ovariectomized and Ovariectomized Sprague-Dawley Rats. Phytother. Res. 2008;22:873–877. doi: 10.1002/ptr.2381. [DOI] [PubMed] [Google Scholar]
- 114.Majid A.K., Ahmed Z., Khan R. Effect of Pumpkin Seed Oil on Cholesterol Fractions and Systolic/Diastolic Blood Pressure. Food Sci. Technol. 2020;40:769–777. doi: 10.1590/fst.03720. [DOI] [Google Scholar]
- 115.Proboningsih J., Wirjatmadi B., Kuntoro K., Adriani M. Expression of VCAM in Male Wistar Rats (Rattus Norvegicus) with Hypercholesterolemia Supplemented with Pumpkin Seeds (Cucurbita Moschata Duch) Extract. Health Notions. 2018;2:648–654. doi: 10.33846/hn.v2i6.214. [DOI] [Google Scholar]
- 116.Bai Y., Zhang M., Chandra Atluri S., Chen J., Gilbert R.G. Relations between Digestibility and Structures of Pumpkin Starches and Pectins. Food Hydrocoll. 2020;106:105894. doi: 10.1016/j.foodhyd.2020.105894. [DOI] [Google Scholar]
- 117.Adams G.G., Imran S., Wang S., Mohammad A., Kok S., Gray D.A., Channell G.A., Morris G.A., Harding S.E. The hypoglycaemic effect of pumpkins as anti-diabetic and functional medicines. Food Res. Int. 2011;44:862–867. doi: 10.1016/j.foodres.2011.03.016. [DOI] [Google Scholar]
- 118.Barakat L.A.A., Mahmoud R.H. The Antiatherogenic, Renal Protective and Immunomodulatory Effects of Purslane, Pumpkin and Flax Seeds on Hypercholesterolemic Rats. N. Am. J. Med. Sci. 2011;3:411–417. doi: 10.4297/najms.2011.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kushawaha D., Yadav M., Chatterji S., Srivastava A., Watal G. α-Amylase and α-Glucosidase Inhibitory Activity Assessment of Cucurbita Maxima Seeds—A LIBS Based Study. Intern. J. Phytomed. 2016;8:312. doi: 10.5138/09750185.1906. [DOI] [Google Scholar]
- 120.Gill N.S., Bali M. Isolation of Anti Ulcer Cucurbitane Type Triterpenoid from the Seeds of Cucurbita Pepo. Res. J. Phytochem. 2011;5:70–79. doi: 10.3923/rjphyto.2011.70.79. [DOI] [Google Scholar]
- 121.Bardaa S., Ben Halima N., Aloui F., Ben Mansour R., Jabeur H., Bouaziz M., Sahnoun Z. Oil from Pumpkin (Cucurbita Pepo L.) Seeds: Evaluation of Its Functional Properties on Wound Healing in Rats. Lipids Health Dis. 2016;15:73. doi: 10.1186/s12944-016-0237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gutierrez R. Review of Cucurbita Pepo (Pumpkin) Its Phytochemistry and Pharmacology. Med. Chem. 2016;6:1. doi: 10.4172/2161-0444.1000316. [DOI] [Google Scholar]
- 123.Chonoko U.G., Rufai A.B. Phytochemical Screening and Antibacterial Activity of Cucurbita Pepo (Pumpkin) against Staphylococcus Aureus and Salmonella Typhi. Bayero J. Pure Appl. Sci. 2011;4:145–147. doi: 10.4314/bajopas.v4i1.30. [DOI] [Google Scholar]
- 124.Ahmad G., Khan A.A. Pumpkin: Horticultural Importance and Its Roles in Various Forms; a Review. Inter. J. Hortic. Agric. 2019;4:1–6. doi: 10.15226/2572-3154/4/1/00124. [DOI] [Google Scholar]
- 125.Asif M., Naqvi S.A.R., Sherazi T.A., Ahmad M., Zahoor A.F., Shahzad S.A., Hussain Z., Mahmood H., Mahmood N. Antioxidant, Antibacterial and Antiproliferative Activities of Pumpkin (Cucurbita) Peel and Puree Extracts—An in Vitro Study. Pak. J. Pharm. Sci. 2017;30:1327–1334. [PubMed] [Google Scholar]
- 126.Rezig L., Chouaibi M., Msaada K., Hamdi S. Chemical Composition and Profile Characterisation of Pumpkin (Cucurbita Maxima) Seed Oil. Ind. Crops Prod. 2012;37:82–87. doi: 10.1016/j.indcrop.2011.12.004. [DOI] [Google Scholar]
- 127.Baek I.-H., Cho H.-S., Said N.S., Olawuyi I.F., Kim K.-R., Lee W.-Y. Physicochemical and Nutritional Characteristics of Vegan Protein Bars Formulated with Sweet Potato and Rice Protein. Inter. J. Food Sci. Technol. 2024;59:5664–5674. doi: 10.1111/ijfs.17294. [DOI] [Google Scholar]
- 128.Alazragi R. Protective Effect of Pumpkin Seed Oil against Hepatotoxicity and Nephrotoxicity in Rats Administered High Doses of Aspartame. Med. Sci. 2019;23:799–809. [Google Scholar]
- 129.Sarkar S., Buha D. Effect of Ripe Fruit Pulp Extract of Cucurbita Pepo Linn. in Aspirin Induced Gastric and Duodenal Ulcer in Rats. Indian. J. Exp. Biol. 2008;46:639–645. [PubMed] [Google Scholar]
- 130.Narayan S., Sasmal D., Mazumder P.M. Evaluation of the Wound Healing Effect of Herbal Ointment Formulated with Salvia Splendens (Scarlet Sage) Int. J. Pharm. Pharm. Sci. 2011;3:195–199. [Google Scholar]
- 131.Alonso J.L., Domínguez H., Garrote G., González-Muñoz M.J., Gullón B., Moure A., Santos V., Vila C., Yáñez R. Biorefinery Processes for the Integral Valorization of Agroindustrial and Forestal Wastes Procesos de Biorrefinería Para La Valorización Integral de Residuos Agroindustriales y Forestales. CyTA—J. Food. 2011;9:282–289. doi: 10.1080/19476337.2011.598949. [DOI] [Google Scholar]
- 132.Gullón B., Eibes G., Moreira M.T., Dávila I., Labidi J., Gullón P. Antioxidant and Antimicrobial Activities of Extracts Obtained from the Refining of Autohydrolysis Liquors of Vine Shoots. Ind Crops Prod. 2017;107:105–113. doi: 10.1016/j.indcrop.2017.05.034. [DOI] [Google Scholar]
- 133.Rațu R.N., Veleșcu I.D., Stoica F., Usturoi A., Arsenoaia V.N., Crivei I.C., Postolache A.N., Lipșa F.D., Filipov F., Florea A.M., et al. Application of Agri-Food By-Products in the Food Industry. Agriculture. 2023;13:1559. doi: 10.3390/agriculture13081559. [DOI] [Google Scholar]
- 134.Handore A.V., Khandelwal S.R., Ghayal M.S., Handore D.V. Adopting a Circular Bio-Economy: The Biorefinery Concept. In: Bandh S.A., Malla F.A., editors. Biofuels in Circular Economy. Springer Nature; Singapore: 2022. pp. 183–200. [Google Scholar]
- 135.Jităreanu A.F., Mihăilă M., Alecu C.-I., Robu A.-D., Ignat G., Costuleanu C.L. The Relationship between Environmental Factors, Satisfaction with Life, and Ecological Education: An Impact Analysis from a Sustainability Pillars Perspective. Sustainability. 2022;14:10679. doi: 10.3390/su141710679. [DOI] [Google Scholar]
- 136.Jimenez-Lopez C., Fraga-Corral M., Carpena M., García-Oliveira P., Echave J., Pereira A.G., Lourenço-Lopes C., Prieto M.A., Simal-Gandara J. Agriculture Waste Valorisation as a Source of Antioxidant Phenolic Compounds within a Circular and Sustainable Bioeconomy. Food Funct. 2020;11:4853–4877. doi: 10.1039/D0FO00937G. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.