Abstract
Transcript patterns elicited in response to hosts can reveal how fungi recognize suitable hosts and the mechanisms involved in pathogenicity. These patterns could be fashioned by recognition of host-specific topographical features or by chemical components displayed or released by the host. We investigated this in the specific locust pathogen Metarhizium anisopliae var. acridum. Only host (Schistocerca gregaria) cuticle stimulated the full developmental program of germination and differentiation of infection structures (appressoria). Cuticle from beetles (Leptinotarsa decimlineata) repressed germination while cuticle from hemipteran bugs (Magicicada septendecim) allowed germination but only very low levels of differentiation, indicating that the ability to cause disease can be blocked at different stages. Using organic solvents to extract insects we identified a polar fraction from locusts that allowed appressorial formation against a flat plastic (hydrophobic) surface. Microarrays comprising 1,730 expressed sequence tags were used to determine if this extract elicits different transcriptional programs than whole locust cuticle or nonhost extracts. Of 483 differentially regulated genes, 97% were upregulated. These included genes involved in metabolism, utilization of host cuticle components, cell survival and detoxification, and signal transduction. Surprisingly, given the complex nature of insect epicuticle components and the specific response of M. anisopliae var. acridum to locusts, very similar transcript profiles were observed on locust and beetle extracts. However, the beetle extract cluster was enriched in genes for detoxification and redox processes, while the locust extract upregulated more genes for cell division and accumulation of cell mass. In addition, several signal transduction genes previously implicated in pathogenicity in plant pathogens were only upregulated in response to locust extract, implying similarities in the regulatory circuitry of these pathogens with very different hosts.
Many fungal insect pathogens, such as Metarhizium anisopliae, infect host cuticles via spores that adhere and germinate to form a series of infection structures during penetration. In the presence of nutrients and water, conidia of M. anisopliae form germ tubes. The germ tube continues undifferentiated hyphal growth on a soft surface or if nutrient quality and quantity is not conducive to differentiation. On a host cuticle, however, apical elongation terminates and the germ tube swells distally to form an appressorium, a major site of adhesion and for production of enzymes that help breach host cuticle and establish a nutritional relationship with the host (33, 38). The formation of appressoria by strains of M. anisopliae var. anisopliae with a broad host range can also be induced efficiently by a hard hydrophobic surface (i.e., polystyrene) in the presence of low levels of complex nitrogenous nutrients. However, pathogens with a narrow host range such as M. anisopliae var. acridum (specific for acridids) germinate poorly under these conditions and do not produce infection structures, suggesting they may be adapted to conditions pertaining to their hosts (36). The mechanisms for recognizing host-related triggers and the processes responsible for appressorium initiation are likely involved in the expression of virulence factors and parasitic specialization. The nature of the inductive triggers has not been determined, but while the protein and chitin composition of insect procuticle appears similar in all insects, the epicuticular components are extremely heterogeneous, even within the same genus, and therefore have the potential to lead to different pathogen responses to particular insects (7, 34).
A particular reason for focusing on M. anisopliae var. acridum is that it is being developed as a promising biological control agent of desert locusts, Schistocerca gregaria, and other orthopteran pests (30). These applied interests would obviously benefit from an understanding of the molecular and biochemical interactions between the pathogen and its hosts, particularly if genetic modifications are to be designed to enhance pathogen attributes (30). In this study we investigated the conditions required for M. anisopliae var. acridum strain ARSEF 324 to produce infection structures and developed a model system that allowed control of environmental conditions and inductive stimuli independent of host behavior. Having studied the biological significance of cuticular components for triggering appressoria, we employed microarrays to initiate a detailed investigation of the molecular mechanisms involved.
MATERIALS AND METHODS
Organism and growth.
M. anisopliae var. acridum ARSEF 324 originally isolated from Austracris guttulosa in Australia was obtained from the U.S. Department of Agriculture Entompathogenic Fungus Collection in Ithaca, N.Y. Cultures were maintained on potato dextrose agar.
Behavior of M. anisopliae var. acridum ARSEF 324 germinating on insect wings.
The membranous hind wings of locusts (S. gregaria), beetles (Leptinotarsa decimlineata), and 17-year periodical cicadas (Magicicada septendecim) were dissected away, surface sterilized in 5% sodium hypochlorite (5 min), and rinsed with 4 changes (5 min each) of sterile distilled water. Wings were placed on water agar (1.5%, wt/vol) plates, dried in a sterile-airflow cabinet and inoculated with 200 μl distilled water containing about 10,000 conidia. Following incubation (up to 24 h) at 27.5°C, cuticles were examined microscopically.
Extraction of cuticular surface lipids.
Groups of 5 or more adult locusts (S. gregaria), cicadas (M. septendecim), or an equivalent weight of beetles (L. decemlineata) were killed by freezing, freeze-dried, and immersed in three- to fourfold excess of organic solvents (dichloromethane, hexane or methanol) and gently shaken for 10 min to extract cuticular lipids (17). All types of lipid tend to dissolve in dichloromethane, which is of intermediate polarity. As it was desirable to test the nonpolar lipids separately from the polar ones, hexane (a nonpolar solvent) and methanol (a polar solvent) were also used to extract locusts. Hexane removes hydrocarbons, glycerides, sterol esters and other nonpolar lipids from cuticles virtually free of nonlipid contaminants (16). Methanol does not extract these but extracts acylglycerols, free fatty acids, and phospholipids. However, polar solvents may also extract amino acids, peptides, and carbohydrates (16). The extracts were collected in glass petri dishes, dried in a hood, and suspended in absolute ethanol. Samples of the ethanol suspension were evaporated on preweighed aluminum dishes to determine the dry weight of extract and the balance was stored at −20°C. To obtain the water-soluble fraction of the epicuticle, a methanol extract from locust was evaporated and the water-soluble components taken up with a small volume of water.
Behavior of M. anisopliae var. acridum ARSEF 324 germinating against plastic.
To test the chemical requirements for strain differentiation against a smooth hydrophobic surface, conidia were induced to germinate by growing them in 5.5-cm polystyrene petri dishes containing 2 ml of water supplemented with 0.0125% yeast extract (YE) and/or insect cuticle extracts. Various amounts of each extract were pipetted onto glass coverslips and evaporated, leaving a white greasy layer on one side. The coverslips were then placed in the polystyrene petri dishes with the lipid layer facing up. One hundred spores from each of four replicates were scored microscopically to assess germination and differentiation frequency. The adherence of conidia or germlings to polystyrene surfaces was determined by applying a jet of water from a pipette and counting the spores retained. The kinase inhibitors H89 and PD-98059 (purchased from Biomol Research Laboratories) were added to cultures from stock solutions in dimethyl sulfoxide.
RNA extraction and cDNA microarray analysis.
Mycelia from 36-h Sabouraud dextrose broth (SDB) cultures were harvested and washed with sterile distilled water. The mycelium was transferred to minimal medium (MM, containing 0.1% wt/vol KH2PO4; 0.05% wt/vol MgSO4 and 50% vol/vol tap water) or MM containing intact locust cuticle (10 mg/ml), dichloromethane extracts from locusts (locust extract), beetles (L. decimlineata) (beetle extract), or cicadas (cicada extract), or the water-soluble fraction of methanolic extracts from locusts (i.e., locust polar lipid) (all at 2 mg/ml). Cultures were shaken (100 rpm) for 6 h at 27°C. Total RNA was extracted from mycelium using an RNeasy Plant minikit plus the treatment with DNase I (QIAGEN).
The RNA from SDB cultures was used as the reference sample for microarray analysis. Hybridizations were conducted using slides printed with 1,730 cDNA clones from M. anisopliae var. anisopliae ARSEF 2575 and a few genes from M. anisopliae var. acridum absent from the library of M. anisopliae var. anisopliae (15, 40). The M. anisopliae var. acridum genes sequenced to date are less than 5% divergent from their M. anisopliae var. anisopliae counterparts, even though these are mostly secreted products encoding ecological traits that would be expected to diverge more than housekeeping genes (1, 14) so we did not envision any problems with genes from this strain being able to hybridize to the arrays.
Microarray data were analyzed as described before (40) with a TIGR TM4 system (http://www.tigr.org/software/tm4/). Briefly, data normalization was performed based on the assumption that all spots within each block have the same standard deviation for Log 2 ratios (29). The geometric mean of in-slide replicates was calculated with the MIDAS implemented process function after assigning a unique identification number for each clone. One-class significance analysis of microarray (SAM) test (39) was conducted to identify the genes whose expressions were differentially regulated. For this analysis, the exchangeability factor s0 was set at the fifth percentile with δ, 1.0. Hybridizations were conducted in triplicate with RNA from different experiments.
RESULTS
Behavior of M. anisopliae var. acridum ARSEF 324 germinating on insect cuticles.
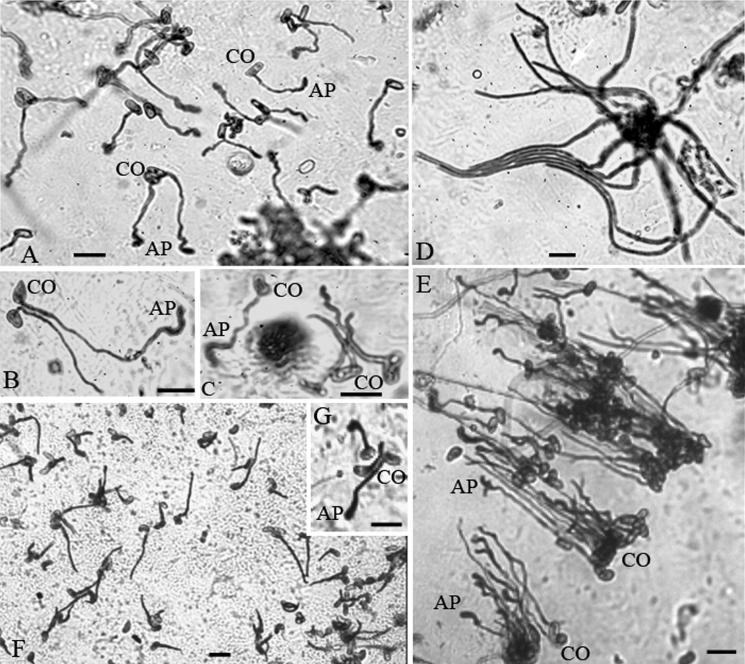
At 24 h, 73% of conidia had germinated on locust wings but production of appressoria was patchy. In some regions almost 100% of germlings had terminal swellings at the end of short germ tubes (Fig. 1A), while over other regions of the cuticle there was extensive hyphal growth with appressorial production usually being preceded by pronounced curling of germ tubes (Fig. 1B and C). Long hyphae often grew together forming orderly aggregations with the same orientation (Fig. 1C) but we saw no evidence that hyphae in close proximity attract each other. Hyphae often crossed over other hyphae that were at an angle to their direction of growth (Fig. 1D) but anastomosis was not observed.
FIG. 1.
Micrographs of M. anisopliae var. acridum germinating on locust (S. gregaria) wings (A to E) or cicada (M. septendecim) wings (F and G) for 24 h. CO, conidium; AP, appressorium. Bar, 10 μm.
M. anisopliae var. acridum, unlike M. anisopliae var. anisopliae (35), was never observed forming compound appressoria, i.e., multicellular appressoria comprising cells from multiple germlings. Thus, even when hyphae were close together and with the same directional growth each hyphae terminated in a simple appressorium (Fig. 1E). Subterminal appressoria (lateral to germ tubes) developed from 16 h. Appressorium production was not related to any discernible physical feature. Thus, hyphae were frequently deflected by hair sockets and other surface structures and changed direction of growth but they did not produce appressoria against hair sockets as observed for M. anisopliae var. anisopliae (35).
At 24 h, 54% of conidia had germinated on cicada wings, often with extensive hyphal growth (Fig. 1F). Appressoria were rarely observed. In 45 fields of view containing several thousand germlings we observed only 36. Interestingly, these almost always occurred in groups (Fig. 1G), suggesting that small regions of this nonhost cuticle possessed conditions conducive to differentiation. Only 4% of the conidia had germinated on beetle cuticle by 24 h and we did not observe appressoria.
Behavior of M. anisopliae var. acridum germinating against plastic.
M. anisopliae var. acridum did not adhere or germinate in water alone, but yeast extract was sufficient to stimulate both adhesion and germination of M. anisopliae var. acridum spores, although not differentiation of appressoria (Table 1). Since most analyses of locust S. gregaria lipids have used dichloromethane extracts (17), dichloromethane extracts from locust, cicada and beetle were tested for their effects on M. anisopliae var. acridum with and without YE (Table 1). Extract from beetle cuticle stimulated adhesion compared to water alone but not germination. In fact, combined with YE, the beetle extract repressed germination compared to YE alone, suggesting the existence of an antifungal component in the beetle cuticle (Table 1). This inhibitory effect was not observed with M. anisopliae var. anisopliae strain ARSEF 2575 (unpublished data), indicating greater physiological plasticity by this broad- host-range strain.
TABLE 1.
Effects of water, yeast extract, and different insect dichloromethane extracts on spore adhesion (A), germination (G), and appressorium differentiation (D)
| Treatmenta | % Change
|
|||||
|---|---|---|---|---|---|---|
| 15 h
|
24 h
|
|||||
| A | G | D | A | G | D | |
| Water | 0 | 0 | 0 | 0 | 0 | 0 |
| YE | 95 | 36 | 0 | 100 | 82 | 0 |
| Beetle extract | 21 | 0 | 0 | 27 | <1 | 0 |
| Beetle extract + YE | 100 | 3 | 0 | 100 | 57 | 0 |
| Cicada extract | 37 | 11 | 0 | 50 | 48 | 0 |
| Cicada extract + YE | 100 | 73 | 0 | 100 | >99 | 19 |
| Locust extract | 42 | 23 | 1 | 98 | 78 | 7 |
| Locust extract + YE | 100 | 83 | 3 | 100 | >99 | 63 |
Ethanol containing 1 mg of beetle, locust, or cicada dichloromethane extract was evaporated on coverslips that were placed in 5.5-cm petri dishes containing 2 ml of water or 0.0125% yeast extract (YE) and 10,000 conidia of M. ansiopliae var. acridum ARSEF 324. Percent germination and differentiation frequencies were determined from counts on the petri dish surface overlaid by the coverslip. Adhesion was determined on separate petri dishes not containing coverslips.
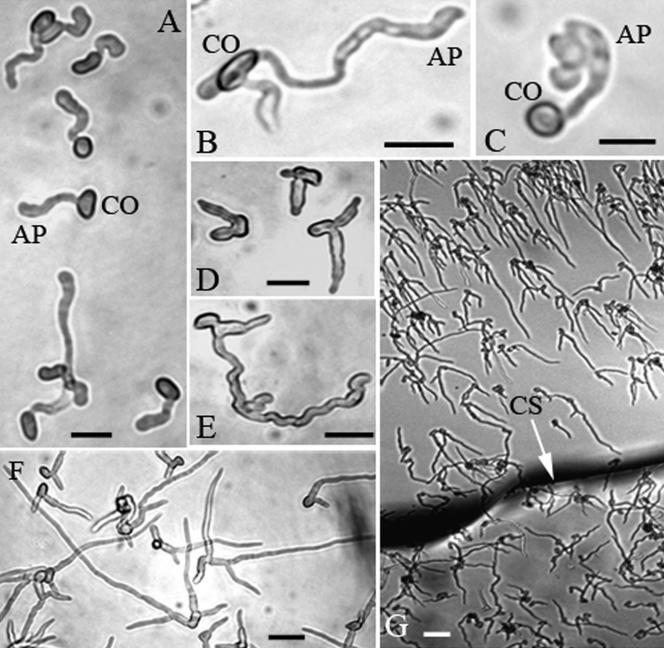
The dichloromethane extracts from locusts and cicadas allowed germination without addition of YE, and when combined with YE both extracts stimulated higher levels (P < 0.01) of germination compared to YE alone. Unlike cicada extract, locust extract stimulated differentiation of small appressoria morphologically very similar to appressoria formed on locust wings (Fig. 2A). Cicada extract combined with YE also induced formation of appressoria, but 8-fold fewer than when locust extract was combined with YE. However, whether growing on locust extract or cicada extract, during the first 24 h appressoria only formed on the petri dish surface overlaid by the coverslip (some appressoria had formed outside the coverslip by 48 h). The germ tubes and appressoria remained attached to the hydrophobic petri dish even after removing the coverslip.
FIG. 2.
Micrographs of M. anisopliae var. acridum germinated on polystyrene for 24 h with insect extracts. Germlings grown with dichloromethane (A) or methanol (B) extracts from locusts showing terminal hyphal swellings. C, production of lobate appressoria when the methanol extract was combined with 0.0125% yeast extract. D and E, branching of germlings in the water-soluble fraction (1 mg/ml) of the methanol extract; F, extensive hyphal growth at 4 mg/ml that may demonstrate directional growth towards the coverslip (G). Panels A to F show growth on the area of the petri dish overlaid by the coverslip. CO, conidium; AP, appressorium; CS, coverslip. Bar, 10 μm.
The nonpolar hexane extract from locusts induced very low levels of germination by 24 h (Table 2). However, when supplemented with YE, germination levels were similar to that in YE alone, indicating that it was not repressing germination. The hexane extract with or without YE did not induce production of appressoria, even when conidia were germinating directly on the lipid layer of the coverslip. In contrast, the methanol fraction allowed germination without addition of YE and stimulated differentiation of small appressoria on the area of the petri dish overlaid by the coverslip (Fig. 2B). Supplementing the methanol extract with YE greatly increased both the size of appressoria and the frequency of lobate appressoria formed by short, swollen side branches from the original appressorial cell (Fig. 2C). This suggests that YE does not contain inducers of appressorial differentiation but provides nutritional resources allowing the maturation of larger cells.
TABLE 2.
Effects of locust organic extracts on spore adhesion (A), germination (G), and appressorium differentiation (D)
| Treatmenta | % Change
|
|||||
|---|---|---|---|---|---|---|
| 15 h
|
24 h
|
|||||
| A | G | D | A | G | D | |
| YE | 95 | 39 | 0 | 100 | 79 | 0 |
| Dichloromethane | 40 | 28 | 3 | 97 | 81 | 9 |
| Dichloromethane + YE | 100 | 87 | 9 | 100 | >99 | 66 |
| Dichloromethane + YE + H89 (5 μM) | 84 | 61 | 0 | 100 | 87 | 0 |
| Dichloromethane + YE + PD98059 (10 nM) | 80 | 22 | 0 | 95 | 65 | 0 |
| Hexane | 0 | 0 | 0 | 50 | 10 | 0 |
| Hexane + YE | 95 | 55 | 0 | 100 | 80 | 0 |
| Methanol | 100 | 91 | 3 | 98 | >99 | 47 |
| Methanol + YE | 100 | 93 | 53 | 100 | >99 | 63 |
| LPL (1 mg/ml) | 95 | 67 | 0 | 100 | >99 | 13 |
| LPL (2 mg/ml) | 100 | 94 | 4 | 100 | >99 | 17 |
| LPL (4 mg/ml) | 100 | 95 | 0 | 100 | >99 | 0 |
| LPL (1 mg/ml) + YE | 95 | 85 | 3 | 100 | >99 | 8 |
Ethanol containing 1 mg of locust dichloromethane, hexane, or methanol extract was evaporated on coverslips that were placed in 5.5-cm petri dishes containing 2 ml of water or 0.0125% yeast extract (YE) and 10,000 conidia of M. anisopliae var. acridum ARSEF 324. The effects of YE alone and the water-soluble fraction of the methanol extract (locust polar lipid [LPL]) were tested using ethanol-washed cover slips. Percent germination and differentiation frequencies were determined from counts on the petri dish surface overlaid by the coverslip. Adhesion was determined on separate petri dishes not containing coverslips.
Since the inducer(s) appears to be confined to the polar fraction, we wished to determine whether the inducer was a polar lipid or a nonlipid contaminant. The methanol solvent was removed by evaporation and water-soluble components were taken up in a small volume of water. This water-soluble fraction retained the ability to stimulate high levels of adhesion and germination (Table 2). However, the germlings showed unusual development. In particular, branching of germlings was more numerous and compact than observed with methanol or dichloromethane extracts (Fig. 2D and E) or on locust cuticle and fewer appressoria were produced under coverslips. At high concentrations (4 mg/ml) the water-soluble fraction stimulated extensive hyphal growth under coverslips with even fewer appressoria (Fig. 2F). In 3 out of 5 replicates we also observed extensive directional growth by germ tubes towards the coverslip (Fig. 2G).
Effect of cAMP on appressoria formation.
Because cyclic AMP (cAMP) is sufficient to induce appressorial formation in many specific plant pathogens (2), we tested the effect of external cAMP application on conidial development of M. anisopliae var. acridum in YE and YE plus dichloromethane extracts. Cyclic AMP added at 5, 10, or 20 mM produced no change in germination frequencies and cAMP did not induce appressorial formation under conditions in which they do not normally form, such as in YE culture, on a hydrophilic glass surface, or not overlaid by a coverslip. Cyclic AMP exerts its effects principally through the activation of cAMP-dependent protein kinase A (PKA) (2). In spite of exogenous cAMP having no apparent effect, both germination and differentiation were inhibited by the PKA inhibitor H89, suggesting that cAMP/PKA is one of multiple pathways required to trigger differentiation. Consistent with this a highly selective mitogen-activated protein kinase kinase inhibitor PD-98059 was also very potent at inhibiting either germination or differentiation (Table 2).
Microarray analysis reveals distinctive gene transcription responses to locust cuticle, to lipid extracts and to minimal medium.
Mycelium grown in shake cultures to prevent contact stimuli caused by settling was used as the source of RNA to probe slides with 1,730 printed genes (40). These microarrays were used for direct comparisons of gene expression levels as M. anisopliae var. acridum responded to nutrient deprivation (MM), intact locust cuticle, dichloromethane extracts from locusts, beetles, or cicadas, and the water-soluble fraction of a methanol extract from locusts (locust polar lipid, locust polar lipid) (Fig. 3).
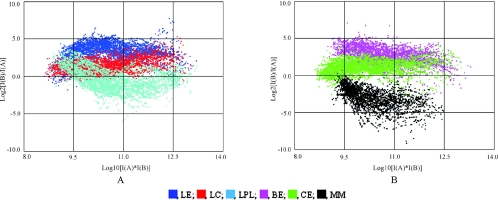
FIG. 3.
Raw intensity (RI) graph of overall gene expression profiles from microarray analysis of locust-related extracts (A) and the others (B). I(A) and I(B) represent the intensity data from channel A (Cy3) and channel B (Cy5), respectively. LE, locust dichloromethane extracts; LC, locust cuticle; LPL, locust polar lipid; BE, beetle extract; CE, cicada extracts; MM, minimal medium.
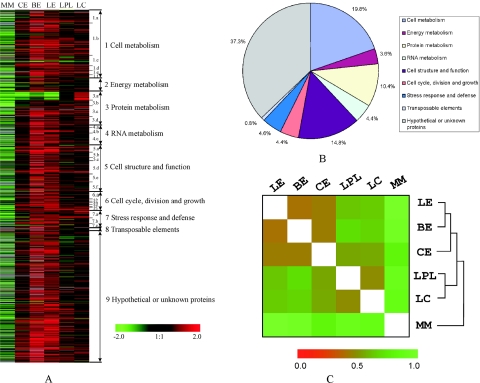
A SAM test identified 483 genes (28% of those arrayed) that were differentially up (471 genes) or down (12 genes) regulated in one or more of the host related medium 6 h after transfer of mycelium from nutrient rich SDB. In contrast, most genes were downregulated during nutrient deprivation (Fig. 3B; Fig. 4A). The printed genes were classified based on their potential cellular function into 9 functional groups (40), as presented in Fig. 4A. Viewed broadly, of the 483 differentially regulated genes, 19.8% were involved in cell metabolism, 14.8% in cell structure and 10.4% in protein metabolism. Other significantly varied genes were evenly dispersed among the 6 remaining functional groups. In addition, 37.3% of genes with hypothetical or unknown function also showed differential expression between experiments, sometimes being upregulated very sharply in one or other host related medium (Fig. 4A and B).
FIG. 4.
Transcriptional profiling and characterization. A, transcriptional profiles for the entire array; B, functional distribution of genes differently expressed between treatments; and C, experimental clustering analysis. LE, locust extract; BL, beetle extract; CL, cicada extract; LPL, locust polar lipid; LC, locust cuticle; MM, minimal medium.
Based on the Euclidean distances estimated from the transcriptional profiles in different medium, MM (nutrient deprivation) is divergent from the host-related medium while these clustered into two distinct groups: expression patterns in the three dichloromethane extracts (locust extract, beetle extract and cicada extract) cluster together, while locust polar lipid clusters with locust cuticle (Fig. 4C).
The large-scale upregulation of gene expression produced by cuticular components implies regulation by several modulators, consistent with which insect cuticle components activated a series of genes involved in signal transduction and transcriptional regulation. Of particular interest, putative signal transduction genes previously implicated in pathogenicity in M. anisopliae and/or other fungi (37) were either upregulated in all host-related media, all three dicloromethane extracts, or just in locust extract (Fig. 5A). The exceptions were CN807989, which is similar to a pathogenicity transmembrane protein from Magnaporphe grisea that is upregulated in all media, including MM, and adenylate cyclase (AJ251971), which showed minimal changes in regulation. Otherwise, PKA (AJ273657) and CN809265, similar to a virulence factor in Gibberella moniformis that functions as a two component signaling response regulator for transmitting extracellular signals (6), were upregulated in all media except MM, while mitogen-activated protein kinase kinase (AJ272796) was upregulated (>2-fold) in all three dicloromethane extracts but not in locust polar lipid, locust cuticle, or MM.
FIG. 5.
Transcriptional profiling and characterization. Differentially expressed genes involved in signaling pathways (A; *, indicates whose genes transcriptional expression varied significantly between the treatments); genes differentially expressed between locust extract and beetle extract (B) and between locust extract and locust cuticle (C). The blocks in gray show the intensity values (Cy3 and Cy5) have been rescaled as zero after background filtering and normalization.
Activation of adenylate cyclase is mediated by heterotrimic GTP-binding proteins (Gα, Gβ, and Gγ). A G protein α subunit was only upregulated in locust extract (ca. 6-fold), while the β subunit, also involved in regulating infection-related morphogenesis in Magnaporphe grisea (26), was downregulated >4-fold in the three dichloromethane extracts and MM, but was upregulated in locust cuticle and locust polar lipid by 3.3- and 1.6-fold, respectively. Most significantly, several homologs for regulators of infection structure formation in other fungi were only upregulated in locust extract. This resulted in signal transduction components upregulated in beetle extract and cicada extract clustering separately from locust extract (Fig. 5A). Thus, CN808063, which is similar to MAS1 protein, required for appressorial formation in M. grisea (19), is upregulated 2.7-fold in locust extract but downregulated 4.4-fold in beetle extract and 1.6-fold in cicada extract. Likewise, CN808339 that is similar to a CAP20-like protein involved in virulence, penetration, and appressorium formation in Blumeria graminis (18) was upregulated in locust extract and downregulated in other media.
The downstream target genes of many signaling pathways are still poorly understood. However, the transcriptome in beetle extract possessed some unique features as shown in Fig. 5b, which lists genes preferentially responding to locust extract but not to beetle extract and vice versa. The beetle extract cluster does not represent a defined functional category but is enriched in genes associated with detoxification and redox processes, including the antioxidant defense protein thioredoxin AJ273832 (upregulated 2.3-fold in beetle extract but downregulated in locust extract), aldehyde dehydrogenase CN809673 (upregulated 6.5-fold), and formate dehydrogenase CN809022 (upregulated 20-fold). It also contains genes for reallocation of resources such as a sugar transport protein (AJ273888), a sterol carrier protein (CN809167), and both high- and low-affinity copper transporters (AJ273995 and CN808791) that were differentially upregulated 25- and 40-fold, respectively. Besides signal transduction genes, those differentially upregulated in locust extract included some for cell wall synthesis (e.g., CN808518) and cell cycle regulation (e.g., AJ274064), implying more rapid cell division and accumulation of cell mass (Fig. 5B).
We also identified clusters of genes specifically induced by either growth on intact locust cuticle or its dichloromethane extract (Fig. 5C). Genes differentially upregulated during growth on intact locust cuticle included the M. anisopliae homolog of protein disulfide isomerase (CN808269) along with calnexin (AJ273132) and several chaperones in the heat shock class (AJ272997, AJ273036, AJ273534, AJ273662, and CN809938).
Some of the enzymes upregulated in locust cuticle have toxic substrates known to be present in procuticle. Thus, a putative dioxygenase (AJ272846) with homologs that cleave aromatic hydrocarbon components of phenolics was upregulated. Like other fungi, M. anisopliae var. acridum contains multiple glutathione S-transferases, multifunctional detoxification enzymes that conjugate glutathione to aliphatic, aromatic or heterocyclic agents. One of these activities (CN809045) was sharply upregulated in locust cuticle (28-fold as ca. 2.6-fold in locust extract), while a second (CN808753) was upregulated 4.4-fold in locust extract (1.8-fold in locust cuticle) and the third (CN809169) was upregulated approximately 1.3-fold in both locust cuticle and locust extract. Two flavohemoglobins (CN808105 and AJ273710) were also differentially upregulated in locust cuticle.
Locust cuticle is largely insoluble and even though present in medium at 5-fold greater amounts than locust extract is likely to be a less accessible nutrient source. Some differences in transcription probably reflect this. Thus, sulfur amino acid synthesis was upregulated in locust cuticle, as shown by upregulation of both methionine permease (CN807990) and homocysteine synthase (CN809047), the latter being a key enzyme in the pathway from methionine to cysteine. Exogenous methionine is reported to repress transcription of homocysteine synthase in Aspergillus nidulans (31). A greater abundance of free amino acids in locust extract compared with locust cuticle is also indicated by higher levels of expression of amino acid catabolizing genes. These include glutaminase A (AJ273512), which converts glutamine to glutamic acid, and ornithine transaminase (CN809353), which is specifically induced by arginine in A. nidulans (12).
The product of ornithine transaminase is a semialdehyde which is converted to proline. Proline can be converted back to glutamate by the sequential action of proline oxidase and delta 1-pyrroline-5-carboxylate dehydrogenase (5) and a proline oxidase homolog (AJ274200) is upregulated >6-fold in locust extract compared to 1.7-fold in locust cuticle. Glutamate can also be metabolized via the GABA (γ-aminobutyric acid) shunt. However, both GABA permease (CN808046) and the required enzyme for GABA metabolism, GABA transaminase (AJ272688) were upregulated in locust extract. These are inducible activities in other fungi (20, 32), suggesting rather surprisingly that GABA is a significant source of nitrogen in locust extract but not locust cuticle.
Ferulic acid esterases are produced by plant-pathogenic fungi and are involved in the degradation of pectin and xylan, from which they release aromatic acids on cereal-derived substrates. The expression of a putative homolog (AJ273114) in locust extract implies the presence of a hydrophobic substrate with bulky substituents of the benzene ring (9). The ability of strain 324 to use at least some lipoidal components of the dichloromethane extract was suggested by upregulation of several classes of lipases. Most of these were similarly regulated in locust extract and locust cuticle. The exceptions included a lipase (AJ274323) and a nitroalkane dioxygenase (CN809138) that converts nitroalkanes to the corresponding aldehyde and nitrite. They are >5-fold upregulated in locust extract. Nitrite reductase (CN808073) is also sharply upregulated in locust extract (Fig. 5C).
DISCUSSION
One of the outstanding features of M. anisopliae is its ability to parasitize a wide range of different insect species. Many workers have characterized the host range of different isolates in attempts to characterize strains on the basis of pathogenicity (28). Most of the information on why isolates attack some hosts and not others is concerned with the effects of different host components on adhesion and germination (35). This focus was reasonable given that these are the earliest stages of infection. Consistent with it, beetle cuticle and extracts repress germination of M. anisopliae var. acridum, although allowing low levels of adhesion. However, there are many stages in the process of infection where the ability of an isolate to cause disease may be influenced, and so a widening of our knowledge in this area is needed.
The inability of M. anisopliae var. acridum to infect cicadas is apparently determined at the stage of appressorial formation. M. anisopliae var. acridum adheres and germinates on cicada cuticle but forms few small appressoria suggesting differences in stimulation on the locust and cicada cuticles. The patchy distribution of infection structures produced by M. anisopliae var. acridum on cicada cuticles also suggests that the fungus recognizes specific signals from the structures or chemistry of the cuticle surface that vary with site, and that it behaves differently as a reaction to these signals. These stimuli are likely to be chemical rather than physical as the ability of an extract from locust cuticles to stimulate abundant appressorial formation against a flat surface excludes an important role for topographical features on the locust cuticle in initiating appressoria formation.
Spores of most plant pathogens will germinate in water alone. Like many other entomopathogenic fungi (4), M. anisopliae var. acridum apparently requires water-soluble nutrients from the host surface to germinate and develop. The extent to which parasitic specificity of entomopathogenic fungi is determined by cuticular lipids is uncertain, with apparent contradictions in the literature. In part this probably reflects lack of precision in the chemical nomenclature or incomplete methodology description (21) as well as strain differences. Strains with multiple hosts are likely to respond to multiple nonspecific signals. It is highly improbable that diverse insects secrete a single universal signaling chemical to which all fungi respond. Individual strains may sense one signal or a set of specific signals within a complex cocktail of insect chemicals and would respond according to what is secreted by any given host insect.
Evidence for a mixture of signals in the locust extract was provided by the response of M. anisopliae var. acridum to different fractions. Total extraction with dichloromethane was very effective at inducing infection structure formation under coverslips but M. anisopliae var. acridum was largely unresponsive to the nonpolar lipid fraction that comprises the bulk of the locust epicuticle (17). The polar components extracted with methanol elicited a response similar to that obtained with dichloromethane but its water-soluble fraction (locust polar lipid) produced not only appressoria but a bushy type of hyphal branching pattern. At high levels (4 mg/ml) locust polar lipid produced extensive hyphal growth at the expense of appressorial production. This is consistent with observations on M. anisopliae var. anisopliae (33) that suggest that a primary role of the appressoria is to establish a nutritional relationship with the host and is not necessary in the presence of a sufficiency of nutrients. The directional growth that occurred towards the coverslip was reminiscent of directional growth shown on locust cuticle except that appressoria were not produced. Directional growth could be adaptive if it increased the probability that hyphae will contact other host related signals.
In spite of different, presumably selectable responses to insects, the ultimate physiological and morphological responses of most M. anisopliae strains in terms of appressorial formation are similar. However, several differences were noted between the behavior of M. anisopliae var. acridum and M. anisopliae var. anisopliae on cuticle surfaces (33). Failure of M. anisopliae var. acridum to produce compound appressoria may not be of great functional significance as the simple M. anisopliae var. acridum appressoria have developed at a similar time they will probably also penetrate at a similar time, resulting in an effective multiple invasion. However, they do imply genetic differences that are employed during penetration events. Potentially related to our observation that M. anisopliae var. acridum does not anastomatize and form compound appressoria, CN808914 related (E, 10−101) to Het-C (heterokaryon incompatibility) from Neurospora crassa was sharply upregulated (>5-fold) in dichloromethane extracts. Podospora anerina uses common signal transduction pathways in regulating development and vegetative incompatibility, including the G protein α subunit (23, 24). Thus, potential relationships can be anticipated between fungal appressorium differentiation and vegetative incompatibility controls.
Aside from the G protein β subunit, all the signal transduction genes previously implicated in pathogenicity in plant pathogens that we had homologs for in the M. anisopliae var. anisopliae arrays were upregulated in locust extract. Thus, although the signals that induce germination and differentiation are different, similar signal transduction pathways may mediate these signals in plant and insect pathogens. Fluctuations in the relative levels of both Ca2+ and cAMP effect changes in the cytoskeleton and cell wall of M. anisopliae var. anisopliae during formation of appressoria (34, 37). However, while cAMP is also sufficient to induce appressorium formation in many specific plant pathogens (2) this was not the case for M. anisopliae var. acridum. Evidence for commonalities was provided by the inhibitor studies with H89 and PD-98059 as PKA and mitogen-activated protein kinases have been implicated in developmental processes in many other fungal pathogens (10).
The mitogen-activated protein kinase cascade is a highly conserved module that mediates new gene expression in response to external signals in a series of developmental processes in fungi including germination, appressorium formation, and pathogenicity (41). Branching and the curvilinear mode of growth that often preceded differentiation both in vivo and in vitro imply an effect on the Spitzenkorfer (the vesicle-generating apparatus at the hyphal tip) that by its presence and position orients cell wall component disposition of the hyphal apex and controls the direction of growth (3). Previously, we suggested that the transition from polarized hyphal growth to germ tube expansion (appressoria) might be linked to a reduced displacement rate or dispersal of the Spitzenkorfer, redirecting cell wall synthesis from the apical tip to the entire cell surface (34). Vesicles are transported along microtubules and phosphorylation of mitogen-activated protein kinase is involved in regulating the organization of microtubules (27). The Ca2+-dependent phosphorylation of three Metarhizium proteins recognized by antiserum to mitogen-activated protein kinase (34) suggests a possible mechanism for both Ca2+- and mitogen-activated protein kinase on differentiation.
Our microarray data indicate that induction of transcription is an overriding feature of the response of M. anisopliae var. acridum to insect-related medium with relatively little transcriptional repression observed. Before this study we had suspected that host recognition is determined by regulatory controls that allow expression of pathogenicity genes that are not expressed on nonhosts. The similar transcriptional responses of M. anisopliae var. acridum grown on inductive (for appressoria) locust extract and noninductive beetle extract is evidence that specialization to locust hosts has not eliminated a substantial stereotypical response to epicuticular components. As beetle extract represses germination and growth of M. anisopliae var. acridum, it contains components that inhibit development and the differentially regulated transcripts equivalently expressed in both locust extract and beetle extract cannot be sufficient to overcome these inhibitors. It is possible that genes specifically upregulated by locust extract are essential for development.
Based on the overall transcriptional profiles for the total array of 1,730 cDNAs, locust extract and beetle extract cluster more closely with each other than with cicada extract (Fig. 4C) but locust extract forms an outgroup when cluster analysis is limited to coregulated genes involved in signal transduction (Fig. 5A). This correlates with differences between locust extract and beetle extract or cicada extract in their ability to induce appressorial formation, consistent with perception and regulatory factors in M. anisopliae var. acridum controlling infection related differentiation. Among these regulatory elements, some of the biggest expression differences were observed for a Mas1-like protein (CN808063) and a CAP20-like protein (CN808339) that were both upregulated in locust extract while being downregulated in beetle extract and cicada extract (Fig. 4A). Deletion of their homologs in Magnaporthe grisea and Colletotrichum gloeosporiodies, respectively, allows appressorial formation but greatly reduces penetration and virulence (18, 42), and they may have similar effects in other plant pathogens (19).
Other differences between the transcriptional profiles induced by locust extract and beetle extract occurred principally among genes involved in detoxification, oxidant defense and transport. These differential shifts in gene expression occurred in the context of equivalent expression of other genes in the same functional categories in both locust extract and beetle extract. Thus, many genes involved in detoxification, oxidant defense and transport were comparably induced by incubating in locust extract and in beetle extract suggesting that both extracts contain a cocktail of inductive and repressive components. The overall favorable chemistry of the locust extract was shown by differential upregulation of genes for cell wall synthesis (CN808518) and cell cycle regulation (AJ274064) implying more rapid cell division and accumulation of cell mass.
The locust polar lipid and intact cuticle (locust cuticle) induced a similar transcriptional response that may reflect similarities in composition between the water-soluble fraction of the epicuticle and the water-soluble fraction released by whole insect cuticle in liquid medium. The larger number of genes whose expression is altered by the dichloromethane extracts compared to locust cuticle or locust polar lipid indicates that their direct and indirect effects on the cell are broader. However, several clusters of genes were specifically induced by growth on intact locust cuticle including chaperones and foldases (Fig. 5C). A number of studies have shown that these are upregulated in response to stress or secretion of foreign proteins (8). Thus, in Trichoderma reesei the levels of protein disulfide isomerase is a function of the quantity of total secreted protein being produced (13). The M. anisopliae var. acridum homolog (AJ273116) was upregulated in locust cuticle (>3-fold).
One of the major groups of secreted proteins is the proteases. Consistent with the proteinaceous nature of the procuticle 6 out of 8 genes encoding secreted subtilisin proteinases were expressed at higher levels in locust cuticle (Table S1 in the supplemental material). An exception was subtilisin Pr1B, which was upregulated 13-fold in beetle extract compared with 3.6- and 1.5-fold on locust extract and locust cuticle, respectively. This unique profile implies that this subtilisin has a distinctive role. Some of the enzymes upregulated in locust cuticle such as dioxygenase and glutathione S-transferases have toxic substrates known to be present in procuticle.
Two flavohemoglobins (CN808105 and AJ273710) were also differentially upregulated in locust cuticle. Fungal flavohemoglobins are produced to counteract nitrosative stress (22) and as such promote fungal virulence to mammals (11). Their upregulation in locust cuticle suggests that if insects also employ nitrosative challenge, their pathogens have the necessary enzymatic defenses. Several of the enzymes differentially expressed in locust polar lipid, locust extract, and locust cuticle would affect intracellular levels of proline. Proline acts as a signaling/regulatory molecule inversely controlling cell division (25), and it would be interesting to determine the linkage, if any, between intracellular levels of proline and the higher percentage of appressorium differentiation in locust extract (Table 2).
In conclusion, we have demonstrated that at least four different signals affect appressorial formation in a specific insect pathogen: a polar cuticle fraction from an appropriate host, nutrient levels, a hydrophobic surface, and the unknown signal imparted by the coverslip. The cuticle fraction elicits an active transcriptional response, and we have identified a list of inducible genes that respond to this fraction and intact locust cuticle. The gene set defined in this study will now permit rigorous comparison of gene expression programs by M. anisopliae var. anisopliae strains with widely different hosts. Such studies could address the origin of intraspecies differences and correlate these differences with the underlying metabolic and biosynthetic differences that define different host ranges, identify the mechanisms by which novel pathogens emerge with different host ranges and identify targets for restricting or broadening host ranges.
Supplementary Material
Acknowledgments
This work was supported by USDA grants (2003-351-07-13658 and 2003-353-02-13588).
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Bagga, S., G. Hu, S. E. Screen, and R. J. St. Leger. 2004. Reconstructing the diversification of subtilisins in the pathogenic fungus Metarhizium anisopliae. Gene 324:159-169. [DOI] [PubMed] [Google Scholar]
- 2.Barhoom, S., and A. Sharon. 2004. cAMP regulation of “pathogenic” and “saprophytic” fungal spore germination. Fungal Genet. Biol. 41:317-326. [DOI] [PubMed] [Google Scholar]
- 3.Bartnicki-Garcia, S., F. Hergert, and G. Gierz. 1989. Computer simulation of morphogenesis: mathematical basis for the hyphal tip growth. Protoplasma 153:46-57. [Google Scholar]
- 4.Boucias, D. G., and J. C. Pendland. 1984. Nutritional requirements for conidial germination of several host range pathotypes of the entomopathogenic fungus Nomuraea rileyi. J. Invertebr. Pathol. 43:288-292. [Google Scholar]
- 5.Brandriss, M. C., and B. Magasanik. 1997. Genetics and physiology of proline utilization in Saccharomyces cerevisiae: enzyme induction by proline. J. Bacteriol. 140:498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catlett, N. L., O. C. Yoder, and B. G. Turgeon. 2003. Whole-genome analysis of two-component signal transduction genes in fungal pathogens. Eukaryot. Cell. 2:1151-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charnley, A. K. 1984. Physiological aspects of destructive pathogenesis in insects by fungi: A speculative review, p. 229-270. In J. M. Anderson, A. D. M. Rayner and D. W. H. Walton (ed.), Invertebrate-microbial interactions. British Mycological Society Symposium 6. Cambridge University Press, London, U.K.
- 8.Conesa, A., P. J. Punt, N. van Luijk, and C. A. van den Hondel. 2001. The secretion pathway in filamentous fungi: a biotechnological view. Fungal Genet. Biol. 33:155-171. [DOI] [PubMed] [Google Scholar]
- 9.Crepin, V. F., C. B. Faulds, and I. F. Connerton. 2004. Functional classification of the microbial feruloyl esterases. Appl. Microbiol. Biotechnol. 63:647-652. [DOI] [PubMed] [Google Scholar]
- 10.Dean, R. A. 1997. Signal pathways and appressorium morphogenesis. Annu. Rev. Phytopathol. 35:211-234. [DOI] [PubMed] [Google Scholar]
- 11.De Jesus-Berrios, M., L. Liu, J. C. Nussbaum, G. M. Cox, J. S. Stamler, and J. Heitman. 2003. Enzymes that counteract nitrosative stress promote fungal virulence. Curr. Biol. 13:1963-1968. [DOI] [PubMed] [Google Scholar]
- 12.Dzikowska, A., M. Kacprzak, R. Tomecki, M. Koper, C. Scazzocchio, and P. Weglenski. 2003. Specific induction and carbon/nitrogen repression of arginine catabolism gene of Aspergillus nidulans-functional in vivo analysis of the otaA promoter. Fungal Genet. Biol. 38:175-186. [DOI] [PubMed] [Google Scholar]
- 13.Foreman, P. K., D. Brown, L. Dankmeyer, R. Dean, S. Diener, N. S. Dunn-Coleman, F. Goedegebuur, T. D. Houfek, G. J. England, A. S. Kelley, H. J. Meerman, T. Mitchell, C. Mitchinson, H. A. Olivares, P. J. Teunissen, J. Yao, and M. Ward. 2003. Transcriptional regulation of biomass-degrading enzymes in the filamentous fungus Trichoderma reesei. J. Biol. Chem. 278:31988-31997. [DOI] [PubMed] [Google Scholar]
- 14.Freimoser, F. M., S. Screen, S. Bagga, G. Hu, and R. J. St. Leger. 2003. Expressed sequence tag (EST) analysis of two subspecies of Metarhizium anisopliae reveals a plethora of secreted proteins with potential activity in insect hosts. Microbiology 149:239-247. [DOI] [PubMed] [Google Scholar]
- 15.Freimoser, F. M., G. Hu, and R. J. St. Leger. 2005. Variation in gene expression patterns as the insect pathogen Metarhizium anisopliae adapts to different host cuticle or nutrient deprivation in vitro. Microbiology 151:361-371. [DOI] [PubMed] [Google Scholar]
- 16.Gilby, A. R. 1980. Chemical methods (lipids), p. 217-252. In T. A. Miller (ed.), Cuticle techniques in arthropods. Springer-Verlag, New York, N.Y.
- 17.Heifetz, Y., I. Boekhoff, H. Breer, and S. W. Applebaum. 1997. Cuticular hydrocarbons control behavioural phase transition in Schistocerca gregaria nymphs and biochemical responses in antennae. Insect Biochem. Mol. Biol. 27:563-568. [Google Scholar]
- 18.Hwang, C. S., M. A. Flaishman, and P. E. Kolattukudy. 1995. Cloning of a gene expressed during appressorium formation by Colletotrichum gloeosporioides and a marked decrease in virulence by disruption of this gene. Plant Cell 7:183-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irie, T., H. Matsumura, R. Terauchi, and H. Saitoh. 2003. Serial Analysis of Gene Expression (SAGE) of Magnaporthe grisea: genes involved in appressorium formation. Mol. Genet. Genomics 270:181-189. [DOI] [PubMed] [Google Scholar]
- 20.Kumar, S., N. S. Punekar, V. SatyaNarayan, and K. V. Venkatesh. 2000. Metabolic fate of glutamate and evaluation of flux through the 4-aminobutyrate (GABA) shunt in Aspergillus niger. Biotechnol. Bioeng. 67:575-584. [PubMed] [Google Scholar]
- 21.Lecuona, R., J.-L. Clement, G. Riba, C. Joulie, and P. Juarez. 1997. Spore germination and hyphal growth of Beauveria sp. on insect lipids. J. Econ. Entomol. 90:118-123. [Google Scholar]
- 22.Liu, L., M. Zeng, A. Hausladen, J. Heitman, and J. S. Stamler. 2000. Protection from nitrosative stress by yeast flavohemoglobin. Proc. Natl. Acad. Sci. USA 97:4672-4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loubradou, G., J. Begueret, and B. Turcq. 1997. A mutation in an HSP90 gene affects the sexual cycle and suppresses vegetative incompatibility in the fungus Podospora anserina. Genetics 147:581-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loubradou, G., J. Begueret, and B. Turcq. 1999. MOD-D, a G alpha subunit of the fungus Podospora anserina, is involved in both regulation of development and vegetative incompatibility. Genetics 152:519-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maggio, A., S. Miyazaki, P. Veronese, T. Fujita, J. I. Ibeas, B. Damsz, M. L. Narasimhan, P. M. Hasegawa, R. J. Joly, and R. A. Bressan. 2002. Does proline accumulation play an active role in stress-induced growth reduction? Plant J. 31:699-712. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura, M., G. Park, and J. R. Xu. 2003. The G-beta subunit MGB1 is involved in regulating multiple steps of infection-related morphogenesis in Magnaporthe grisea. Mol. Microbiol. 50:231-243. [DOI] [PubMed] [Google Scholar]
- 27.Nixon, R. A., I. Fischer, and S. E. Lewis. 1990. Synthesis, axonal transport, and turnover of the high molecular weight microtubule-associated protein MAP 1A in mouse retinal ganglion cells: tubulin and MAP 1A display distinct transport kinetics. J. Cell Biol. 110:437-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowierski, R. M., Z. Zeng, S. Jaronski, F. Delgado, and W. Swearingen. 1996. Analysis and Modeling of Time-Dose-Mortality of Melanoplus sanguinipes, Locusta migratoria migratorioides, and Schistocerca gregaria (Orthoptera: Acrididae) from Beauveria, Metarhizium, and Paecilomyces isolates from Madagascar. J. Invertebr. Pathol. 67:236-252. [DOI] [PubMed] [Google Scholar]
- 29.Quackenbush, J. 2002. Microarray data normalization and transformation. Nat. Genet. 32(Suppl.):496-501. [DOI] [PubMed] [Google Scholar]
- 30.Roberts, D. W., and R. J. St. Leger. 2004. Metarhizium spp., cosmopolitan insect-pathogenic fungi: Mycological aspects. Adv. Appl. Microbiol. 54:1-70. [DOI] [PubMed] [Google Scholar]
- 31.Sienko, M., J. Topczewski, and A. Paszewski. 1998. Structure and regulation of cysD, the homocysteine synthase gene of Aspergillus nidulans. Curr. Genet. 33:136-144. [DOI] [PubMed] [Google Scholar]
- 32.Solomon, P. S., and R. P. Oliver. 2002. Evidence that gamma-aminobutyric acid is a major nitrogen source during Cladosporium fulvum infection of tomato. Planta 214:414-420. [DOI] [PubMed] [Google Scholar]
- 33.St. Leger, R. J., T. M. Butt, M. S. Goettel, R. S. Staples, and D. W. Roberts. 1989. Production in vitro of appressoria by the entomopathogenic fungus Metarhizium anisopliae. Exp. Mycol. 13:274-288. [Google Scholar]
- 34.St. Leger, R. J., R. M. Cooper, and A. K. Charnley. 1991. Characterization of chitinase and chitobiase produced by the entomopathogenic fungus Metarhizium anisopliae J. Invertebr. Pathol. 58:15-426. [Google Scholar]
- 35.St. Leger, R. J. 1991. Integument as a barrier to microbial infections, p. 286-308. In A. Retnakaran and K. Binnington (ed.), The physiology of insect epidermis. CIRO, Canberra, Australia.
- 36.St. Leger, R. J., B. May, L. L. Allee, D. C. Frank, R. C. Staples, and D. W. Roberts. 1992. Genetic differences in allozymes and in formation of infection structures among isolates of the entomopathogenic fungus Metarhizium anisopliae. J. Invertebr. Pathol. 60:89-101. [Google Scholar]
- 37.St. Leger, R. J. 1993. Biology and mechanisms of invasion of deuteromycete fungal pathogens, p. 211-229. In N. C. Beckage, S. N. Thompson and B. A. Federici (ed.), Parasites and pathogens of insects, vol. 2. Academic Press, New York, N.Y.
- 38.St. Leger, R. J., L. Joshi, M. J. Bidochka, N. W. Rizzo, and D. W. Roberts. 1996. Characterization and ultrastructural localization of chitinases from Metarhizium anisopliae, M. flavoviride, and Beauveria bassiana during fungal invasion of host (Manduca sexta) cuticle. Appl. Environ. Microbiol. 62:907-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98:5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, C.-S., G. Hu, and R. J. St. Leger. Differential gene expression by Metarhizium anisopliae growing in root exudate and host (Manduca sexta) cuticle or hemolymph reveals mechanisms of physiological adaptation. Fungal Genet. Biol., in press. [DOI] [PubMed]
- 41.Xu, J.-R. 2000. MAP kinases in fungal pathogens. Fungal Genet. Biol. 31:137-152. [DOI] [PubMed] [Google Scholar]
- 42.Xue, C., G. Park, W. Choi, L. Zheng, R. A. Dean, and J. R. Xu. 2002. Two novel fungal virulence genes specifically expressed in appressoria of the rice blast fungus. Plant Cell 14:2107-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.