Abstract
Borna disease virus (BDV) infection triggers an immune-mediated encephalomyelitis and results in a persistent infection. The immune response in the acute phase of the disease is characterized by a cellular response in which CD8+ T cells are responsible for the destruction of virus-infected brain cells. CD4+ T cells function as helper cells and support the production of antiviral antibodies. Antibodies generated in the acute phase of the disease against the nucleoprotein and the phosphoprotein are nonneutralizing. In the chronic phase of the disease, neutralizing antibodies directed against the matrix protein and glycoprotein are synthesized. In the present work, the biological role of the neutralizing-antibody response to BDV was further investigated. By analyzing the blood of rats infected intracerebrally with BDV, a highly neurotropic virus, nucleic acid could be detected between 30 and 50 days after infection. Neutralizing antibodies were found between 60 and 100 days after infection. Furthermore, we produced hybridomas secreting BDV-specific neutralizing monoclonal antibodies. These antibodies, directed against the major glycoprotein (gp94) of BDV, were able to prevent Borna disease if given prophylactically. These data suggest that the late appearance of BDV-specific neutralizing antibodies is due to the presence of BDV in the blood of chronically infected rats. Furthermore, these antibodies have the potential to neutralize the infectious virus when given early, which is an important finding with respect to the development of a vaccine.
Immunological control of infection with noncytopathic viruses such as human immunodeficiency virus, hepatitis B and C viruses (HBV, HCV) in humans, lymphocytic choriomeningitis virus (LCMV) in mice, or Borna disease virus (BDV) in rats is mediated by the cellular immune system (3, 21, 27, 28, 43), whereas neutralizing antibodies appear rather late after infection (1, 23, 25). Nevertheless, at least for LCMV it was shown in a transgenic mouse model that early inducible neutralizing antibodies enhanced virus clearance in the blood and spleen (33). In the immune defense against cytopathic viruses (e.g., poliovirus, rabies virus, vesicular stomatitis virus, and influenza virus) virus-neutralizing antibodies play the dominant role and are usually produced very early after infection (11, 19, 22, 41).
Control of viral infections of the central nervous system (CNS) is limited due to specific properties of this organ. The blood-brain barrier (BBB) is a unique barrier that controls the transition of cells and molecules into the brain. It was shown that only activated T cells are able to cross the intact BBB and that antibodies are excluded from entering the CNS by the BBB (18, 40, 42). In the brain, virus-specific nonneutralizing and neutralizing antibodies can be found, and the latter in particular are important in the control and elimination of viral infection of the CNS (10, 24).
After experimental infection of rats with the highly neurotropic BDV, a nonsegmented single-stranded RNA virus with negative polarity (for a review, see reference 37), the virus spreads intra-axonally and can be detected in the CNS during the acute and chronic phases of the disease (5, 16, 36). BDV replicates preferentially in neurons, astrocytes, and ependymal cells (6, 7, 9); however, evidence of infection in the periphery and in the autonomic nervous system has been presented (5, 6, 30, 36). After infection, lymphocytic infiltrations can be detected in the cortex and hippocampus of infected rats, characterized as CD4+ and CD8+ T cells and macrophages (9). Earlier work clearly showed that virus-specific CD8+ T cells function as effector cells and that the presence of major histocompatibility complex class I-restricted lysis parallels the severe degeneration during the acute phase and precedes cortical brain atrophy in the chronic phase of disease (4, 15, 29, 35). CD4+ T cells function as helper cells and support the synthesis of BDV-specific antibodies (13, 26, 30, 35). After experimental BDV infection of rats, nonneutralizing antibodies directed against the nucleoprotein (p40) and against the phosphoprotein (p24) can be detected in the sera after 2 weeks. In the chronic disease phase, neutralizing antibodies directed against the two glycosylated proteins (glycoprotein gp94 and matrix protein gp18) are detectable (14, 36, 38). In the brains of BDV-infected rats, antibodies and plasma cells can be found around day 30 after experimental infection (9, 14). After immunization of mice with purified nucleoprotein or phosphoprotein, hybridomas could be obtained that secrete BDV-specific monoclonal antibodies (39). Furthermore, monoclonal antibodies directed against the unglycosylated form of the matrix protein (p14) are available and have neutralizing activity (20). Nevertheless, no monoclonal antibodies directed against the major glycoprotein (gp94) have been generated so far to prove their in vivo biological activity. In the work presented here, monoclonal antibodies directed against the glycoprotein gp94 were established that were able to neutralize BDV.
MATERIALS AND METHODS
Experimental animals, virus, and infection.
Male and female Lewis rats were obtained from the animal-breeding facilities at the Bundesforschungsanstalt für Viruskrankheiten, Tübingen. Rats were infected intracerebrally (i.c.) in the left brain hemisphere with 0.05 ml of BDV (Giessen strain He/80 [17]) corresponding to 5 × 103 focus-forming units (FFU). The vaccinia virus (VV)-BDV recombinants (VV-p40, VV-gp18, and VV-gp94) were obtained from J. C. de la Torre, The Scripps Research Institute, La Jolla, Calif.
Clinical evaluation.
Experimental animals were examined daily. The disease symptoms were scored on an arbitrary scale from 0 to 3 based on the general state of health of the rats (0.5, ruffled fur and hunched back back) and the appearance of neurologic symptoms (1, slight incoordination and fearfulness; 2, distinct ataxia and slight paresis; 3, marked paresis and paralysis) by two independent investigators.
Detection of BDV-specific, nonneutralizing antibodies.
Antisera were tested in a solid-phase enzyme-linked immunosorbent assay using a 1:1,000 dilution of a brain homogenate from BDV-infected rats as coating antigens and by Western blot analysis with a 10% brain homogenate from BDV-infected rats. The tests were performed as described earlier (26).
Detection of BDV-neutralizing antibodies.
Virus neutralization was performed in a focus reduction assay. BDV (50 FFU) was incubated with serial twofold dilutions of heat-inactivated serum (at 56°C for 30 min) or hybridoma supernatant (at 37°C for 90 min). Then CRL1405 cells were added to the reaction mixture. After 7 days of incubation, the cells were fixed with 4% formaldehyde–phosphate-buffered saline (PBS) and permeabilized with 1% Triton X-100–PBS. Viral antigen was demonstrated in an immunohistochemical reaction using mouse anti-BDV-specific monoclonal antibodies. Nonspecific binding of immunological reagents was blocked by incubation of plates with 10% fetal calf serum–PBS. The reaction of monoclonal antibodies with cells was detected by a secondary anti-species biotin-labeled antibody (Dianova, Hamburg, Germany) and by streptavidin-peroxidase conjugate (Dianova). The reaction was visualized with ortho-phenylendiamine and H2O2 (Sigma, Taufkirchen, Germany). The dilution required to reduce the 50 FFU by 50% was defined as the neutralization titer.
Generation of BDV-specific monoclonal antibodies.
Sera of chronically infected Lewis rats were tested for the presence of BDV-neutralizing antibodies. Thereafter, seropositive rats were boosted with the VV-BDV recombinant VV-gp94 4 or 7 days before fusion of spleen cells with the mouse myeloma cell line P3X63Ag8. Prewarmed polyethylene glycol 4000 (Merck, Mannheim, Germany) was added for 1 min to ensure equal numbers of spleen cells and mouse myeloma cells. The cells were rested for 1 min at room temperature and the polyethylene glycol was carefully diluted with 10 ml of prewarmed Iscove modified Dulbecco medium containing glutamine and gentamicin. Thereafter, the cells were again rested for 10 min at room temperature, centrifuged for 10 min at 200 × g, and resuspended in selection medium (Iscove modified Dulbecco medium plus 10% fetal calf serum containing 10−4 M hypoxanthine [Sigma], 4 × 10−7 M aminopterin [Sigma], and 1.6 × 10−5 M thymidine [Sigma]). Then 2 × 104 fused cells per well of 96-well microtiter plates were cultivated together with macrophages that were isolated 1 day prior to fusion.
All hybridoma supernatants were screened by Western blot analysis, neutralization assay, and flow cytometry. Neutralizing monoclonal antibodies were selected according to their neutralizing ability and their specificity for the BDV glycoprotein gp94.
Infectivity assay.
The virus infectivity of organ homogenates of BDV-infected rats was determined on CRL1405 cells. The cells were cultured for 7 days in the presence of organ homogenates from infected rats in flat-bottom 96-well microtiter plates. Thereafter, cells were fixed with 4% paraformaldehyde–PBS, and the immunohistochemical staining of BDV-specific antibodies was performed as described for the BDV neutralization assay.
Antigen detection.
Tissue homogenates were used as antigens in Western blot analysis. The homogenates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% polyacrylamide). Proteins were transferred from gels onto a protein binding membrane (Immobilon-P membrane; Millipore). Nonspecific binding of immunological reagents was blocked by incubation of the membrane with blocking solution (PBS–0.05% Tween 20 containing 0.2% bovine serum albumin and 10% fetal calf serum). The reaction of monoclonal or polyclonal antibodies specific for viral proteins with membrane-bound proteins was detected by a secondary anti-species biotin-labeled antibody (Dianova) and by streptavidin-peroxidase conjugate (Dianova). The reaction was visualized with chloronaphthol and H2O2.
In situ hybridization.
Digoxigenin-labeled RNA probes complementary to BDV nucleoprotein p40, phosphoprotein p24, or matrix protein gp18 mRNAs were used. Brains from experimental animals were frozen in isopentane at −150°C. Sections (5 μm) were mounted on slides and fixed in 4% formaldehyde–PBS. After treatment with 0.1 N HCl and acetic acid, hybridization was carried out overnight at 65°C with 20 ng of probe per slide. The slides were washed with 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) followed by 2× SSC at hybridization temperature. The slides were incubated with an alkaline phosphatase-labeled anti-digoxigenin antibody and then placed overnight in a 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium (BCIP-NBT) solution.
Flow cytometry.
Persistently BDV-infected cells were either treated with ortho-Permeafix (Ortho, Neckargemünd, Germany) for 40 min at room temperature to permeabilize the cells (intracellular staining) or used untreated (cell surface staining). Cells (5 × 105) were incubated with 50 μl of hybridoma supernatant for 25 min at 4°C. The cells were washed once with fluorescence-activated cell sorter buffer (PBS, 2% fetal calf serum, 10 mM EDTA). Bound antibodies were detected by a biotin-labeled anti-species antibody (Sigma) (25 min at 4°C) and a streptavidin-fluorescein isothiocyanate (FITC) conjugate (Dianova) (25 min at 4°C) and analyzed in a flow cytometer (FACscan; Becton Dickinson).
To determine the specificity of the neutralizing monoclonal antibodies, rat astrocytes (F10 cells) were infected with the VV-BDV recombinants VV-p40, VV-gp18, and VV-gp94 (multiplicity of infection, 5 to 10) (6 h at 37°C). The cells were then fixed and permeabilized with ortho-Permeafix, and the intracellularly expressed BDV-specific proteins were detected by the neutralizing antibodies as described above.
Immunohistochemistry.
BDV was stained on cryostat sections for the presence of BDV-specific antigen. Brains were frozen in isopentane at −150°C. All antibodies were diluted 1:500 or 1:1,000 in PBS. Monoclonal antibodies were reacted with an avidin-biotin complex with peroxidase as the marker enzyme and 3,3-diaminobenzidine as the substrate. To avoid reactions of anti-mouse secondary antibody with rat immunoglobulins, a commercially rat-absorbed horse anti-mouse antibody was used. All avidin-biotin complex reagents were purchased from Vector (Burlingame, Calif.).
Antibody production.
Hybridomas, secreting monoclonal antibodies, were cultured using the CELLine system (Integra Biosciences, Fernwald, Germany). This procedure gave rise to antibody concentrations of 1 mg/ml of supernatant, which was suitable for the experiments. Furthermore, the antibodies were purified on a protein A-Sepharose column (Pharmacia, Freiburg, Germany).
RNA isolation and reverse transcription-PCR.
A 1-ml volume of EDTA-blood was mixed with 1 ml of Trizol (Life Technology, Karlsruhe, Germany) and was further processed as recommended by the manufacturer.
(i) Reverse transcription.
RNA (1 μg) was transcribed into cDNA at 42°C for 1 h using 50 U of Expand reverse transcriptase (Roche, Mannheim, Germany), 100 mM dithiothreitol, 20 U of RNase Inhibitor (Pharmacia Biotech Products), 10 mM deoxynucleoside triphosphate mix (Pharmacia), and 0.5 μg of BDV-p24 specific primer (BV1865R).
(ii) Conditions for PCR.
BDV cDNA was detected by first-round and nested PCR using primers located in the p24 gene of BDV. First-round amplification was performed using hot-start PCR in a total volume of 50 μl containing 1 to 5 μl of cDNA, 50 ng of each primer, 20 mM deoxynucleoside triphosphate mix (Pharmacia), 5 μl of 10× PCR buffer (Roche), and 0.5 μl of Taq polymerase (5 U/μl) (Roche). Amplification was carried out for 40 cycles (94°C, 80 s; 58°C, 90 s; 72°C, 90 s) in a Trio thermocycler (Biometra, Göttingen, Germany). Specific primers for p24, BV1387F (5′-TGACCAACCAGTAGACCA-3′) and BV1865R (5′-GTCCCATTCATCCGTTGTC-3′), were used. Nested PCR was performed identically to first-round PCR, using 1 μl of 1:100-diluted first-round PCR product as template and the p24-specific primers BV1443F (5′-TCAGACCCAGACCAGCGAA-3′) and BV1834R (5′-AGCTGGGGATAAATGCGCG-3′). Amplification products were analysed by electrophoresis in a 1% agarose gel containing 0.3 μg of ethidium bromide per ml.
RESULTS
Presence of nucleic acid and BDV-specific neutralizing antibodies in the blood of chronically infected rats.
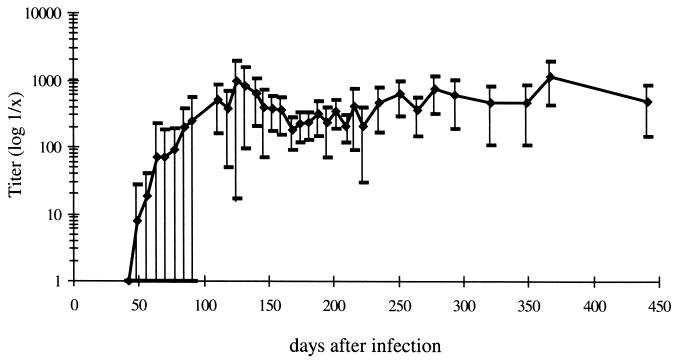
Previous reports have shown that BDV-specific neutralizing antibodies appear very late after infection and are involved in controlling the tropism of the virus (14, 36). Furthermore, it has been shown that BDV-specific nucleic acid can be detected in the blood of chronically infected rats (34). However, no information on the time point of the first appearance of both BDV-specific neutralizing antibodies and nucleic acid in the blood is available. Therefore, rats were infected i.c. with BDV and every 10 days the blood and sera of these animals were tested for the appearance of BDV-specific nucleic acid and BDV-specific neutralizing antibodies. As shown in Table 1, as early as 30 days postinfection (p.i.), nucleic acid, encoding the BDV phosphoprotein, was detected in the blood. Testing revealed that all animals had BDV-specific nucleic acid in the blood by day 50 p.i. In this experiment, the first BDV-specific neutralizing antibodies were detectable in the sera by day 70 p.i. and all rats had synthesized BDV-specific neutralizing antibodies by day 110. In another experiment, where again BDV-specific nucleic acid was found between days 30 and 50 p.i., the first BDV-specific neutralizing antibodies were detected as early as day 50 p.i. in some rats. Neutralizing-antibody titers varied in individual animals, but all infected rats had BDV-specific neutralizing-antibody titers for as long as 450 days pi. (Fig. 1).
TABLE 1.
Appearance of BDV-specific nucleic acid and BDV-specific neutralizing antibodies in the blood of chronically BDV-infected rats
| Day p.i. | No. of rats with BDV-specific nucleic acid/total no. | No. of rats with BDV-specific neutralizing antibodies/total no. |
|---|---|---|
| 20 | 0/7 | 0/7 |
| 30 | 1/7 | 0/7 |
| 40 | 5/7 | 0/7 |
| 50 | 7/7 | 0/7 |
| 60 | 7/7 | 1/7 |
| 70 | 7/7 | 1/7 |
| 80 | 7/7 | 3/7 |
| 90 | 7/7 | 3/7 |
| 100 | 7/7 | 6/7 |
| 110 | 7/7 | 7/7 |
FIG. 1.
Mean value (⧫) of the BDV neutralization titer. Values represent the highest and lowest titers in a total of 15 rats at indicated time points.
Generation of BDV-specific neutralizing monoclonal antibodies.
To further investigate the role of neutralizing antibodies after BDV-infection, plasma-myeloma cell fusions were carried out to establish hybridoma cultures secreting BDV-specific neutralizing monoclonal antibodies. Fusion experiments with lymphocytes isolated from the spleens, blood, or brains of BDV-infected rats and used as a source of plasma cells did not result in hybridomas secreting neutralizing antibodies, regardless of whether acute or chronically infected animals were used (data not shown). Since the frequency of activated plasma cells might be very low in the chronic phase of BD, infected rats were boosted with VV-BDV recombinant viruses carrying either the matrix (VV-gp18) or the glycoprotein (VV-gp94) of BDV (Table 2). Spleen cells were used 4 days after the recombinant VV booster injection and fused with mouse myeloma cells. Since neutralizing antibodies recognize conformational epitopes, supernatants from hybridoma cultures were tested exclusively in BDV neutralization assays. As shown in Table 2, no stable hybridomas secreting BDV-specific antibodies were obtained after fusion of spleen cells of rats that were boosted with VV-gp18. In contrast, after VV-gp94 booster infection, four hybridoma cultures producing BDV-specific neutralizing antibodies were established (Table 2). After 3 weeks in culture, one hybridoma lost the ability to secrete antibodies. Therefore, after six rounds of subcloning, only three hybridomas synthesizing BDV-specific monoclonal neutralizing antibodies could be established (H12, B1, and E6) (Table 3). Using the CELLine system, we were able to produce antibodies in concentrations of 1 mg/ml in cell culture supernatant. The neutralizing activity was measured in a neutralization assay and standardized to an antibody concentration of 1 μg/ml; furthermore, immunoglobulin (Ig) subclasses were determined (Table 3).
TABLE 2.
Hybridomas obtained in fusion experiments from chronically BDV-infected rats boosted with VV recombinants containing BDV glycoproteins
| Day p.i. | Booster | No. of fused spleen cells | Total no. of hybridomas | No. of hybridomas secreting BDV-specific neutralizing antibodies |
|---|---|---|---|---|
| 132 | VV-gp18 | 6.0 × 107 | 69 | 0 |
| 218 | VV-gp18 | 3.0 × 107 | 161 | 0 |
| 238 | VV-gp18 | 3.0 × 107 | 86 | 0 |
| 88 | VV-gp94 | 1.4 × 108 | 24 | 1 |
| 132 | VV-gp94 | 6.5 × 107 | 111 | 1 |
| 218 | VV-gp94 | 3.0 × 107 | 192 | 0 |
| 238 | VV-gp94 | 3.0 × 107 | 100 | 2 |
TABLE 3.
Neutralizing activity of BDV-specific monoclonal antibodies
| Hybridoma | Ig subclass | Neutralization titera |
|---|---|---|
| H12 | IgG2c | 640 |
| B1 | IgG2b | 80 |
| E6 | IgG2b | 80 |
Values are standardized to an antibody concentration of 1 μg/ml.
Characterization of BDV-neutralizing monoclonal antibodies.
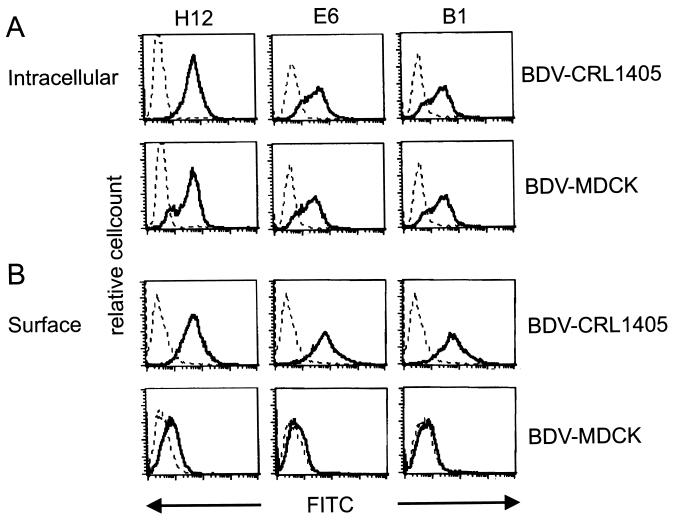
To characterize the specificity of the antibodies secreted from hybridomas, intracellular staining was performed in persistently infected cell lines (BDV-MDCK and BDV-CRL1405) using flow cytometric analysis. As shown in Fig. 2A, antibodies H12, E6, and B1 reacted with BDV-infected cells, while no staining was found when the antibodies were incubated with noninfected cell lines (data not shown). Staining with antibody H12 was more intense than that with E6 and B1. Furthermore, surface staining of BDV-infected cells was tested, without prior permeabilization and fixation. A positive reaction was found on BDV-CRL1405 cells, whereas no reaction could be observed on BDV-MDCK cells (Fig. 2B). Additional experiments revealed that persistently infected Lewis astrocytes (BDV-F10) and skin fibroblasts (BDV-LEW) showed staining patterns comparable to the reaction on BDV-CRL1405 cells (data not shown). Antibody H12 again showed more intense straining than did E6 and B1.
FIG. 2.
Intracellular (A) and surface (B) staining of either BDV-CRL or BDV-MDCK cells incubated with 1 μg of different monoclonal antibodies directed against BDV gp94 (H12, E6, and B1) (solid line) or BDV-CRL and BDV-MDCK cells incubated with secondary FITC-labeled goat anti-rat antibody alone (dotted line).
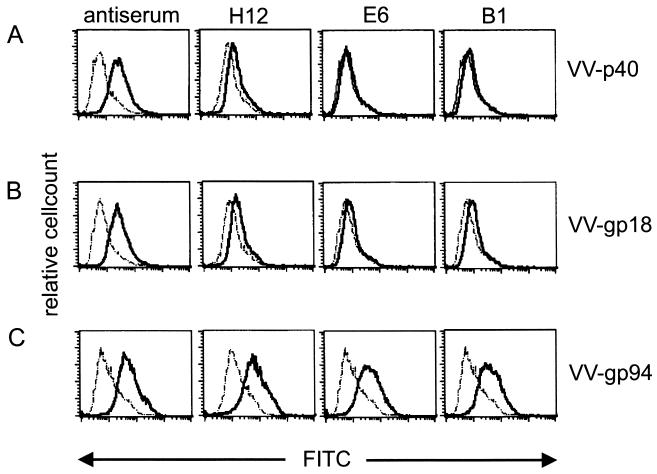
These data reveal the BDV specificity of the neutralizing monoclonal antibodies. To verify the specificity for the glycoprotein, CRL1405 cells were infected with VV-p40 (carrying the nucleoprotein of BDV), VV-gp18, or VV-gp94. As shown in Fig. 3, a BDV-specific hyperimmune serum with neutralizing activity used as a control reacted with the surface of all VV-BDV-infected cells. No staining could be observed when antibodies H12, E6, or B1 were used on VV-p40- or VV-gp18-infected cells. In contrast, using VV-gp94-infected cells, all antibodies showed a clear shift to a higher fluorescence intensity. The fact that the neutralizing monoclonal antibodies did not stain VV-p40- or VV-gp18-infected cells also demonstrates that the staining on VV-gp94-infected cells is due to the recognition of the BDV glycoprotein G and not due to VV proteins (Fig. 3).
FIG. 3.
Intracellular staining of CRL cells infected with different VV-BDV recombinants and stained with either a BDV-specific hyperimmune serum or the different monoclonal antibodies H12, E6, and B1 (solid line) or with secondary FITC-labeled goat anti-rat antibody alone (dotted lines).
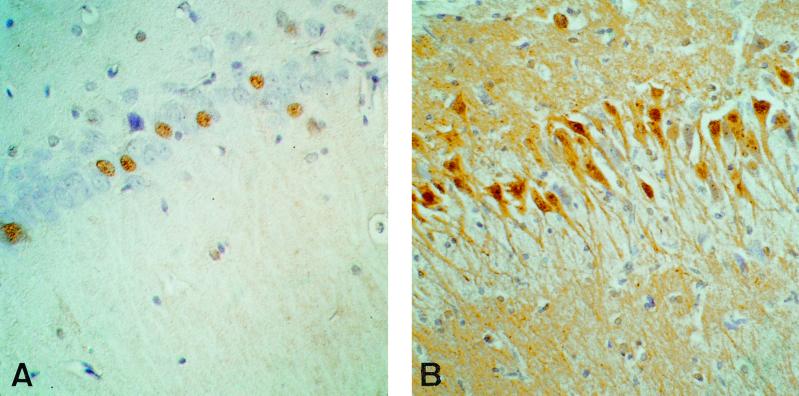
In addition, we tested the ability of H12 to recognize BDV antigen in immunohistochemistry. Therefore, sections of the brains of BDV-infected rats 28 days after infection were stained. As shown in Fig. 4, antibody H12 is able to detect BDV-glycoprotein G in rat brains (Fig. 4A). However, compared to the commonly used monoclonal antibody 38/17C1 (39) directed against the nucleoprotein p40, only relatively few cells were stained (Fig. 4B).
FIG. 4.
Immunohistochemistry from the hippocampal area of a BDV-infected rat using H12 directed against the glycoprotein gp94 (A) and 38/17C1 directed against the nucleoprotein p40 (B). Magnification, ×100.
Effect of BDV-specific monoclonal neutralizing antibodies in BDV-infected rats.
After having established monoclonal antibodies with BDV glycoprotein specificity and neutralizing capacity in vitro, it was of great biological importance to test the function of these antibodies in vivo. Since antibody H12 had the highest neutralizing activity in vitro, this antibody was chosen for most in vivo experiments. In addition, E6 and B1 were used in some experiments and essentially gave the same results as those obtained with H12 (data not shown). To determine whether monoclonal neutralizing antibody H12 can protect against i.c. BDV infection, 1 mg of antibody was given 3 days and 1 day before and once weekly after BDV infection. The intravenously transfused antibodies were detectable in the blood for at least 1 week after application by using neutralization assays. Compared to control animals that received no treatment, antibody-treated animals developed clinical disease symptoms, BDV-specific antibodies, and encephalitis at comparable time points and with comparable intensity to those for control animals (data not shown). Since antibodies are not able to pass an intact BBB, this result was plausible and a model infection needed to be used that would allow the antibody to neutralize the virus before it reaches the brain. Therefore, we used the intrafootpad inoculation of BDV as described by Carbone et al. (5).
In a first experiment, 6 × 104 FFU was inoculated into the left hind foot of Lewis rats and the onset of the first clinical disease symptoms was observed as early as 28 to 35 days later. This rather early onset of disease makes the model of footpad injection more convenient for testing the protective effects of the transfused neutralizing monoclonal antibody. First, 1 mg of antibody H12 was injected 1 h and once a week after BDV inoculation. Control animals developed the first clinical disease symptoms on day 23, and all rats had developed severe BD, including pareses and paralyses, by day 28. In antibody-treated rats, the first clinical symptoms such as ataxia could be observed by day 28. At this time point, all control and antibody-treated rats were sacrificed and the brains and spinal cords were tested for the presence of viral antigen, nucleic acid, and infectious virus. As shown in Table 4, viral antigen and infectious virus could be detected in all control animals in the forebrain, the cerebellum, and the thoracal and lumbar part of the spinal cord. Furthermore, viral nucleic acid was found in the forebrain and the cerebellum and was not tested in the spinal cord. In contrast, no viral antigen or infectious virus was found in rats treated with BDV-neutralizing antibody H12 but in situ hybridization revealed infected cells in the cerebellum and, to a lesser extent, in the forebrain (Table 4). In control animals the first BDV-specific nonneutralizing antibodies were found by day 28, while in antibody-treated rats only monoclonal antibody H12 was found as tested by neutralization assay and isotype-specific enzyme-linked immunosorbent assay (data not shown). These experiments show that antibody treatment starting 1 h after BDV infection is not sufficient to prevent disease but only delays virus replication and onset of neurological disease for a few days. These experiments were repeated twice with a total of nine animals and gave comparable results (data not shown).
TABLE 4.
Presence of virus nucleic acid in BDV-infected rats treated with anti-gp94 neutralizing monoclonal antibody after infection
| Viral component | Area found | Viral component presence in:
|
|
|---|---|---|---|
| Control rats | Antibody-treated ratsa | ||
| Antigenb | Forebrain | +, +, + | |
| Cerebellum | +, +, + | −, −, − | |
| Thoracal spinal cord | +, +, + | −, −, − | |
| Lumbar spinal cord | +, +, + | −, −, − | |
| Nucleic acidc | Forebrain | +, +, + | +, +, + |
| Cerebellum | +, +, + | +, +, + | |
| Infectious virus (log10 virus titer) | Forebrain | 4.3, 3.4, 4.8 | <1.8, <1.8, <1.8 |
| Cerebellum | 3.7, 3.4, 3.2 | <1.8, <1.8, <1.8 | |
| Thoracal spinal cord | 5.0, 4.3, 4.9 | <1.8, <1.8, <1.8 | |
| Lumbar spinal cord | 4.4, 4.6, 4.5 | <1.8, <1.8, <1.8 | |
Treatment was given 1 h and every 7 days after BDV infection. Rats were killed 28 days after infection.
Detected by Western blotting.
Detected by in situ hybridization.
To test whether prophylactic treatment could prevent disease, in the next set of experiments antibody H12 was injected 1 h before and every 7th day after BDV infection. Again, three animals were used as controls and three were treated with 1 mg of H12. The first disease symptoms could be observed in control rats by day 25. By day 28, all three rats showed severe BD. No disease symptoms could be seen in antibody-treated rats. Again, the animals were killed by day 28 and the brains and spinal cords were tested for the presence of virus as described above. In the control group all animals had viral antigen and infectious virus in the brains and spinal cords, whereas in treated animals no antigen or infectious virus could be detected in the brains or spinal cords. Nevertheless, no BDV-specific nucleic acid was detectable in the forebrain, and BDV-specific nucleic acid was found in the cerebellum, although in one animal only a few infected cells could be detected (Table 5).
TABLE 5.
Partial absence of virus nucleic acid in the nervous tissue of BDV-infected rats treated with anti-gp94 neutralizing monoclonal antibody after infection
| Viral component | Area found | Viral component presence in:
|
|
|---|---|---|---|
| Control rats | Antibody-treated ratsa | ||
| Antigenb | Forebrain | +, +, + | |
| Cerebellum | +, +, + | −, −, − | |
| Thoracal spinal cord | +, +, + | −, −, − | |
| Lumbar spinal cord | +, +, + | −, −, − | |
| Nucleic acidc | Forebrain | +, +, + | −, −, − |
| Cerebellum | +, +, + | +,d +, + | |
| Infectious virus (log10 virus titer) | Forebrain | <1.8, 4.3, 4.5 | <1.8, <1.8, <1.8 |
| Cerebellum | <1.8, 3.7, 4.2 | <1.8, <1.8, <1.8 | |
| Thoracal spinal cord | 3.6, 5.0, 4.3 | <1.8, <1.8, <1.8 | |
| Lumbar spinal cord | 5.0, 4.3, 4.2 | <1.8, <1.8, <1.8 | |
Treatment was given 1 h prior to and once a week after BDV infection. Rats were killed 28 days after infection.
Detected by Western blotting.
Detected by in situ hybridization.
Only a few infected cells.
Since antibody treatment around the time of infection only delayed the onset of BD but did not prevent disease, the neutralizing monoclonal antibody H12 was injected 3 days and 1 day before and, in addition, every 7th day after BDV infection. Again, control animals first developed clinical symptoms on days 18 to 21 and by day 24 all animals had severe BD. In contrast, antibody-treated animals did not show any disease symptoms until the end of the observation period on day 35. Control animals developed BDV-specific nonneutralizing antibodies directed against the nucleoprotein and phosphoprotein as detected by Western blot analysis (data not shown), whereas in the sera of treated rats no anti-nucleoprotein and -phosphoprotein antibodies could be detected. On day 35, antibody-treated animals were sacrificed and the brains and spinal cords were tested for the presence of virus. As shown in Table 6, BDV was present in the brains and spinal cords of control animals whereas no BDV-specific antigen, infectious virus, or BDV-specific nucleic acid was detectable in antibody-treated rats.
TABLE 6.
Absence of virus in nervous tissue in BDV-infected rats treated with anti-gp94 neutralizing monoclonal antibody prior to infection
| Viral component | Area found | Viral component presence in:
|
|
|---|---|---|---|
| Control rats | Antibody-treated ratsa | ||
| Antigenb | Forebrain | +, +, + | |
| Cerebellum | +, +, + | −, −, − | |
| Thoracal spinal cord | +, +, + | −, −, − | |
| Lumbar spinal cord | +, +, + | −, −, − | |
| Nucleic acidc | Forebrain | +, +, + | −, −, − |
| Cerebellum | +, +, + | −, −, − | |
| Infectious virus (log10 virus titer) | Forebrain | 6.8, 4.6, 7.5 | <1.8, <1.8, <1.8 |
| Cerebellum | 3.9, 3.6, 4.3 | <1.8, <1.8, <1.8 | |
| Thoracal spinal cord | 5.3, 4.9, 4.9 | <1.8, <1.8, <1.8 | |
| Lumbar spinal cord | 3.3, 3.9, 4.3 | <1.8, <1.8, <1.8 | |
Treatment was given 3 days and 1 day prior to and every 7 days after BDV infection until day 28 p.i. Rats were killed on day 35 p.i.
Detected by Western blotting.
Detected by in situ hybridization.
Thus, if BDV-specific neutralizing monoclonal antibodies directed against the glycoprotein gp94 were given prophylactically before intrafootpad infection with BDV, they were able to prevent BDV-induced encephalitis in rats.
DISCUSSION
In the present communication we show that BDV-specific neutralizing antibodies can be detected in the blood after BDV-specific nucleic acid is found. Furthermore, we show that BDV-specific neutralizing antibodies directed against the glycoprotein (gp94) are able to control virus infection in vivo and prevent disease.
In rats, nonneutralizing antibodies can be detected around 2 weeks after BDV infection whereas neutralizing antibodies appear only at late times after infection (e.g., 50 to 70 days after infection). As shown by Sierra-Honigmann et al., BDV-specific nucleic acid can be found outside the CNS in organs and in blood in chronically infected rats (34). Our data support this earlier finding but in addition show a correlation between the appearance of virus and BDV-specific neutralizing antibodies in the blood of BDV-infected rats. Therefore, one might speculate that the gp94 distribution in blood cells may be more accessible to immune recognition than that in neural cells. Nevertheless, this speculation will be difficult to prove, since the number of infected cells in the blood is limited. In addition, we cannot exclude that priming of gp94-specific B cells occurs in cervical lymph nodes following replication in the brain. Neutralizing antibodies have been detected after infection with noncytopathic viruses, although they usually appear late after infection (1, 2, 13, 23, 25, 32, 36). Recently, it was demonstrated that late after LCMV infection of mice, at a point where neutralizing antibodies could be found, LCMV was detected in very small numbers (8).
After immunization of chronically BDV-infected rats with VV recombinants expressing the glycoprotein of BDV, a strong BDV-specific neutralizing-antibody response was detected. Fusion of the spleen cells from these rats with myeloma cells resulted in three stable hybridoma cultures secreting BDV-neutralizing monoclonal antibodies detected by the BDV neutralization assay. The fact that the number of hybridomas secreting neutralizing antibodies was very small might indicate that the frequency of plasma cells capable of producing neutralizing antibody activity in vivo is rather limited. Since we could also demonstrate a direct correlation between the presence of BDV-nucleic acid and the succeeding neutralizing antibody response, this finding might be interpreted in the same way. In our hands, only nested reverse transcription-PCR of blood from chronically infected rats was successful in detecting footprints of virus in the blood, whereas classical virus titer determination is far too insensitive. If the amount of glycoprotein associated with the virus particles is the limiting factor for the induction of a neutralizing-antibody response, it is conceivable that the frequency of B cells secreting glycoprotein-specific antibodies is low. Since it is generally accepted that BDV does not significantly replicate outside the nervous system in immunocompetent hosts, the presence of a few virus particles in the blood is the only source of viral glycoprotein in the periphery (34, 36).
By using flow cytometry we were able to detect BDV glycoprotein intracellularly in cells expressing only gp94 through recombinant VV with the three monoclonal antibodies H12, E6, and B1, and we could demonstrate that these antibodies are able to recognize gp94 in BDV-infected cells. In addition, we tested H12 by immunohistochemistry and found that this antibody detects infected cells in the hippocampus of BDV-infected rats. Interestingly, only very few cells were stained even in the hippocampus, where the largest numbers of glycoprotein gp94-positive cells had been detected by use of a hyperimmune serum (31). Interestingly, in differentiated, resting neurons, we found staining in the nucleus. This finding again provides evidence that the BDV glycoprotein is expressed only at low levels in infected brain tissue, which also might explain the relatively late appearance of anti-gp94 neutralizing antibodies and thereby their limited role during infection.
Furthermore, it was of interest to determine the functional role of BDV-specific neutralizing monoclonal antibodies in vivo. Experimental BDV infection is carried out either i.c. or intranasally (i.n.). The i.c. route turned out not to be suitable to investigate the role of neutralizing antibodies since antibodies cannot cross an intact BBB (40). The same is true for the i.n. route, where the virus has a direct neural route from the nasal mucosa to the brain. Therefore, it was not unexpected that antibody application to i.c.- or i.n.-infected rats had no effect. Consequently, we had to chose a route of infection that would allow the neutralization of infectious virus before it enters the CNS. Therefore, we used intrafootpad inoculation of BDV, originally described by Carbone et al. (5), while the antibody was given intravenously. If H12 was given 1 h and every 7 days after BDV infection, the presence of virus in the brain and the onset of disease was significantly delayed but could not be prevented. If the antibody treatment started 1 h before infection and was repeated every 7 days the difference between antibody-treated and untreated rats was even more pronounced. Whereas untreated rats developed disease between days 25 and 28, antibody-treated animals did not develop disease until the end of the observation period on day 28. In untreated animals BDV was detected in the brains and spinal cords, whereas in antibody-treated rats no infectious virus or antigen was found in the CNS. Nevertheless, by in situ hybridization we were able to find BDV-infected cells in the cerebellum. Therefore, we hypothesize that these animals would have developed disease if they had not been killed. These findings can be interpreted in two ways. First, the systemic application of the neutralizing monoclonal antibody only caused partial neutralization. Therefore, virus particles escaping neutralization were still capable of infecting the local nerves, possibly replicating in Schwann cells (7), and getting access to the brain. Second, the virus gains access to the nerves before infection is successfully blocked by transfused antibodies. In this case, intra-axonal virus transport, which has been demonstrated in BDV infection (5), would be hampered by neutralizing antibodies, resulting in a delayed appearance in the brain. However, so far viral particles have never been demonstrated in peripheral nerves. Since BDV is thought to spread along nerve fibers from neuron to neuron by unknown means (5, 12) and since virus replicates in Schwann cells (7), it cannot be formally excluded that virus particles might be neutralized by transfused antibodies either before or after they enter the brain.
However, the use of neutralizing antibodies against BDV for postexposure treatment appears not to be successful, unlike the case for rabies virus infection (10). In contrast to the previous treatment, experiments with antibodies given 3 days and 1 day prior to infection and every 7 days until day 28 after treatment resulted neither in disease nor in detectable BDV in the brains or spinal cords of the animals. The fact that only prophylactic treatment with the neutralizing monoclonal antibody resulted in the absence of virus in the spinal cords and in the brains of the animals indicates that the virus was neutralized at the site of inoculation and that the initial infection was blocked. This interpretation is supported by the lack of viral antigen in the sciatic nerve. Therefore, it appears unlikely that the virus is cleared from infected tissue as it has been shown for other viral infections of the nervous system (10, 24). Furthermore, the presented data also do not provide evidence for a limitation of viral spread within the nervous system by the neutralizing antibody used in this study. Although we did not look for viral footprints at early time points after infection, the finding in the sciatic nerve and the efficacy of neutralizing antibodies solely in prophylaxis argue against an initial infection of the peripheral nervous system and a subsequent clearance of the virus.
Together, these results show that antibodies can neutralize BDV in vivo and therefore can prevent virus spread and consequently BD. Nevertheless, these experiments also show that the time point at which the antibodies were given and the viral route are critical for the outcome.
From these results, one might hypothesize that during BDV infection the virus leaks out of the CNS and can be detected in the blood. Here, the complete viral particle is accessible to the immune system, and consequently neutralizing antibodies against the major glycoprotein, gp94, of the virus can be produced to control infection. In contrast, if the virus is tissue associated, in particular in the CNS, where it replicates efficiently, most abundantly synthesized viral proteins are presented to the immune system, resulting in a strong CD8+ and CD4+ T-cell response and in the production of nonneutralizing antibodies directed predominantly against the nucleoprotein and the phosphoprotein of BDV (14, 26, 30).
An implication of our findings is that this above-described mechanism of control of virus infection in persistent BDV infection might be similar in other infections with noncytopathic viruses (e.g., human immunodeficiency virus or HBV) and also could explain the late appearance of neutralizing antibodies in these infections. Furthermore, our study could lead to the use of gp94 as a candidate vaccine immunogen in BDV infection.
ACKNOWLEDGMENTS
We thank Arvind Batra for help with flow cytometric analysis.
The work was supported in part by the Deutsche Forschungsgemeinschaft grant Sti 71/2-2 (to L.S. and O.P.), grant Pl 256/1-1 (to O.P. and L.S.), and grant Sti 71/3-1 (to L.S.). E.F. is a recipient of a grant from the Schweizer Nationalfonds (SNF) (83EU-048814).
REFERENCES
- 1.Alberti A, Cavalletto D, Pontisso P, Chemello L, Tagariello G, Belussi F. Antibody response to pre-S2 and hepatitis B virus induced liver damage. Lancet. 1988;i:1421–1424. doi: 10.1016/s0140-6736(88)92237-4. [DOI] [PubMed] [Google Scholar]
- 2.Battegay M, Moskophidis D, Waldner H, Brundler M A, Fung-Leung W P, Mak T W, Hengartner H, Zinkernagel R M. Impairment and delay of neutralizing antiviral antibody responses by virus-specific cytotoxic T cells. J Immunol. 1993;151:5408–5415. [PubMed] [Google Scholar]
- 3.Bilzer T, Stitz L. Immune-mediated brain atrophy. CD8+ T cells contribute to tissue destruction during borna disease. J Immunol. 1994;153:818–823. [PubMed] [Google Scholar]
- 4.Bilzer T, Stitz L. Immunopathogenesis of virus diseases affecting the central nervous system. Crit Rev Immunol. 1996;16:145–222. doi: 10.1615/critrevimmunol.v16.i2.20. [DOI] [PubMed] [Google Scholar]
- 5.Carbone K M, Duchala C S, Griffin J W, Kincaid A L, Narayan O. Pathogenesis of Borna disease in rats: evidence that intra-axonal spread is the major route for virus dissemination and the determinant for disease incubation. J Virol. 1987;61:3431–3440. doi: 10.1128/jvi.61.11.3431-3440.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone K M, Moench T R, Lipkin W I. Borna disease virus replicates in astrocytes, Schwann cells and ependymal cells in persistently infected rats: location of viral genomic and messenger RNAs by in situ hybridization. J Neuropathol Exp Neurol. 1991;50:205–214. doi: 10.1097/00005072-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Carbone K M, Trapp B D, Griffin J W, Duchala C S, Narayan O. Astrocytes and Schwann cells are virus-host cells in the nervous system of rats with Borna disease. J Neuropathol Exp Neurol. 1989;48:631–644. doi: 10.1097/00005072-198911000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Ciurea A, Klenerman P, Hunziker L, Horvath E, Odermatt B, Ochsenbein A F, Hengartner H, Zinkernagel R M. Persistence of lymphocytic choriomeningitis virus at very low levels in immune mice. Proc Natl Acad Sci USA. 1999;96:11964–11969. doi: 10.1073/pnas.96.21.11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deschl U, Stitz L, Herzog S, Frese K, Rott R. Determination of immune cells and expression of major histocompatibility complex class II antigen in encephalitic lesions of experimental Borna disease. Acta Neuropathol (Berlin) 1990;81:41–50. doi: 10.1007/BF00662636. [DOI] [PubMed] [Google Scholar]
- 10.Dietzschold B, Kao M, Zheng Y M, Chen Z Y, Maul G, Fu Z F, Rupprecht C E, Koprowski H. Delineation of putative mechanisms involved in antibody-mediated clearance of rabies virus from the central nervous system. Proc Natl Acad Sci USA. 1992;89:7252–7256. doi: 10.1073/pnas.89.15.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietzschold B, Tollis M, Lafon M, Wunner W H, Koprowski H. Mechanisms of rabies virus neutralization by glycoprotein-specific monoclonal antibodies. Virology. 1987;161:29–36. doi: 10.1016/0042-6822(87)90167-x. [DOI] [PubMed] [Google Scholar]
- 12.Gosztonyi G, Dietzschold B, Kao M, Rupprecht C E, Ludwig H, Koprowski H. Rabies and borna disease. A comparative pathogenetic study of two neurovirulent agents. Lab Investig. 1993;68:285–295. [PubMed] [Google Scholar]
- 13.Hatalski C G, Hickey W F, Lipkin W I. Humoral immunity in the central nervous system of Lewis rats infected with Borna disease virus. J Neuroimmunol. 1998;90:128–136. doi: 10.1016/s0165-5728(98)00066-6. [DOI] [PubMed] [Google Scholar]
- 14.Hatalski C G, Kliche S, Stitz L, Lipkin W I. Neutralizing antibodies in Borna disease virus-infected rats. J Virol. 1995;69:741–747. doi: 10.1128/jvi.69.2.741-747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hausmann J, Hallensleben W, de la Torre J C, Pagenstecher A, Zimmermann C, Pircher H, Staeheli P. T cell ignorance in mice to Borna disease virus can be overcome by peripheral expression of the viral nucleoprotein. Proc Natl Acad Sci USA. 1999;96:9769–9774. doi: 10.1073/pnas.96.17.9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herzog S, Kompter C, Frese K, Rott R. Replication of Borna disease virus in rats: age-dependent differences in tissue distribution. Med Microbiol Immunol (Berlin) 1984;173:171–177. doi: 10.1007/BF02122108. [DOI] [PubMed] [Google Scholar]
- 17.Herzog S, Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol (Berlin) 1980;168:153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- 18.Hickey W F, Hsu B L, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 19.Katrak K, Mahon B P, Minor P D, Mills K H. Cellular and humoral immune responses to poliovirus in mice: a role for helper T cells in heterotypic immunity to poliovirus. J Gen Virol. 1991;72:1093–1098. doi: 10.1099/0022-1317-72-5-1093. [DOI] [PubMed] [Google Scholar]
- 20.Kliche S, Briese T, Henschen A H, Stitz L, Lipkin W I. Characterization of a Borna disease virus glycoprotein, gp18. J Virol. 1994;68:6918–6923. doi: 10.1128/jvi.68.11.6918-6923.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koziel M J, Dudley D, Wong J T, Dienstag J, Houghton M, Ralston R, Walker B D. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J Immunol. 1992;149:3339–3344. [PubMed] [Google Scholar]
- 22.Lefrancois L, Lyles D S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. II. Monoclonal antibodies of nonneutralizing and cross-reactive epitopes of Indiana and New Jersey serotypes. Virology. 1982;121:168–174. doi: 10.1016/0042-6822(82)90126-x. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann-Grube F. Lymphocytic choriomeningitis virus. Virol Monogr. 1971;10:1–173. [Google Scholar]
- 24.Levine B, Hardwick J M, Trapp B D, Crawford T O, Bollinger R C, Griffin D E. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 25.Moore J P, Cao Y, Ho D D, Koup R A. Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J Virol. 1994;68:5142–5155. doi: 10.1128/jvi.68.8.5142-5155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nöske K, Bilzer T, Planz O, Stitz L. Virus-specific CD4+ T cells eliminate borna disease virus from the brain via induction of cytotoxic CD8+ T cells. J Virol. 1998;72:4387–4395. doi: 10.1128/jvi.72.5.4387-4395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pantaleo G. Immunology of HIV infection. Res Immunol. 1997;148:417–419. doi: 10.1016/s0923-2494(97)82875-1. [DOI] [PubMed] [Google Scholar]
- 28.Peters M, Vierling J, Gershwin M E, Milich D, Chisari F V, Hoofnagle J H. Immunology and the liver. Hepatology. 1991;13:977–994. [PubMed] [Google Scholar]
- 29.Planz O, Bilzer T, Sobbe M, Stitz L. Lysis of major histocompatibility complex class I-bearing cells in Borna disease virus-induced degenerative encephalopathy. J Exp Med. 1993;178:163–174. doi: 10.1084/jem.178.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Planz O, Bilzer T, Stitz L. Immunopathogenic role of T-cell subsets in Borna disease virus-induced progressive encephalitis. J Virol. 1995;69:896–903. doi: 10.1128/jvi.69.2.896-903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richt J A, Furbringer T, Koch A, Pfeuffer I, Herden C, Bause-Niedrig I, Garten W. Processing of the Borna disease virus glycoprotein gp94 by the subtilisin-like endoprotease furin. J Virol. 1998;72:4528–4533. doi: 10.1128/jvi.72.5.4528-4533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robert-Guroff M, Brown M, Gallo R C. HTLV-III-neutralizing antibodies in patients with AIDS and AIDS-related complex. Nature. 1985;316:72–74. doi: 10.1038/316072a0. [DOI] [PubMed] [Google Scholar]
- 33.Seiler P, Kalinke U, Rulicke T, Bucher E M, Bose C, Zinkernagel R M, Hengartner H. Enhanced virus clearance by early inducible lymphocytic choriomeningitis virus-neutralizing antibodies in immunoglobulin-transgenic mice. J Virol. 1998;72:2253–2258. doi: 10.1128/jvi.72.3.2253-2258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sierra-Honigmann A M, Rubin S A, Estafanous M G, Yolken R H, Carbone K M. Borna disease virus in peripheral blood mononuclear and bone marrow cells of neonatally and chronically infected rats. J Neuroimmunol. 1993;45:31–36. doi: 10.1016/0165-5728(93)90160-z. [DOI] [PubMed] [Google Scholar]
- 35.Sobbe M, Bilzer T, Gommel S, Nöske K, Planz O, Stitz L. Induction of degenerative brain lesions after adoptive transfer of brain lymphocytes from Borna disease virus-infected rats: presence of CD8+ T cells and perforin mRNA. J Virol. 1997;71:2400–2407. doi: 10.1128/jvi.71.3.2400-2407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stitz L, Nöske K, Planz O, Furrer E, Lipkin W I, Bilzer T. A functional role for neutralizing antibodies in Borna disease: influence on virus tropism outside the central nervous system. J Virol. 1998;72:8884–8892. doi: 10.1128/jvi.72.11.8884-8892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stitz L, Rott R. Borna disease virus (Bornaviridae) In: Granoff A, Webster R G, editors. Encyclopedia of virology. New York, N.Y: Academic Press, Inc.; 1999. pp. 167–173. [Google Scholar]
- 38.Stoyloff R, Bode L, Borchers K, Ludwig H. Neutralization of Borna disease virus depends upon terminal carbohydrate residues (alpha-d-Man, beta-d-GlcNAc) of glycoproteins gp17 and gp94. Intervirology. 1998;41:135–140. doi: 10.1159/000024926. [DOI] [PubMed] [Google Scholar]
- 39.Thiedemann N, Presek P, Rott R, Stitz L. Antigenic relationship and further characterization of two major Borna disease virus-specific proteins. J Gen Virol. 1992;73:1057–1064. doi: 10.1099/0022-1317-73-5-1057. [DOI] [PubMed] [Google Scholar]
- 40.Torsteinsdottir S, Georgsson G, Gisladottir E, Rafnar B, Palsson P A, Petursson G. Pathogenesis of central nervous system lesions in visna: cell-mediated immunity and lymphocyte subsets in blood, brain and cerebrospinal fluid. J Neuroimmunol. 1992;41:149–158. doi: 10.1016/0165-5728(92)90065-s. [DOI] [PubMed] [Google Scholar]
- 41.Webster R G. The immune response to influenza virus. I. Effect of the route and schedule of vaccination on the time course of the immune response, as measured by three serological methods. Immunology. 1965;9:501–519. [PMC free article] [PubMed] [Google Scholar]
- 42.Wekerle H, Linington C, Lassmann H, Meyermann R. Cellular immune reactivity within the CNS. Trends Neurosci. 1986;9:271–277. [Google Scholar]
- 43.Zinkernagel R M. Virus-induced immunopathology. In: Nathanson N, editor. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 163–179. [Google Scholar]