Abstract
Purpose
Acute kidney injury (AKI) is a common clinical critical condition that has become a significant healthcare burden. In recent years, the relationship between AKI and mitochondria has attracted increasing attention. Protecting mitochondria or restoring their function has emerged as a novel therapeutic strategy for alleviating AKI. This study aims to analyze and summarize the current status, research trends, and hotspots in this field, providing references and directions for future research.
Methods
AKI and mitochondria-related literature from the Web of Science core collection were retrieved and collected. Bibliometric and visualization analyses were conducted using Microsoft Excel 2021, bibliometric tools (VosViewer, Citespace 6.3.R1, and the bibliometrix R package), R 4.3.2, and SCImagoGraphica software.
Results
A total of 2433 publications were included in this study. The number of annual publications in this field has increased year by year. China and the United States are the two most productive countries. Central South University is the most influential research institution in terms of research output, and Parikh SM, Schnellmann RG, and Dong Z are the most influential authors in this field. KIDNEY INTERNATIONAL, JOURNAL OF THE AMERICAN SOCIETY OF NEPHROLOGY, and AMERICAN JOURNAL OF PHYSIOLOGY-RENAL PHYSIOLOGY are the most influential journals. Initially, the research focused on keywords such as oxidative stress, ischemia-reperfusion injury, apoptosis, inflammation, and autophagy. In recent years, new research hotspots have emerged, including ferroptosis, aging, mitochondrial quality control, messenger RNA, mitochondrial-targeted antioxidants, extracellular vesicles, and nanodrug delivery.
Conclusion
Research on the relationship between mitochondria and AKI has broad developing prospects, and targeting mitochondrial regulation will become a focus of future AKI prevention and treatment research.
Keywords: acute kidney injury, mitochondria, bibliometric analysis, visualization, VOSviewer, citespace
Introduction
Acute Kidney Injury (AKI) represents a clinical syndrome primarily distinguished by the abrupt onset of a sharp decline in renal function, featuring a notable incidence rate and correlation with severe clinical outcomes.1,2 Hospitalized individuals exhibit AKI at rates as high as 20%, with intensive care units reporting incidences exceeding 50%, establishing AKI as a primary factor in inpatient mortality.3 Additionally, AKI is acknowledged as a significant precursor to chronic kidney disease (CKD), imposing substantial healthcare costs.4,5
The pathogenesis of AKI is intricate, involving numerous pathological mechanisms such as oxidative stress, inflammatory injury, immune response, calcium overload, and apoptosis. However, these factors alone do not fully elucidate the complexity of AKI’s pathophysiology. In recent years, the association between AKI and mitochondria has garnered increasing attention. Mitochondria, often referred to as the “powerhouses” of cells, frequently exhibit dysfunction in AKI, playing a pivotal role in the disease’s progression by influencing various cellular processes, including membrane permeability, energy metabolism, and signal transduction. Tubular epithelial cells, the primary targets in AKI, harbor abundant mitochondria with high energy demands but limited glycolytic capacity, hence predominantly relying on mitochondrial aerobic respiration as their primary energy source.6–8 In AKI models, mitochondrial injury, particularly in proximal tubular cells, is characterized by heightened levels of reactive oxygen species (ROS) and diminished ATP levels, alongside morphological alterations such as swelling, disrupted cristae, and compromised membrane integrity.9–11 Preserving mitochondrial integrity or reinstating their function has emerged as a novel therapeutic strategy to mitigate AKI.12–14
Over the past decade, the volume of publications exploring the connection between mitochondria and AKI has steadily increased. However, there remains a lack of quantitative analysis in this field from a bibliometric perspective. Bibliometrics employs mathematical and statistical methodologies to analyze aspects such as publication count, citation rates, author collaborations, and journal distribution, thereby illuminating research focal points, trajectories, and scholarly impact. Unlike reviews, bibliometrics offers the advantage of providing quantitative insights to assist researchers in understanding the evolution of a specific research area, predicting future trends, and identifying potential collaborators and research institutions. This study conducted a bibliometric analysis of all literature preceding 2024 related to mitochondrial dysfunction and AKI. The analysis captures the current landscape, future research directions, and prevalent areas of interest in the field, providing guidance and a roadmap for future investigations.
Methods
To query the Web of Science Core Collection database, the following search string was used: [TS = (acute renal failure OR ARF OR acute renal injury OR acute kidney failure OR acute kidney injury OR AKI) AND (Mitochondrion OR Mitochondria OR Mitochondrial)]. The search covered the database’s inception to December 31, 2023. Certain article types were excluded, such as Meeting Abstracts, Editorial Material, Proceeding Papers, Corrections, Retracted Publications, Letters, and Publications with an Expression of Concern. Only Articles, Review Articles, Book Chapters, and Early Accesses were considered. Ultimately, only original articles published within the specified timeframe were included in the search results.
The document was exported locally for further analysis. Microsoft Excel 2021 was utilized, along with bibliometric tools including VOSviewer, Citespace R6.3.1, the bibliometrix R package (version R4.3.2), and SCImagoGraphica for visual analysis based on authors, countries, journals, institutions, and keywords.
Microsoft Excel 2019 was employed to analyze the annual publication trends in the field. VOSviewer facilitated the examination of publication volumes and collaboration relationships among countries/regions, while SCImagoGraphica was used to generate a world map illustrating the distribution of publications via gradient colors. Within the Bibliometrix R package, visualizations and analyses were conducted on citation frequency for countries/journals/literature, collaborations between countries/institutions, and trend topics. Institutional collaboration timelines and keyword timelines were created using the analysis and mapping capabilities of Cite Space 6.1.6.
Results
Global Publications and Trends
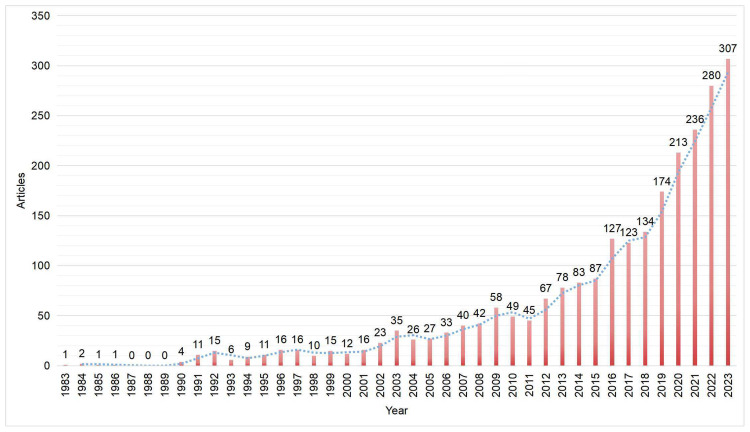
The initial search yielded 2560 articles, which were filtered down to 2433 for final analysis. The annual publication output showed a general upward trend from 2011 to 2021, as illustrated in Figure 1. The first article was published in 1983, with publication numbers remaining relatively low (n < 100) until 2015, indicating an initial stage. However, starting from 2011, the number of articles surged rapidly, reaching 307 in 2023, nearly five times the count compared to 2011. Overall, this trend suggests increasing scholarly involvement in future research. Exploring AKI from the perspective of mitochondrial function shows significant promise.
Figure 1.
Global trends in the number of articles.
Countries/Regions, Institutions, and Authors
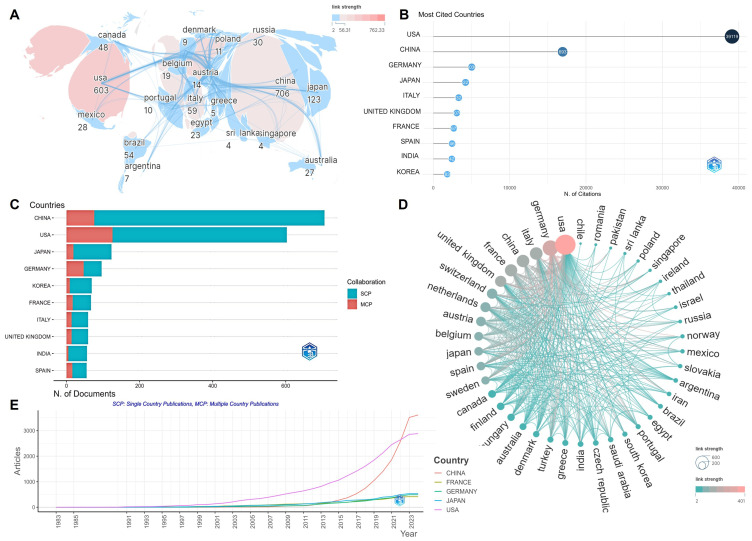
According to the visual analysis, research institutions from 82 countries/regions have contributed to the field. China led with 706 articles, followed by the United States with 603, collectively comprising over half of the global publication count (Figure 2A). However, the United States excelled in citation counts with 39,119 citations, ranking first, while China had 16,924 citations (Figure 2B). In terms of international collaboration, Asian countries like China, Japan, and South Korea exhibited relatively fewer collaborations, with more independent publications (Figure 2C), whereas countries like the United States, Germany, and France demonstrated closer collaborative relationships (Figure 2D). From a temporal perspective, the United States was the most influential country until 2022, after which China emerged as the leader (Figure 2E). Despite China’s significant lead in publication volume, strengthening international cooperation may yield greater benefits.
Figure 2.
Visualization results of countries/regions. (A) National/regional cooperation depicted with node size representing the number of articles and color depth indicating collaboration intensity. (B) Top ten countries with the highest citation counts. (C) Countries of corresponding authors. (D) In the country cooperation network, node size reflects collaboration intensity. (E) Country production trends over time.
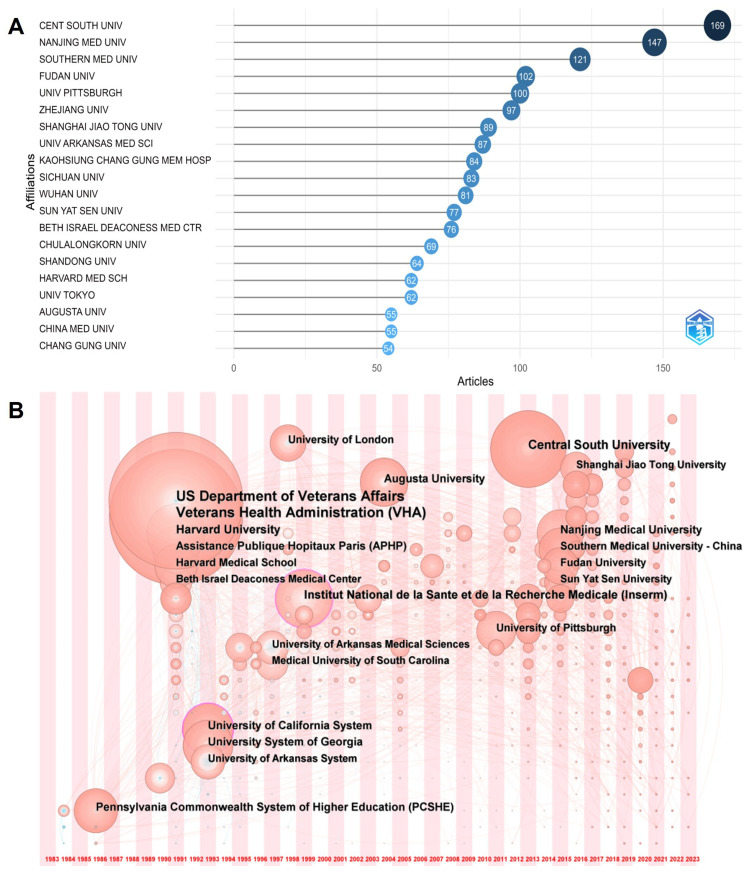
There are 2803 institutions involved in the research, with Central South University, Nanjing Medical University, Southern Medical University, Fudan University, and the University of Pittsburgh being the top publishing institutions (Figure 3A). The collaboration network timeline indicates early research initiation by institutions in the United States, which have since become influential (Figure 3B). Institutions in China have shown increasing research activities since 2010, contributing to China’s leadership in publication volume but lagging in citation counts compared to the United States.
Figure 3.
Visualization results of institutions. (A) Most relevant institutions. (B) Institution cooperation network timeline from 1983 to 2023.
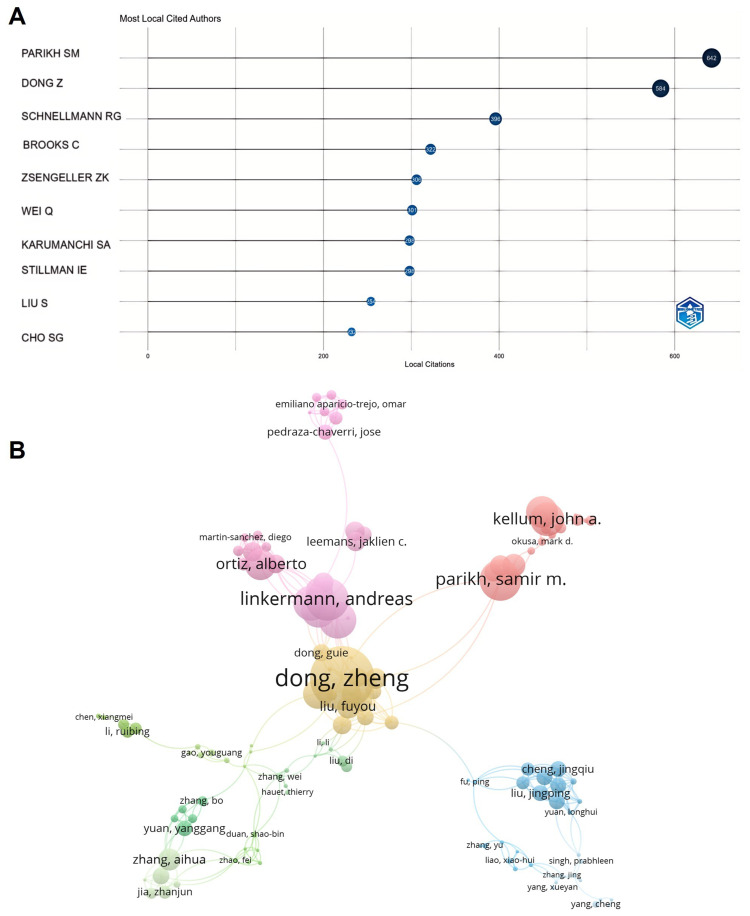
There are 9912 authors involved in the research, with Parikh SM and Schnellmann RG from the United States, and Dong Z from Central South University in China, being the most cited authors (Figure 4A). This underscores their significant influence in the research field. Author collaboration analysis reveals a global distribution of authors engaging in cooperation, forming collaborative teams of considerable scale (Figure 4B).
Figure 4.
Visualization results of author cooperation. (A) Most locally cited authors. (B) Author cooperation network.
Journals/Cited Journals
A total of 772 journals have reported on research within the field, with the top five journals being the AMERICAN JOURNAL OF PHYSIOLOGY-RENAL PHYSIOLOGY, KIDNEY INTERNATIONAL, INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES, JOURNAL OF THE AMERICAN SOCIETY OF NEPHROLOGY, and FRONTIERS IN PHARMACOLOGY (Table 1). Among these, KIDNEY INTERNATIONAL, JOURNAL OF THE AMERICAN SOCIETY OF NEPHROLOGY, and AMERICAN JOURNAL OF PHYSIOLOGY-RENAL PHYSIOLOGY have received the most citations, establishing them as highly influential journals in the field (Table 1).
Table 1.
Journals/Cited Journals
| Rank | Sources | Articles | Times of cited |
|---|---|---|---|
| 1 | AMERICAN JOURNAL OF PHYSIOLOGY-RENAL PHYSIOLOGY | 109 | 4191 |
| 2 | KIDNEY INTERNATIONAL | 72 | 6514 |
| 3 | INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES | 61 | 997 |
| 4 | JOURNAL OF THE AMERICAN SOCIETY OF NEPHROLOGY | 61 | 5559 |
| 5 | FRONTIERS IN PHARMACOLOGY | 39 | 512 |
| 6 | PLOS ONE | 39 | 2108 |
| 7 | OXIDATIVE MEDICINE AND CELLULAR LONGEVITY | 38 | 902 |
| 8 | RENAL FAILURE | 34 | 507 |
| 9 | BIOMEDICINE & PHARMACOTHERAPY | 29 | 454 |
| 10 | SCIENTIFIC REPORTS | 26 | 1154 |
Cited Articles
The top 10 most cited articles focus on mitochondrial autophagy, oxidative stress, ferroptosis, and mitochondrial energetics (Table 2). The most cited study, “Mitochondria ROS and mitophagy in acute kidney injury”, underscores the significance of mitochondrial autophagy in maintaining mitochondrial quality and its relevance to AKI development and progression.
Table 2.
Cited Articles
| Rank | Paper | DOI | Total Citations | TC per Year TC | Normalized TC |
|---|---|---|---|---|---|
| 1 | XIE Y, 2016, CELL DEATH DIFFER | 10.1038/cdd.2015.158 | 2058 | 228.67 | 26.93 |
| 2 | BASILE DP, 2012, COMPR PHYSIOL | 10.1002/cphy.c110041 | 735 | 56.54 | 9.84 |
| 3 | LINKERMANN A, 2014, PROC NATL ACAD SCI U S A | 10.1073/pnas.1415518111 | 721 | 65.55 | 8.87 |
| 4 | BHARGAVA P, 2017, NAT REV NEPHROL | 10.1038/nrneph.2017.107 | 658 | 82.25 | 13.32 |
| 5 | BROOKS C, 2009, J CLIN INVEST | 10.1172/JCI37829 | 582 | 36.38 | 8.72 |
| 6 | BINDU S, 2020, BIOCHEM PHARMACOL | 10.1016/j.bcp.2020.114147 | 566 | 113.20 | 16.56 |
| 7 | GOMEZ H, 2014, SHOCK | 10.1097/SHK.0000000000000052 | 537 | 48.82 | 6.60 |
| 8 | IYER SS, 2009, PROC NATL ACAD SCI U S A | 10.1073/pnas.0908698106 | 524 | 32.75 | 7.85 |
| 9 | GOLOMB BA, 2008, AM J CARDIOVASC DRUGS | 10.2165/0129784-200,808,060-00004 | 506 | 29.76 | 7.14 |
| 10 | VARSHAVSKY A, 2011, PROTEIN SCI | 10.1002/pro.666 | 501 | 35.79 | 6.01 |
Research Hotspots
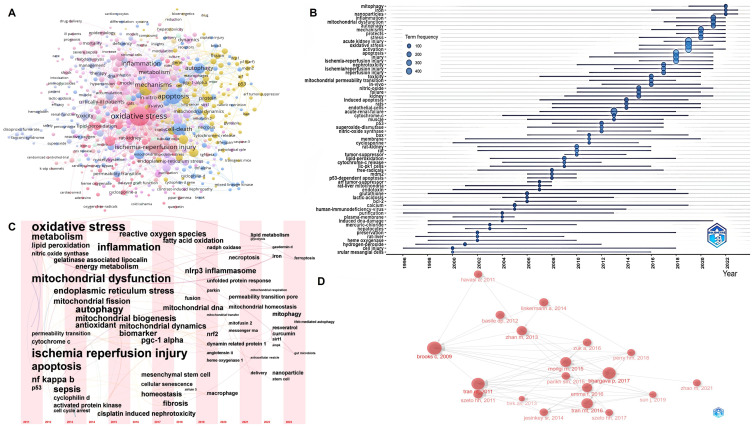
Keyword cluster analysis reveals interconnected clusters around major keywords such as oxidative stress, ischemia-reperfusion injury, apoptosis, inflammation, and autophagy, forming a complex network within the field (Figure 5A). The evolution of research, focusing from broader biological processes to specific cellular organelles and molecular mechanisms, underscores the deepening understanding of AKI pathophysiology (Figure 5B–D). Advancements in exploring specific molecular mechanisms and treatment methods have made research areas focus on cellular senescence, Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha (PGC-1α), ferroptosis, mitochondrial quality control, mitochondria-targeted antioxidants, natural products, extracellular vesicles, and nanoparticle drug delivery, etc (Table 3).
Figure 5.
Research Hotpots. (A) Clustering network of keywords. (B) Trend topics from 2000 to 2023. (C) Historiograph of articles. (D) Keyword network timeline from 2011 to 2023.
Table 3.
Summary of Current Research Directions and Findings
| Research Area | Research Direction | Keywords | Key Findings | Future Directions | References |
|---|---|---|---|---|---|
| Cell Biology | Cellular Senescence | Cellular senescence, p53, cell cycle arrest | Senescence of renal tubular epithelial cells accelerates CKD progression. Mitochondrial overfusion is linked to DRP1 downregulation. Lack of TREM1/3 cells causes cell cycle arrest and decreased mitochondrial metabolism. | Develop drugs targeting mitochondrial metabolism to delay senescence and advance preclinical and clinical research. | [15–18] |
| Ferroptosis | Ferroptosis, lipid peroxidation | Succinate: quinone oxidoreductase degradation leads to mitochondrial dysfunction, aggravating ferroptosis and AKI. PKM2 regulates glycolysis, maintaining mitochondrial integrity. | Study drug interventions in ferroptosis and promote translational applications. | [19–21] | |
| Mitochondrial Quality Control | Biogenesis, dynamics, mitophagy, fusion, fission, PGC-1α, NRF1, MFN1, PINK1, Parkin, SIRT1 | Mitochondrial biogenesis driven by PGC-1α promotes nuclear respiratory factors and TFAM expression. Fusion and fission integrate mitochondria into networks. Damaged mitochondria are cleared by mitophagy via PINK1-Parkin pathway. | Develop drugs regulating mitochondrial quality and conduct preclinical studies. | [8,22–29] | |
| Emerging Therapies | Pharmacotherapy | Antioxidants, MitoQ, Szeto-Schiller (SS), SS-31, MitoTEMPO, SkQR1 |
Antioxidants like MitoQ and SS peptides protect mitochondrial membranes, clear ROS, and inhibit permeability transition. Mitochondrial acetate increases ATP levels, reducing AKI tubular necrosis. | Improve drug bioavailability and stability, and advance clinical applications. | [30–35] |
| Natural Products | Curcumin, Resveratrol | Curcumin protects kidneys by regulating immune system, clearing ROS, reducing apoptosis, and improving mitochondrial function. Resveratrol enhances antioxidant responses and mitochondrial homeostasis. | Study mechanisms of natural products and conduct translational research. | [36–39] | |
| Nanomedicine | liposomes, nanoparticles, drug delivery systems | Curcumin derivatives target mitochondria via KIM-1 receptor-mediated endocytosis and TPP action. New technologies like nanoparticles and liposomes show potential in improving bioavailability and stability. | Optimize nano-drug delivery systems and promote preclinical and clinical applications. | [40–43] | |
| Cell Therapy | Mesenchymal stem cells (MSCs), exosomes | MSCs reduce mitochondrial fission and enhance biogenesis. Fibroblast-derived vesicles promote mitophagy, inhibit pyroptosis, and improve kidney function. | Optimize cell therapy and advance preclinical and clinical applications. | [44–46] |
Discussion
Research results
The study offers a comprehensive analysis of the evolution, current state, and emerging trends in research concerning the relationship between mitochondria and AKI over the past two decades. Since 2011, researchers have increasingly focused on the role of mitophagy in the pathogenesis of AKI, indicating a deepening understanding of AKI. Furthermore, the scope of research has expanded beyond mitochondrial dysfunction to include factors such as epigenetic regulation and renal repair mechanisms during AKI.
Both China and the United States have made substantial research contributions, with the United States leading in citation counts. Historically, institutions in the United States, notably Harvard University, held considerable influence, but since 2010, several institutions from China have contributed to a surge in publication volume. While China leads in publication volume, it trails behind the United States in citation counts, suggesting a need for enhanced international collaboration. Other countries like Japan, Germany, South Korea, France, Italy, the United Kingdom, India, and Spain also contribute to the field’s development, albeit to a lesser extent.
The top 10 journals publishing research on mitochondria and AKI span a range of disciplines, from nephrology to molecular sciences and pharmacology, reflecting the interdisciplinary nature of the field. This diverse publication landscape underscores the high level of academic interest, indicating the necessity for further research to advance strategies for the prevention, diagnosis, and treatment of AKI. Global collaboration among research institutions is encouraged to foster innovation and address the multifaceted challenges posed by AKI.
Parikh SM and Schnellmann RG from the United States, along with Dong Z from Central South University in China, are the most frequently cited authors, indicating their significant influence in this research field. Parikh’s team primarily focuses on changes in mitochondrial metabolism during AKI, particularly mitochondrial biogenesis and energy metabolism.47 They underscore the potential protective roles of the mitochondrial biogenesis regulator PGC1α and nicotinamide adenine dinucleotide in mitigating renal stress.48,49 Schnellmann’s research particularly highlights the role of mitochondria in drug-induced AKI. His team has investigated the effects of lasmiditan, a 5-hydroxytryptamine (5-HT) 1F receptor agonist, and Formoterol PLGA-PEG Nanoparticles on mitochondria in renal proximal tubules.50–53 Dong Z’s research focuses on mitochondrial regulation, cellular autophagy, and epigenetics in AKI. The team’s studies have showcased the rapid regulation of mitochondrial dynamics in AKI and and have delved into the role of mitochondrial quality control in renal injury and repair.8,54,55
The top 10 most cited articles primarily focus on areas such as mitochondrial autophagy, oxidative stress, ferroptosis, and mitochondrial energetics. Among them, the most cited study is “Mitochondrial ROS and mitophagy in acute kidney injury”, which underscores mitochondrial autophagy as a pivotal mechanism for maintaining mitochondrial quality, responsible for eliminating damaged mitochondria, and closely associated with the occurrence and development of AKI.56 Lin et al’s research revealed that PINK1-Parkin-mediated mitochondrial autophagy can mitigate mitochondrial reactive oxygen species (ROS) and consequent activation of the NLRP3 inflammasome, thereby averting apoptosis of renal tubular epithelial cells and tissue damage.57 Oxidative stress is another focus of the research.58 Research indicates that mitochondrial ROS can exacerbate kidney injury by impeding mitochondrial transcription factor A-mediated maintenance of mitochondrial DNA (mtDNA), resulting in reduced mitochondrial energy metabolism and heightened cytokine release. Additionally, ferroptosis, recognized as a newly regulated form of cell death, has gained considerable attention.59 These studies underscore the active research surrounding the role of mitochondria in AKI. Future investigations may center on achieving a more precise understanding of the mechanisms involved, identifying novel therapeutic targets, and exploring potential clinical applications.
Hot Spots and Trends
By analyzing literature and key terms through the Historiograph Network, we gain insight into research hotspots and trends. Since 2011, research keywords have shifted from macro-level to micro-level focuses, including ischemia-reperfusion, sepsis, cisplatin-induced nephrotoxicity, and contrast-induced AKI. Techniques like gene knockout, overexpression, and siRNA interference are now used to study molecular mechanisms in greater depth. Early research focused on broad biological processes such as oxidative stress and apoptosis, but has since narrowed to organelle-level mechanisms and therapies, especially mitochondrial quality control. Emerging hotspots include mRNA, mitochondrial-targeted antioxidants, extracellular vesicles, and nanoparticle drug delivery, reflecting a deeper understanding of AKI mechanisms. The specific analysis and summary are as follows.
As research on cell death and injury in AKI progresses, the focus has shifted from apoptosis to cellular senescence and ferroptosis. Senescence of renal tubular epithelial cells is a crucial factor in the progression of AKI to CKD and is closely linked to mitochondrial dysfunction.15–18 Impaired mitochondrial function weakens defenses against ferroptosis, leading to increased levels of lipid peroxidation.19 Recent evidence suggests that mitochondria could be potential therapeutic targets for reducing ferroptosis and improving AKI prognosis.20,21 The mitochondrial quality control system aids renal tubular epithelial cells in coping with stress and restoring homeostasis.8 In cases of mild injury, protein quality control, antioxidant defenses, and mtDNA repair play significant roles, while mitochondrial biogenesis, dynamics, and mitophagy are more critical during severe injury.22–24Increasing evidence indicates that dysregulation of mitochondrial quality control contributes significantly to the pathogenesis of AKI and suboptimal kidney repair.25–29 The gut microbiota has also emerged as a new focal point in AKI research. Dysbiosis can promote the progression of AKI to CKD through mechanisms such as hypoxia induction, mitochondrial ROS production, aryl hydrocarbon receptor activation, and uremic toxin generation.60–64 Continued research is essential to uncover these mechanisms, which will help in identifying novel therapeutic targets and improving clinical outcomes for AKI patients.
Mitochondria-targeted therapy shows immense potential in the treatment of AKI.65 However, most therapeutic research is still in the preclinical stage, focusing on testing efficacy in experimental animal models and exploring treatment mechanisms. Initial approaches involve the use of lipophilic antioxidant molecules coupled with triphenylphosphonium ions, such as MitoQ, MitoTEMPO, or SkQR1, among others. Szeto-Schiller (SS) peptides are selectively targeted to the inner mitochondrial membrane. SS-31 has been extensively studied in AKI models, protecting the cristae, preventing mitochondrial swelling, scavenging mitochondrial ROS, and inhibiting the opening of mitochondrial permeability transition pores.30,31 Mitochondrial Acidic 5 is a derivative of the plant hormone indole-3-acetic acid, which can increase cellular ATP levels and reduce AKI tubular necrosis.32,33 The Schnellmann team primarily searched formoterol and 5-HT2 agonists, which can increase PGC-1α expression and promote mitochondrial biogenesis.34,35
Natural compounds have also shown promising effects in the prevention and treatment of AKI. In particular, curcumin, a polyphenolic compound with multiple biological activities, stands out. It protects the kidneys by regulating the immune system, scavenging reactive oxygen species, reducing cell apoptosis, and improving mitochondrial function. It can activate Nuclear factor erythroid 2–related factor 2, Heme oxygenase 1, and PGC-1α to reduce ROS, upregulate OPA1, and downregulate DRP1 to restore mitochondrial homeostasis.36 The stepwise targeted curcumin derivative Ser@TPP@CUR can be specifically internalized by renal tubular epithelial cells through endocytosis mediated by the Kidney Injury Molecule-1 receptor. Subsequently, it actively distributes in mitochondria under the action of the mitochondrial-targeting moiety TPP.40 New technologies such as curcumin nanoparticles, liposomes, and polymer conjugates show immense potential in enhancing the bioavailability and stability of curcumin.41–43
Additionally, cell therapies such as mesenchymal stem cells and extracellular vesicles show promise in experimental AKI models. Mesenchymal Stem Cells have been shown to reduce mitochondrial fission and enhance mitochondrial biogenesis.44,45 The latest research indicates that extracellular vesicles derived from fibroblastic reticular cells selectively bind to primary renal tubular cells, promoting mitochondrial autophagy, inhibiting cell pyroptosis, and improving kidney function by inhibiting NLRP3 inflammasome activation.46 With further understanding of the mechanisms of these therapeutic approaches and the advancement of related clinical studies, it is hoped that more effective treatments for AKI will be developed in the future.
Overall, medical research on AKI has made significant progress over the past decade. However, due to the complexity of AKI mechanisms, applying related theories in clinical treatment has not yielded the expected results. Therefore, researchers still face major challenges and need to further explore the mechanisms of AKI to achieve more effective treatment strategies.
Limitations
Literature metrics analysis relies on specific technical tools and algorithms. Different literature metrics analyses depend on specialized technical tools and algorithms. Different algorithms may be utilized in various literature analysis software to compute citation frequency, impact factor, or collaboration networks, resulting in some level of variability in research findings. Furthermore, in our study, we performed thorough data preprocessing and cleansing to reduce the influence of erroneous and duplicate data. Nevertheless, this procedure may entail subjective assessments, such as determining which literature to examine and defining keywords, potentially introducing subjectivity into the analysis outcomes.
Conclusion
Based on the WoSCC database, a bibliometric analysis was conducted on 2433 studies published before 2024 regarding the relationship between mitochondria and AKI. The results show that the number of papers on it has been increasing annually over the past 20 years, with China and the United States being the leading countries in this field. Moreover, Central South University is the research institution with the greatest impact on research outcomes, and Parikh SM, Schnellmann RG, and Dong Z are the most influential authors in this field. KIDNEY INTERNATIONAL, JOURNAL OF THE AMERICAN SOCIETY OF NEPHROLOGY, and AMERICAN JOURNAL OF PHYSIOLOGY-RENAL PHYSIOLOGY are the most influential journals. Initially, the research focused on keywords such as oxidative stress, ischemia-reperfusion injury, cell apoptosis, inflammation, and autophagy. In recent years, new research hotspots have emerged, including ferroptosis, aging, mitochondrial quality control, messenger ribonucleic acid, mitochondrial-targeted antioxidants, extracellular vesicles, and nanodrug delivery. In summary, research on the relationship between mitochondria and AKI has promising prospects, and targeted regulation of mitochondria will be a focus of future research on the prevention and treatment of AKI.
Funding Statement
This work is funded by the National Nature Science Foundation of China (82274482, 82104830) and Natural Science Foundation of Sichuan, China (2022YFS0398).
Abbreviations
AKI, Acute Kidney Injury; CKD, Chronic Kidney Disease; ROS, Reactive Oxygen Species; mtDNA, Mitochondrial DNA; PGC-1α, Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha; NLRP3, Nod-like receptor protein 3; PINK1, PTEN-induced kinase 1; DRP1, Dynamin-related protein 1; TFAM, Mitochondrial Transcription Factor A; SS, Szeto-Schiller.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1949–1964. doi: 10.1016/S0140-6736(19)32563-2 [DOI] [PubMed] [Google Scholar]
- 2.Ostermann M, Basu RK, Mehta RL. Acute kidney injury. Intensive Care Med. 2023;49(2):219–222. doi: 10.1007/s00134-022-06946-0 [DOI] [PubMed] [Google Scholar]
- 3.Wu C, Zhang Y, Nie S, et al. Predicting in-hospital outcomes of patients with acute kidney injury. Nat Commun. 2023;14(1):3739. doi: 10.1038/s41467-023-39474-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joannidis M, Meersch-Dini M, Forni LG. Acute kidney injury. Intensive Care Med. 2023;49(6):665–668. doi: 10.1007/s00134-023-07061-4 [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Xing G, Wang L, et al. Acute kidney injury in China: a cross-sectional survey. Lancet. 2015;386(10002):1465–1471. doi: 10.1016/S0140-6736(15)00344-X [DOI] [PubMed] [Google Scholar]
- 6.Lan R, Geng H, Singha PK, et al. Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J Am Soc Nephrol. 2016;27(11):3356–3367. doi: 10.1681/asn.2015020177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Li Z, Zhang H, et al. Mitochondrial metabolism and targeted treatment strategies in ischemic-induced acute kidney injury. Cell Death Disc. 2024;10(1):69. doi: 10.1038/s41420-024-01843-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang C, Cai J, Yin X-M, Weinberg JM, Venkatachalam MA, Dong Z. Mitochondrial quality control in kidney injury and repair. Nat Rev Nephrol. 2021;17(5):299–318. doi: 10.1038/s41581-020-00369-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emma F, Montini G, Parikh SM, Salviati L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat Rev Nephrol. 2016;12(5):267–280. doi: 10.1038/nrneph.2015.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao M, Wang Y, Li L, et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Resea Paper Theran. 2021;11(4):1845–1863. doi: 10.7150/thno.50905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srinivasan S, Guha M, Kashina A, Avadhani NG. Mitochondrial dysfunction and mitochondrial dynamics-The cancer connection. Biochimica et Biophysica Acta. 2017;1858(8):602–614. doi: 10.1016/j.bbabio.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry HM, Huang L, Wilson RJ, et al. Dynamin-Related Protein 1 Deficiency Promotes Recovery from AKI. J Am Soc Nephrol. 2018;29(1):194–206. doi: 10.1681/asn.2017060659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szeto HH, Liu S, Soong Y, et al. Mitochondria Protection after Acute Ischemia Prevents Prolonged Upregulation of IL-1β and IL-18 and Arrests CKD. J Am Soc Nephrol. 2017;28(5):1437–1449. doi: 10.1681/asn.2016070761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu D, Jin F, Shu G, et al. Enhanced efficiency of mitochondria-targeted peptide SS-31 for acute kidney injury by pH-responsive and AKI-kidney targeted nanopolyplexes. Biomaterials. 2019;211:57–67. doi: 10.1016/j.biomaterials.2019.04.034 [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Zhang H, Yi X, et al. Cellular senescence of renal tubular epithelial cells in acute kidney injury. Cell Death Disc. 2024;10(1):62. doi: 10.1038/s41420-024-01831-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo J, Chiang W-C. Mitophagy in aging and longevity. IUBMB Life. 2022;74(4):296–316. doi: 10.1002/iub.2585 [DOI] [PubMed] [Google Scholar]
- 17.Dalle Pezze P, Nelson G, Otten EG, et al. Dynamic Modelling of Pathways to Cellular Senescence Reveals Strategies for Targeted Interventions. PLOS Computat Biology. 2014;10(8):e1003728. doi: 10.1371/journal.pcbi.1003728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tammaro A, Scantlebery AML, Rampanelli E, et al. TREM1/3 Deficiency Impairs Tissue Repair After Acute Kidney Injury and Mitochondrial Metabolic Flexibility in Tubular Epithelial Cells. Original Research. 2019:10. doi: 10.3389/fimmu.2019.01469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo R, Duan J, Pan S, et al. The Road from AKI to CKD: molecular Mechanisms and Therapeutic Targets of Ferroptosis. Cell Death Dis. 2023;14(7):426. doi: 10.1038/s41419-023-05969-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai F, Li D, Xie Y, et al. Sulfide:Quinone oxidoreductase alleviates ferroptosis in acute kidney injury via ameliorating mitochondrial dysfunction of renal tubular epithelial cells. Redox Biol. 2024;69:102973. doi: 10.1016/j.redox.2023.102973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie W, He Q, Zhang Y, et al. Pyruvate kinase M2 regulates mitochondrial homeostasis in cisplatin-induced acute kidney injury. Cell Death Dis. 2023;14(10):663. doi: 10.1038/s41419-023-06195-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popov L-D. Mitochondrial biogenesis: an update. J Cell & Mol Med. 2020;24(9):4892–4899. doi: 10.1111/jcmm.15194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adebayo M, Singh S, Singh AP, Dasgupta S, Dasgupta SJFjopotFoASfEB. Mitochondrial fusion and fission: the fine-tune balance for cellular homeostasis. FASEB. 2021;35(6):e21620. doi: 10.1096/fj.202100067R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurtado KA, Schnellmann RG. Mitophagy regulates mitochondrial number following pharmacological induction of mitochondrial biogenesis in renal proximal tubule cells. Brief Research Rep. 2024;15. doi: 10.3389/fphar.2024.1344075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Zhu J, Liu Z, et al. The PINK1/PARK2/optineurin pathway of mitophagy is activated for protection in septic acute kidney injury. Redox Biol. 2021;38:101767. doi: 10.1016/j.redox.2020.101767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Slikke EC, Star BS, van Meurs M, Henning RH, Moser J, Bouma HR. Sepsis is associated with mitochondrial DNA damage and a reduced mitochondrial mass in the kidney of patients with sepsis-AKI. Critical Care. 2021;25(1):36. doi: 10.1186/s13054-020-03424-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Zhu P, Li R, Ren J, Zhou H. Fundc1-dependent mitophagy is obligatory to ischemic preconditioning-conferred renoprotection in ischemic AKI via suppression of Drp1-mediated mitochondrial fission. Redox Biol. 2020;30:101415. doi: 10.1016/j.redox.2019.101415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran M, Tam D, Bardia A, et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121(10):4003–4014. doi: 10.1172/JCI58662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Qin Y, Liu B, et al. PGC-1α-Mediated Mitochondrial Quality Control: molecular Mechanisms and Implications for Heart Failure. Review. 2022:10. doi: 10.3389/fcell.2022.871357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szeto HH, Liu S, Soong Y, et al. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22(6):1041–1052. doi: 10.1681/asn.2010080808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birk AV, Liu S, Soong Y, et al. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24(8):1250–1261. doi: 10.1681/asn.2012121216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki T, Yamaguchi H, Kikusato M, et al. Mitochonic Acid 5 (MA-5), a Derivative of the Plant Hormone Indole-3-Acetic Acid, Improves Survival of Fibroblasts from Patients with Mitochondrial Diseases. Tohoku J Exp Med. 2015;236(3):225–232. doi: 10.1620/tjem.236.225 [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T, Yamaguchi H, Kikusato M, et al. Mitochonic Acid 5 Binds Mitochondria and Ameliorates Renal Tubular and Cardiac Myocyte Damage. J Am Soc Nephrol. 2016;27(7):1925–1932. doi: 10.1681/asn.2015060623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cameron RB, Gibbs WS, Miller SR, et al. Proximal Tubule β (2)-Adrenergic Receptor Mediates Formoterol-Induced Recovery of Mitochondrial and Renal Function after Ischemia-Reperfusion Injury. J Pharm Experim Therapeu. 2019;369(1):173–180. doi: 10.1124/jpet.118.252833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harmon JL, Wills LP, McOmish CE, et al. 5-HT2 Receptor Regulation of Mitochondrial Genes: unexpected Pharmacological Effects of Agonists and Antagonists. J Pharm Experim Therapeu. 2016;357(1):1–9. doi: 10.1124/jpet.115.228395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai Y, Huang C, Zhou M, et al. Role of curcumin in the treatment of acute kidney injury: research challenges and opportunities. Phytomedicine. 2022;104:154306. doi: 10.1016/j.phymed.2022.154306 [DOI] [PubMed] [Google Scholar]
- 37.Aranda-Rivera AK, Cruz-Gregorio A, Aparicio-Trejo OE, Pedraza-Chaverri J. Mitochondrial redox signaling and oxidative stress in kidney diseases. Biomolecules. 2021;11(8):1–29. doi: 10.3390/biom11081144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singer M, Deutschman C. S, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3. J Am Med Assoc. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Guan Y. Resveratrol Rescues Kidney Mitochondrial Function Following Hemorrhagic Shock. Shock. 2015;44(2):173–180. doi: 10.1097/SHK.0000000000000390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan X, Tan X-Y, Y-X L, et al. A Stepwise Targeting Curcumin Derivative, Ser@TPP@CUR, for Acute Kidney Injury. ACS Med Chem Lett. 2022;13(4):554–559. doi: 10.1021/acsmedchemlett.1c00585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao M, Guo J, Tian C, et al. Dual-targeted nanoparticles with removing ROS inside and outside mitochondria for acute kidney injury treatment. Nanomed Nanotech Biol Med. 2024;55:102725. doi: 10.1016/j.nano.2023.102725 [DOI] [PubMed] [Google Scholar]
- 42.Wang D-W, S-J L, Tan X-Y, et al. Engineering of stepwise-targeting chitosan oligosaccharide conjugate for the treatment of acute kidney injury. Carbohydr Polym 2021;256:117556. doi: 10.1016/j.carbpol.2020.117556 [DOI] [PubMed] [Google Scholar]
- 43.Lan T, Guo H, Lu X, et al. Dual-Responsive Curcumin-Loaded Nanoparticles for the Treatment of Cisplatin-Induced Acute Kidney Injury. Biomacromolecules. 2022;23(12):5253–5266. doi: 10.1021/acs.biomac.2c01083 [DOI] [PubMed] [Google Scholar]
- 44.Zhao L, Hu C, Zhang P, Jiang H, Chen J. Mesenchymal stem cell therapy targeting mitochondrial dysfunction in acute kidney injury. J Transl Med. 2019;17(1):142. doi: 10.1186/s12967-019-1893-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torrico S, Hotter G, Játiva S. Development of Cell Therapies for Renal Disease and Regenerative Medicine. Int J Mol Sci. 2022;23(24):15943. doi: 10.3390/ijms232415943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Hu C, Zhai P, et al. Fibroblastic reticular cell-derived exosomes are a promising therapeutic approach for septic acute kidney injury. Kidney Int. 2024;105(3):508–523. doi: 10.1016/j.kint.2023.12.007 [DOI] [PubMed] [Google Scholar]
- 47.Clark AJ, Parikh SM. Mitochondrial Metabolism in Acute Kidney Injury. Seminars Nephrology Mar. 2020;40(2):101–113. doi: 10.1016/j.semnephrol.2020.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parikh SM. Metabolic Stress Resistance in Acute Kidney Injury: evidence for a PPAR-Gamma-Coactivator-1 Alpha-Nicotinamide Adenine Dinucleotide Pathway. Nephron. 2019;143(3):184–187. doi: 10.1159/000500168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poyan Mehr A, Parikh SM. PPARγ-Coactivator-1α, Nicotinamide Adenine Dinucleotide and Renal Stress Resistance. Nephron. 2017;137(4):253–255. doi: 10.1159/000471895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith JA, Stallons LJ, Collier JB, Chavin KD, Schnellmann RG. Suppression of Mitochondrial Biogenesis through Toll-Like Receptor 4–Dependent Mitogen-Activated Protein Kinase Kinase/Extracellular Signal-Regulated Kinase Signaling in Endotoxin-Induced Acute Kidney Injury. J J Pharm Experimental Therap. 2015;352(2):346–357. doi: 10.1124/jpet.114.221085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hurtado KA, Janda J, Schnellmann RG. Lasmiditan promotes recovery from acute kidney injury through induction of mitochondrial biogenesis. Am J Physiol Renal Physiol. 2023;324(1):F56–F63. doi: 10.1152/ajprenal.00249.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurtado KA, Janda J, Schnellmann RG. Lasmiditan restores mitochondrial quality control mechanisms and accelerates renal recovery after ischemia-reperfusion injury. Biochem Pharmacol. 2023;218:115855. doi: 10.1016/j.bcp.2023.115855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vallorz EL, Blohm-Mangone K, Schnellmann RG, Mansour HM. Formoterol PLGA-PEG Nanoparticles Induce Mitochondrial Biogenesis in Renal Proximal Tubules. AAPS J. 2021;23(4):88. doi: 10.1208/s12248-021-00619-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119(5):1275–1285. doi: 10.1172/jci37829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang M, Liu K, Luo J, ZJTAjop D. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol. 2010;176(3):1181–1192. doi: 10.2353/ajpath.2010.090594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su L, Zhang J, Gomez H, Kellum JA, Peng Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy. 2023;19(2):401–414. doi: 10.1080/15548627.2022.2084862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin Q, Li S, Jiang N, et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019;26:101254. doi: 10.1016/j.redox.2019.101254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jomová K, Raptová R, Alomar SY, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol. 2023;97(2499):–2574. doi: 10.1007/s00204-023-03562-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie Y, Hou W, Song X, et al. Ferroptosis: process and function. Cell death and differentiation. Mar. 2016;23(3):369–379. doi: 10.1038/cdd.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saranya GR, Viswanathan P. Gut microbiota dysbiosis in AKI to CKD transition. Biomed. Pharmacother. 2023;161:114447. doi: 10.1016/j.biopha.2023.114447 [DOI] [PubMed] [Google Scholar]
- 61.Lim YJ, Tonial NC, Hartjes ED, Haig A, Velenosi TJ, Urquhart BL. Metabolomics for the identification of early biomarkers of nephrotoxicity in a mouse model of cisplatin-induced acute kidney injury. Biomed Pharm. 2023;163:114787. doi: 10.1016/j.biopha.2023.114787 [DOI] [PubMed] [Google Scholar]
- 62.Jackson DN, Theiss AL. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes. 2020;11(3):285–304. doi: 10.1080/19490976.2019.1592421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mafra D, Borges NA, Lindholm B, Stenvinkel P. Mitochondrial dysfunction and gut microbiota imbalance: an intriguing relationship in chronic kidney disease. Mitochondrion. 2019;47:206–209. doi: 10.1016/j.mito.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 64.Ma L, Zhang L, Li J, et al. The potential mechanism of gut microbiota-microbial metabolites-mitochondrial axis in progression of diabetic kidney disease. Mol Med. 2023;29(1):148. doi: 10.1186/s10020-023-00745-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szeto HH. Pharmacologic Approaches to Improve Mitochondrial Function in AKI and CKD. J Am Soc Nephrol. 2017;28(10):2856–2865. doi: 10.1681/asn.2017030247 [DOI] [PMC free article] [PubMed] [Google Scholar]