Abstract
Glycosylation is the most structurally diverse form of post-translational modification (PTM) of proteins that affects a myriad of cellular processes. As a pivotal regulator of protein homeostasis, glycosylation notably impacts the function of proteins, spanning from protein localization and stability to protein-protein interactions. Aberrant glycosylation is a hallmark of cancer, and extensive studies have revealed the multifaceted roles of glycosylation in tumor growth, migration, invasion and immune escape Over the past decade, glycosylation has emerged as an immune regulator in the tumor microenvironment (TME). Here, we summarize the intricate interplay between glycosylation and the immune system documented in recent literature, which orchestrates the regulation of the tumor immune response through endogenous lectins, immune checkpoints and the extracellular matrix (ECM) in the TME. In addition, we discuss the latest progress in glycan-based cancer immunotherapy. This review provides a basic understanding of glycosylation in the tumor immune response and a theoretical framework for tumor immunotherapy.
Keywords: glycosylation, immune response, endogenous lectins, immune checkpoint, extracellular matrix, immunotherapy
Introduction
Glycosylation is a common form of protein and lipid modification by the covalent attachment of saccharides to proteins and lipids. The major types of glycosylation in mammals include N-glycosylation, O-glycosylation, O-GlcNAcylation, GPI-anchored glycoproteins, and glycosaminoglycans [1]. N-glycosylation, in which N-glycans are attached to asparagine within the conserved N-X-S/T sequon (where X represents any amino acid excluding proline) in peptides, frequently occurs in membrane proteins [1]. All N-glycans possess a core pentasaccharide; however, the diversity of monosaccharides and the complexity of linking patterns result in different types of N-glycans, which are divided into high-mannose, hybrid, and complex types [2]. In addition to N-glycosylation, O-glycosylation and O-GlcNAcylation occur at serine/threonine (S/T) residues [1]. O-glycosylation often refers to the glycosylation of glycans initiated by N-acetylgalactosamine (GalNAc). Biosynthesis of N-glycans is initiated in the endoplasmic reticulum (ER) and further elongated in the Golgi apparatus, while the entire synthesis of O-glycans occurs in the Golgi apparatus [3]. In contrast to N-glycans and O-glycans, O-GlcNAcylation is a unique monosaccharide β-N-acetylglucosamine (GlcNAc) modification that predominantly occurs in intracellular proteins and is mediated solely by O-GlcNAc transferase (OGT) and the O-GlcNAcase (OGA) [4].
Glycosylation functions as a critical regulator of multiple physiopathological processes, including signal transduction and communication, invasion, cell–matrix interactions and immune modulation [5]. Therefore, deregulation of glycosylation is closely associated with tumor development and progression. Accumulating evidence highlights that aberrant glycosylation plays a pivotal role in tumor immunity through influencing the interaction of glycosylation receptors, lectins and ligands on the cell surfaces of tumor cells and immune cells. For example, tumor cells secrete galectins to promote regulatory T cells (Tregs) and T-cell exhaustion by impairing TME homeostasis [ 6– 9]. The binding of sialylated glycans and sialic acid-binding immunoglobulin-like lectin (siglec) receptors remodeled the immunosuppressive TME through activating the tumor-promoting phenotype of tumor-associated macrophages (TAMs), repressing the activation of natural killer (NK) cells and dendritic cell-mediated antigen presentation and subsequent T-cell responses [ 10– 14]. Aberrant glycosylation also regulates the interaction between immune checkpoint molecules and their corresponding ligands by affecting their stability and/or binding affinity [15]. Thus, in-depth analysis of the role of glycosylation in the tumor immune response and how aberrant glycan structures modulate tumor-mediated immune evasion may provide new and viable strategies for potential immunotherapy.
In this review, we focus on the role of glycosylation in antitumor immunity, particularly its regulatory effects on endogenous lectins, a wide range of immune checkpoints and the ECM in the TME. We summarize the mechanisms by which aberrant glycosylation modulates the tumor immune response, ranging from influencing endogenous lectins and immune checkpoints to influencing their interactions with other molecules, as well as the function of immune cells in the TME. In addition, we also review the current glycan-based cancer immunotherapies and aim to pave the way for future investigations on targeted glycosylation to augment the efficacy of immunotherapy.
Endogenous Lectins and Tumor Immunity
Accumulating evidence has demonstrated that endogenous lectins play vital roles in the tumor immune response by binding to specific glycan structures in the TME. Among them, siglecs and galectins are the main types of lectins and play critical roles in antitumor immunity. Here, we summarize the functions of the Siglec and galectin families in the immune response.
Sialic acid-siglec interactions
Tumor cells are coated with large amounts of sialic acids present at the termini of glycans. These sialic acids are added to N-glycans or O-glycans through sialyltransferases in the linked forms of α2,3, α2,6, and α2,8 [16]. Siglecs, which belong to the immunoglobulin-like lectin family normally expressed on innate and adaptive immune cells, recognize and bind to sialic acid-containing glycans (sialoglycans). Based on their evolutionary relevance, siglecs are divided into two prominent subsets. Siglec-1, siglec-2 (CD22), siglec-4, and siglec-15 are conserved receptors, while others are highly variable CD33-related siglecs (siglec-3, siglec7, siglec-9, siglec-10, etc.) [17]. The immunoregulatory effects exerted by siglecs rely on their intracellular structural domains. Most siglecs contain immunoreceptor tyrosine-based inhibitory motifs (ITIMs) within their intracellular regions, which, in turn, recruit tyrosine phosphatases such as Src homology 2 domain-containing protein tyrosine phosphatase 1 (SHP-1) and SHP-2 to transduce inhibitory signals [18]. Therefore, the elevated levels of sialylation in cancer cells enhance their affinity for binding to siglecs, potentially dampening the immune response and facilitating immune evasion.
Siglec-7
Siglec-7 is an inhibitory receptor constitutively expressed in NK cells and tends to bind disialyl-T antigen with α2,3- and α2,6-linked sialic acids [ 12, 19, 20] ( Table 1). Hudak et al. [10] used glycopolymers end-functionalized with phospholipids to reshape the sialylation state of cancer cells, which resulted in the engagement of siglec-7 and impaired the killing function of NK cells. The protein ligands on target cells that bind to Siglec-7 include CD43 [21], CD45 [20], P-selectin glycoprotein ligand-1 (PSGL-1) [ 11, 20], MUC2 [19], and MUC5AC [19] ( Table 1). Accumulating evidence has revealed that inhibiting the interactions between Siglec-7 and its ligands enhances the anticancer activity of cytotoxic immune cells. For example, Wisnovsky and his colleagues reported that blocking the interaction between CD43 and Siglec-7 enhanced the cytotoxic activity of NK cells toward leukemia cells [21]. In multiple myeloma (MM), PSGL-1 is overexpressed and acts as a sialic acid-derived ligand for Siglec-7. Targeting the interaction between PSGL-1 and Siglec-7 could increase NK cell-mediated cytotoxicity [11]. These findings demonstrate that selective blockade of the interactions between Siglec-7 and its ligands is a promising strategy for cancer therapy.
Table 1 Glycans and protein ligands of siglecs in tumors
|
Siglec |
Cell |
Glycan ligand |
Protein ligand |
Reference |
|
Siglec-7 |
NK, Mono, Macro, T |
Disialyl-T, α2,3/6 sia |
CD43, CD45, CD162/PSGL-1, MUC2, MUC5AC |
|
|
Siglec-9 |
NK, Mono, Neu, DC, TAM, T |
sLex, sTn, α2,3 sia |
ST-MUC1, MUC16, LGALS3BP |
|
|
Siglec-10 |
TAM, T, NK, |
α2,3/6 sia |
CD24, CD52 |
|
|
Siglec-15 |
BMDM |
sTn, α2,3/6 sia |
CD44, CD11b |
Siglec-9
Siglec-9 is another inhibitory receptor that is widely expressed in immune cells, particularly in myeloid cells, including monocytes, neutrophils and macrophages. Glycan ligands that contain sialic acid α2,3 linked to galactose are preferred ( Table 1). Several studies have demonstrated that Siglec-9 recognizes specific antigens, such as sialyl Lewis X (sLex) [ 22, 23], sialyl Tn (sTn) [ 23, 24], sialyl-T-MUC1 (ST-MUC1) [ 25, 26], and MUC16 [ 27, 28] ( Table 1). The binding of ST-MUC1 to siglec-9 activates the MEK-ERK pathway in macrophages, leading to the induction of a TAM phenotype in macrophages by increasing the expression of suppressive proteins such as indoleamine 2,3-dioxygenase (IDO), CD163, CD206, and programmed cell death ligand 1 (PD-L1) to inhibit T-cell activity [ 25, 26]. The expression of siglec-9 is frequently upregulated in glioblastoma (GBM) and is positively correlated with poor prognosis in GBM patients. Single-cell RNA sequencing and spatial transcriptomics analyses of GBM patients who did not respond to anti-PD-1 therapy revealed high and continuous expression of the SIGLEC9 gene in TAM subpopulations [29]. Blocking siglec-9 in combination with an anti-PD-1 antibody has shown potential for enhancing immune checkpoint blockade (ICB) immunotherapy for GBM. Similar observations have been made in high-grade serous ovarian cancer (HGSOC), where high expression of siglec-9 on TAMs correlates with CD8 + T-cell exhaustion and increased expression of immune checkpoint molecules [30]. Blocking siglec-9 leads to the suppression of SHP-1 phosphorylation, resulting in TAM repolarization and the restoration of CD8 + T-cell cytotoxicity.
Siglec-10
Siglec-10 recognizes glycans that carry α2,3- or α2,6-linked sialic acids and is mainly expressed on the cell surfaces of TAMs, T cells and NK cells ( Table 1). Recent research has revealed that CD24, a glycosylphosphatidylinositol (GPI)-anchored protein in tumor cells, represses the innate immune response by binding to siglec-10 in a sialic acid-dependent manner [ 31, 32]. Soluble CD52 containing sialylated N-glycans is another ligand for siglec-10. It inhibits T-cell activation by dampening the phosphorylation of T-cell receptor-associated kinases, including lymphocyte-specific protein tyrosine kinase (Lck) and the zeta chain of T-cell receptor-associated protein kinase 70 (Zap70), when binding to siglec-10 [33].
Siglec-15
Siglec-15 is primarily expressed by myeloid cells and macrophages and plays central roles in suppressing antigen-specific T-cell proliferation, thereby contributing to immune evasion [34]. As an immunomodulator, siglec-15 remodels the TME to promote primary tumor metastasis in a manner dependent on sTn [35] and branched α2,3/6 di-sialylated biantennary glycans [22] ( Table 1). A recent study proposed that osteoclasts upregulate Siglec-15 to suppress T-cell activity, contributing to the establishment of an immunosuppressive TME and facilitating secondary metastasis in breast cancer [36]. Previous studies have indicated that CD44 on tumor cells and CD11b on T cells can act as ligands for siglec-15, suggesting that siglec-15 may interact with different ligands from various cell types, further influencing its role in tumor progression and immune regulation [ 37, 38].
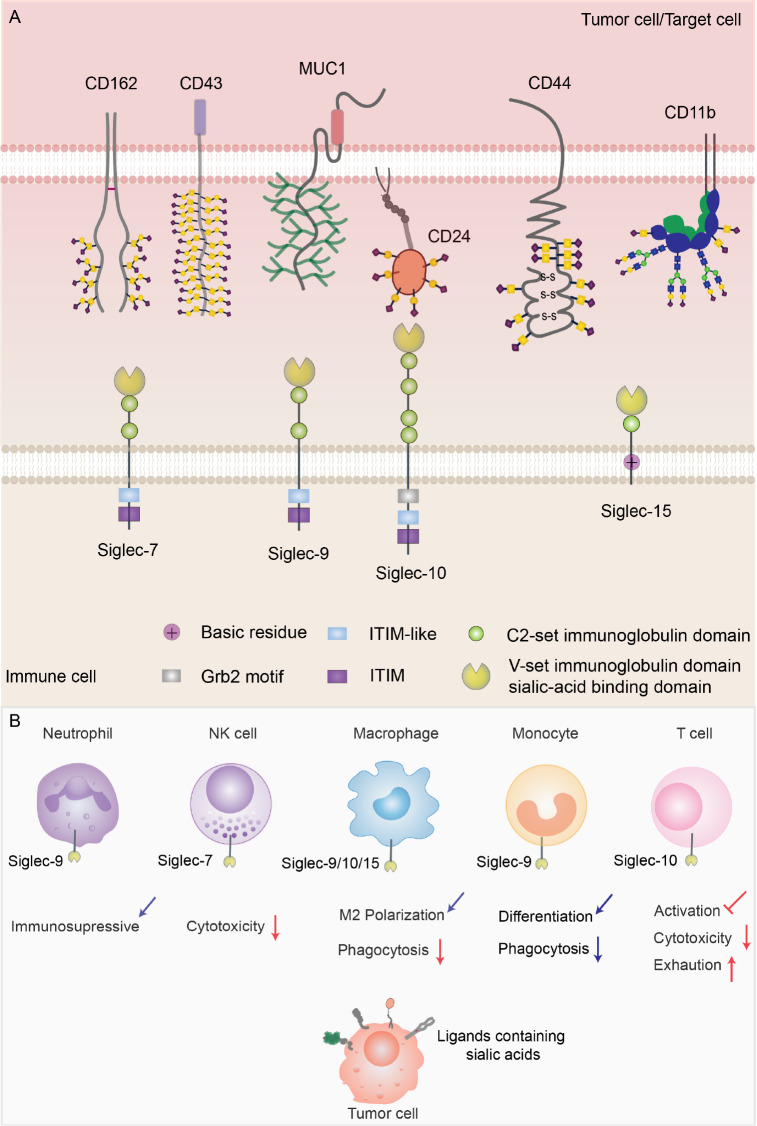
Thus, an increasing number of studies have shown that hypersialylation on the tumor cell surface contributes to tumor immune evasion by binding to siglecs present on immune cell surfaces. The interaction of siglecs and protein ligands with sialylation inhibits the antitumor activity of immune cells ( Figure 1). Consequently, targeting glycosylated cell-surface antigens is emerging as an attractive strategy for cancer immunotherapy.
Figure 1 .
Ligands of siglecs and the effects on immune cells mediated by their interactions
(A) Structures of siglec7/9/10/15 and their binding ligands on tumor cells or target cells. (B) Potential effects on immune cells caused by the interaction between siglec 7/9/10/15 and glycosylated ligands on tumor cells.
Glycans and galectins
Galectins, a family of β-galactoside-binding proteins, mainly recognize N-acetyllactosamine (LacNAc) epitopes on glycosylated receptors through their carbohydrate recognition domains (CRDs). Based on the structure of CRDs, galectins can be categorized into three subgroups: prototypical galectins (GAL1, GAL2, GAL5, GAL7, GAL10, GAL11, GAL13, GAL14, and GAL15), tandem repeat galectins (GAL4, GAL6, GAL8, GAL9 and GAL12), and chimeric galectins (GAL3) [39]. Prototypical galectins possess two identical CRDs, while tandem repeat galectins contain two different CRDs. Chimeric galectins have only one GAL3 member with one CRD, which is linked to a nonlectin N-terminal domain (NTD) responsible for GAL3 oligomerization [40]. Dysregulation of galectins is observed in different cancers and affects the tumor immune response through glycosylation-dependent or glycosylation-independent mechanisms. Among them, GAL1, GAL3, and GAL9, in particular, have been implicated in immune evasion. In this section, we focus on the functions of GAL1, GAL3, and GAL9 during the immune response.
GAL1
GAL1, a highly conserved galectin encoded by the LGALS1 gene, is associated with diverse glycosylated ligands, such as CD7, CD43, CD45, CD69, melanoma cell adhesion molecule (MCAM/CD146), and vascular endothelial growth factor receptor (VEGFR) [ 41, 42] ( Table 2). GAL1 is abundant in the TME, where it is involved in immune regulation by supporting tumor cells in evading immune surveillance. MCAM is the main GAL1 ligand in melanoma and binds to GAL1 mostly through N-glycans [42]. Many studies have explored how GAL1 assists tumor cells in acquiring immune privilege by impacting T-cell function. Initially, GAL1 was considered a crucial factor in the apoptosis of tumor-specific effector T cells. Due to the interaction between GAL1 and its ligands with poly-N-acetyllactosamine (LacNAc), T-cell homeostasis is disrupted, which is characterized by T-cell apoptosis, inactivation, and decreased proinflammatory cytokines [43]. In addition, lung cancer cell-derived GAL1 increases the amount of CD4 +CD25 +FOXP3 + Tregs, an immunosuppressive T-cell subset in the TME [44]. Similarly, the depletion of Gal1 reduces the infiltration of Tregs within tumors, decreases the level of the T-cell regulatory molecule linker for the activation of T cells (LAT), and eliminates the immunosuppressive function of Tregs in breast cancer [6]. In addition to CD4 +CD25 +FOXP3 + Tregs, CD8 +CD122 +PD-1 + Tregs identified in colorectal cancer (CRC) have been associated with Gal-1. Notably, heightened GAL1 expression and elevated CD8 + Treg scores correlate with poor prognosis in CRC patients. Studies in CRC models have demonstrated that targeting GAL1 results in a decreased number of CD8 +CD122 +PD-1 + Tregs and reduced tumor growth [45].
Table 2 Glycan and protein ligands of galectins in tumors
|
Galectin |
Glycan ligand |
Protein ligand |
Reference |
|
GAL-1 |
Terminal LacNAc residues |
CD7, CD43, CD45, CD69, VEGFR, MCAM/CD146 |
|
|
GAL-3 |
Wide range of LacNAc residues |
MUC1, CD45, CD71, LAG-3, α3β1 integrin, CD98 |
|
|
GAL-9 |
Prefers poly-LacNAc repeats |
TIM-3, PD-1, VISTA, CD44, Dectin-1, Dectin-2, ERMAP |
GAL1 can not only directly modulate T cells within tumors but also regulate other cells, such as endothelial cells, macrophages, and cancer-associated fibroblasts (CAFs), to form a barrier against T-cell infiltration or dysregulate T-cell function. Endothelial cells are reprogrammed by GAL1 and increase the expression of PD-L1, which leads to T-cell exclusion [7]. Hypoxia is a common feature observed in numerous tumors. This hypoxia-induced milieu triggers a reciprocal activation mechanism between GAL1 and hypoxia-inducible factor-1 (HIF), facilitated by H-Ras [46]. Under hypoxic conditions, heightened lactate production promotes the differentiation of myeloid-derived suppressor cells (MDSCs) into TAMs. Within pancreatic ductal adenocarcinoma (PDAC), over-activated pancreatic stellate cells (PSCs) secrete substantial amounts of GAL1 and interleukin-6 (IL-6) [47]. GAL1 synergizes with lactate to activate HIF, promoting the differentiation of MDSCs into TAMs [48]. On the other hand, IL-6 recruits MDSCs via the IL-6/JAK/STAT3 pathway or collaborates with lactate to induce the production of vascular endothelial growth factor (VEGF). Additionally, the increased levels of reactive oxygen species (ROS) resulted from hypoxia and lactate enhance the immunosuppressive activity of TAMs [40]. In summary, GAL1 promotes immune system evasion and induces T-cell dysfunction through the regulation of TAMs and MDSCs.
GAL3
In addition to CRD, GAL3 has a unique NTD because of its special ability to form pentamers. GAL3 can interact with internal or other modified LacNAc in branched N-glycans [39]. Further insights into the roles of extracellular GAL3 come from its influence on immune cells, especially T cells and macrophages. Tumor-derived GAL3 induces the apoptosis of T cells by interacting with the glycosylated receptors CD45 and CD71 [49]. The presence of GAL3 in the TME dampens immune synapse formation by hampering the mobility of T-cell receptors during T-cell activation [50]. GAL3 also binds to lymphocyte activation gene 3 (LAG-3), an inhibitory immune checkpoint, thereby suppressing CD8 + T-cell function [8]. In addition, GAL3 interacts with the glycoprotein α3β1 integrin on γδ T cells to prevent γδ T-cell proliferation [51]. In macrophages, GAL3 shows distinct expression patterns in different subtypes. Interferon-γ (IFN-γ) and lipopolysaccharide (LPS) stimulate M1 macrophages to express and release lower level of GAL3, while IL-4/IL-13-mediated M2 macrophages exhibit increased GAL3 biosynthesis and secretion [52]. Secreted GAL3 binds to the surface glycoprotein CD98 on macrophages to boost immunosuppressive M2 macrophage proliferation [53].
GAL9
GAL9, which belongs to the “tandem repeat galectins” family, consists of two homologous but distinct CRDs, namely, the N-terminal CRD (N-CRD) and the C-terminal CRD (C-CRD). Compared with GAL1 and GAL3, GAL9 prefers poly-LacNAc structures [39] ( Table 2). Recent research has shed light on the immunomodulatory role of GAL9 in the TME. The binding of GAL9 to the T-cell suppressive marker mucin domain-3 (TIM-3) triggers T-cell apoptosis and exhaustion [9]. In addition to TIM-3, CD44 was reported to be a potential receptor for GAL9 expressed on activated T cells. Extracellular GAL9 collaborates with transforming growth factor β (TGF-β) to interact with CD44-TGF-βRI, which promotes forkhead box protein P3 (FOXP3) expression, stabilizes FXOP3, and enhances the inhibitory function of induced Tregs (iTregs) [54]. In addition to its effects on T cells, GAL9 also plays a critical role in modulating myeloid cells. In PTEN-deficient GBM cells, GAL9 secretion is increased, activating TIM-3 on macrophages and downstream pathways that accelerate macrophage M2 polarization, leading to angiogenesis and supporting tumor cell growth [55]. Moreover, GAL9 interacts with innate immune receptors, namely, dendritic cell-associated C-type lectin-1 (Dectin-1) and dendritic cell-associated C-type lectin-2 (Dectin-2), which are primarily expressed on dendritic cells (DCs) and macrophages [56]. The binding of GAL9 to Dectin-1 results in macrophage differentiation and the suppression of adaptive immune responses, thereby facilitating the progression of PDAC [56]. On the other hand, GAL9 binds to Dectin-2 and ERMAP, which are expressed on Kupffer cells and cancer cells, respectively, to form a complex that signals Kupffer cells to engulf tumor cells more efficiently [57]. These findings highlight the diverse roles of GAL9 in modulating the immune response and tumor progression in the TME, providing potential targets for therapeutic interventions.
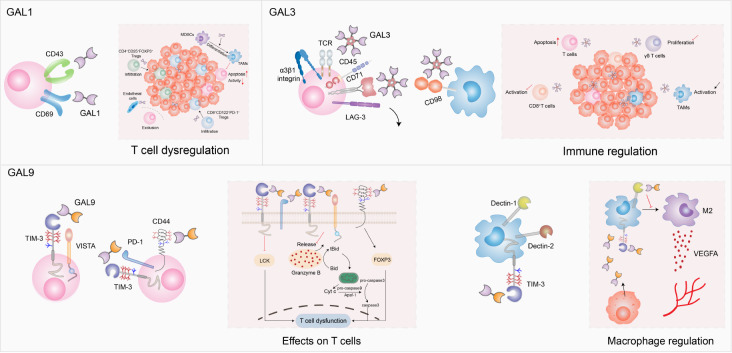
Overall, GAL1, GAL3, and GAL9 are key players in mediating immune escape in cancer through various mechanisms, highlighting their potential as therapeutic targets in cancer immunotherapy ( Figure 2). Understanding the roles of galectins in tumor immune evasion is crucial for developing effective strategies to overcome immune suppression.
Figure 2 .
Interactions between galectins and their ligands regulate tumor cells and the immune system in the TME
GAL1 plays a key role in T-cell regulation by influencing T-cell infiltration and function directly or indirectly. GAL3 mainly binds to cell surface receptors on T cells, such as CD45, CD71, and LAG-3, which inhibits T-cell activation and proliferation and induces apoptosis. GAL9 engages with immune checkpoint molecules, including TIM-3, VISTA, and PD-1, leading to T-cell dysregulation. GAL9 is also involved in macrophage polarization to modulate the TME.
Glycosylation of B7 Family Proteins and Tumor Immune Escape
Recent studies have shown that certain members of the B7 family, which are transmembrane proteins found on activated antigen-presenting cells (APCs), are highly expressed in tumor cells and play central roles in tumor immune evasion. To date, B7 families include ten members: B7-1 (CD80), B7-2 (CD86), B7-H1 (PD-L1 or CD274), B7-DC (CD273 or PD-L2), B7-H2 (ICOSLG or CD275), B7-H3 (CD276), B7-H4 (B7S1, B7x or VTCN1), B7-H5 (VISTA), B7-H6 and B7-H7 [63] ( Table 3). Most B7 family proteins are glycoproteins, and an increasing number of studies have highlighted the importance of how alterations in glycosylation affect proteins themselves and the TME. Here, the roles of PD-L1, PD-L2, B7-H3 and B7-H4 glycosylation in the tumor immune response are discussed ( Figure 3).
Table 3 Protein ligands for B7 family and the glycosyltransferases involved in B7 family regulation
|
B7 family |
Transferase |
Protein ligand |
Reference |
|
PD-L1 |
STT3A/STT3B, B3GNT3, B4GALT1, MAN2A1, FUT8 |
PD-1 |
|
|
PD-L2 |
FUT8 |
PD-1 |
|
|
B7-H3 |
FUT8 |
TLT-2, IL20RA, PLA2R1 |
|
|
B7-H4 |
STT3A, UGGT1 |
– |
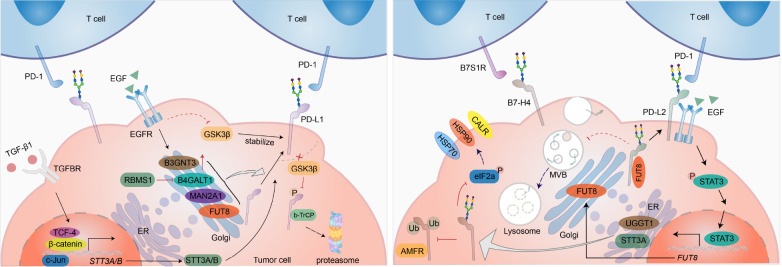
Figure 3 .
Regulation of B7 family glycosylation
The B7 family is a group of highly N-glycosylated proteins abnormally expressed by tumor cells. Upregulated signal transduction promotes the aberrant expression of glycosyltransferases and alters the glycosylation patterns of B7 family proteins, which strengthens their stability and interaction with their ligands, thus contributing to tumor invasion.
PD-L1
B7H1, also referred to as PD-L1, has emerged as a pivotal target in cancer immunotherapy. Tumor cells frequently exhibit elevated PD-L1 expression, impeding the activation of tumor-specific T cells by binding to PD-1, resulting in T-cell exhaustion and immune evasion. PD-L1 is composed of an extracellular region (including an immunoglobulin V-like (IgV) domain and an immunoglobulin C-like (IgC) domain), a transmembrane domain (TM), and an intracellular C-terminal domain [ 64, 65]. The extracellular domain (ECD) of PD-L1 contains 4 N-glycosylated sites, N35, N192, N200, and N219, which are critical for its stability and interaction with PD-1 [ 66, 67]. Compelling evidence has demonstrated that alterations in enzymatic activity during the synthesis and processing of N-glycans can lead to the erroneous formation of these glycans on PD-L1, impacting PD-L1-mediated tumor immune evasion. Epithelial–mesenchymal transition (EMT) upregulates the expression of the N-glycosyltransferase STT3 through β-catenin, leading to the stability of STT3-dependent PD-L1 N-glycosylation and contributing to cancer stem-like cell (CSC) immune evasion [68]. Mannosidase α class II member 1 (MAN2A1) is a critical enzyme that catalyzes the transformation of precursor high-mannose N-glycans to the mature complex type. Knockdown of MAN2A1 diminishes the binding of PD-L1 to PD-1, promoting T-cell infiltration in tumors and enhancing the therapeutic efficacy of anti-PD-L1 therapy [69]. Glycogen synthase kinase 3β (GSK3β) also plays a vital role in PD-L1 glycosylation and stability. GSK3β induces PD-L1 glycosylation to block the binding of b-TrCP to PD-L1, which inhibits PD-L1 phosphorylation and proteasomal degradation of PD-L1, thereby promoting PD-L1 expression and tumor immune evasion [ 66, 70]. In addition, many glycosyltransferases and glycosidases localized in the Golgi are responsible for the processing and branching of N-glycans on PD-L1. EGFR activation increases the expression of β-1,3-N-acetylglucosaminyltransferase 3 (B3GNT3), leading to the stabilization of PD-L1, the interaction with PD-1, and the inhibition of PD-L1 internalization and degradation [71]. Another Golgi membrane protein, β-1,4-galactosyltransferase 1 (B4GALT1), also participates in regulating PD-L1 expression. B4GALT1 modulates PD-L1 N-glycosylation, preventing PD-L1 degradation. On the other hand, B4GALT1 stabilizes the TAZ protein via glycosylation, which in turn facilitates PD-L1 transcription [72]. Zhang et al. [73] also showed that elevating the mRNA stability of B4GALT1 by RNA binding motif single strand interacting protein 1 (RBMS1) promotes B4GALT1 expression, thereby enhancing PD-L1 glycosylation and dampening the antitumor effect of cytotoxic T cells. Our laboratory demonstrated that O-GlcNAc modified hepatocyte growth factor-regulated tyrosine kinase substrate (HGS), a key component of the endosomal sorting machinery, and subsequently repressed its interaction with intracellular PD-L1, resulting in impaired lysosomal degradation of PD-L1 and promoting tumor immune evasion [74]. Taken together, a comprehensive understanding of the glycosylation of PD-L1 would greatly contribute to the development of new immunotherapeutic strategies.
PD-L2
PD-L2 is a second ligand for PD-1, whose binding affinity for PD-1 is 2- to 6-fold greater than that of PD-L1 [75]. Like PD-L1, PD-L2 is a negative regulator of T-cell activation and plays a vital role in immune tolerance [76]. PD-L2 has four N-glycosylated sites, N64, N157, N163, and N189, of which N157, N163, and N189 are responsible for the PD-1 interaction rather than N64 [77]. In many solid tumors, including head and neck squamous cell carcinoma (HNSCC), PD-L2 is reportedly overexpressed and is an independent factor for poor outcomes [78]. Glycosylated PD-L2 interacts with EGFR, resulting in EGFR/STAT3 activation and decreased binding affinity of cetuximab for EGFR. STAT3 activation promotes fucosyltransferase 8 (FUT8) transcription, which is essential for PD-L2 glycosylation, as well as inhibiting PD-L2 ubiquitination and preventing PD-L2 from accessing endosomal sorting complexes required for transport (ESCRT)-mediated lysosomal degradation [77].
B7-H3
B7-H3 is a highly glycosylated immune checkpoint molecule expressed on malignant cells and immune cells that possesses 8 N-glycosylated sites, N91, N104, N189, N215, N309, N322, N407, and N433 [79]. The abundant N-glycans on B7H3 may endow it with diverse functions and biological significance. FUT8 catalyzes core fucosylation at N-linked glycans, contributing to membrane B7H3 expression and immunosuppression in triple-negative breast cancer(TNBC). FUT8 knockdown promotes T-cell proliferation and activation, and combined treatment with a 2-fluoro-L-fucose (2F-Fuc) inhibitor and an anti-PD-L1 antibody improves the efficacy of anti-PD-L1 immunotherapy [80]. In addition, B7H3 can interact with putative receptors on the T-cell surface, such as the triggering receptor expressed on myeloid cells (TREM)-like transcript 2 (TLT-2), interleukin-20 receptor subunit α (IL20RA), and phospholipase A2 receptor 1 (PLA2R1) [79]. However, the impact of glycosylation on the interaction of B7H3 with these ligands and the mechanisms by which it further affects T-cell function remain to be elucidated.
B7-H4
B7-H4, also known as B7S1, B7x, and VTCN1, were identified as B7 family members in 2003 [81]. B7-H4 is normally expressed by specialized APCs but is also expressed in solid tumors. It has been reported that B7-H4 induces T cell dysfunction by interacting with B7S1R, a B7-H4 receptor on effector T cells, to increase eomesodermin (Eomes) expression, thus initiating T-cell exhaustion [82]. Extensively glycosylated B7-H4 is overexpressed in breast cancer (BC) and ovarian cancer (OV) and has 5 putative N-glycosylated sites, N112, N140, N156, N160, and N255. In TNBC, STT3 oligosaccharyltransferase complex catalytic subunit A (STT3A) and glycoprotein glycosyltransferase 1 (UGGT1) participate in B7-H4 glycosylation, which interferes with E3 ligase autocrine motility factor receptor (AMFR)-mediated B7-H4 ubiquitination. Furthermore, glycosylated B7-H4 inhibits eukaryotic translation initiation factor 2 subunit alpha (eIF2a) phosphorylation, thereby interrupting the exposure of cells to calreticulin (CALR) and the heat shock proteins, including heat shock protein 70 (HSP70) and heat shock protein 90 (HSP90), and suppressing cancer cell immunogenicity [83].
ECM and Tumor Immunity
The ECM is an important but long-understood cellular component in tissues. Structurally, the BM is composed of the basement membrane (BM) and the stromal ECM. The BM includes laminins and collagen IV, which serve as a separating line between the layer containing endothelial and epithelial cells and the stromal ECM. The stromal ECM varies in different tissues and is characterized by alterations in components such as collagens, glycosaminoglycans (GAGs), proteoglycans, fibrillar proteins, and cytokines, which influence the molecular, physical, and mechanical features of the stromal ECM. In healthy tissues, a suitable niche is formed for the interconnectivity between adjacent cells (immune cells, fibroblasts) and the ECM. In contrast, tumor cells stimulate fibroblasts, macrophages, and other cells to be accomplices, resulting in the deposition of collagens, proteoglycans, and hyaluronic acid (HA) and ultimately the formation of an immunosuppressive environment. Here, we provide an overview of the significance and complexity of ECM components in cell-matrix interactions, TME reshaping, and tumor immune regulation.
Collagens in tumor immune regulation
Collagens, the most abundant ECM constituents, are recognized to be critical for shaping the ECM. The synthesis of collagen initiates COL gene expression, followed by modifications, including proline hydroxylation and hydroxylysine glycosylation, in the rough ER (RER), which is imperative for the correct folding of procollagens. Once appropriately folded, procollagens are translocated to the outer space and become mature [86]. Collagens are predominantly expressed by CAFs in the TME. During cancer progression, the accumulation and activation of CAFs are usually observed, and the deposition of the collagen matrix is increased, providing a narrow way for immune cell infiltration [ 87, 88]. Even in the presence of high concentrations of C-X-C motif chemokine ligand 10 (CXCL10) or C-X-C motif chemokine ligand 4 (CXCL4), a T-cell chemoattractant, the infiltration of T cells in the TME is blocked due to the hypercrosslinked collagens and dense glycoproteins surrounding the tumor cells [89]. Furthermore, the collagen arrangement around tumor epithelial cell regions and vascular regions orients the migratory trajectory of T cells [90]. Collagen degradation by TAMs is also directly related to high collagen density and an immunosuppressive microenvironment. Mechanistically, TAMs ingest collagen fragments via the mannose receptor (MRC1), resulting in elevated levels of inducible nitric oxide synthase (iNOS) and reactive nitrogen species (RNS). TAM-derived RNS activate PSCs, which leads to the increased deposition of fibrillar collagens in the TME [91].
The emerging understanding is that collagens within the TME serve as ligands capable of interacting with receptors on immune cells, thus influencing tumor immunity. Various receptors participate in such interactions, including integrins, discoidin domain receptors 1 and 2 (DDR1 and DDR2), and leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) [92]. These collagen-receptor interactions lead to the modulation of tumor immunity and impact immune cell functions. For example, cancer cell-secreted collagen-I homotrimers exhibit notable resistance to cleavage by matrix metalloproteinases (MMPs) through binding to integrin α3β1 and activating the FAK-AKT-MAPK signaling pathway in PDAC cells, thereby promoting immunosuppression and tumor progression [ 93, 94]. DDR1 and DDR2, which belong to the receptor tyrosine kinase (RTK) family, are expressed by both tumor cells and some immune cells. DDR1 receptors on PDAC cells interact with collagens, inducing C-X-C motif chemokine ligand 5 (CXCL5) production, which in turn promotes the aggregation of tumor-associated neutrophils and the formation of neutrophil extracellular traps (NETs) [95]. The interaction of DDR1 with collagens in breast cancer cells leads to collagen matrix alignment and hinders immune cell infiltration [96]. In addition, LAIR-1, a coinhibitory receptor expressed on various immune cells, interacts with collagens to suppress immune cell activation through its intracellular ITIMs [ 97, 98]. Increased LAIR-1 expression and LAIR-1-dependent CD8 + T-cell exhaustion in lung cancer are induced by the interaction between integrin β1 (CD18) and collagens [99].
Proteoglycans (PGs) in tumor immune regulation
Proteoglycans (PGs) constitute a heterogeneous array of molecules characterized by a core protein covalently linked to one or more glycosaminoglycans (GAGs). These GAGs are classified into four categories: chondroitin sulfate (CS)/dermatan sulfate (DS), keratan sulfate (KS), heparan sulfate (HS), and HA. GAGs serve as receptive sites within the TME, facilitating the binding of soluble ligands such as cytokines, chemokines, growth factors, and cell surface receptors. Delineated by their cellular localization, PGs can be divided into four groups: intracellular PGs, cell surface PGs, pericellular PGs, and extracellular PGs. Here, we summarize the pivotal roles of these PG subsets in tumor immunity.
SRGN is a unique intracellular PG found in hematopoietic cells, endothelial cells, fibroblasts, and some malignant cells that binds to inflammatory mediators to maintain them inside storage granules and secretory vesicles [100]. As exemplified by the role of SRGN in cytotoxic T lymphocytes (CTLs), it is a vector that carries granzyme B (GZMB), assisting in transporting GZMB to kill target cells [101]. SRGN can be secreted directly or by exosomes into the TME and then interacts with target ligands or receptors to regulate tumor immunity. In MM, SRGN specifically inhibits the classical and lectin pathways to protect tumor cells from attack by the complement system by binding to complement component 1q (C1q) and mannose-binding lectins (MBL) [102].
Cell surface PGs include syndecans, chondroitin sulfate proteoglycan 4 (CSPG-4), betaglycan, and glypicans, which are expressed in a variety of cancers. Syndecans consist of four members: syndecan-1, syndecan-2, syndecan-3, and syndecan-4, with HS predominantly attached to the ectodomain protein core. They also act as soluble heparan sulfate PGs (HSPGs). Syndecan-1 suppresses T-cell-mediated inflammation by binding to T-cell-specific chemokines with HS chains. A study proposed that syndecan-1 engages VEGFR2 and very late antigen-4 (VLA-4) and then causes VLA-4 S988 phosphorylation, which leads to immunosuppression [103]. Syndecan-2 expressed by tumor-associated stromal cells promotes the activation of TGF-β-mediated immunosuppressive genes, such as PD-L1 and C-X-C chemokine receptor type 4 (CXCR4) [104]. Syndecan-4 is the main HSPG expressed on the DC surface. It interacts with dendritic cell-associated heparan sulfate proteoglycan-integrin ligand (DC-HIL) via its HS chains, whose major function is to suppress T-cell activation [105]. Other cell surface PGs, such as CSPG-4, betaglycan, and glypicans, may play dual roles in modulating the TME and antitumor immunity [106].
Perlecan and arginine are multidomain HSPGs in pericellular regions that are expressed by tumor-associated stromal cells and cancer cells. The molecular states of these cells are disrupted by MMPs, sulfatases, and heparanases in the TME, which destroy the hostile stroma and reconstruct a tolerant stroma that is suitable for tumor progression and escape [107].
Extracellular PGs can be further divided into two groups: small leucine-rich PGs (biglycan, decorin, and lumican) and large extracellular PGs (versican and HA) [106]. Among small leucine-rich PGs, biglycan and decorin are usually acknowledged to play antagonistic roles in modulating the tumor immune response [108]. Biglycan facilitates the binding of TGF-β to its receptors, which initiates downstream signaling pathways and generates a protumor TME. In contrast, decorin binds to toll-like receptor (TLR) 2/4 on macrophages to promote tumor necrosis factor-α (TNF-α), C-C motif chemokine ligand 2 (CCL2), and interleukin-12 (IL-12) secretion [109]. Versican is a large matrix PG that engages with HA and linked proteins as well as cell surface proteins in the TME, such as CD44, P selectins, L selectins, and Toll-like receptor 2 (TLR2) [ 110, 111]. Tumor-derived versican dampens DC function by binding to TLR2 and forming a positive feedback loop characterized by the upregulation of IL-10/IL-6 receptors, resulting in tumor immunosuppression. HA is another important ECM component whose metabolic changes are strongly correlated with tumor-associated immune suppression. Hyaluronan synthases (HASs) catalyze the synthesis of HA, and hyaluronidase (Hyal) causes high molecular weight (HMW) HA to be fragmented into low molecular weight (LMW) HA. It appears that LMW-HA occurs in abundance in the TME because of increased Hyal expression or activity [112]. Increasing HA synthesis results in a high level of Hyal expression and ROS/NOS production, leading to HA fragmentation. On the one hand, LMW-HA bolsters the migration and proliferation of cancer cells by binding to CD44, RHAMM, and TLR2/4 [113]. On the other hand, LMW-HA formation activates fibroblasts and attracts tumor-associated neutrophils (TANs) and TAMs. Tumor-recruited Hyal2 + myeloid cells are activated in the TME to break down HA through the translocation of Hyal2 to the cell membrane, which is dependent on the CD44 signaling pathway [114]. In turn, such crosstalk promotes the differentiation of Hyal2 + myeloid cells into immunosuppressive PD-L1 + TAMs, thereby creating an immune exclusion environment [115].
Taken together, these results highlight the essential contribution of the ECM in modulating cancer-immune crosstalk. Regardless of the form, matrices containing collagens and proteoglycans link soluble factors in the TME to surface receptors on cells, which affects downstream signaling and mediates TME remodeling, thus impacting the tumor immune response. The delineated mechanisms underscore promising avenues for future therapeutic interventions aimed at targeting the ECM to modulate tumor immunity.
Glycan-targeted Immunotherapies
In recent years, immunotherapy has led to a boom in cancer treatment research. In comparison to traditional radio- and chemotherapies, immunotherapies such as immune checkpoint blockade have made substantial progress and elevated therapeutic efficacy. Nonetheless, most patients still show low or even no response to the current existing immunotherapies. Aided by fruitful findings on how glycosylation contributes to the tumor immune response, it is promising that new strategies combined with targeting glycans will be propitious for overcoming the drawbacks of current immunotherapies. Focusing on three areas, namely, lectins, immune checkpoints and the ECM, we outline recent advances in glycosylation-targeted therapeutics ( Table 4).
Table 4 Agents for glycan-targeted immunotherapies.
|
Category |
Target |
Agent |
Type |
Status |
Reference |
|
Siglecs |
Siglec7 |
1E8 |
mAb |
Preclinical |
|
|
Siglec9 |
AL009 |
Fc-fusion protein |
Preclinical |
||
|
Siglec15 |
NC318 |
mAb |
Phase II |
||
|
Sialic acid |
E-602, E-705 |
Antibody-sialidases conjugates |
Phase I/II, preclinical |
||
|
Peracetyl-3Fax-Neu5Ac, 5-N-triazole substituted sialosides, 9-N-sulfonamide substituted sialosides |
Sialic acid analogue |
Preclinical |
|||
|
Galectins |
GAL1 |
OTX008, 6DBF7, DB16, DB21 |
Inhibitor |
Preclinical |
|
|
GM-CT-01 |
Inhibitor |
Phase I/II |
|||
|
F8.G7 |
mAb |
Preclinical |
|||
|
GAL3 |
GCS-100 |
Inhibitor |
Phase II |
||
|
GAL9 |
P4D2 |
mAb |
Preclinical |
||
|
B7 family |
PD-L1-PD-1 |
2-DG |
Glucose analogue |
Phase I |
|
|
BMS1166 |
Inhibitor |
Preclinical |
|||
|
STM108, STM418, MW11-h317, MAb059c |
Glycan-targeted mAb |
Preclinical |
|||
|
B7H3 |
2F-Fuc |
Fucose analogue |
Preclinical |
||
|
B7H4 |
NGI-1 |
Inhibitor |
Preclinical |
||
|
ECM |
HA |
Hyaluronidase |
Enzyme |
Preclinical |
|
|
Collagen receptor |
PRTH-101 |
mAb |
Preclinical |
Lectins
Siglecs
Due to their expression patterns and immune-modulating functions, siglecs have emerged as appealing targets for cancer immunotherapy. One approach to block the immunosuppressive effects mediated by siglecs is through antibody-based therapies. For example, NC318, a monoclonal antibody (mAb) in a phase II clinical trial targeting siglec-15, exhibited remarkable efficacy by conferring prolonged and sustained relief to 54% of patients with refractory solid tumors [34]. Other monoclonal antibodies aimed at siglec-7/9 are undergoing preclinical development and have already demonstrated potent efficacy in reducing tumor burdens in murine models [116]. In addition, derivative antibodies, including Fc-fusion proteins, antibody-drug conjugates (ADCs), and antibody-enzyme fusions, herald a new era of therapeutics by regulating the siglec-sialic acid immune axis. AL009, an engineered Fc-fusion protein with siglec-9 ECD, competes with multiple inhibitory siglecs, thereby blocking their binding to corresponding sialic acid receptors [117]. Antibody-enzyme fusions, employing enzyme-antibody glycan-ligand editing (EAGLE) technology, combine sialidases with human mAbs, which selectively bind to tumor cells and erase overexpressed sialoglycans, thus potentiating the immune response. The drug candidate E602 is composed of two engineered sialidases and an antibody Fc fragment [118]. Although it can reverse the antitumor response both innately and adaptively in phase I clinical trials, the lack of specificity is an obstacle that limits its successful clinical translation. To overcome this drawback, anti-PD-L1 (E-705) or anti-HER2 with sialidases is designed to recognize specific tumor cells or immune cells to simultaneously remove sialic acids [ 118, 119].
Another complementary strategy involves the utilization of sialic acid analogues capable of disrupting de novo sialic acid synthesis and impeding the interaction between siglecs and sialic acids. Generally, sialic acid mimetics exhibit enhanced affinity toward the ligand-binding domain of siglecs. Modified sialic acids, such as peracetyl-3Fax-Neu5Ac, can either inhibit the production of cytidine monophosphate-N-acetyl-neuraminic acid (CMP-Neu5Ac) or sialyltransferases, leading to a reduction in sialic acid on the cell surface glycocalyx [120]. Additional small molecule inhibitors, including 5-N-triazole-substituted sialosides and 9-N-sulfonamide-substituted sialosides, play competitive roles in the interaction of most inhibitory siglecs [ 121, 122]. Beyond adopting these analogues individually, the application of liposomes, nanoparticles, and polymers as supporting materials to generate multivalent mimetics markedly enhances the avidity of siglecs and outcompetes natural ligands [ 123, 124].
Galectins
Within the intricate landscape of tumor immunology, the indispensability of galectins in facilitating tumor immune evasion has spurred investigations into the use of galectin inhibitors as adjuncts to immune checkpoint blockade. OTX008, an inhibitory agent of GAL1, binds to the GAL1 amphipathic β-sheet conformation rather than the CRD conformation and markedly decreases tumor cell proliferation, invasion, and angiogenesis both in vitro and in vivo [ 125– 127]. Similarly, anginex 6DBF7 and its derivatives DB16 and DB21 are noncompetitive allosteric inhibitors of Gal-1 and remain in the nascent stages of preclinical validation [128]. GM-CT-01, an α-galactomannan that targets a large surface area of GAL1, has shown no side effects on metastatic colorectal cancer patients in phase I or II clinical studies [129]. GCS-100, which is isolated from the peel and pulp of citrus fruits and modified with high pH and temperature, blocks GAL3 and has been demonstrated to potentiate antitumor effects in myeloma therapy [130]. GAL1 can also compromise anti-PD-1/PD-L1 therapy by regulating T cells or reprogramming other cells to disrupt T-cell homeostasis in the TME [ 7, 40, 43]. The highly specific neutralizing GAL1 mAb F8. G7 may reverse adverse outcomes [131]. Additionally, immune checkpoint molecules such as lymphocyte activation gene-3 (LAG-3), cytotoxic T-lymphocyte antigen 4 (CTLA-4), TIM-3, and PD-1 are binding partners for GAL3 or GAL9. Combined treatment with an anti-GAL3 or anti-GAL9 antibody (P4D2) and ICB has the potential to potentiate antitumor immunity [ 132– 134].
B7 family
Tumor cells highly express immunosuppressive molecules, partially the B7 family, commonly modified by a unique type of glycan. Aberrant glycosylation of these molecules, which is driven by dysregulated signaling pathways in tumor cells, plays a crucial role in immune evasion and tumor progression. Based on the mechanism underlying the regulation of B7 family glycosylation, novel strategies have been investigated to address cancer therapies in recent years.
Focusing on the diverse alterations of N-glycosylation in the B7 family, agents, including small molecules, carbohydrate analogues, and glycan-targeted mAbs, have accelerated the development of immunotherapies and provided new insights into treatment. The small molecule inhibitor BMS1166 blocks the transport of PD-L1 from the ER to the Golgi apparatus and its further glycosylation, preventing PD-L1/PD-1-mediated immune suppression [135]. PD-L1 deglycosylation mediated by the glucose analogue 2-deoxy-glucose (2-DG) inhibits the PD-1/PD-L1 interaction and promotes antitumor immunity via combined therapy with gefitinib (an EGFR inhibitor) [136]. N-linked glycosylation inhibitor-1 (NGI-1), a reversible inhibitor of STT3A/B, reduces the glycosylation of B7-H4, thereby contributing to its protein stability and immunosuppressive effects in TNBC [83]. In addition, FUT8-mediated core fucosylation promotes tumor immune evasion by inhibiting the degradation of B7-H3 in TNBC. The combination of the fucose analogue 2F-Fuc [80] with an anti-PD-1 antibody synergistically promotes an antitumor immune response. Moreover, the concomitant administration of O-GlcNAc, which inhibits OSMI4, along with an anti-PD-L1 antibody further bolsters the antitumor immune response [74]. Thus, these inhibitors are instrumental in enhancing current cancer therapies in future clinical trials.
Glycan-targeted mAbs are also feasible approaches for tumor immunotherapy. STM108 is a mAb that recognizes the B3GNT3-mediated poly-LacNAc moiety modification on the N192/N200 sites of glycosylated PD-L1. When STM108 binds to PD-L1, the interaction between PD-1 and PD-L1 is blocked, and PD-L1 internalization is induced [70]. In turn, PD-1 is an N-glycosylated ligand for PD-L1 and PD-L2. The N-glycans at the N49, N58, N74 and N116 sites are important for the stability and binding of PD-L1. Compared with anti-PD-1 antibodies (nivolumab and pembrolizumab) approved by the FDA, the mAb STM418, which targets the PD-1 N58 site, exhibits greater binding affinity to PD-1, which strongly attenuates the PD-1/PD-L1 or PD-1/PD-L2 interaction, thereby enhancing antitumor efficacy [137]. Other mAbs targeting the PD-1 N58 site, such as MW11-H317 and MAb059c, also show specific binding with the PD-1 N-glycosylated antigen, resulting in a T-cell-mediated immune response and effective inhibition of tumor growth in a mouse model [ 138, 139].
ECM
As mentioned before, the tumor-associated ECM is a barrier to immune cell infiltration. Increased HA synthesis in the TME contributes to the malignant phenotypes of many cancers. The adoption of hyaluronidase in preclinical BC and melanoma models enhances the penetration of anti-PD-L1 antibodies and cancer vaccines, thus increasing therapeutic effectiveness [ 140, 141]. Furthermore, HA-mediated ECM eradication therapy enlarges the population of antigen-specific CD8 + T cells, DCs, and macrophages in tumors and stimulates DC maturation, which induces powerful antitumor effects in cancer therapy [ 141, 142]. Blockade of ECM-binding receptors provides opportunities for cancer intervention. Several studies have demonstrated that targeting LAIR-1 in tumor models fosters immune cell activation [ 99, 143, 144]. LAIR-2 is a natural receptor that has a greater affinity for collagens [143]. Taking advantage of this mechanism, the LAIR-2 Fc fusion protein is designed to shut down the signaling pathway mediated by LAIR-1, showing notable antitumor effects [ 144, 145]. The ECD of DDR1 mediates collagen fibril alignment by binding with collagen and impeding immune cell permeability, revealing the role of DDR1-ECD in immunosuppression [96]. Recently, the humanized DDR1 antibody PRTH-101 has been proven to destroy collagen fiber alignment and increase CD8 + T-cell infiltration in tumor-bearing mice [146]. Accompanied by the accelerating focus on ECM-binding immune receptors, drugs targeting these receptors or combined therapies will continue to be developed in the future.
Tumor vaccines and chimeric antigen receptor-redirected T cells (CAR-T cells) can serve as novel approaches for targeting the intratumoral ECM. In a polyoma middle T oncoprotein mouse breast cancer model, vaccination targeting the extradomain A (EDA) of fibronectin increased macrophage infiltration and decreased tumor metastasis [147]. Fibroblast activation protein (FAP) is a key regulator of ECM remodeling and is overexpressed by CAFs. Moreover, FAP-specific CAR-T cells dramatically induce ECM degradation and suppress PDAC progression [148]. However, due to the rareness of cancer-specific ECM targets, there is still a long way to go in terms of clinical translation for tumor vaccines and CAR therapy.
Conclusions and Prospective
Glycosylation is the most abundant and diverse form of PTM in eukaryotic cells. Sugar donors, glycosyltransferases, glycosidases, and receptors are indispensable components in the process of glycosylation, which guarantees that glycans are synthesized precisely. Fourteen glycosylation pathways, at least 173 glycosyltransferases, and other enzymes result in complex glycan structures and multiple functions of glycoproteins. The emerging field of glycobiology has shed light on the intricate interplay between glycosylation and the immune response. Aberrant glycosylation has been recognized as a hallmark of cancer. Compared with normal cells, many types of glycan alterations, including increased or incomplete glycan synthesis, hypersialylation, and elevated fucosylation, occur in tumor cells. These changes provide a more thorough profile of the tumor immune response, immune regulation, and cancer progression. The striking roles of glycans in the tumor immune response have been uncovered in the last decade and have significantly broadened the scope of cancer biology. Here, we summarize recent studies depicting how glycosylation can influence endogenous lectins, the B7 family, and the ECM, thereby remodeling the TME and protecting tumor cells from attack by the immune system. In addition, we summarize the latest advancements in tumor immunotherapy based on aberrant glycosylation, thereby providing a robust impetus for drug development and clinical interventions.
Despite substantial advancements in comprehending the pivotal role of glycosylation in the tumor immune response, research in this area still faces some challenges. For example, the complexity and diversity of glycosylation patterns pose major difficulties in elucidating the precise mechanisms by which specific glycosylation contributes to tumor progression and immune evasion. Additionally, while the interaction between glycosylation and the immune system in the tumor microenvironment has garnered considerable attention, the translation of such insights into clinical applications, such as effective glycan-based cancer immunotherapies, remains a formidable task due to the lack of specific tools for comprehensive glycan analysis. However, with the development of mass spectrometry (MS)-based glycoproteomic technology, glycan microarrays, and glycan sequencing at the single-molecule level, the precise differences in glycan structure and the resulting abnormalities in protein functions have gradually been revealed in recent years. In addition, combining multiomics data and glycomics data will accelerate the understanding of abnormal glycosylation processes in cancer development. Furthermore, it is foreseeable that the progressive acquisition and understanding of new knowledge regarding tumor glycobiology will facilitate the development of small molecule inhibitors, glycan-targeted antibodies, and other therapeutic strategies.
Supporting information
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 92353303 to W.Y., and 32201045 to Q.Z).
References
- 1.Schjoldager KT, Narimatsu Y, Joshi HJ, Clausen H. Global view of human protein glycosylation pathways and functions. Nat Rev Mol Cell Biol. . 2020;21:729–749. doi: 10.1038/s41580-020-00294-x. [DOI] [PubMed] [Google Scholar]
- 2.Esmail S, Manolson MF. Advances in understanding N-glycosylation structure, function, and regulation in health and disease. Eur J Cell Biol. . 2021;100:151186–151192. doi: 10.1016/j.ejcb.2021.151186. [DOI] [PubMed] [Google Scholar]
- 3.Wandall HH, Nielsen MAI, King‐Smith S, de Haan N, Bagdonaite I. Global functions of O‐glycosylation: promises and challenges in O‐glycobiology. FEBS J. . 2021;288:7183–7212. doi: 10.1111/febs.16148. [DOI] [PubMed] [Google Scholar]
- 4.Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol. . 2017;18:452–465. doi: 10.1038/nrm.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. . 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 6.Dalotto-Moreno T, Croci DO, Cerliani JP, Martinez-Allo VC, Dergan-Dylon S, Méndez-Huergo SP, Stupirski JC, et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. . 2013;73:1107–1117. doi: 10.1158/0008-5472.CAN-12-2418. [DOI] [PubMed] [Google Scholar]
- 7.Nambiar DK, Aguilera T, Cao H, Kwok S, Kong C, Bloomstein J, Wang Z, et al. Galectin-1–driven T cell exclusion in the tumor endothelium promotes immunotherapy resistance. J Clin Invest. . 2019;129:5553–5567. doi: 10.1172/JCI129025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kouo T, Huang L, Pucsek AB, Cao M, Solt S, Armstrong T, Jaffee E. Galectin-3 shapes antitumor immune responses by suppressing CD8+ T cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol Res. . 2015;3:412–423. doi: 10.1158/2326-6066.CIR-14-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. . 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 10.Hudak JE, Canham SM, Bertozzi CR. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat Chem Biol. . 2014;10:69–75. doi: 10.1038/nchembio.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly J, Sarkar S, Natoni A, Stark JC, Riley NM, Bertozzi CR, Carlsten M, et al. Targeting hypersialylation in multiple myeloma represents a novel approach to enhance NK cell-mediated tumor responses. Blood Adv. . 2022;6:3352–3366. doi: 10.1182/bloodadvances.2021006805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith BAH, Deutzmann A, Correa KM, Delaveris CS, Dhanasekaran R, Dove CG, Sullivan DK, et al. MYC-driven synthesis of Siglec ligands is a glycoimmune checkpoint. Proc Natl Acad Sci USA. . 2023;120:e2215376120. doi: 10.1073/pnas.2215376120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez E, Boelaars K, Brown K, Eveline Li RJ, Kruijssen L, Bruijns SCM, van Ee T, et al. Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the Siglec receptors Siglec-7 and Siglec-9. Nat Commun. . 2021;12:1270. doi: 10.1038/s41467-021-21550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmassmann P, Roux J, Buck A, Tatari N, Hogan S, Wang J, Rodrigues Mantuano N, et al. Targeting the Siglec-sialic acid axis promotes antitumor immune responses in preclinical models of glioblastoma. Sci Transl Med. . 2023;15:eadf5302. doi: 10.1126/scitranslmed.adf5302. [DOI] [PubMed] [Google Scholar]
- 15.Xiao L, Guan X, Xiang M, Wang Q, Long Q, Yue C, Chen L, et al. B7 family protein glycosylation: promising novel targets in tumor treatment. Front Immunol. . 2022;13:1088560. doi: 10.3389/fimmu.2022.1088560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanczak MA, Läubli H. Siglec receptors as new immune checkpoints in cancer. Mol Aspects Med. . 2023;90:101112. doi: 10.1016/j.mam.2022.101112. [DOI] [PubMed] [Google Scholar]
- 17.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. . 2014;14:653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. . 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 19.Büll C, Nason R, Sun L, Van Coillie J, Madriz Sørensen D, Moons SJ, Yang Z, et al. Probing the binding specificities of human Siglecs by cell-based glycan arrays. Proc Natl Acad Sci USA. . 2021;118:e2026102118. doi: 10.1073/pnas.2026102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang LY, Liang SY, Lu SC, Tseng HC, Tsai HY, Tang CJ, Sugata M, et al. Molecular basis and role of siglec-7 ligand expression on chronic lymphocytic leukemia b cells. Front Immunol. . 2022;13:840388. doi: 10.3389/fimmu.2022.840388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wisnovsky S, Möckl L, Malaker SA, Pedram K, Hess GT, Riley NM, Gray MA, et al. Genome-wide CRISPR screens reveal a specific ligand for the glycan-binding immune checkpoint receptor Siglec-7. Proc Natl Acad Sci USA. . 2021;118:e2015024118. doi: 10.1073/pnas.2015024118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anwar MT, Kawade SK, Huo YR, Adak AK, Sridharan D, Kuo YT, Fan CY, et al. Sugar nucleotide regeneration system for the synthesis of Bi- and triantennary N-glycans and exploring their activities against siglecs. Eur J Med Chem. . 2022;232:114146. doi: 10.1016/j.ejmech.2022.114146. [DOI] [PubMed] [Google Scholar]
- 23.Lustig M, Chan C, Jansen JHM, Bräutigam M, Kölling MA, Gehlert CL, Baumann N, et al. Disruption of the sialic acid/Siglec-9 axis improves antibody-mediated neutrophil cytotoxicity towards tumor cells. Front Immunol. . 2023;14:1178817. doi: 10.3389/fimmu.2023.1178817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gianchecchi E, Arena A, Fierabracci A. Sialic acid-siglec axis in human immune regulation, involvement in autoimmunity and cancer and potential therapeutic treatments. Int J Mol Sci. . 2021;22:5774. doi: 10.3390/ijms22115774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beatson R, Tajadura-Ortega V, Achkova D, Picco G, Tsourouktsoglou TD, Klausing S, Hillier M, et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat Immunol. . 2016;17:1273–1281. doi: 10.1038/ni.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beatson R, Graham R, Grundland Freile F, Cozzetto D, Kannambath S, Pfeifer E, Woodman N, et al. Cancer-associated hypersialylated MUC1 drives the differentiation of human monocytes into macrophages with a pathogenic phenotype. Commun Biol. . 2020;3:644. doi: 10.1038/s42003-020-01359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y, Liu Q, Xie Y, Zhu J, Zhang S, Ge Y, Guo J, et al. MUC16 stimulates neutrophils to an inflammatory and immunosuppressive phenotype in ovarian cancer. J Ovarian Res. . 2023;16:181. doi: 10.1186/s13048-023-01207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belisle JA, Horibata S, Jennifer GA, Petrie S, Kapur A, André S, Gabius HJ, et al. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol Cancer. . 2010;9:118. doi: 10.1186/1476-4598-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mei Y, Wang X, Zhang J, Liu D, He J, Huang C, Liao J, et al. Siglec-9 acts as an immune-checkpoint molecule on macrophages in glioblastoma, restricting T-cell priming and immunotherapy response. Nat Cancer. . 2023;4:1273–1291. doi: 10.1038/s43018-023-00598-9. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, He M, Zhang C, Cao K, Zhang G, Yang M, Huang Y, et al. Siglec-9 + tumor-associated macrophages delineate an immunosuppressive subset with therapeutic vulnerability in patients with high-grade serous ovarian cancer . J Immunother Cancer. . 2023;11:e007099. doi: 10.1136/jitc-2023-007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.arkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, Krishnan V, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. . 2019;572:392–396. doi: 10.1038/s41586-019-1456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin SS, Gao FH. Molecular mechanism of tumor cell immune escape mediated by CD24/Siglec-10. Front Immunol. . 2020;11:1324. doi: 10.3389/fimmu.2020.01324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toh BH, Kyaw T, Tipping P, Bobik A. Immune regulation by CD52-expressing CD4 T cells. Cell Mol Immunol. . 2013;10:379–382. doi: 10.1038/cmi.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun J, Lu Q, Sanmamed MF, Wang J. Siglec-15 as an emerging target for Next-generation cancer immunotherapy. Clin Cancer Res. . 2021;27:680–688. doi: 10.1158/1078-0432.CCR-19-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angata T, Tabuchi Y, Nakamura K, Nakamura M. Siglec-15: an immune system Siglec conserved throughout vertebrate evolution. Glycobiology. . 2007;17:838–846. doi: 10.1093/glycob/cwm049. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Xu Z, Wu KL, Yu L, Wang C, Ding H, Gao Y, et al. Siglec-15/sialic acid axis as a central glyco-immune checkpoint in breast cancer bone metastasis. Proc Natl Acad Sci USA. . 2024;121:e2312929121. doi: 10.1073/pnas.2312929121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenza MP, Egia-Mendikute L, Antoñana-Vildosola A, Soares CO, Coelho H, Corzana F, Bosch A, et al. Structural insights into Siglec-15 reveal glycosylation dependency for its interaction with T cells through integrin CD11b. Nat Commun. . 2023;14:3496. doi: 10.1038/s41467-023-39119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao C, Wang Q, An J, Chen J, Li X, Long Q, Xiao L, et al. CD44 glycosylation as a therapeutic target in oncology. Front Oncol. . 2022;12:883831. doi: 10.3389/fonc.2022.883831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mariño KV, Cagnoni AJ, Croci DO, Rabinovich GA. Targeting galectin-driven regulatory circuits in cancer and fibrosis. Nat Rev Drug Discov. . 2023;22:295–316. doi: 10.1038/s41573-023-00636-2. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Z, Zhang W, Sha G, Wang D, Tang D. Galectins are central mediators of immune escape in pancreatic ductal adenocarcinoma. Cancers. . 2022;14:5475. doi: 10.3390/cancers14225475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Y, Wang HC, Zhao J, Wu MH, Shih TC. Immunosuppressive roles of galectin-1 in the tumor microenvironment. Biomolecules. . 2021;11:1398. doi: 10.3390/biom11101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yazawa EM, Geddes-Sweeney JE, Cedeno-Laurent F, Walley KC, Barthel SR, Opperman MJ, Liang J, et al. Melanoma cell galectin-1 ligands functionally correlate with malignant potential. J Investig Dermatol. . 2015;135:1849–1862. doi: 10.1038/jid.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection. Cancer Cell. . 2004;5:241–251. doi: 10.1016/S1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 44.Kuo PL, Hung JY, Huang SK, Chou SH, Cheng DE, Jong YJ, Hung CH, et al. Lung cancer-derived galectin-1 mediates dendritic cell anergy through inhibitor of DNA binding 3/IL-10 signaling pathway. J Immunol. . 2011;186:1521–1530. doi: 10.4049/jimmunol.1002940. [DOI] [PubMed] [Google Scholar]
- 45.Cagnoni AJ, Giribaldi ML, Blidner AG, Cutine AM, Gatto SG, Morales RM, Salatino M, et al. Galectin-1 fosters an immunosuppressive microenvironment in colorectal cancer by reprogramming CD8 + regulatory T cells . Proc Natl Acad Sci USA. . 2021;118:e2102950118. doi: 10.1073/pnas.2102950118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuo P, Le QT. Galectin-1 links tumor hypoxia and radiotherapy. Glycobiology. . 2014;24:921–925. doi: 10.1093/glycob/cwu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Q, Tian Y, Zhang J, Zhang H, Gu F, Lu Y, Zou S, et al. Functions of pancreatic stellate cell-derived soluble factors in the microenvironment of pancreatic ductal carcinoma. Oncotarget. . 2017;8:102721–102738. doi: 10.18632/oncotarget.21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cui R, Yue W, Lattime EC, Stein MN, Xu Q, Tan XL. Targeting tumor-associated macrophages to combat pancreatic cancer. Oncotarget. . 2016;7:50735–50754. doi: 10.18632/oncotarget.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce t cell death. J Immunol. . 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- 50.Dimitrijevic Stojanovic M, Stojanovic B, Radosavljevic I, Kovacevic V, Jovanovic I, Stojanovic BS, Prodanovic N, et al. Galectin-3’s complex interactions in pancreatic ductal adenocarcinoma: from cellular signaling to therapeutic potential. Biomolecules. . 2023;13:1500. doi: 10.3390/biom13101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonnermann D, Oberg HH, Lettau M, Peipp M, Bauerschlag D, Sebens S, Kabelitz D, et al. Galectin-3 released by pancreatic ductal adenocarcinoma suppresses γδ T cell proliferation but not their cytotoxicity. Front Immunol. . 2020;11:1328. doi: 10.3389/fimmu.2020.01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novak R, Dabelic S, Dumic J. Galectin-1 and galectin-3 expression profiles in classically and alternatively activated human macrophages. Biochim Biophys Acta Gen Subj. . 2012;1820:1383–1390. doi: 10.1016/j.bbagen.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 53.Farhad M, Rolig AS, Redmond WL. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. OncoImmunology. . 2018;7:e1434467. doi: 10.1080/2162402X.2018.1434467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C, Zhu C, et al. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory t cells. Immunity. . 2014;41:270–282. doi: 10.1016/j.immuni.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ni X, Wu W, Sun X, Ma J, Yu Z, He X, Cheng J, et al. Interrogating glioma-M2 macrophage interactions identifies Gal-9/Tim-3 as a viable target against PTEN-null glioblastoma . Sci Adv. . 2022;8:eabl5165. doi: 10.1126/sciadv.abl5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daley D, Mani VR, Mohan N, Akkad N, Ochi A, Heindel DW, Lee KB, et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat Med. . 2017;23:556–567. doi: 10.1038/nm.4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Liu X, Ge R, Yin Y, Liu Y, Lu W, Huang M, et al. The ligation between ERMAP, galectin-9 and dectin-2 promotes Kupffer cell phagocytosis and antitumor immunity. Nat Immunol. . 2023;24:1813–1824. doi: 10.1038/s41590-023-01634-7. [DOI] [PubMed] [Google Scholar]
- 58.Mori Y, Akita K, Yashiro M, Sawada T, Hirakawa K, Murata T, Nakada H. Binding of Galectin-3, a β-Galactoside-binding lectin, to MUC1 protein enhances phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) and akt, promoting tumor cell malignancy. J Biol Chem. . 2015;290:26125–26140. doi: 10.1074/jbc.M115.651489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piyush T, Chacko AR, Sindrewicz P, Hilkens J, Rhodes JM, Yu LG. Interaction of galectin-3 with MUC1 on cell surface promotes EGFR dimerization and activation in human epithelial cancer cells. Cell Death Differ. . 2017;24:1937–1947. doi: 10.1038/cdd.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang R, Sun L, Li CF, Wang YH, Yao J, Li H, Yan M, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. . 2021;12:832. doi: 10.1038/s41467-021-21099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yasinska IM, Meyer NH, Schlichtner S, Hussain R, Siligardi G, Casely-Hayford M, Fiedler W, et al. Ligand-receptor interactions of galectin-9 and VISTA suppress human T lymphocyte cytotoxic activity. Front Immunol. . 2020;11:580557. doi: 10.3389/fimmu.2020.580557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harris RJ, Willsmore Z, Laddach R, Crescioli S, Chauhan J, Cheung A, Black A, et al. Enriched circulating and tumor-resident TGF-β + regulatory B cells in patients with melanoma promote FOXP3 + Tregs . OncoImmunology. . 2022;11:2104426. doi: 10.1080/2162402X.2022.2104426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang W, Qiu Y, Xie X, Fu Y, Wang L, Cai Z. B7 family members in lymphoma: promising novel targets for tumor immunotherapy? Front Oncol. . 2021;11:647526. doi: 10.3389/fonc.2021.647526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang H, Yao H, Li C, Shi H, Lan J, Li Z, Zhang Y, et al. HIP1R targets PD-L1 to lysosomal degradation to alter T cell-mediated cytotoxicity. Nat Chem Biol. . 2019;15:42–50. doi: 10.1038/s41589-018-0161-x. [DOI] [PubMed] [Google Scholar]
- 65.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. . 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 66.Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, Khoo KH, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. . 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee HH, Wang YN, Xia W, Chen CH, Rau KM, Ye L, Wei Y, et al. Removal of N-Linked glycosylation enhances PD-L1 detection and predicts Anti-PD-1/PD-L1 therapeutic efficacy. Cancer Cell. . 2019;36:168–178.e4. doi: 10.1016/j.ccell.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsu JM, Xia W, Hsu YH, Chan LC, Yu WH, Cha JH, Chen CT, et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat Commun. . 2018;9:1908. doi: 10.1038/s41467-018-04313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi S, Gu S, Han T, Zhang W, Huang L, Li Z, Pan D, et al. Inhibition of MAN2A1 enhances the immune response to anti–PD-L1 in human tumors. Clin Cancer Res. . 2020;26:5990–6002. doi: 10.1158/1078-0432.CCR-20-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li CW, Lim SO, Chung EM, Kim YS, Park AH, Yao J, Cha JH, et al. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell. . 2018;33:187–201.e10. doi: 10.1016/j.ccell.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leng X, Wei S, Mei J, Deng S, Yang Z, Liu Z, Guo C, et al. Identifying the prognostic significance of B3GNT3 with PD-L1 expression in lung adenocarcinoma. Transl Lung Cancer Res. . 2021;10:965–980. doi: 10.21037/tlcr-21-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui Y, Li J, Zhang P, Yin D, Wang Z, Dai J, Wang W, et al. B4GALT1 promotes immune escape by regulating the expression of PD-L1 at multiple levels in lung adenocarcinoma. J Exp Clin Cancer Res. . 2023;42:146. doi: 10.1186/s13046-023-02711-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang J, Zhang G, Zhang W, Bai L, Wang L, Li T, Yan L, et al. Loss of RBMS1 promotes anti-tumor immunity through enabling PD-L1 checkpoint blockade in triple-negative breast cancer. Cell Death Differ. . 2022;29:2247–2261. doi: 10.1038/s41418-022-01012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu Q, Wang H, Chai S, Xu L, Lin B, Yi W, Wu L. O-GlcNAcylation promotes tumor immune evasion by inhibiting PD-L1 lysosomal degradation . Proc Natl Acad Sci USA. . 2023;120:e2216796120. doi: 10.1073/pnas.2216796120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Youngnak P, Kozono Y, Kozono H, Iwai H, Otsuki N, Jin H, Omura K, et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun. . 2003;307:672–677. doi: 10.1016/S0006-291X(03)01257-9. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Chung Y, Bishop C, Daugherty B, Chute H, Holst P, Kurahara C, et al. Regulation of T cell activation and tolerance by PDL2. Proc Natl Acad Sci USA. . 2006;103:11695–11700. doi: 10.1073/pnas.0601347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu Y, Gao Z, Hu R, Wang Y, Wang Y, Su Z, Zhang X, et al. PD-L2 glycosylation promotes immune evasion and predicts anti-EGFR efficacy. J Immunother Cancer. . 2021;9:e002699. doi: 10.1136/jitc-2021-002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qiao Y, Liu C, Zhang X, Zhou Q, Li Y, Xu Y, Gao Z, et al. PD-L2 based immune signature confers poor prognosis in HNSCC. OncoImmunology. . 2021;10:1947569. doi: 10.1080/2162402X.2021.1947569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao B, Li H, Xia Y, Wang Y, Wang Y, Shi Y, Xing H, et al. Immune checkpoint of B7-H3 in cancer: from immunology to clinical immunotherapy. J Hematol Oncol. . 2022;15:153. doi: 10.1186/s13045-022-01364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang Y, Zhang HL, Li ZL, Du T, Chen YH, Wang Y, Ni HH, et al. FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nat Commun. . 2021;12:2672. doi: 10.1038/s41467-021-22618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prasad DVR, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates t cell activation. Immunity. . 2003;18:863–873. doi: 10.1016/S1074-7613(03)00147-X. [DOI] [PubMed] [Google Scholar]
- 82.Li J, Lee Y, Li Y, Jiang Y, Lu H, Zang W, Zhao X, et al. Co-inhibitory molecule B7 superfamily member 1 expressed by tumor-infiltrating myeloid cells induces dysfunction of anti-tumor CD8+ T cells. Immunity. . 2018;48:773–786.e5. doi: 10.1016/j.immuni.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 83.Song X, Zhou Z, Li H, Xue Y, Lu X, Bahar I, Kepp O, et al. Pharmacologic suppression of B7-H4 glycosylation restores antitumor immunity in immune-cold breast cancers. Cancer Discov. . 2020;10:1872–1893. doi: 10.1158/2159-8290.CD-20-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma XM, Luo YF, Zeng FF, Su C, Liu X, Li XP, Lu J. TGF-β1-Mediated PD-L1 glycosylation contributes to immune escape via c-Jun/STT3A pathway in nasopharyngeal carcinoma. Front Oncol. . 2022;12:815437. doi: 10.3389/fonc.2022.815437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mao C, Li J, Feng L, Gao W. Beyond antibody fucosylation: Α-(1,6)-fucosyltransferase (Fut8) as a potential new therapeutic target for cancer immunotherapy. Antibody Ther. . 2023;6:87–96. doi: 10.1093/abt/tbad004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Su H, Karin M. Collagen architecture and signaling orchestrate cancer development. Trends Cancer. . 2023;9:764–773. doi: 10.1016/j.trecan.2023.06.002. [DOI] [PubMed] [Google Scholar]
- 87.Pankova D, Chen Y, Terajima M, Schliekelman MJ, Baird BN, Fahrenholtz M, Sun L, et al. Cancer-associated fibroblasts induce a collagen cross-link switch in tumor stroma. Mol Cancer Res. . 2016;14:287–295. doi: 10.1158/1541-7786.MCR-15-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nissen NI, Karsdal M, Willumsen N. Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J Exp Clin Cancer Res. . 2019;38:115. doi: 10.1186/s13046-019-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hartmann N, Giese NA, Giese T, Poschke I, Offringa R, Werner J, Ryschich E. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin Cancer Res. . 2014;20:3422–3433. doi: 10.1158/1078-0432.CCR-13-2972. [DOI] [PubMed] [Google Scholar]
- 90.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. . 2012;122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.LaRue MM, Parker S, Puccini J, Cammer M, Kimmelman AC, Bar-Sagi D. Metabolic reprogramming of tumor-associated macrophages by collagen turnover promotes fibrosis in pancreatic cancer. Proc Natl Acad Sci USA. . 2022;119:e2119168119. doi: 10.1073/pnas.2119168119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flies DB, Langermann S, Jensen C, Karsdal MA, Willumsen N. Regulation of tumor immunity and immunotherapy by the tumor collagen extracellular matrix. Front Immunol. . 2023;14:1199513. doi: 10.3389/fimmu.2023.1199513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caron J, Han X, Lary C, Sathyanarayana P, Remick S, Ernstoff M, Herlyn M, et al. Targeting the secreted RGDKGE collagen fragment reduces PD‑L1 by a proteasome‑dependent mechanism and inhibits tumor growth. Oncol Rep. . 2023;49:44. doi: 10.3892/or.2023.8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Y, Yang S, Tavormina J, Tampe D, Zeisberg M, Wang H, Mahadevan KK, et al. Oncogenic collagen I homotrimers from cancer cells bind to α3β1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell. . 2022;40:818–834.e9. doi: 10.1016/j.ccell.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deng J, Kang Y, Cheng CC, Li X, Dai B, Katz MH, Men T, et al. DDR1-induced neutrophil extracellular traps drive pancreatic cancer metastasis. JCI Insight. . 2021;6:e146133. doi: 10.1172/jci.insight.146133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun X, Wu B, Chiang HC, Deng H, Zhang X, Xiong W, Liu J, et al. Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature. . 2021;599:673–678. doi: 10.1038/s41586-021-04057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lebbink RJ, de Ruiter T, Adelmeijer J, Brenkman AB, van Helvoort JM, Koch M, Farndale RW, et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med. . 2006;203:1419–1425. doi: 10.1084/jem.20052554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rygiel TP, Stolte EH, de Ruiter T, van de Weijer ML, Meyaard L. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol Immunol. . 2011;49:402–406. doi: 10.1016/j.molimm.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 99.Peng DH, Rodriguez BL, Diao L, Chen L, Wang J, Byers LA, Wei Y, et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8+ T cell exhaustion. Nat Commun. . 2020;11:4520. doi: 10.1038/s41467-020-18298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kolset SO, Tveit H. Serglycin—structure and biology. Cell Mol Life Sci. . 2008;65:1073–1085. doi: 10.1007/s00018-007-7455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Metkar SS, Wang B, Aguilar-Santelises M, Raja SM, Uhlin-Hansen L, Podack E, Trapani JA, et al. Cytotoxic cell granule-mediated apoptosis. Immunity. . 2002;16:417–428. doi: 10.1016/S1074-7613(02)00286-8. [DOI] [PubMed] [Google Scholar]
- 102.Skliris A, Happonen KE, Terpos E, Labropoulou V, Børset M, Heinegård D, Blom AM, et al. Serglycin inhibits the classical and lectin pathways of complement via its glycosaminoglycan chains: implications for multiple myeloma. Eur J Immunol. . 2011;41:437–449. doi: 10.1002/eji.201040429. [DOI] [PubMed] [Google Scholar]
- 103.Jung O, Beauvais DLM, Adams KM, Rapraeger AC. VLA-4 phosphorylation during tumor and immune cell migration relies on its coupling to VEGFR2 and CXCR4 by syndecan-1. J Cell Sci. . 2019;132:jcs232645. doi: 10.1242/jcs.232645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Loftus PG, Watson L, Deedigan LM, Camarillo‐Retamosa E, Dwyer RM, O'Flynn L, Alagesan S, et al. Targeting stromal cell Syndecan‐2 reduces breast tumour growth, metastasis and limits immune evasion. Intl J Cancer. . 2021;148:1245–1259. doi: 10.1002/ijc.33383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chung JS, Sato K, Dougherty II, Cruz Jr PD, Ariizumi K. DC-HIL is a negative regulator of T lymphocyte activation. Blood. . 2007;109:4320–4327. doi: 10.1182/blood-2006-11-053769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deb G, Cicala A, Papadas A, Asimakopoulos F. Matrix proteoglycans in tumor inflammation and immunity. Am J Physiol Cell Physiol. . 2022;323:C678–C693. doi: 10.1152/ajpcell.00023.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cruz LA, Tellman TV, Farach-Carson MC. Flipping the molecular switch: influence of perlecan and its modifiers in the tumor microenvironment [M]//BIRBRAIR A. Tumor Microenvironment: Extracellular Matrix Components–Part A. Cham, Springer International Publishing. 2020: 133–146 . [DOI] [PubMed]
- 108.Diehl V, Huber LS, Trebicka J, Wygrecka M, Iozzo RV, Schaefer L. The role of decorin and biglycan signaling in tumorigenesis. Front Oncol. . 2021;11:801801. doi: 10.3389/fonc.2021.801801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schaefer L, Tredup C, Gubbiotti MA, Iozzo RV. Proteoglycan neofunctions: regulation of inflammation and autophagy in cancer biology. FEBS J. . 2017;284:10–26. doi: 10.1111/febs.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matsumoto K, Shionyu M, Go M, Shimizu K, Shinomura T, Kimata K, Watanabe H. Distinct interaction of versican/PG-M with hyaluronan and link protein. J Biol Chem. . 2003;278:41205–41212. doi: 10.1074/jbc.M305060200. [DOI] [PubMed] [Google Scholar]
- 111.Kawashima H, Hirose M, Hirose J, Nagakubo D, Plaas AHK, Miyasaka M. Binding of a large chondroitin sulfate/dermatan sulfate proteoglycan, versican, to L-selectin, P-selectin, and CD44. J Biol Chem. . 2000;275:35448–35456. doi: 10.1074/jbc.M003387200. [DOI] [PubMed] [Google Scholar]
- 112.Stern R. Hyaluronidases in cancer biology. Semin Cancer Biol. . 2008;18:275–280. doi: 10.1016/j.semcancer.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 113.Liu M, Tolg C, Turley E. Dissecting the dual nature of hyaluronan in the tumor microenvironment. Front Immunol. . 2019;10:947. doi: 10.3389/fimmu.2019.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dominguez-Gutierrez PR, Kwenda EP, Donelan W, O'Malley P, Crispen PL, Kusmartsev S. Hyal2 expression in tumor-associated myeloid cells mediates cancer-related inflammation in bladder cancer. Cancer Res. . 2021;81:648–657. doi: 10.1158/0008-5472.CAN-20-1144. [DOI] [PubMed] [Google Scholar]
- 115.Donelan W, Dominguez-Gutierrez PR, Kusmartsev S. Deregulated hyaluronan metabolism in the tumor microenvironment drives cancer inflammation and tumor-associated immune suppression. Front Immunol. . 2022;13:971278. doi: 10.3389/fimmu.2022.971278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ibarlucea-Benitez I, Weitzenfeld P, Smith P, Ravetch JV. Siglecs-7/9 function as inhibitory immune checkpoints in vivo and can be targeted to enhance therapeutic antitumor immunity . Proc Natl Acad Sci USA. . 2021;118:e2107424118. doi: 10.1073/pnas.2107424118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nalle S, Lam H, Barner C, Long H, Liang S, Rosenthal A, Maslyar D. Abstract 613: AL009 is a multi-Siglec inhibitor engineered to bind myeloid cells that enhances innate and adaptive immunity to cancer. Cancer Res. . 2022;82:613. doi: 10.1158/1538-7445.AM2022-613. [DOI] [Google Scholar]
- 118.Läubli H, Nalle SC, Maslyar D. Targeting the siglec-sialic acid immune axis in cancer: current and future approaches. Cancer Immunol Res. . 2022;10:1423–1432. doi: 10.1158/2326-6066.CIR-22-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith BAH, Bertozzi CR. The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans. Nat Rev Drug Discov. . 2021;20:217–243. doi: 10.1038/s41573-020-00093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rillahan CD, Antonopoulos A, Lefort CT, Sonon R, Azadi P, Ley K, Dell A, et al. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat Chem Biol. . 2012;8:661–668. doi: 10.1038/nchembio.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prescher H, Gütgemann S, Frank M, Kuhfeldt E, Watzl C, Brossmer R. Synthesis and biological evaluation of 9-oxamyl sialosides as Siglec-7 ligands. Bioorg Med Chem. . 2015;23:5915–5921. doi: 10.1016/j.bmc.2015.06.069. [DOI] [PubMed] [Google Scholar]
- 122.Murugesan G, Weigle B, Crocker PR. Siglec and anti-Siglec therapies. Curr Opin Chem Biol. . 2021;62:34–42. doi: 10.1016/j.cbpa.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 123.Angata T, Nycholat CM, Macauley MS. Therapeutic targeting of siglecs using antibody- and glycan-based approaches. Trends Pharmacol Sci. . 2015;36:645–660. doi: 10.1016/j.tips.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lenza MP, Atxabal U, Oyenarte I, Jiménez-Barbero J, Ereño-Orbea J. Current status on therapeutic molecules targeting siglec receptors. Cells. . 2020;9:2691. doi: 10.3390/cells9122691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Astorgues-Xerri L, Riveiro ME, Tijeras-Raballand A, Serova M, Rabinovich GA, Bieche I, Vidaud M, et al. OTX008, a selective small-molecule inhibitor of galectin-1, downregulates cancer cell proliferation, invasion and tumour angiogenesis. Eur J Cancer. . 2014;50:2463–2477. doi: 10.1016/j.ejca.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 126.Koonce N, Griffin R, Dings R. Galectin-1 inhibitor OTX008 induces tumor vessel normalization and tumor growth inhibition in human head and neck squamous cell carcinoma models. Int J Mol Sci. . 2017;18:2671. doi: 10.3390/ijms18122671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gheysen L, Soumoy L, Trelcat A, Verset L, Journe F, Saussez S. New treatment strategy targeting galectin-1 against thyroid cancer. Cells. . 2021;10:1112. doi: 10.3390/cells10051112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dings RPM, Kumar N, Miller MC, Loren M, Rangwala H, Hoye TR, Mayo KH. Structure-based optimization of angiostatic agent 6DBF7, an allosteric antagonist of galectin-1. J Pharmacol Exp Ther. . 2013;344:589–599. doi: 10.1124/jpet.112.199646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Klyosov A, Zomer E, Platt D. DAVANAT® (GM-CT-01) and colon cancer: preclinical and clinical (Phase I and II) studies [M]. 2013: 89–130
- 130.Streetly MJ, Maharaj L, Joel S, Schey SA, Gribben JG, Cotter FE. GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death. Blood. . 2010;115:3939–3948. doi: 10.1182/blood-2009-10-251660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Croci DO, Salatino M, Rubinstein N, Cerliani JP, Cavallin LE, Leung HJ, Ouyang J, et al. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. J Exp Med. . 2012;209:1985–2000. doi: 10.1084/jem.20111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stasenko M, Smith E, Yeku O, Park KJ, Laster I, Lee K, Walderich S, et al. Targeting galectin-3 with a high-affinity antibody for inhibition of high-grade serous ovarian cancer and other MUC16/CA-125-expressing malignancies. Sci Rep. . 2021;11:3718. doi: 10.1038/s41598-021-82686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bertino P, Premeaux TA, Fujita T, Haun BK, Marciel MP, Hoffmann FW, Garcia A, et al. Targeting the C-terminus of galectin-9 induces mesothelioma apoptosis and M2 macrophage depletion. OncoImmunology. . 2019;8:1601482. doi: 10.1080/2162402X.2019.1601482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lv Y, Ma X, Ma Y, Du Y, Feng J. A new emerging target in cancer immunotherapy: galectin-9 (LGALS9) Genes Dis. . 2023;10:2366–2382. doi: 10.1016/j.gendis.2022.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen FF, Li Z, Ma D, Yu Q. Small-molecule PD-L1 inhibitor BMS1166 abrogates the function of PD-L1 by blocking its ER export. OncoImmunology. . 2020;9:1831153–1831163. doi: 10.1080/2162402X.2020.1831153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shao B, Li C W, Lim S O, et al. Deglycosylation of PD-L1 by 2-deoxyglucose reverses PARP inhibitor-induced immunosuppression in triple-negative breast cancer. Am J Cancer Res. 2018, 8: 1837–1846 . [PMC free article] [PubMed]
- 137.Sun L, Li CW, Chung EM, Yang R, Kim YS, Park AH, Lai YJ, et al. Targeting glycosylated PD-1 induces potent antitumor immunity. Cancer Res. . 2020;80:2298–2310. doi: 10.1158/0008-5472.CAN-19-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang M, Wang J, Wang R, Jiao S, Wang S, Zhang J, Zhang M. Identification of a monoclonal antibody that targets PD-1 in a manner requiring PD-1 Asn58 glycosylation. Commun Biol. . 2019;2:392–401. doi: 10.1038/s42003-019-0642-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu J, Wang G, Liu L, Wu R, Wu Y, Fang C, Zhou X, et al. Study of the interactions of a novel monoclonal antibody, mAb059c, with the hPD-1 receptor. Sci Rep. . 2019;9:17830–17839. doi: 10.1038/s41598-019-54231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang H, Han X, Dong Z, Xu J, Wang J, Liu Z. Hyaluronidase with pH‐responsive dextran modification as an adjuvant nanomedicine for enhanced photodynamic‐immunotherapy of cancer. Adv Funct Mater. . 2019;29:1902440. doi: 10.1002/adfm.201902440. [DOI] [Google Scholar]
- 141.Najibi AJ, Shih TY, Zhang DKY, Lou J, Sobral MC, Wang H, Dellacherie MO, et al. Targeting tumor extracellular matrix activates the tumor-draining lymph nodes. Cancer Immunol Immunother. . 2022;71:2957–2968. doi: 10.1007/s00262-022-03212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guan X, Chen J, Hu Y, Lin L, Sun P, Tian H, Chen X. Highly enhanced cancer immunotherapy by combining nanovaccine with hyaluronidase. Biomaterials. . 2018;171:198–206. doi: 10.1016/j.biomaterials.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 143.Lebbink RJ, van den Berg MCW, de Ruiter T, Raynal N, van Roon JAG, Lenting PJ, Jin B, et al. The soluble leukocyte-associated Ig-like receptor (LAIR)-2 antagonizes the collagen/LAIR-1 inhibitory immune interaction. J Immunol. . 2008;180:1662–1669. doi: 10.4049/jimmunol.180.3.1662. [DOI] [PubMed] [Google Scholar]
- 144.Xu L, Wang S, Li J, Li J, Li B. Cancer immunotherapy based on blocking immune suppression mediated by an immune modulator LAIR-1. OncoImmunology. . 2020;9:1740477. doi: 10.1080/2162402X.2020.1740477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ramos MIP, Tian L, de Ruiter EJ, Song C, Paucarmayta A, Singh A, Elshof E, et al. Cancer immunotherapy by NC410, a LAIR-2 Fc protein blocking human LAIR-collagen interaction. eLife. . 2021;10:e62927. doi: 10.7554/eLife.62927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu J, Chiang HC, Xiong W, Laurent V, Griffiths SC, Dülfer J, Deng H, et al. A highly selective humanized DDR1 mAb reverses immune exclusion by disrupting collagen fiber alignment in breast cancer. J Immunother Cancer. . 2023;11:e006720. doi: 10.1136/jitc-2023-006720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Femel J, Huijbers EJM, Saupe F, Cedervall J, Zhang L, Roswall P, Larsson E, et al. Therapeutic vaccination against fibronectin ED-A attenuates progression of metastatic breast cancer. Oncotarget. . 2014;5:12418–12427. doi: 10.18632/oncotarget.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lo A, Wang LCS, Scholler J, Monslow J, Avery D, Newick K, O'Brien S, et al. Tumor-promoting desmoplasia is disrupted by depleting FAP-Expressing stromal cells. Cancer Res. . 2015;75:2800–2810. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.