Abstract
The immunological resistance of a host to viral infections may be strongly influenced by cytokines such as interleukin-12 (IL-12) and gamma interferon (IFN-γ), which promote T helper type 1 responses, and IL-4, which promotes T helper type 2 responses. We studied the role of these cytokines during primary and secondary immune responses against Friend retrovirus infections in mice. IL-4- and IL-12-deficient mice were comparable to wild-type B6 mice in the ability to control acute and persistent Friend virus infections. In contrast, more than one-third of the IFN-γ-deficient mice were unable to maintain long-term control of Friend virus and developed gross splenomegaly with high virus loads. Immunization with a live attenuated vaccine virus prior to challenge protected all three types of cytokine-deficient mice from viremia and high levels of spleen virus despite the finding that the vaccinated IFN-γ-deficient mice were unable to class switch from immunoglobulin M (IgM) to IgG virus-neutralizing antibodies. The results indicate that IFN-γ plays an important role during primary immune responses against Friend virus but is dispensable during vaccine-primed secondary responses.
Cytokines regulate both the initiation and the maintenance of immune responses against foreign antigens. Moreover, they control the types of immune responses generated and therefore the effector mechanisms that ultimately mediate resistance. Experiments using mice with specific cytokine gene inactivations have proven to be useful models for obtaining information about the regulation of immune cells in response to infection. Central to this regulation are CD4+ T helper (Th) cells, which can be subdivided into distinct subsets based on the cytokines they produce. Th1 cells produce gamma interferon (IFN-γ) and predominantly induce cell-mediated immune responses and virus-neutralizing antibody responses of the immunoglobulin G2a (IgG2a) isotype (39). The production of IFN-γ by Th cells is often induced by interleukin-12 (IL-12) which is secreted by antigen-presenting cells (APC) (12). In contrast to Th1 cells, Th2 cells secrete IL-4 and stimulate B-cell proliferation and differentiation to produce predominantly IgG1 and IgE antibodies (39). The balance between Th1 and Th2 cells plays a major role in immunity and pathogenesis for several infectious diseases (10), including possible roles in infections by human immunodeficiency virus (9, 61) and murine AIDS virus (19, 33, 45). However, little is known about the role of cytokines in primary immune responses and vaccine-mediated protection against retroviral infections.
We have previously used the Friend virus (FV) model to investigate basic mechanisms of retroviral immunity. FV is a complex comprised of a replication-competent helper virus known as Friend murine leukemia virus (F-MuLV), which is nonpathogenic in adult mice, and a replication-defective but pathogenic virus, spleen focus-forming virus (32). The latter virus encodes a defective envelope protein, gp55, that binds to the erythropoietin receptor, leading to polyclonal erythroblast proliferation and splenomegaly (30, 31, 38). The infection of adult mice with FV induces acute viremia and splenomegaly of various degrees depending on the genetic background of the mouse strain (7, 24). In susceptible strains, disease progresses to lethal erythroleukemia (46, 49, 70). Both virus-specific cellular and humoral immune responses are essential for recovery from primary FV infection (25, 27, 60, 67). Furthermore, vaccine-induced protection against FV-induced erythroleukemia also requires complex immune responses including CD4+ T cells (Th cells); CD8+ T cells (cytolytic T lymphocytes [CTL]), and B cells (17).
In this study, we analyzed the role of IL-4, IL-12, and IFN-γ in immunity to FV infection in mice with genetic inactivations in each of the cytokine genes. We focused on these cytokines because they are major regulators of Th1 versus Th2 responses (10), which may strongly influence the outcome of disease (48, 63). All mice used for these experiments were on the C57BL/6 (B6) genetic background because of the availability of cytokine genetic inactivations in this mouse strain. One consideration in studying FV-induced disease in B6 mice is that these mice are genetically resistant to FV-induced erythroleukemia due to the Fv2 gene. Fv2 acts in a nonimmunological manner to limit FV-induced polyclonal cell activation and splenomegaly (30, 55). Despite their genetic resistance to FV-induced disease, wild-type B6 mice cannot completely eliminate FV and remain infected with low-level virus for life. Furthermore, B6 mice deficient in specific lymphocyte subsets such as CD4+ or CD8+ T cells develop late-onset lethal erythroleukemia (24, 35, 68). Thus, immune responsiveness at the cellular level is an important factor in the resistance of B6 mice to FV-induced erythroleukemia, and this resistance may involve the production of cytokines. We previously showed that the establishment of persistent FV infections could be prevented by vaccination with a live attenuated Friend helper virus (16). Such vaccine-induced protection from persistent infections was shown to be associated with clearance of infectious centers from the spleen by 2 weeks postchallenge. Therefore, in this study we analyzed the role of IL-4, IL-12, and IFN-γ in vaccine-induced clearance of spleen FV by 2 weeks postchallenge as well as the role of these cytokines in the resolution of primary FV infections.
MATERIALS AND METHODS
Mice.
All experiments were performed with 3- to 6-month old female mice, and all strains except B6-IFN-γ−/− were obtained from the Jackson Laboratory, Bar Harbor, Maine. The C57BL/6-IL-12btm1Jm (B6-IL-12β−/−) mice were N11 generation and were provided by permission from Jeanne Magram and Hoffmann-La Roche, Nutley, N.J. Enzyme-linked immunosorbent assays for FV-specific IFN-γ−/− responses in vitro showed that the B6-IL-12β−/− mice did not make normal IFN-γ−/− responses as reported for this strain (data not shown) (40, 41). The C57BL/6-IL-4tm1Nnt (B6-IL4−/−) mice were produced using B6 embryonic stem cells (43). B6.129S7-Ifngtm1Ts (B6-IFN-γ−/− or B6-GKO) mice were N8 generation backcrosses to B6 and were obtained from Genentech, San Francisco, Calif. (13). Enzyme-linked immunosorbent assays for FV-specific IFN-γ−/− responses in vitro showed that these mice did not make normal IFN-γ−/− responses as reported for this strain (data not shown). All mice were treated in accordance with National Institutes of Health regulations and the guidelines of the Animal Care and Use Committee of Rocky Mountain Laboratories.
Virus challenge and vaccination.
In all virus challenge experiments, mice were injected intravenously with 0.5 ml of phosphate-buffered balanced salt solution containing 2% fetal bovine serum and 3,000 spleen focus-forming units of FV complex. The B-tropic, polycythemia-inducing FV complex used as challenge virus in all experiments was from uncloned virus stocks obtained from 10% spleen cell homogenates as described elsewhere (26). The N-tropic F-MuLV vaccine virus (stock 29-51N) (6) was a 24-h supernatant from infected Mus dunni cells. Mice were vaccinated by intravenous injection of 0.5 ml of phosphate-buffered balanced salt solution containing 2% normal mouse serum and 104 focus-forming units (FFU) of F-MuLV vaccine virus. Disease was followed by palpation for splenomegaly in a blinded fashion as described elsewhere (26).
Viremia and virus-neutralizing antibody assays.
For viremia assays, freshly frozen plasma samples were titrated by focal infectivity assays (65) on susceptible M. dunni cells pretreated with Polybrene (4 μg/ml). Cultures were incubated for 5 days, fixed with ethanol, stained with F-MuLV envelope-specific monoclonal antibody (MAb) 720 (59), and developed with goat anti-mouse peroxidase-conjugated antisera (Cappel, West Chester, Pa.) and aminoethylcarbazole to detect foci. To test plasma samples for virus-neutralizing antibodies, heat-inactivated (56°C, 30 min) samples at titrated dilutions were incubated with virus stock in the presence of complement at 37°C with or without β-mercaptoethanol to distinguish IgG from IgM as previously described (47). The samples were then plated as described for the viremia assay to determine the dilution at which 90% of the virus had been neutralized.
Infectious center assays.
Titrations of single-cell suspensions from infected mouse spleens were plated onto susceptible M. dunni cells (36), cocultivated for 5 days, fixed with ethanol, stained with F-MuLV envelope specific MAb 720 (59), and developed with peroxidase-conjugated goat anti-mouse antibodies and aminoethylcarbazole to detect foci.
Flow cytometric analysis for F-MuLV antigen expression.
Single-cell suspensions were analyzed using a Becton Dickinson FACSCalibur flow cytometer. The cells were stained with tissue culture supernatant containing MAb 34 (8), which is specific for F-MuLV glycosylated Gag protein expressed on the surfaces of infected cells. Development was done with fluorescein isothiocyanate-labeled goat anti-mouse IgG2b-specific antiserum (Caltag Laboratories, Burlingame, Calif.) preabsorbed with mouse cells to remove background activity (109 nucleated spleen cells/ml of serum, 40 min on ice).
RESULTS
Effect of cytokine deficiencies on primary immune responses to FV infection.
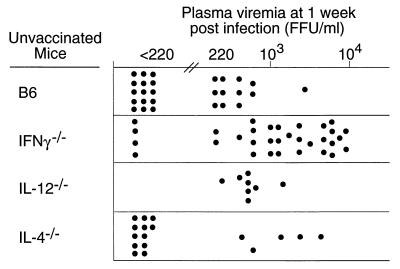
To determine whether Th1- or Th2-type cytokines were important in primary FV-specific immune responses, we first compared virus loads in the plasma of FV-infected B6 wild-type mice with loads in the plasma of IFN-γ−/−, IL-4−/−, and IL-12−/− mice. At 1 week postinfection, less than half of the wild-type B6 mice were viremic, and all but one had levels below 103 FFU per ml of plasma (Fig. 1). In contrast, two-thirds of the IFN-γ−/− mice had viremia levels greater than 103 FFU per ml, and the geometric mean viremia of this group was significantly higher than that in the B6 group (P < 0.01) (Fig. 1). Deficiencies in IL-4 and IL-12 did not have a statistically significant effect on viremia levels at 1 week postinfection. Most of the IL-4−/− mice and most of the B6 mice tested negative. None of the IL-12−/− mice tested negative for viremia, but the levels of viremia were low and not statistically different from values for B6 wild-type controls (Fig. 1). Thus, IFN-γ had a significant influence on plasma viremia.
FIG. 1.
Plasma viremia in mice 1 week after infection with FV. Each dot represents the results from a single mouse. The limit of detection was 220 FFU/ml of plasma. The difference in geometric means (log10) between the B6 control group and the other three groups was analyzed by one-way analysis of variance. Since the control B6 group was used for multiple comparisons, the P values were corrected using Dunnett's multiple-comparisons test. For purposes of statistical analysis where a value must be assigned, the negative mice (<220 FFU/ml) were arbitrarily assigned a value of 102. Only the difference between B6 and IFN-γ−/− mice was statistically significant (P < 0.01).
To determine if the increased viremia in the IFN-γ−/− mice was due to increased virus replication in specific tissues, flow cytometric analysis was performed to detect cell surface viral antigen expression on cells from the spleen, bone marrow, blood, lymph nodes, and thymus. At 1 week postinfection, the IFN-γ−/− mice had significantly higher levels of FV antigen-positive cells in the spleen, bone marrow, and blood than did the B6 mice (Table 1). The increases were only slight in the spleen and blood but averaged more than twice as high in the bone marrow. Infection in the blood and lymph nodes was quite low, and no infection above background levels was observed in the thymus. The approximate doubling of infection levels in the bone marrow suggested that it was a relevant source of virus in IFN-γ−/− mice. These results also suggested that the bone marrow may be particularly sensitive to loss of the antiviral activities of IFN-γ.
TABLE 1.
Percentages of virus antigen-positive cells in various tissues 1 week after infection with FVa
| Mouse | % Antigen-positive cells
|
||||
|---|---|---|---|---|---|
| Spleen | Bone marrow | Blood | Lymph nodes | Thymus | |
| B6 | 5.2 ± 0.9 | 6.4 ± 3.9 | 1.5 ± 0.2 | 3.2 ± 0.6 | 0.3 ± 0.28 |
| IFN-γ−/− | 6.6 ± 0.6 | 14.9 ± 3.6 | 2.3 ± 0.6 | 2.7 ± 0.6 | 0.1 ± 0.10 |
All tissues were examined as single-cell suspensions of nucleated, live cells. Six samples were analyzed for each tissue, and the values shown are means plus or minus standard deviations. The small differences in spleen infection levels and the larger differences in bone marrow infection levels were statistically significant by Student's two-tailed t test (P = 0.0131 and 0.0029, respectively). The differences in blood infection levels were evaluated by the Mann-Whitney test (P = 0.0022) because the standard deviations between the two groups were significantly different, precluding the use of Student's t test.
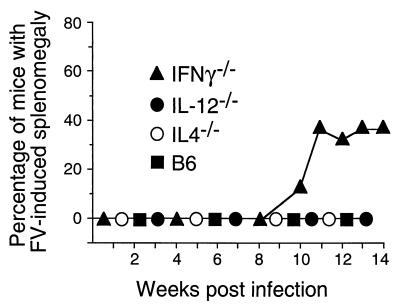
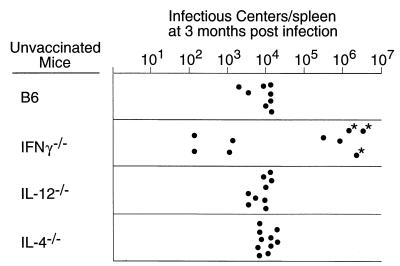
To determine whether the infections in any of the cytokine-deficient mice progressed to leukemia, groups of mice were individually palpated for splenomegaly every week for 12 to 14 weeks following infection. The mice tested in this part of the study were not bled during this time, as bleeding activates hematopoiesis, stimulates virus spread, and can exacerbate disease (72). Also, levels of spleen infectious centers were measured to determine whether the mice had maintained immunological control over spleen virus. Consistent with previous reports (35, 51), none of the B6 mice became grossly splenomegalic over the observation period (Fig. 2). However, all of the mice still harbored persistent virus at the 3-month time point, with approximately 104 infectious centers per spleen (Fig. 3). IL-4−/− and IL-12−/− mice also did not develop gross splenomegaly (Fig. 2), and their levels of persistent infection were comparable to those for wild-type B6 mice (Fig. 3). IFN-γ−/− mice gave interesting results. At the 3-month time point, the IFN-γ−/− mice had diverged into two groups with respect to both splenomegaly and spleen virus levels. One-third of the IFN-γ−/− mice progressed to gross splenomegaly beginning at 10 weeks postinfection (Fig. 3), and five of nine IFN-γ−/− mice had levels of spleen virus more than 10 times higher than levels observed in normal B6 mice (Fig. 3). These results associated lack of IFN-γ with an increased risk of late onset splenomegaly and high levels of spleen infectious centers following FV infection but also suggested that compensatory mechanisms might be at play in some mice which were able to maintain control over virus. The fact that some mice had high levels of infectious centers in the absence of splenomegaly suggests that they may have been caught at an early stage of leukemia development.
FIG. 2.
Splenomegaly in FV-infected mice. Adult mice were infected with FV and monitored for induction and progression of splenomegaly as described elsewhere (26). The IFN-γ−/− group comprised two separate groups of nine and seven mice. The group of nine mice was euthanized at 12 weeks and tested for infectious centers as shown in Fig. 3, while palpations were continued on the remaining seven mice). The IL-12−/− group, IL-4−/− group, and B6 control group consisted of 20, 10, and 10 mice, respectively. Chi square analysis of splenomegaly in B6 versus IFN-γ−/− mice using Fisher's exact test gave a P value of 0.035.
FIG. 3.
Infectious center assays of virus-producing cells in the spleens of mice persistently infected with FV. Each dot represents the results from a single mouse infected 12 to 14 weeks earlier with FV. The limit of detection for this assay was one F-MuLV infectious center per 3 × 107 spleen cells. Asterisks indicate that the mice were splenomegalic. The nine IFN-γ−/− mice tested are from the 12-week time point in Fig. 2.
Effect of cytokine deficiencies on vaccine-induced secondary responses to FV infection.
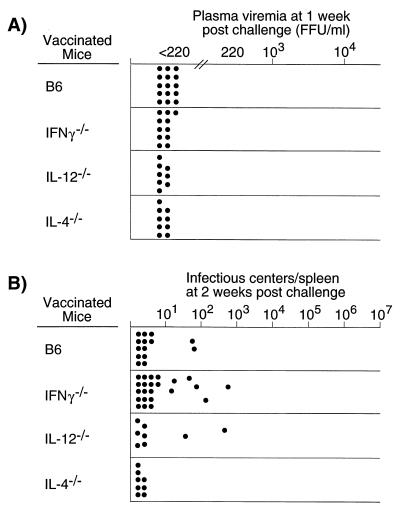
In previous experiments, we used N-tropic F-MuLV helper virus as a vaccine virus to prevent acute viremia (15) and persistent FV infection (16). To determine the effect of IL-4, IL-12, and IFN-γ deficiencies on vaccine protection from acute virus infection, vaccinated and challenged mice were assayed at 1 week for viremia. Since previous experiments showed that prevention of the establishment of persistent FV infection correlated with lack of spleen infectious centers at 2 weeks postinfection, the mice were also assayed for spleen infectious centers at this time point. Vaccinated B6 mice were completely protected from viremia at 1 week postchallenge (Fig. 4A), and only 14% had detectable spleen infectious centers at 2 weeks postchallenge (Fig. 4B). Results for the vaccinated cytokine-deficient mice were quite similar to those for wild-type mice: no measurable viremia (Fig. 4A) and very few or no spleen infectious centers. Thus, IL-4, IL-12, and IFN-γ did not appear essential for the vaccine-induced protection elicited by infection with live attenuated virus.
FIG. 4.
(A) Plasma viremia in vaccinated mice 1 week after challenge with FV. Each dot represents the results from a single mouse. The mice were challenged at 1 month postvaccination. The limit of detection was 220 FFU/ml of plasma. (B) Spleen infectious centers in vaccinated mice 2 weeks after challenge with FV. Each dot represents the results from a single mouse. The limit of detection for this assay was one infectious center per 3 × 107 cells (approximately one-third of a spleen).
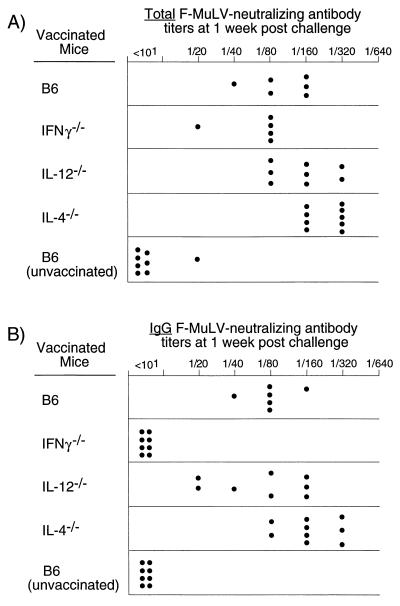
IL-4, IL-12, and IFN-γ all potentially influence antibody responses against pathogens, especially with regard to the classes of antibodies which develop in response to infection (10, 58). Therefore, we compared the antibody responses in cytokine-deficient mice with those in wild-type mice in terms of total virus-neutralizing antibody titers and ability to switch from IgM to IgG. At 1 week postchallenge, all groups of mice had similar total virus-neutralizing antibody titers, with slightly higher levels in the IL-4−/− mice (Fig. 5A). In contrast, IFN-γ−/− mice had no detectable virus-neutralizing IgG antibodies (Fig. 5B). Thus, the lack of IFN-γ in the knockout mice prevented class switching from IgM to IgG antibody, but class switching was not critical for vaccine-induced protection against FV since the IFN-γ−/− mice appeared to be completely protected.
FIG. 5.
Virus-neutralizing antibody titers in mice vaccinated with F-MuLV and subsequently challenged with FV. Virus-neutralizing antibody titers from wild-type mice (B6) were compared with those from IL-4−/−, IL-12−/−, and IFN-γ−/− mice. Plasma samples were taken 1 week after FV challenge of vaccinated mice. Titers of total FV-neutralizing antibodies (A) and FV-neutralizing IgG antibodies (B) are shown. The neutralizing antibody titer was considered to be the highest dilution at which greater than 75% of the input virus was neutralized. The difference between the log2 geometric mean total Ig titer of the vaccinated B6 control group was compared to the values for three cytokine-deficient groups by one-way analysis of variance using Dunnett's multiple-comparisons correction for comparing a control group to several experimental groups. Only the difference between B6 (log2 geometric mean = 6.6) and IL-4−/− (log2 geometric mean = 7.9) mice was statistically significant (P < 0.05). The same statistical analysis was done for the IgG virus-neutralizing antibody titers. The difference between B6 (log2 geometric mean = 6.3) and IFN-γ−/− (log2 geometric mean = 2.3) mice was statistically significant (P < 0.01), as was the difference between B6 and IL-4−/− mice (log2 geometric mean = 7.4, P < 0.05).
DISCUSSION
Of the three cytokine-deficient mouse strains analyzed in this study, only the IFN-γ−/− mice were defective in the ability to control FV, as evidenced by high levels of viremia at 1 week postinfection high levels of infectious centers and FV-induced splenomegaly in some of the mice at 3 months postinfection. These results are consistent with other studies showing protective roles of IFN-γ for a number of viruses, including ectromelia virus (34), lymphocytic choriomeningitis virus (LCMV) (21, 37, 50), herpes simplex virus (5, 66, 73), adenovirus (71), cytomegalovirus (29), hepatitis B virus (22, 23), and the retrovirus complex designated murine AIDS virus (19, 20). IFN-γ has been shown to exert its antiviral activities through a number of direct and indirect mechanisms. Direct mechanisms include induction of cellular resistance to infection (14), inhibition of virus replication through noncytopathic effects (23, 34), and induction of apoptosis (18). Indirect mechanisms of IFN-γ antiviral activities include up-regulation of major histocompatibility complex class I and class II molecules (12, 71), activation of APC (4), and the induction of Th1-type T-cell responses, which are generally associated with protection from viral infections (10, 58).
Th1 responses are characterized by CTL activity and the production of IgG2a antibodies, both of which have been shown to be important mediators of anti-FV immunity (3, 25, 60). In addition, major histocompatibility complex-dependent recovery from FV infection has been correlated with IFN-γ production by CD4+ cells (56). Thus, the dependence of the primary anti-FV immune response on IFN-γ suggests that protective immunity against FV is associated with IFN-γ-dependent Th1 responses. The lack of effect from IL-4 deficiency on recovery from primary FV infection is also consistent with a protective Th1 response, since IL-4 down-regulates Th1 responses and up-regulates Th2 responses which are generally not beneficial to viral immunity (1, 10, 44, 58, 64).
There was a dichotomy within the IFN-γ−/− group regarding the ability to maintain control over long-term FV replication. Approximately half of the mice maintained FV at quite low levels in the spleen, while the remaining mice had very high levels (Fig. 3). In addition, approximately one-third of the IFN-γ−/− mice developed FV-induced splenomegaly (Fig. 2). Thus, it appears that some mice were able to compensate for the lack of IFN-γ whereas others were not. However, these findings must be interpreted in light of the role of IFN-γ in regulating erythropoiesis (57). Because IFN-γ inhibits colony formation by erythroid precursor cells (42, 69), the cells that are primary targets for infection by FV, it is very likely to have effects on FV-induced disease progression that are distinct from its antiviral properties. In fact, it is possible that lack of IFN-γ disrupts the complex network of humoral and microenvironmental factors regulating hematopoiesis in ways that are in opposition to the antiviral activities of IFN-γ, resulting in imbalances that could resolve either in favor of virus replication or in favor of virus control. Alternative courses of resolution could explain the divergent data obtained for the IFN-γ−/− mice at 3 months postinfection. Another possibility is that a genetic resistance factor other than IFN-γ might be segregating within IFN-γ−/− mice. The IFN-γ-targeted mutation was bred from mouse strain 129 into the B6 genetic background for eight generations, and so the genetic material in these mice should be 99.6% identical. The consistency of the results from the IFN-γ−/− mice in all assays at early time points argues against this possibility but does not exclude a genetic influence.
Although IL-12 is often involved in eliciting IFN-γ responses (10), the FV-specific IFN-γ response in vivo was apparently not dependent on IL-12, and no effects from IL-12 deficiency were observed in this study. It is possible that we missed some effects because they fell below the detection limits of our assays, but IL-12 was clearly not a major factor even in unvaccinated mice. However, in in vitro analysis of CD4+ T cells obtained from infected IL-12−/− mice, we did find poor production of IFN-γ in response to stimulation with FV (data not shown). Thus, it appears that there was a compensatory mechanism involved in production of IFN-γ in vivo, possibly through IL-18 (52) or IFN-α/β (11). These findings are in accordance with results from other viral systems such as mouse hepatitis virus (62), LCMV (11, 53), and vesicular stomatitis virus (53), in which IL-12−/− mice showed levels of virus replication and antibody responses similar to those of wild-type mice. Thus, it appears that many viruses can induce protective IFN-γ responses in the absence of IL-12.
Although IFN-γ played an important role in the primary response to FV infection, there was no apparent requirement for IFN-γ in vaccine-induced protection. Similar results have been obtained in the LCMV model, where DNA-vaccinated IFN-γ−/− mice developed CTL and non-IgG2a antibody and were protected as well as wild-type mice (28). However, the dependence on IFN-γ for vaccine protection can vary from model to model, depending on the virus and the vaccine. For example, in the mouse model for influenza A, IFN-γ−/− mice immunized with an attenuated virus developed CTL and antibodies but were not protected as well as wild-type mice (2). The fact that we did not see an effect from IFN-γ deficiency in the vaccinated mice does not indicate that it played no role but merely shows that it was not essential for protection given the parameters that we studied. These results further indicate the existence of mechanisms which can compensate for lack of IFN-γ. Since IFN-γ has been shown to play an important role in primary responses against a large number of viruses, including FV, and also plays important roles in secondary immune responses against some viruses, it seems likely that vaccines designed to elicit IFN-γ responses and Th1-type immunity would have a better probability of success than those that do not.
Some reports have shown that IL-4, which is involved in down-regulating Th1-type immunity, is detrimental to protection from viral infections. For example, virus-encoded IL-4 or exogenously administered IL-4 significantly delayed clearance of vaccinia virus, respiratory syncytial virus, and influenza virus (1, 44, 64). Consistent with the idea that Th1 responses are involved in protective secondary immune responses to FV, vaccinated IL-4−/− mice, which cannot down-regulate Th1 responses, had slightly but significantly higher virus-neutralizing antibody titers than wild-type mice (Fig. 5). As detailed previously, the antibody response is an essential aspect of vaccine-induced immunity to FV. While no difference in protection from acute disease or persistent infection was demonstrable in the present studies, the results suggest that vaccines designed to avoid eliciting IL-4 secretion might enhance virus-neutralizing antibody responses. This might be especially helpful in cases where it is difficult to generate antibody responses (54).
ACKNOWLEDGMENTS
We are grateful to Bruce Chesebro, Donald Lodmell, and Sue Priola for critical comments on the manuscript.
U.D. is supported by the Deutsche Krebsforschungszentrum Heidelberg, Nachwuchsfoerderprogramm Infektiologie.
REFERENCES
- 1.Bachmann M F, Schorle H, Kuhn R, Muller W, Hengartner H, Zinkernagel R M, Horak I. Antiviral immune responses in mice deficient for both interleukin-2 and interleukin-4. J Virol. 1995;69:4842–4846. doi: 10.1128/jvi.69.8.4842-4846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bot A, Bot S, Bona C A. Protective role of gamma interferon during the recall response to influenza virus. J Virol. 1998;72:6637–6645. doi: 10.1128/jvi.72.8.6637-6645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britt W J, Chesebro B. Use of monoclonal anti-gp70 antibodies to mimic the effects of the Rfv-3 gene in mice with Friend virus-induced leukemia. J Immunol. 1983;130:2363–2367. [PubMed] [Google Scholar]
- 4.Buchmeier N A, Schreiber R D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci USA. 1985;82:7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantin E, Tanamachi B, Openshaw H. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J Virol. 1999;73:3418–3423. doi: 10.1128/jvi.73.4.3418-3423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesebro B, Britt W, Evans L, Wehrly K, Nishio J, Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of Friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983;127:134–148. doi: 10.1016/0042-6822(83)90378-1. [DOI] [PubMed] [Google Scholar]
- 7.Chesebro B, Miyazawa M, Britt W J. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu Rev Immunol. 1990;8:477–499. doi: 10.1146/annurev.iy.08.040190.002401. [DOI] [PubMed] [Google Scholar]
- 8.Chesebro B, Wehrly K, Cloyd M, Britt W, Portis J, Collins J, Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: Friend-specific and FMR-specific antigens. Virology. 1981;112:131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- 9.Clerici M, Shearer G M. A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 10.Constant S L, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 11.Cousens L P, Peterson R, Hsu S, Dorner A, Altman J D, Ahmed R, Biron C A. Two roads diverged: interferon alpha/beta- and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J Exp Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curfs J H, Meis J F, Hoogkamp-Korstanje J A. A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev. 1997;10:742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 14.Dhawan S, Heredia A, Lal R B, Wahl L M, Epstein J S, Hewlett I K. Interferon-gamma induces resistance in primary monocytes against human immunodeficiency virus type-1 infection. Biochem Biophys Res Commun. 1994;201:756–761. doi: 10.1006/bbrc.1994.1765. [DOI] [PubMed] [Google Scholar]
- 15.Dittmer U, Brooks D M, Hasenkrug K J. Characterization of a live-attenuated retroviral vaccine demonstrates protection via immune mechanisms. J Virol. 1998;72:6554–6558. doi: 10.1128/jvi.72.8.6554-6558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dittmer U, Brooks D M, Hasenkrug K J. Protection against establishment of retroviral persistence by vaccination with a live attenuated virus. J Virol. 1999;73:3753–3757. doi: 10.1128/jvi.73.5.3753-3757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dittmer U, Brooks D M, Hasenkrug K J. Requirement for multiple lymphocyte subsets in protection against retroviral infection by a live attenuated vaccine. Nat Med. 1999;5:189–193. doi: 10.1038/5550. [DOI] [PubMed] [Google Scholar]
- 18.Drapier J C, Hibbs J B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in l-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988;140:2829–2838. [PubMed] [Google Scholar]
- 19.Gazzinelli R T, Giese N A, Morse H C., III In vivo treatment with interleukin 12 protects mice from immune abnormalities observed during murine acquired immunodeficiency syndrome (MAIDS) J Exp Med. 1994;180:2199–2208. doi: 10.1084/jem.180.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gazzinelli R T, Makino M, Chattopadhyay S K, Snapper C M, Sher A, Hugin A W, Morse H C., III CD4+ subset regulation in viral infection. Preferential activation of Th2 cells during progression of retrovirus-induced immunodeficiency in mice. J Immunol. 1992;148:182–188. [PubMed] [Google Scholar]
- 21.Guidotti L G, Borrow P, Brown A, McClary H, Koch R, Chisari F V. Noncytopathic clearance of lymphocytic choriomeningitis virus from the hepatocyte. J Exp Med. 1999;189:1555–1564. doi: 10.1084/jem.189.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidotti L G, Chisari F V. To kill or to cure: options in host defense against viral infection. Curr Opin Immunol. 1996;8:478–483. doi: 10.1016/s0952-7915(96)80034-3. [DOI] [PubMed] [Google Scholar]
- 23.Guidotti L G, Rochford R, Chung J, Shapiro M, Purcell R, Chisari F V. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 24.Hasenkrug K J. Lymphocyte deficiencies increase susceptibility to Friend virus-induced erythroleukemia in Fv-2 genetically resistant mice. J Virol. 1999;73:6468–6473. doi: 10.1128/jvi.73.8.6468-6473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasenkrug K J, Brooks D M, Chesebro B. Passive immunotherapy for retroviral disease: influence of major histocompatibility complex type and T-cell responsiveness. Proc Natl Acad Sci USA. 1995;92:10492–10495. doi: 10.1073/pnas.92.23.10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasenkrug K J, Brooks D M, Robertson M N, Srinivas R V, Chesebro B. Immunoprotective determinants in Friend murine leukemia virus envelop protein. Virology. 1998;248:66–73. doi: 10.1006/viro.1998.9264. [DOI] [PubMed] [Google Scholar]
- 27.Hasenkrug K J, Chesebro B. Immunity to retroviral infection: the Friend virus model. Proc Natl Acad Sci USA. 1997;94:7811–7816. doi: 10.1073/pnas.94.15.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassett D E, Zhang J, Whitton J L. Plasmid DNA vaccines are effective in the absence of IFNgamma. Virology. 1999;263:175–183. doi: 10.1006/viro.1999.9957. [DOI] [PubMed] [Google Scholar]
- 29.Heise M T, Virgin H W., IV The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J Virol. 1995;69:904–909. doi: 10.1128/jvi.69.2.904-909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoatlin M E, Kabat D. Host-range control of a retroviral disease: Friend erythroleukemia. Trends Microbiol. 1995;3:51–57. doi: 10.1016/s0966-842x(00)88875-7. [DOI] [PubMed] [Google Scholar]
- 31.Hoatlin M E, Kozak S L, Lilly F, Chakraborti A, Kozak C A, Kabat D. Activation of erythropoietin receptors by Friend viral gp55 and by erythropoietin and down-modulation by the murine Fv-2r resistance gene. Proc Natl Acad Sci USA. 1990;87:9985–9989. doi: 10.1073/pnas.87.24.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kabat D. Molecular biology of Friend viral erythroleukemia. Curr Top Microbiol Immunol. 1989;148:1–42. doi: 10.1007/978-3-642-74700-7_1. [DOI] [PubMed] [Google Scholar]
- 33.Kanagawa O, Vaupel B A, Gayama S, Koehler G, Kopf M. Resistance of mice deficient in IL-4 to retrovirus-induced immunodeficiency syndrome (MAIDS) Science. 1993;262:240–242. doi: 10.1126/science.8211142. [DOI] [PubMed] [Google Scholar]
- 34.Karupiah G, Xie Q W, Buller R M, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 35.Kitagawa M, Matsubara O, Kasuga T. Dynamics of lymphocytic subpopulations in Friend leukemia virus-induced leukemia. Cancer Res. 1986;46:3034–3039. [PubMed] [Google Scholar]
- 36.Lander M R, Chattopadhyay S K. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ecotropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J Virol. 1984;52:695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leist T P, Eppler M, Zinkernagel R M. Enhanced virus replication and inhibition of lymphocytic choriomeningitis virus disease in anti-gamma interferon-treated mice. J Virol. 1989;63:2813–2819. doi: 10.1128/jvi.63.6.2813-2819.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J-P, D'Andrea A D, Lodish H F, Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature (London) 1990;343:762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- 39.Liblau R S, Singer S M, McDevitt H O. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 40.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C Y, Ferrante J, Stewart C, Sarmiento U, Faherty D A, Gately M K. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 41.Magram J, Sfarra J, Connaughton S, Faherty D, Warrier R, Carvajal D, Wu C Y, Stewart C, Sarmiento U, Gately M K. IL-12-deficient mice are defective but not devoid of type 1 cytokine responses. Ann NY Acad Sci. 1996;795:60–70. doi: 10.1111/j.1749-6632.1996.tb52655.x. [DOI] [PubMed] [Google Scholar]
- 42.Means R T, Jr, Krantz S B, Luna J, Marsters S A, Ashkenazi A. Inhibition of murine erythroid colony formation in vitro by interferon gamma and correction by interferon receptor immunoadhesin. Blood. 1994;83:911–915. [PubMed] [Google Scholar]
- 43.Metwali A, Elliott D, Blum A M, Li J, Sandor M, Lynch R, Noben-Trauth N, Weinstock J V. The granulomatous response in murine schistosomiasis mansoni does not switch to Th1 in IL-4-deficient C57BL/6 mice. J Immunol. 1996;157:4546–4553. [PubMed] [Google Scholar]
- 44.Moran T M, Isobe H, Fernandez-Sesma A, Schulman J L. Interleukin-4 causes delayed virus clearance in influenza virus-infected mice. J Virol. 1996;70:5230–5235. doi: 10.1128/jvi.70.8.5230-5235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morawetz R A, Doherty T M, Giese N A, Hartley J W, Muller W, Kuhn R, Rajewsky K, Coffman R, Morse H C., III Resistance to murine acquired immunodeficiency syndrome (MAIDS) Science. 1994;265:264–266. doi: 10.1126/science.8023146. [DOI] [PubMed] [Google Scholar]
- 46.Moreau-Gachelin F, Tavitian A, Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukemia. Nature (London) 1988;331:277–280. doi: 10.1038/331277a0. [DOI] [PubMed] [Google Scholar]
- 47.Morrison R P, Earl P L, Nishio J, Lodmell D L, Moss B, Chesebro B. Different H-2 subregions influence immunization against retrovirus and immunosuppression. Nature (London) 1987;329:729–732. doi: 10.1038/329729a0. [DOI] [PubMed] [Google Scholar]
- 48.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 49.Munroe D G, Peacock J W, Benchimol S. Inactivation of the cellular p53 gene is a common feature of Friend virus-induced erythroleukemia: relationship of inactivation to dominant transforming alleles. Mol Cell Biol. 1990;10:3307–3313. doi: 10.1128/mcb.10.7.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nansen A, Jensen T, Christensen J P, Andreasen S O, Ropke C, Marker O, Thomsen A R. Compromised virus control and augmented perforin-mediated immunopathology in IFN-gamma-deficient mice infected with lymphocytic choriomeningitis virus. J Immunol. 1999;163:6114–6122. [PubMed] [Google Scholar]
- 51.Odaka T. Inheritance of susceptibility to Friend mouse leukemia virus. IV. Persistence of Friend leukemia virus in C57BL/6 mice. Jpn J Exp Med. 1967;37:71–72. [PubMed] [Google Scholar]
- 52.Okamoto I, Kohno K, Tanimoto T, Ikegami H, Kurimoto M. Development of CD8+ effector T cells is differentially regulated by IL-18 and IL-12. J Immunol. 1999;162:3202–3211. [PubMed] [Google Scholar]
- 53.Oxenius A, Karrer U, Zinkernagel R M, Hengartner H. IL-12 is not required for induction of type 1 cytokine responses in viral infections. J Immunol. 1999;162:965–973. [PubMed] [Google Scholar]
- 54.Parren P W H I, Moore J P, Burton D R, Sattentau Q J. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS. 1999;13(Suppl. A):S137–S162. [PubMed] [Google Scholar]
- 55.Persons D A, Paulson R F, Loyd M R, Herley M T, Bodner S M, Bernstein A, Correll P H, Ney P A. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat Genet. 1999;23:159–165. doi: 10.1038/13787. [DOI] [PubMed] [Google Scholar]
- 56.Peterson K E, Iwashiro M, Hasenkrug K J, Chesebro B. Major histocompatibility complex class I gene controls the generation of gamma interferon-producing CD4+ and CD8+ T cells important for recovery from Friend retrovirus-induced leukemia. J Virol. 2000;74:5363–5367. doi: 10.1128/jvi.74.11.5363-5367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raefsky E L, Platanias L C, Zoumbos N C, Young N S. Studies of interferon as a regulator of hematopoietic cell proliferation. J Immunol. 1985;135:2507–2512. [PubMed] [Google Scholar]
- 58.Ramshaw I A, Ramsay A J, Karupiah G, Rolph M S, Mahalingam S, Ruby J C. Cytokines and immunity to viral infections. Immunol Rev. 1997;159:119–135. doi: 10.1111/j.1600-065x.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 59.Robertson M N, Miyazawa M, Mori S, Caughey B, Evans L H, Hayes S F, Chesebro B. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and western blotting. J Virol Methods. 1991;34:255–271. doi: 10.1016/0166-0934(91)90105-9. [DOI] [PubMed] [Google Scholar]
- 60.Robertson M N, Spangrude G J, Hasenkrug K, Perry L, Nishio J, Wehrly K, Chesebro B. Role and specificity of T-cell subsets in spontaneous recovery from Friend virus-induced leukemia in mice. J Virol. 1992;66:3271–3277. doi: 10.1128/jvi.66.6.3271-3277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romagnani S, Maggi E. Th1 versus Th2 responses in AIDS. Curr Opin Immunol. 1994;6:616–622. doi: 10.1016/0952-7915(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 62.Schijns V E, Haagmans B L, Wierda C M, Kruithof B, Heijnen I A, Alber G, Horzinek M C. Mice lacking IL-12 develop polarized Th1 cells during viral infection. J Immunol. 1998;160:3958–3964. [PubMed] [Google Scholar]
- 63.Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 64.Sharma D P, Ramsay A J, Maguire D J, Rolph M S, Ramshaw I A. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol. 1996;70:7103–7107. doi: 10.1128/jvi.70.10.7103-7107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sitbon M, Nishio J, Wehrly K, Lodmell D, Chesebro B. Use of a focal immunofluorescence assay on live cells for quantitation of retroviruses: distinction of host range classes in virus mixtures and biological cloning of dual-tropic murine leukemia viruses. Virology. 1985;141:110–118. doi: 10.1016/0042-6822(85)90187-4. [DOI] [PubMed] [Google Scholar]
- 66.Stanton G J, Jordan C, Hart A, Heard H, Langford M P, Baron S. Nondetectable levels of interferon gamma is a critical host defense during the first day of herpes simplex virus infection. Microb Pathog. 1987;3:179–183. doi: 10.1016/0882-4010(87)90094-5. [DOI] [PubMed] [Google Scholar]
- 67.Super H J, Brooks D, Hasenkrug K J, Chesebro B. Requirement for CD4+ T cells in the Friend murine retrovirus neutralizing antibody response: evidence for functional T cells in genetic low-recovery mice. J Virol. 1998;72:9400–9403. doi: 10.1128/jvi.72.11.9400-9403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van der Gaag H C, Axelrad A A. Friend virus replication in normal and immunosuppressed C57BL/6 mice. Virology. 1990;177:837–839. doi: 10.1016/0042-6822(90)90561-5. [DOI] [PubMed] [Google Scholar]
- 69.Wang C Q, Udupa K B, Lipschitz D A. Interferon-gamma exerts its negative regulatory effect primarily on the earliest stages of murine erythroid progenitor cell development. J Cell Physiol. 1995;162:134–138. doi: 10.1002/jcp.1041620116. [DOI] [PubMed] [Google Scholar]
- 70.Wendling F, Tambourin P E. Oncogenicity of Friend-virus-infected cells: determination of origin of spleen colonies by the H-2 antigens as genetic markers. Int J Cancer. 1978;22:479–486. doi: 10.1002/ijc.2910220418. [DOI] [PubMed] [Google Scholar]
- 71.Yang Y, Xiang Z, Ertl H C, Wilson J M. Upregulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7561. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoosook C, Steeves R, Lilly F. Fv-2r-mediated resistance of mouse bone-marrow cells to Friend spleen focus-forming virus infection. Int J Cancer. 1980;26:101–106. doi: 10.1002/ijc.2910260116. [DOI] [PubMed] [Google Scholar]
- 73.Yu Z, Manickan E, Rouse B T. Role of interferon-gamma in immunity to herpes simplex virus. J Leukoc Biol. 1996;60:528–532. doi: 10.1002/jlb.60.4.528. [DOI] [PubMed] [Google Scholar]